Abstract
Context: Mutations in the Kir6.2 subunit (KCNJ11) of the ATP-sensitive potassium channel (KATP) underlie neonatal diabetes mellitus. In severe cases, Kir6.2 mutations underlie developmental delay, epilepsy, and neonatal diabetes (DEND). All Kir6.2 mutations examined decrease the ATP inhibition of KATP, which is predicted to suppress electrical activity in neurons (peripheral and central), muscle, and pancreas. Inhibitory sulfonylureas (SUs) have been used successfully to treat diabetes in patients with activating Kir6.2 mutations. There are two reports of improved neurological features in SU-treated DEND patients but no report of such improvement in adulthood.
Objective: The objective of the study was to determine the molecular basis of intermediate DEND in a 27-yr-old patient with a KCNJ11 mutation (G53D) and the patient’s response to SU therapy.
Design: The G53D patient was transferred from insulin to gliclazide and then to glibenclamide over a 160-d period. Motor function was assessed throughout. Electrophysiology assessed the effect of the G53D mutation on KATP activity.
Results: The G53D patient demonstrated improved glycemic control and motor coordination with SU treatment, although glibenclamide was more effective than gliclazide. Reconstituted G53D channels exhibit reduced ATP sensitivity, which is predicted to suppress electrical activity in vivo. G53D channels coexpressed with SUR1 (the pancreatic and neuronal isoform) exhibit high-affinity block by gliclazide but are insensitive to block when coexpressed with SUR2A (the skeletal muscle isoform). High-affinity block by glibenclamide is present in G53D channels coexpressed with either SUR1 or SUR2A.
Conclusion: The results demonstrate that SUs can resolve motor dysfunction in an adult with intermediate DEND and that this improvement is due to inhibition of the neuronal but not skeletal muscle KATP.
Developmental delay, Epilepsy, and Neonatal Diabetes (DEND) is associated with mutations in the Kir6.2 subunit gene (KCNJ11) of the KATP channel, which causes suppression of electrical activity in neurons, muscle, and pancreas. While inhibitory sulfonylureas (SUs) have been used to successfully treat the neurological features in infants with DEND, there are no reports of such improvement in adulthood. This study of an adult with intermediate DEND demonstrates that SUs can resolve motor function, and that this improvement is due to inhibition of neuronal and not skeletal muscle KATP.
The ATP-sensitive K+-channel (KATP) regulates insulin secretion from the pancreatic β-cell by coupling changes in metabolism (represented by the concentration ratio of ATP to ADP) with changes in electrical activity. At low blood glucose levels, KATP channels provide the dominant membrane conductance in the pancreatic β-cell and maintain the cell in a hyperpolarized, nonsecreting state. However, as blood glucose levels rise, the intracellular ATP to ADP ratio increases, causing in succession, closure of KATP channels, membrane depolarization, voltage-driven influx of Ca2+, and Ca2+-dependent insulin release. Disruption of KATP activity is predicted to impair insulin release and result in inappropriate serum insulin levels at all blood glucose concentration.
It is now well established that KATP overactivity contributes to neonatal diabetes mellitus, a disease characterized by an insulin-requiring hyperglycemia diagnosed before 6 months of age (1,2). Specifically, heterozygous activating mutations in KATP (both the Kir6.2 and SUR1 subunits) are causal in permanent (PNDM) and transient (TNDM) forms of neonatal diabetes mellitus in humans (1,3,4,5,6,7,8,9,10). In approximately one third of the cases, KATP mutations are associated with developmental delay (both motor and intellectual), epilepsy, and neonatal diabetes (DEND syndrome), and extrapancreatic symptoms are likely the result of overactive KATP in muscle, peripheral nerves, and/or brain (11). In intermediate DEND the patient exhibits developmental delay and neonatal diabetes but not epilepsy, and in most cases, this form of DEND is associated with the V59M mutation. Significantly, sulfonylurea (SU) compounds that specifically inhibit KATP and thereby stimulate insulin secretion have proven a valuable alternative to insulin therapy in treating the hyperglycemia in patients with activating KATP mutations (12). To date, 91% of patients (68 of 75) have successfully switched from insulin to oral sulfonylureas with improvement or maintenance of glycemic control, although the success rate is lower in patients with DEND symptoms (77%; 10 of 14) (5,12,13,14,15). Notably, an infant and a 3-yr-old patient with DEND syndrome have exhibited improved motor features with SU treatment, in addition to resolution of the diabetes (13,14).
The mature KATP channel exists as a hetero-octomer of four pore-forming Kir6.2 subunits (KCNJ11) and four regulatory sulfonylurea receptors (SURs; e.g. ABCC8) (17,18,19). The Kir6.2 subunit contains the binding site for inhibitory ATP (20,21), whereas the SUR subunit confers channel stimulation by Mg nucleotides (22) and inhibition to SU drugs (23). With respect to SU block, an added level complexity arises from the existence of multiple SUR isoforms (SUR1, SUR2A, and SUR2B), which exhibit differential tissue expression and SU sensitivities (24). The skeletal muscle KATP (Kir6.2 + SUR2A) channel, for example, is less sensitive to both glibenclamide and gliclazide block than the pancreatic/neuronal KATP (Kir6.2 + SUR1) (24,25). High-affinity gliclazide block is absent in Kir6.2+SUR2A channels, whereas high-affinity glibenclamide block is reduced approximately 3-fold relative to Kir6.2+SUR1 channels.
In the present study, we describe a patient with intermediate DEND who at 27 yr of age was found to carry a heterozygous mutation in Kir6.2 (G53D). The patient was weaned from insulin onto gliclazide and then glibenclamide, resulting in good glycemic control, although gliclazide treatment required supplemental insulin. Importantly, motor features were improved on both SUs as assessed by increased muscle coordination and an enhanced gait. Mechanistically, the G53D mutation causes severe loss of ATP sensitivity of the KATP channel, which is predicted to suppress insulin secretion by the pancreatic β-cell by preventing electrical activity, and calcium influx. Importantly, the G53D mutation does not appreciably alter SU sensitivity of reconstituted channels, compared with wild-type KATP. When coexpressed with the skeletal muscle SUR2A isoform, channels are predictably insensitive to gliclazide but exhibit high-affinity gliclazide block when coexpressed with the pancreatic and neuronal SUR1 isoform. Conversely, glibenclamide inhibits both skeletal muscle KATP and neuronal/pancreatic KATP similarly. Taken together, these data indicate that an adult (intermediate) DEND patient with an activating Kir6.2 mutation can still benefit from SU therapy both in terms of glycemic control and improved motor function. Moreover, the combined functional and clinical data suggest that improved motor function is due to inhibition of KATP in central nerves and not the skeletal muscle KATP.
Patient and Methods
Genetics and molecular biology
Total genomic DNA was isolated from nonnucleated, whole blood using the DNeasy tissue isolation kit (QIAGEN, Valencia, CA). The intronless KCNJ11 gene was amplified in three overlapping fragments by the PCR and directly sequenced as described previously (4). The identified G53D mutation was engineered into Kir6.2 cDNA of pCMV6B using the Quikchange site-directed mutagenesis kit (Stratagene, La Jolla, CA), and confirmed by direct sequencing.
Clinical studies: recoding of neurological examination before and after transfer from insulin to SUs
Upon detection of a KCNJ11 mutation (G53D), an attempt was made to transfer the patient from insulin therapy to oral treatment with SU with close monitoring of metabolic control. With the aim of documenting any change in motor performance induced by SU(s), three movies of a standard neurological examination were recorded: 1) during insulin therapy (basal), 2) at the end of 20 d of therapy with high doses of gliclazide (720 mg/d) (first oral treatment), and 3) at the end of 25 d with high doses of glibenclimide (45 mg/d) (second oral treatment) (Fig. 1A). There was no washout between SU treatments. Informed consent was obtained from the patient.
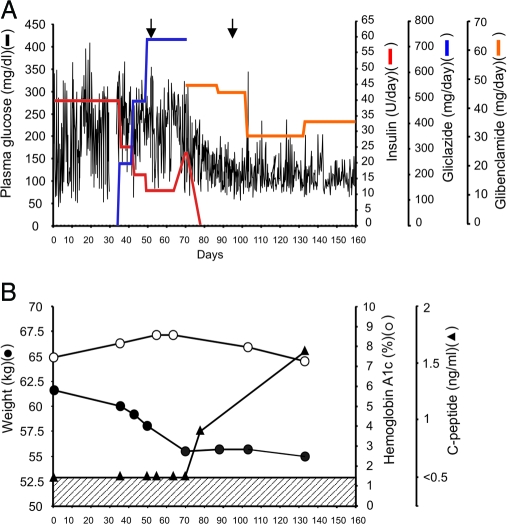
Figure 1.
SU trial and assessment in a patient with a heterozygous KCNJ11 mutation G53D. A, Blood glucose values (black lines) vs. insulin dose (red lines), vs. gliclazide dose (blue lines), and vs. glibenclamide dose (orange line) measured daily over a period of 160 d in the G53D patient. The insulin dose was progressively decreased from 40 U/d to 11 U/d during gliclazide, but it was raised again to 23 U/d immediately before commencement of glibenclamide therapy. Arrows denote days in which motor function testing was documented on video as presented in supplemental Figs. S1 and S2. B, Measurements of weight (closed circle), hemoglobin A1c levels (open circle), and C-peptide levels (closed triangle) on days indicated over the same time period as in B. C-peptide is undetectable below a concentration of 0.8 ng/ml as indicated by hatched region.
Expression of wild-type and mutant KATP channels in COSm6 cells
COSm6 cells were plated at a density of approximately 2.5 × 105 cells/well (30 mm 6 well dishes) and cultured in DMEM plus 10 mm glucose, supplemented with fetal calf serum (10%). COSm6 cells were transfected with cDNA using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN). Total DNA [0.3 μg of Kir6.2(WT) or Kir6.2(G53D) plus 0.5 μg of SUR1(WT) or SUR2A(WT) plus 0.3 μg green fluorescent protein as a marker for transfection] was mixed with FuGENE 6 and preincubated for 1 h. Cells were incubated in the presence of the transfection mixture for 12–24 h and then plated on sterile glass coverslips overnight before patch-clamp experiments.
Electrophysiological methods
Patch-clamp experiments were performed at room temperature in an oil-gate chamber that allowed the solution bathing the exposed surface of the isolated patch to be changed rapidly. COSm6 cells that fluoresced green under UV illumination were selected for patch clamping 3–5 d after transfection. Membrane patches were voltage clamped using a CV-4 headstage, an Axopatch 1-D amplifier, a Digidata 1322A digitizer board (all from Axon Instruments, Union City, CA), and a MP-225 micromanipulator (Sutter Instrument Co., Novato, CA). All currents were measured at a membrane potential of −50 mV (pipette voltage = +50 mV). Data were collected using the pClamp 8.2 software suite (Axon Instruments) and Microsoft Excel (Redmond, WA). Bath and pipette control solutions (KINT) contained (in millimoles): 150 KCl, 10 HEPES, and 1 EGTA (pH 7.4). Where indicated, ATP was added to the bathing solution as dipotassium salts. Gliclazide was dissolved in KINT from a 100 mm stock solution in 100 mm KOH.
Macroscopic 86Rb+ efflux assays
COSm6 cells in 12-well plates were incubated for 24 h in culture medium containing 86RbCl (1 μCi/ml) 2 d after transfection. Before measurement of 86Rb+ efflux, cells were washed twice with Ringer’s solution (basal) [in millimoles: 118 NaCl, 2.5 CaCl2, 1.2 KH2PO4, 4.7 KCl, 25 NaHCO3, 1.2 MgSO4, 10 HEPES (pH 7.4)] or Ringer’s solution plus metabolic inhibition (1 mm 2-deoxy-d-glucose and 2.5 μg/ml oligomycin). At selected time points, the solution was collected from the cells and replaced with fresh solution; after completion of the assay, cells were lysed with 1% sodium dodecyl sulfate and removed. Collected samples were assayed in a scintillation solution. Raw data are shown as percent 86Rb+ efflux relative to total counts (including counts from all time points and the lysate).
The rate constant of KATP-specific 86Rb+ efflux (k2) was obtained by fitting the data with a single-exponential equation:
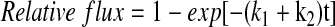 |
1 |
where the apparent rate constant for nonspecific efflux (k1) was obtained from untransfected cells. As indicated, glibenclamide was added to Ringer’s solution plus metabolic inhibition from a 1 mm stock solution in dimethylsulfoxide. For analysis of glibenclamide sensitivity, raw data were fit with a single Hill plot (equation 2 below) with zero flux defined as flux obtained in 1 μm glibenclamide.
Results are presented as mean ± sem. Statistical tests and P values are noted in figure legends where appropriate.
Quantitative analysis of ATP and SU inhibition
The ATP dose response was quantified by fitting the raw data with a Hill equation:
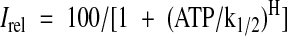 |
2 |
where Irel is the current relative to that in the absence of ATP, ATP is the ATP concentration, k1/2 is the half-maximal inhibitory ATP concentration, and H is the Hill coefficient, which was fixed at 1.3. Dose-response curves for gliclazide inhibition were described by the sum of two Hill equations (reflecting two components of inhibition) (26):
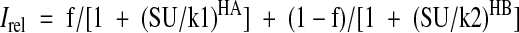 |
3 |
where f is the fraction of high-affinity block, SU is the SU concentration, k1 and k2 are the half-maximal concentrations of high- and low-affinity block, respectively, and H is the Hill coefficient for either the high-affinity (HA) or low-affinity block (HB).
Results
Genotyping and clinical description of G53D patient
In the present study, the female patient was born at 41 wk after an uneventful pregnancy (but intraunterine developmental delay as recorded by ultrasound), with a birth weight of 2550 g (less than the third centile). Thirty-six days after birth, the patient was transferred to the local neonatal intensive care unit because of failure to thrive. She presented with dehydration, hyperglycemia (28.7 mm),but no ketoacidosis (pH 7.34). Therapy was started with regular insulin at the dose of 0.9 U/kg·d and then reduced to 0.65 U/kg·d. Type 1 diabetes autoantibodies were not assessed at the time of diabetes diagnosis, although both islet cell antibodies and glutamic acid decarboxylase autoantibodies tests were negative 10 yr after the onset of the disease. At physical examination a mild dysmorphic feature (ocular hypertelorism) was present. A mild developmental delay was noted because she was unable to sit unaided at the age of 6–7 months. The patient began speaking at the age of 20 months and attended public school between the ages of 6 and 13 yr with a backup teacher. Patient growth was normal, with menarche at 12 yr of age. As an adult of 27 yr of age (supplemental video S1, left panel, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org), she exhibited a slow gait and performed poorly on numerous coordination tests (walking on heels or toes, walking on a single line, hopping on one leg, and touching finger to nose). All physical observations were indicative of a mild ataxia. Sequencing of the patient’s KCNJ11 (Kir6.2) gene revealed a heterozygous mutation, G53D. DNA samples were not available from either parent; however, neither parent was diabetic, which would indicate that the G53D mutation arose de novo. The G53D mutation has previously been reported in a patient with intermediate DEND, which included seizures during infancy and an abnormal electroencephalogram (9).
Improved motor features after transfer from insulin to SU therapy
After the genotyping the G53D patient was progressively transferred from insulin (0.65 UI/kg·d) to the SU gliclazide and then to glibenclamide (Fig. 1A). Gliclazide therapy had only a slight effect on glycemic control, although the insulin requirement was progressively reduced from 40 U/d to 11 U/d during the treatment period. Thus, despite a maximum dose of gliclazide (720 mg/d), insulin therapy could not be discontinued. In contrast, excellent glycemic control with complete weaning from insulin was attained with glibenclamide therapy (30 mg/d). During this period, hemoglobin A1c values progressively decreased from 8.4% (d 36) to the current level of 5.5% (d 370). C-peptide levels were undetectable during gliclazide therapy (lower limit of detection ≥ 0.1 ng/ml), and patient lost body weight (from 61.6 to 56.2 kg; Fig. 1B). Serum C-peptide became detectable on glibenclamide therapy with no further loss of body weight. Importantly, improved motor function, as confirmed by a neurologist, was observed when the G53D patient was switched from insulin (40 U/d) to gliclazide (after 20 d on 720 mg/d) [supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org; compare left video panel (before) to right video panel (after) gliclazide treatment]. A minor further improvement over gliclazide was observed after switching to glibenclamide (after 25 d on 25 mg/d) [supplemental Fig. S2, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org; compare left panel (gliclazide) with right panel (glibenclamide)].
Increased on-cell activity of G53D channels correlates with a reduced ATP sensitivity
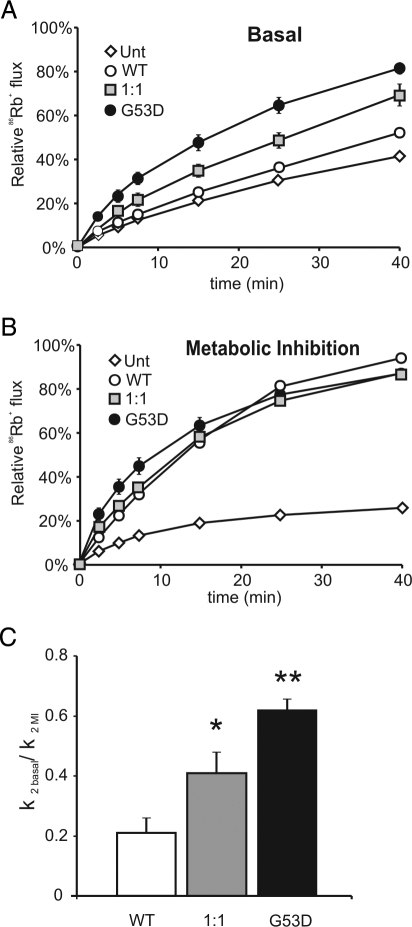
Homology modeling with known K+-channel structures places the G53 residue in the cytoplasmic N terminus of Kir6.2 but outside the ATP-binding pocket itself (27). Residue G53 resides in the slide-helix region of the channel that is implicated in channel gating. To examine the molecular consequences of the G53D mutation, a mutant Kir6.2 subunit containing the G53D mutation was coexpressed with the wild-type SUR1 subunit, and channel activity in intact cells was screened by 86Rb+-efflux. As shown in Fig. 2, A and B, efflux from cells transfected with wild-type KATP channels was comparatively low under basal conditions but was significantly activated by metabolic inhibition (with oligomycin and 2-deoxyglucose to lower cellular ATP to ADP ratio). By contrast, Rb+-efflux from cells transfected with mutant G53D subunits was already elevated under basal conditions and, by comparison with wild-type, only modestly increased with metabolic inhibition. Heteromeric G53D channels were generated by transfecting with 1:1 mixtures of WT Kir6.2 and G53D cDNAs. This approach is expected to recapitulate the properties of native channels from PNDM patients with heterozygous Kir6.2 mutations. Heteromeric G53D channels showed an intermediate basal flux between wild-type and homomeric G53D channels. Quantitative estimates of KATP conductances (see Patient and Methods) confirm significantly higher basal conductances (relative to maximal conductances obtained during metabolic inhibition) for both homomeric and heteromeric G53D channels, compared with wild-type channels (Fig. 2C). In the pancreatic β-cell, the increased basal flux is expected to suppress electrical activity and reduce insulin secretion and can account for the diabetes in the G53D patient.
Figure 2.
Increased basal activity in intact cells expressing KATP (G53D) channels. Relative efflux of 86Rb+ as a function of time in basal conditions (A) or in the presence of metabolic inhibition (B) for reconstituted wild-type (WT) and mutant KATP channels, and untransfected controls (Unt). All transfections were done in parallel. Graphs show compiled data (mean ± sem) from four to six experiments. C, Rate constants for KATP-dependent efflux (k2) in basal conditions relative to metabolic inhibition (MI) for WT, heteromeric, or homomeric KATP channels. Data were fit with a double-exponential equation to obtain rate constants for KATP-dependent efflux, k2 (see Patient and Methods). *, P < 0.05 and **, P < 0.001, compared with wild-type KATP by unpaired Student’s t test.
To examine the correlation between increased KATP activity in intact cells and ATP sensitivity, the latter was measured directly in excised membrane patches (Fig. 3, A–C). Both heteromeric (G53D + WT) and homomeric (G53D) channels coexpressed with SUR1 exhibited a significant decrease in ATP inhibition, between 5- and 20-fold, respectively, compared with wild-type channels [K1/2,ATP = 9.3 μm (WT, n = 25 patches), 46.6 μm (G53D: WT, n = 4 patches), 189.8 μm (G53D, n = 15 patches)] (Fig. 3D). The average KATP current per membrane patch was not significantly different between homomeric WT and G53D patches (WT= 601 ± 201 pA/patch; G53D = 507 ± 175 pA/patch; n = 18).
Figure 3.
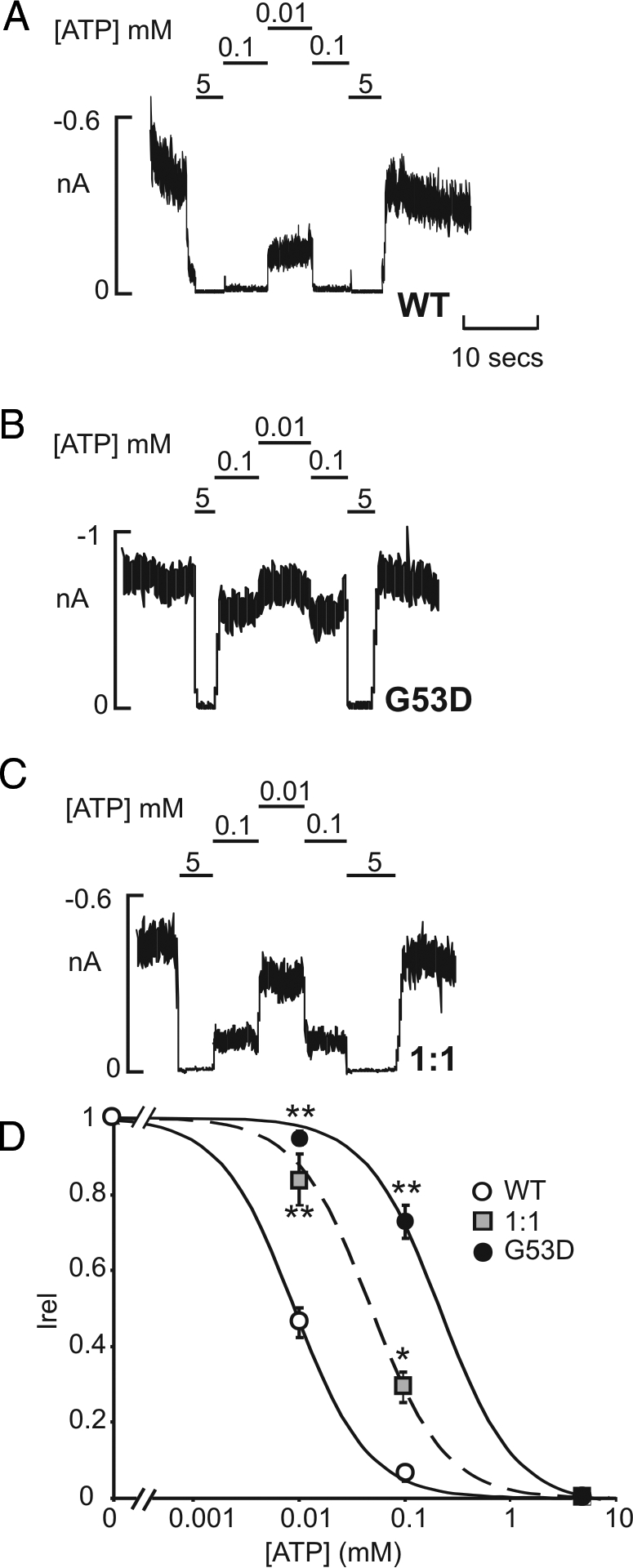
Reduced ATP sensitivity of reconstituted homomeric and heteromeric KATP channels containing G53D subunits. Representative currents recorded from inside-out membrane patches from COS cells expressing wild-type [Kir6.(WT) + SUR1] (A), homomeric [Kir6.(G53D) + SUR1] (B), or heteromeric [Kir6.(G53D) + Kir6.2(WT) + SUR1] (C) KATP channels at −50 mV in Kint solution (see Patient and Methods). Patches were exposed to differing ATP, and baseline current was determined by exposure to ATP (5 mm). D, Steady-state dependence of membrane current on ATP [relative to current in zero ATP (Irel)] for wild-type and G53D-containing channels. Data points represent the mean ± sem (n = 4–9 patches). The fitted lines correspond to least squares fits of a Hill equation (see Patient and Methods). *, P < 0.01 and **, P < 0.005, compared with wild-type KATP by unpaired Student’s t test.
The G53D mutation does not alter SU sensitivity
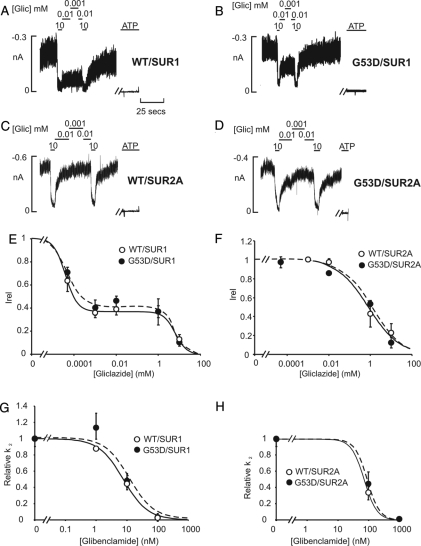
Good glycemic control and improved motor function was observed when the G53D patient was switched from insulin to gliclazide or glibenclamide. However, Kir6.2 mutations can significantly alter SU sensitivity (10,28). To examine the effect of the G53D mutation on SU sensitivity and to determine whether the improved motor function is likely to be neuronal (Kir6.2 + SUR1) or skeletal muscle (Kir6.2 + SUR2A) in origin, membrane patches from transfected cells were exposed to varying concentration of gliclazide. Consistent with previous reports, Kir6.2 + SUR2A channels do not exhibit high-affinity gliclazide block, whereas Kir6.2 + SUR1 channels exhibit both high and low affinities of inhibition (Fig. 4, A, C, and E) (25). Importantly, the gliclazide sensitivities are not altered in G53D-containing channels (Fig. 4, B, D, and F). Similarly, the G53D mutation does not alter glibenclamide sensitivities, compared with wild-type channels (Fig. 4, G and H). In this case both SUR1- and SUR2A-containing channels are inhibited similarly by the drug. Taken together, these results suggest that improved motor function in the G53D patient is the result of inhibition of the SUR1-based neuronal KATP rather than the SUR2A-based skeletal muscle KATP.
Figure 4.
Unaltered SU sensitivity of KATP currents from COS cells expressing G53D-containing channels. A, Representative currents recorded from inside-out membrane patches from COS cells expressing Kir6.2 WT or G53D, coexpressed with either SUR1 (A and B) or SUR2A (C and D) at −50 mV. Patches were exposed to differing gliclazide, and baseline current was determined by addition of either ATP (5 mm). E and F, Steady-state dependence of membrane current on gliclazide [relative to current in zero tolbutamide (Irel)] for wild-type (WT) and mutant G53D channels. Data points represent the mean ± sem (n = 3–7 patches). The fitted lines correspond to least-square fits of a double-Hill equation (see Patient and Methods). For WT + SUR1 channels: the half-inhibitory concentration of the high-affinity site (Ki1/2, high) = 4.1 μm and Hill coefficient of high-affinity site (HA) = 1.5, and the low-affinity site (Ki1/2, low) = 6.8 mm and Hill coefficient of low-affinity site (HB) = 1.5, the fraction of the high-affinity site (f) = 0.63. For G53D + SUR1 channels: Ki1/2, high = 5 μm, and HA = 1.2, Ki1/2, low = 6.6 mm, and HB = 2, and f = 0.60. For WT + SUR2A channels: Ki1/2, low = 0.8 mm, and H = 0.6. For G53D + SUR2A channels: Ki1/2, low = 1.2 mm, and H = 0.6. G and H, Steady-state dependence of 86Rb+ efflux on glibenclamide for WT and G53D channels coexpressed with either SUR1 (G) or SUR2A (H) in metabolic inhibition (oligomycin and 2-deoxyglucose). Intact cells were exposed to differing glibenclamides, and baseline current was calculated in 1 μm glibenclamide. This concentration of glibenclamide saturates the high-affinity component of block (28). Data points represent the mean ± sem (n = 3–5 experiments). Data were fit with a Hill equation (see Patient and Methods). For WT + SUR1: Ki1/2 = 7.6 nm, and H = 1.2; G53D + SUR1: Ki1/2 = 11.1 nm, and H = 1.1. For WT + SUR2A: Ki1/2 = 73.2 nm, and H = 2.1; G53D + SUR2A: Ki1/2 = 92.3 nm, and H = 2.2.
Discussion
Improvement of neurological features in the G53D patient with SU therapy
In the present study, we identified a Kir6.2 mutation (G53D) in an adult patient with intermediate DEND that is responsive to SU treatment (gliclazide and glibenclamide), although with different efficacies of the two drugs in terms of metabolic control (glibenclamide more effective than glicalzide). When switched to SUs, the patient exhibited a significant improvement in muscle tone, coordination, and gait, as assessed by a neurologist and documented in the supplemental videos (videos S1 and S2). This improvement is most likely a consequence of SU therapy as it coincided with the transfer to SUs and has been maintained up to 12 months. Over this same period, a significant improvement in hemoglobin A1c levels was observed (8% on d 36, 7.1% on d 133, and 5.5% on d 370). Whereas we cannot preclude a contribution from the reduced hypoglycemic episodes, the most likely explanation for the improved neurological features is that the SUs are acting on KATP in neurons and/or muscle. In the skeletal muscle, KATP is composed of Kir6.2 and almost exclusively the SUR2A isoform (23,30,31). In contrast, neuronal KATP (central and peripheral) is comprised primarily of the Kir6.2 and SUR1 isoforms (32,33). As shown above, skeletal muscle KATP channels are insensitive to gliclazide block at therapeutic concentrations (peak concentration = 0.2–0.6 μm, free gliclazide in serum) (34), whereas neuronal channels are highly gliclazide sensitive. These results suggest that the improved motor features result from inhibition of neuronal and not skeletal muscle KATP.
The successful transfer to sulfonylureas in our G53D patient is consistent with previous clinical reports of patients with the common V59M mutation. To date all patients (12 of 12) tested with intermediate DEND due to V59M mutation have been successfully switched to SUs with good glycemic control (5,12,15). In one infant (28 months) with the V59M mutation, improved motor features was also noted, in addition to resolution of the diabetes (13). Conversely, a 9-yr-old patient with a V59M mutation did not show amelioration of motor dysfunction (15). Thus, improvement of motor features does necessarily accompany the improved glycemic control in intermediate DEND patients who undergo SU therapy.
Theoretically inhibition of overactive KATP in the presynaptic neurons could restore neurotransmitter release and underlie the improved motor function observed in the G53D patient. The SUs could be targeting KATP in peripheral neurons and/or cross the blood brain-barrier and act on KATP in the central neurons of the brain. However, the observations that the patient does not exhibit muscle weakness and that the mild ataxia is improved with SU therapy are consistent with SU action in the brain. In rodent studies, SUs have been shown to accumulate in the brain but at subtherapeutic concentrations (36). Consistent with our conclusion that overactive KATP in the neurons underlies the motor features of the DEND patient, a recent report has described a DEND phenotype in a patient with an activating mutation in the SUR1 isoform, which is expressed in neurons, but not muscle (37).
A potential role for skeletal muscle KATP in peripheral insulin sensitivity
As has been observed previously for PNDM patients with Kir6.2 mutations, significantly higher doses of SUs were required to treat the diabetes of the G53D patient than are typically used to treat type 2 diabetes in adults (12). This anomaly may reflect, in part, the fact that SU block is less efficient when nucleotide inhibition on Kir6.2 is decreased (26,38). In the case of gliclazide, the patient could not be weaned completely from insulin despite a high therapeutic dose of the drug (720 mg/d, or three times the maximal dose used in type 2 diabetes). Conversely, insulin was successfully discontinued on subsequent transfer to glibenclamide (45 mg/d progressively reduced to 35 mg/d, approximately twice the maximal dose used to treat type 2 diabetes). Because a washout period between the two drugs was not performed, a residual effect of gliclazide (half-life ∼10 h) on glibenclamide therapy cannot be excluded. However, we believe that this is unlikely as insulin was discontinued after 6 d of glibenclamide treatment, a point at which gliclazide is completely cleared from the plasma. Because the G53D mutation does not alter SU sensitivities in intact cells (Fig. 4, G and H), the dosing discrepancy may reflect differences in the pharmacokinetics of the two drugs with respect to bioavailability and/or relative efficacy. Indeed, measurable levels of C-peptide observed with glibenclamide, but not gliclazide, is consistent with a greater insulinotropic effect of the former.
Alternatively, it is intriguing to speculate that the drug differences may also reflect the negative consequence of increased skeletal muscle KATP activity on whole-body glucose disposal. Increasing evidence implicates a role for the skeletal muscle KATP (Kir6.2 and SUR2A) in glucose uptake. An increase in glucose uptake was recently reported in isolated skeletal muscle and adipose tissue from Kir6.2 and SUR2A null mice (31,39), and inhibitory SUs have previously been shown to directly increase both glucose uptake and peripheral insulin sensitivity (16,40). These data implicate an antagonistic association between skeletal muscle KATP activity and insulin sensitivity. The prediction is that KATP overactivity in skeletal muscle, expected in neonatal diabetes mellitus patients with activating Kir6.2 mutations, may actually reduce insulin sensitivity and contribute to the diabetes. The better metabolic control observed with glibenclamide vs. gliclazide in the G53D patient may reflect the specific ability of glibenclamide, but not gliclazide, to inhibit skeletal muscle KATP, (Fig. 4) and, improve insulin sensitivity. Currently it is unknown whether peripheral insulin insensitivity compounds the primary β-cell defect in carriers of activating Kir6.2 mutations. Interestingly, a recent control-based study demonstrated impaired insulin sensitivity in four, nonobese PNDM patients with the activating R201H mutation (29,35). If peripheral insulin insensitivity is indeed a common feature of KATP-induced diabetes, this could have significant clinical implications and alter pharmacological strategies to treat the diabetes both in terms of the type of sulfonylurea used and the dosing.
Correlating Kir6.2 overactvity with disease severity
Previous studies have demonstrated that other mutations of the G53 residue in Kir6.2 (G53S, G53R) decrease ATP sensitivity and underlie TNDM (7). In the current study, we described functional properties of the DEND-causing G53D mutation. ATP sensitivity is decreased approximately 20-fold in G53D homomeric channels, compared with an approximately 5-fold decrease previously reported for both the TNDM-associated G53S and G53R mutations (7). These data are consistent with the premise that the greater the channel overactivity, the greater the suppression of glucose-sensing by the pancreatic β-cell, and the more severe the diabetes (PNDM/DEND vs. TNDM). Theoretically, all Kir6.2 activating mutations should underlie a diabetic phenotype; in general, however, only the more severe activating mutations are associated with DEND symptoms (although extrapancreatic tissues might respond before the pancreas). This suggests that extrapancreatic tissues (muscle, nerve, and brain) are less sensitive to changes in KATP activity than the pancreatic β-cell and that a threshold of channel overactivity must be reached before neurological features become evident.
Conclusion
The present study highlights the potential added benefit of treating DEND patients with SUs and mechanistically attributes this improved motor function with targeting of the skeletal muscle KATP. There are a few other reports of improved neurological features in DEND patients with activating Kir6.2 mutations and no report of such improvement in an adult. Future clinical studies are required to evaluate the effectiveness of different sulfonylureas to treat neurological features in both adult and infant populations with DEND syndrome due to Kir6.2 mutations.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grant DK069445 (to C.G.N.) and National Institutes of Health Diabetes Research and Training Grant DK-20579. F.B. and F.C. are members of the Early Onset Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetology.
Disclosure Information: All authors have nothing to declare.
First Published Online December 11, 2007
Abbreviations: DEND, Developmental delay, epilepsy, and neonatal diabetes; HA, high-affinity site; HB, low-affinity site; k1, nonspecific efflux; k2, 86Rb+ efflux; KATP, ATP-sensitive potassium channel; KINT, control solution; PNDM, permanent diabetes mellitus; SU, sulfonylurea; SUR, sulfonylurea receptor; TNDM, transient neonatal diabetes mellitus.
References
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, Frayling TM, Temple IK, Mackay D, Shield JP, Sumnik Z, van Rhijn A, Wales JK, Clark P, Gorman S, Aisenberg J, Ellard S, Njolstad PR, Ashcroft FM, Hattersley AT 2004 Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 350:1838–1849 [DOI] [PubMed] [Google Scholar]
- Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG 2000 Targeted overactivity of ββ cell K(ATP) channels induces profound neonatal diabetes. Cell 100:645–654 [DOI] [PubMed] [Google Scholar]
- Zung A, Glaser B, Nimri R, Zadik Z 2004 Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J Clin Endocrinol Metab 89:5504–5507 [DOI] [PubMed] [Google Scholar]
- Massa O, Iafusco D, D’Amato E, Gloyn AL, Hattersley AT, Pasquino B, Tonini G, Dammacco F, Zanette G, Meschi F, Porzio O, Bottazzo G, Crino A, Lorini R, Cerutti F, Vanelli M, Barbetti F 2005 KCNJ11 activating mutations in Italian patients with permanent neonatal diabetes. Hum Mutat 25:22–27 [DOI] [PubMed] [Google Scholar]
- Sagen JV, Raeder H, Hathout E, Shehadeh N, Gudmundsson K, Baevre H, Abuelo D, Phornphutkul C, Molnes J, Bell GI, Gloyn AL, Hattersley AT, Molven A, Sovik O, Njolstad PR 2004 Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: patient characteristics and initial response to sulfonylurea therapy. Diabetes 53:2713–2718 [DOI] [PubMed] [Google Scholar]
- Vaxillaire M, Populaire C, Busiah K, Cave H, Gloyn AL, Hattersley AT, Czernichow P, Froguel P, Polak M 2004 Kir6.2 mutations are a common cause of permanent neonatal diabetes in a large cohort of French patients. Diabetes 53:2719–2722 [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, Temple IK, Mackay DJ, Shield JP, Freedenberg D, Noyes K, Ellard S, Ashcroft FM, Gribble FM, Hattersley AT 2005 Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet 14:925–934 [DOI] [PubMed] [Google Scholar]
- Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, Scharfmann R, Bryan J, Aguilar-Bryan L, Vaxillaire M, Froguel P 2006 Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med 355:456–466 [DOI] [PubMed] [Google Scholar]
- Flanagan SE, Edghill EL, Gloyn AL, Ellard S, Hattersley AT 2006 Mutations in KCNJ11, which encodes Kir6.2, are a common cause of diabetes diagnosed in the first 6 months of life, with the phenotype determined by genotype. Diabetologia 49:1190–1197 [DOI] [PubMed] [Google Scholar]
- Koster JC, Remedi MS, Dao C, Nichols CG 2005 ATP and sulfonylurea sensitivity of mutant ATP-sensitive K+ channels in neonatal diabetes: implications for pharmacogenomic therapy. Diabetes 54:2645–2654 [DOI] [PubMed] [Google Scholar]
- Slingerland AS, Hattersley AT 2005 Mutations in the Kir6.2 subunit of the KATP channel and permanent neonatal diabetes: new insights and new treatment. Ann Med 37:186–195 [DOI] [PubMed] [Google Scholar]
- Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Sovik O, Polak M, Hattersley AT 2006 Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med 355:467–477 [DOI] [PubMed] [Google Scholar]
- Slingerland AS, Nuboer R, Hadders-Algra M, Hattersley AT, Bruining GJ 2006 Improved motor development and good long-term glycaemic control with sulfonylurea treatment in a patient with the syndrome of intermediate developmental delay, early-onset generalised epilepsy and neonatal diabetes associated with the V59M mutation in the KCNJ11 gene. Diabetologia 49:2559–2563 [DOI] [PubMed] [Google Scholar]
- Shimomura K, Horster F, Wet HD, Flanagan SE, Ellard S, Hattersley AT, Wolf NI, Ashcroft F, Ebinger F 2007 A novel mutation causing DEND syndrome. Neurology 69:1342–1349 [DOI] [PubMed] [Google Scholar]
- Tonini G, Bizzarri C, Bonfanti R, Vanelli M, Cerutti F, Faleschini E, Meschi F, Prisco F, Ciacco E, Cappa M, Torelli C, Cauvin V, Tumini S, Iafusco D, Barbetti F 2006 Sulfonylurea treatment outweighs insulin therapy in short-term metabolic control of patients with permanent neonatal diabetes mellitus due to activating mutations of the KCNJ11 (KIR6.2) gene. Diabetologia 49:2210–2213 [DOI] [PubMed] [Google Scholar]
- Pulido N, Romero R, Suarez AI, Rodriguez E, Casanova B, Rovira A 1996 Sulfonylureas stimulate glucose uptake through GLUT4 transporter translocation in rat skeletal muscle. Biochem Biophys Res Commun 228:499–504 [DOI] [PubMed] [Google Scholar]
- Shyng S, Nichols CG 1997 Octameric stoichiometry of the KATP channel complex. J Gen Physiol 110:655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement JPT, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J 1997 Association and stoichiometry of K(ATP) channel subunits. Neuron 18:827–838 [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Seino S 1997 Subunit stoichiometry of the pancreatic β-cell ATP-sensitive K+ channel. FEBS Lett 409:232–236 [DOI] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM 1997 Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature 387:179–183 [DOI] [PubMed] [Google Scholar]
- Tanabe K, Tucker SJ, Matsuo M, Proks P, Ashcroft FM, Seino S, Amachi T, Ueda K 1999 Direct photoaffinity labeling of the Kir6.2 subunit of the ATP-sensitive K+ channel by 8-azido-ATP. J Biol Chem 274:3931–3933 [DOI] [PubMed] [Google Scholar]
- Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, Gonzalez G, Aguilarbryan L, Permutt MA, Bryan J 1996 Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272:1785–1787 [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S 1996 A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron 16:1011–1017 [DOI] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Seino S, Ashcroft FM 1998 Tissue specificity of sulfonylureas: studies on cloned cardiac and beta-cell K(ATP) channels. Diabetes 47:1412–1418 [DOI] [PubMed] [Google Scholar]
- Gribble FM, Ashcroft FM 1999 Differential sensitivity of β-cell and extrapancreatic K(ATP) channels to gliclazide. Diabetologia 42:845–848 [DOI] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Ashcroft FM 1997 The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J Physiol 504:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antcliff JF, Haider S, Proks P, Sansom MS, Ashcroft FM 2005 Functional analysis of a structural model of the ATP-binding site of the KATP channel Kir6.2 subunit. EMBO J 24:229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P, Antcliff JF, Lippiat J, Gloyn AL, Hattersley AT, Ashcroft FM 2004 Molecular basis of Kir6.2 mutations associated with neonatal diabetes plus neurological features. Proc Natl Acad Sci USA 101:17539–17544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skupien J, Malecki MT, Mlynarski W, Klupa T, Wanic K, Gach A, Solecka I, Sieradzki J 2006 Assessment of insulin sensitivity in adults with permanent neonatal diabetes mellitus due to mutations in the KCNJ11 gene encoding Kir6.2. Rev Diabet Stud 3:17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico D, Mele A, Lundquist AL, Desai RR, George AL, Camerino DC 2006 Hybrid assemblies of ATP-sensitive K+ channels determine their muscle-type-dependent biophysical and pharmacological properties. Proc Natl Acad Sci USA 103:1118–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, Burant CF 2001 Disruption of Sur2-containing KATP channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci USA 98:11760–11764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GA, Hudson AL, Henderson G 1994 Localization of high affinity [3H]glibenclamide binding sites within the substantia nigra zona reticulata of the rat brain. Neuroscience 61:285–292 [DOI] [PubMed] [Google Scholar]
- Liss B, Bruns R, Roeper J1999 Alternative sulfonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurons. EMBO J 18:833–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Kimura M, Sakoguchi T, Hase A, Matsuoka A, Kaneko S 1984 Pharmacokinetics of gliclazide in healthy and diabetic subjects. J Pharm Sci 73:1684–1687 [DOI] [PubMed] [Google Scholar]
- Malecki MT, Skupien J, Klupa T, Wanic K, Mlynarski W, Gach A, Solecka I, Sieradzki J2007 Transfer to sulphonylurea therapy in adult subjects with permanent neonatal diabetes due to KCNJ11-activating [corrected] mutations: evidence for improvement in insulin sensitivity. Diabetes Care 30:147–149 [DOI] [PubMed] [Google Scholar]
- Takanaga H, Murakami H, Koyabu N, Matsuo H, Naito M, Tsuruo T, Sawada Y 1998 Efflux transport of tolbutamide across the blood-brain barrier. J Pharm Pharmacol 50:1027–1033 [DOI] [PubMed] [Google Scholar]
- Proks P, Arnold AL, Bruining J, Girard C, Flanagan SE, Larkin B, Colclough K, Hattersley AT, Ashcroft FM, Ellard S 2006 A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet 15: 1793–1800 [DOI] [PubMed] [Google Scholar]
- Masia R, Koster JC, Tumini S, Chiarelli F, Colombo C, Nichols CG, Barbetti F 2007 An ATP-binding mutation (G334D) in KCNJ11 is associated with a sulfonylurea-insensitive form of developmental delay, epilepsy, and neonatal diabetes. Diabetes 56:328–336 [DOI] [PubMed] [Google Scholar]
- Miki T, Minami K, Zhang L, Morita M, Gonoi T, Shiuchi T, Minokoshi Y, Renaud JM, Seino S 2002 ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 283:1178–1184 [DOI] [PubMed] [Google Scholar]
- Pulido N, Casla A, Suarez A, Casanova B, Arrieta FJ, Rovira A 1996 Sulphonylurea stimulates glucose uptake in rats through an ATP-sensitive K+ channel dependent mechanism. Diabetologia 39:22–27 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.