Abstract
Context: TSH is a known thyroid growth factor, but the pathogenic role of TSH in thyroid oncogenesis is unclear.
Objective: The aim was to examine the relationship between preoperative TSH and differentiated thyroid cancer (DTC).
Design: The design was a retrospective cohort.
Setting, Participants: Between May 1994 and January 2007, 1198 patients underwent thyroid surgery at a single hospital. Data from the 843 patients with preoperative serum TSH concentration were recorded.
Main Outcome Measures: Serum TSH concentration was measured with a sensitive assay. Diagnoses of DTC vs. benign thyroid disease were based on surgical pathology reports.
Results: Twenty-nine percent of patients (241 of 843) had DTC on final pathology. On both univariate and multivariable analyses, risk of malignancy correlated with higher TSH level (P = 0.007). The likelihood of malignancy was 16% (nine of 55) when TSH was less than 0.06 mIU/liter vs. 52% (15 of 29) when 5.00 mIU/liter or greater (P = 0.001). When TSH was between 0.40 and 1.39 mIU/liter, the likelihood of malignancy was 25% (85 of 347) vs. 35% (109 of 308) when TSH was between 1.40 and 4.99 mIU/liter (P = 0.002). The mean TSH was 4.9 ± 1.5 mIU/liter in patients with stage III/IV disease vs. 2.1 ± 0.2 mIU/liter in patients with stage I/II disease (P = 0.002).
Conclusions: The likelihood of thyroid cancer increases with higher serum TSH concentration. Even within normal TSH ranges, a TSH level above the population mean is associated with significantly greater likelihood of thyroid cancer than a TSH below the mean. Shown for the first time, higher TSH level is associated with advanced stage DTC.
This study shows that higher serum TSH levels in patients with thyroid nodules proving to be thyroid cancer are associated with more advanced stages of disease at surgery.
Although the mortality rate has remained stable, the incidence of thyroid cancer is rising. Between 1973 and 2002, there was a 2.4-fold increase in thyroid cancer and a 2.9-fold increase in papillary thyroid cancer (PTC) (1). The American Cancer Society estimates that in 2007 there will be 33,550 new cases of thyroid cancer diagnosed in the United States (2). Although this recent rise in thyroid cancer is likely related to improved imaging modalities causing both length and lead-time bias, it does reopen the question of whether there are modifiable risk factors for thyroid cancer development.
Well-differentiated thyroid cancers express TSH receptors (3,4). Although oncogenes and other growth factors are involved in thyroid cancer growth and development (5,6), it seems probable that TSH can act as a cancer stimulus. This hypothesis is supported by improved survival in thyroid cancer patients treated with suppressive doses of levothyroxine (7) and by cases of tumor growth post-T4 withdrawal or recombinant TSH (8).
With the underlying hypothesis that TSH, a known thyroid growth factor, may have a fundamental role in thyroid cancer development, we looked at the association between preoperative TSH and differentiated thyroid cancer (DTC).
Subjects and Methods
Between May 1994 and January 2007, 1198 patients underwent thyroid surgery at our institution. Demographic and pathologic data from the 843 patients with preoperative serum TSH concentration and either DTC or benign thyroid disease were recorded. TSH levels were checked within 3 months of surgery, and these levels were analyzed in relationship to DTC on final pathology. The cohort included 681 women and 162 men. One-hundred eight of the 843 patients were prescribed levothyroxine preoperatively. Because of the era of this study, levothyroxine was prescribed for nodule suppression and overt hypothyroidism.
There were predominantly four surgeons performing thyroid surgeries during this 13-yr time period. Of the 1198 thyroid surgeries, 603 were unilateral resection and 593 were total thyroidectomies. Unilateral resections were primarily performed for follicular adenomas; large benign nodules; and, depending on the surgeon, low-risk DTC. Total thyroidectomies were primarily performed for thyroid cancer and large compressive goiters. Fourteen of the 108 patients on levothyroxine underwent a previous unilateral resection and returned for completion thyroidectomy.
The final diagnostic outcomes were the presence or absence of DTC on final pathology. Age, gender, nodule number, nodule size, preoperative fine-needle aspiration (FNA), serum TSH as a continuous variable, TSH within designated ranges, and presence or absence of pathological Hashimoto’s thyroiditis were assessed in relationship to the diagnostic outcome. Univariate and multivariable logistic regression analyses were used to identify pre- and perioperative factors associated with thyroid malignancy. P < 0.05 was considered significant. Means were reported as mean ± sem. All analyses were performed using SAS statistical software (version 9.1; SAS Institute Inc., Cary, NC).
Results
Patient and tumor characteristics
A disproportionate number of women relative to men underwent thyroid surgery for both benign and malignant causes. In women especially, the overwhelming number of these surgeries were for benign disease. Of the 681 female patients, 178 (26%) had malignancy on final pathology vs. 63 of the 162 male patients (39%) (P = 0.001).
In addition, patients with malignancy were significantly younger than those without malignancy. Mean age of the patients with malignancy was 46 ± 1 and the mean age of the patients without malignancy was 50 ± 0.7 yr (P = 0.008). Mean nodule size was 2.1 ± 0.1 cm in those patients with cancer, compared with 2.8 ± 0.08 cm in those without cancer (P = 0.0001). There was no association between solitary vs. multiple nodules and likelihood of cancer, and there was no relationship between pathological Hashimoto’s thyroiditis and cancer on final pathology (Table 1).
Table 1.
Patient and tumor characteristics
| Malignancy | No malignancy | P value | |
|---|---|---|---|
| n | 241 | 602 | |
| Gender | |||
| Male | 63 | 99 | 0.001 |
| Female | 178 | 503 | 0.001 |
| Mean age | 46 ± 1 | 50 ± 0.7 | 0.008 |
| Mean nodule size (cm) | 2.1 ± 0.1 | 2.8 ± 0.08 | 0.0001 |
| No. of nodules | |||
| None | 6 | 10 | |
| Solitary | 113 | 306 | 0.3 |
| Multiple | 122 | 286 | 0.3 |
| Hashimoto’s thyroiditis on final pathology | 56 | 118 | 0.2 |
As expected in an iodine-sufficient region, PTC comprised 87% of all thyroid malignancies (223 of 256). Seven percent of thyroid malignancies (18 of 256) were secondary to follicular thyroid cancer (FTC) or Hürthle cell cancer. The remaining 6% of thyroid malignancies (15 of 256) were medullary thyroid cancer, anaplastic thyroid cancer, and lymphoma. These cancers were considered unlikely to be TSH responsive and thus were not included in the analysis.
TSH and likelihood of malignancy
Preoperative mean TSH was significantly higher in patients with malignancy vs. benign pathology. Mean TSH in the 241 patients with DTC was 2.5 mIU/liter ± 0.3 vs. 1.6 mIU/liter ± 0.1 in those 602 patients with benign diagnoses (P = 0.0001). If all 108 patients prescribed levothyroxine preoperatively were removed from the analysis, the mean TSH was 2.5 mIU/liter ± 0.3 in patients with malignancy vs. a mean TSH of 1.4 mIU/liter ± 0.1 in patients with benign thyroid pathology (P = 0.0001). When the subset of patients with FNA cytology suspicious for malignancy was analyzed, there was a trend for higher TSH with malignancy vs. benign final pathology. In the seven patients with malignancy on final pathology, mean TSH was 3.7 ± 2.3 vs. 1.4 ± 0.4 mIU/liter in the 11 patients with benign pathology (P = 0.2) (Table 2).
Table 2.
Mean preoperative TSH
| Malignancy | No malignancy | P value | |
|---|---|---|---|
| All patients | |||
| n | 241 | 602 | |
| Mean TSH (mIU/liter) | 2.5 ± 0.3 | 1.6 ± 0.1 | 0.0001 |
| Excluding patients on levothyroxine | |||
| n | 212 | 523 | |
| Mean TSH (mIU/liter) | 2.5 ± 0.3 | 1.4 ± 0.07 | 0.0001 |
| FNA suspicious for malignancy | |||
| n | 7 | 11 | |
| Mean TSH (mIU/liter) | 3.7 ± 2.3 | 1.4 ± 0.4 | 0.2 |
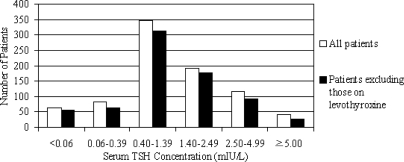
To decrease the likelihood of patients with markedly elevated TSH skewing the data, the patients were subdivided into TSH ranges based on cutoff values predetermined in population studies (9,10,11,12). A TSH of 0.40 mIU/liter was chosen as the lower end of normal range and 1.40 mIU/liter was chosen as the median TSH (9,10,11). Because there is an association between presence of thyroid antibodies and the likelihood of developing overt hypothyroidism with TSH above 2.0 or 2.5 mIU/liter (12), 2.5 mIU/liter was also chosen as a cutoff between ranges. A TSH of less than 0.06 was considered maximally suppressed and a TSH of 5.0 or greater was considered above the upper end of normal TSH (11). Similar to the population at large, the distribution of patients loosely followed a bell-shaped curve (Fig. 1). The majority of patients (n = 655) were euthyroid based on TSH alone.
Figure 1.
The distribution of patients based on TSH range followed a bell-shaped curve. The majority of patients (n = 655) had serum TSH concentrations within normal range of 0.40–4.99 mIU/liter.
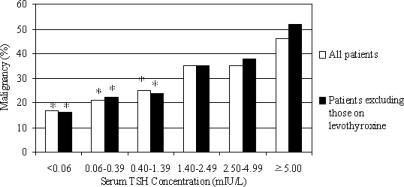
As TSH increased, the likelihood of malignancy on final pathology rose. The likelihood of malignancy was 11% (11 of 65) when TSH was less than 0.06 mIU/liter vs. 46% (19 of 41) when 5.0 mIU/liter or greater (P = 0.001). When the patients on levothyroxine were removed from the analysis, the same pattern of escalating cancer incidence with higher TSH persisted (Fig. 2). Interestingly, even within normal range of TSH, there was a significantly increased risk of DTC as TSH increased. When TSH was between 0.40 and 1.39 mIU/liter, the likelihood of malignancy was 25% (85 of 347) vs. 35% (109 of 308) when TSH was between 1.40 and 4.99 mIU/liter (P = 0.002) (Table 3). Both as a continuous variable and based on divided ranges, higher TSH was independently associated with malignancy on univariate and multivariable analysis (P = 0.007). Relative to a TSH less than 0.06 mIU/liter, on multivariable analysis of patients not on levothyroxine, risk of DTC was 2.5-fold higher if TSH was between 1.40 and 2.49 mIU/liter, 3.5-fold higher when TSH was 2.50–4.99 mIU/liter, and 4.5-fold higher when TSH was 5.00 mIU/liter or greater (Table 4).
Figure 2.
On univariate and multivariable analyses, TSH was an independent predictor of malignancy (P = 0.007). As TSH rises, the risk of malignancy increases. When patients on levothyroxine were excluded from the analyses, TSH was still an independent predictor of thyroid cancer. *, P < 0.05 relative to TSH of 5.00 mIU/liter or greater.
Table 3.
Euthyroid patients
| TSH range (mIU/liter) | No. of patients | No. with malignancy | P value |
|---|---|---|---|
| 0.40–1.39 | 347 | 85 (25%) | 0.002 |
| 1.40–4.99 | 308 | 109 (35%) |
Table 4.
Independent risk factors for diagnosis of thyroid cancer defined by multivariable logistic regression analysis considering gender, age, nodule size, and preoperative serum TSH concentration in patients not on levothyroxine
| Variable | Adjusted odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Male gender | 2.32 | 1.51–3.58 | <0.0001 |
| Age | 0.98 | 0.97–0.99 | 0.01 |
| Nodule size | 0.77 | 0.69–0.87 | <0.0001 |
| TSH (mIU/liter) | |||
| <0.06 | 1 | ||
| 0.06–0.39 | 1.65 | 0.59–4.60 | 0.34 |
| 0.40–1.39 | 1.39 | 0.59–3.27 | 0.44 |
| 1.40–2.49 | 2.50 | 1.04–6.04 | 0.04 |
| 2.50–4.99 | 3.52 | 1.37–9.02 | 0.009 |
| ≥5.00 | 4.56 | 1.35–15.45 | 0.01 |
Of the 241 cases of DTC, 23% were incidental microcarcinomas. When a subset analysis was performed comparing benign tumors less than 1 cm (n = 73) to DTC less than 1 cm (n = 55), the escalating risk of malignancy with higher TSH persisted up to the range of TSH of 5.0 mIU/liter or greater (Table 5).
Table 5.
Nodules < 1 cm in size (n = 128)
| TSH range (mIU/liter) | No. of patients | No. with DTC (%) |
|---|---|---|
| <0.06 | 12 | 3 (25) |
| 0.06–0.39 | 11 | 4 (36) |
| 0.4–1.39 | 44 | 17 (39) |
| 1.4–2.49 | 32 | 16 (50) |
| 2.5–4.99 | 21 | 13 (61) |
| ≥5.00 | 8 | 2 (25) |
TSH and cancer stage
Two hundred thirty-nine of the 241 patients with DTC were staged according to tumor node metastasis. The overwhelming number of surgical patients had stage I and II DTC (204 of 239). Only 15% (35 of 239) had stage III and IV DTC. The patients with advanced disease (stage III and IV) had a significantly higher mean TSH relative to those with stage I and II disease. The mean TSH of those with advanced disease was 4.9 ± 1.5 vs. 2.1 ± 0.2 mIU/liter (P = 0.002). Of the 35 patients with advanced DTC, 23 patients had TSH levels above the mean population TSH of 1.4 mIU/liter vs. only 12 had TSH levels less than 1.4 mIU/liter (Table 6).
Table 6.
TSH and cancer stage
| TNM stage | No. of patients | Mean TSH | P value |
|---|---|---|---|
| I and II | 204 | 2.1 ± 0.24 | 0.002 |
| III and IV | 35 | 4.9 ± 1.59 |
TNM, Tumor node metastasis.
Discussion
The relationship between serum TSH concentration and thyroid malignancy is still being defined. There are several reasons that the role of TSH in cancer development has been minimized. First, TSH receptor mutations in regions functionally associated with increased signal transduction do not commonly occur in thyroid cancer (13). Second, in vitro studies have demonstrated that other factors, such as IGF-I, may play a more dominant role in cancer growth (5,6). Finally, there is an inverse relationship between TSH receptor mRNA level and cancer aggressiveness (4). Despite these negative data, there is clear evidence of improved survival with aggressive thyroid hormone suppression in high-risk cancer patients and improved survival with modest thyroid hormone suppression in stage II thyroid cancer patients (7). It is improbable that TSH acts alone in cancer induction because thyroid cancer has been known to occur at a range of TSH values, including in the suppressed contralateral lobe of hyperfunctioning nodules (14). However, it is possible that chronic TSH stimulation plays a role in cancer pathogenesis.
Supportive of the TSH receptor’s role in cancer are the data on autoimmune thyroid disease and thyroid cancer. Although not all studies are in agreement, a metaanalysis of 10 studies showed a 2.77-fold increased incidence of thyroid cancer in patients with antibody evidence of Hashimoto’s thyroiditis, compared with control population (15). Similar to the Hashimoto’s controversy, there is great debate over the association between Graves’ disease and thyroid cancer incidence and aggressiveness (3,6,16,17,18,19). Although still an ongoing debate, some experts conclude that TSH receptor stimulation is associated with increased cancer incidence and aggressiveness (6). Because Hashimoto’s is often associated with progression to hypothyroidism and thus elevated TSH and because Graves’ disease is associated with TSH receptor stimulation, it is possible that the link between autoimmune thyroid disease and thyroid cancer is the TSH receptor.
Both benign nodules and well-differentiated thyroid cancer express TSH receptors (4). The role of the TSH receptor has been more extensively evaluated in benign nodules. It is unlikely that TSH suppression reduces benign nodule size; however, it may prevent the development of new nodules and decrease rate of growth (5,20,21,22,23). In addition, analysis of benign nodules indicates that there may be a pathogenetic role of TSH because suppressive doses of levothyroxine result in beneficial cytologic changes including an increase in colloid nodules from those nodules previously classified hypercellular or adenomatous (22).
Three previous studies attempted to evaluate TSH as a predictor of thyroid cancer. The initial two studies analyzed TSH values below normal vs. within normal range. In the first study, there was a nonsignificant trend toward decreased cancer risk if TSH was below normal range (24). In the second study, there was a significant reduction in risk if TSH was below normal range (25). Boelaert et al. published the third study (26), which was an analysis of a cohort of 1500 patients who underwent FNA between 1984 and 2002 (26). This analysis found increased odds of malignancy in those patients with solitary nodules and TSH greater than 0.9 mIU/liter. Although there was a trend toward increased cancer risk with rising TSH range, no significant difference existed between normal range TSH levels above 0.9 mIU/liter due to the small number of malignancies (n = 120). All three of the previous studies were performed in the United Kingdom and the incidence of FTC relative to PTC was higher than what would be anticipated for a U.S. population. In the study by Boelaert et al., 37% of cases were either FTC or Hürthle cell cancer. PTC comprised 52% of the cases. This is much lower than the 88% prevalence of PTC in the United States (1). It is not clear whether this reduced PTC incidence and raised FTC incidence is related to dietary iodine consumption, but it does make it more difficult to extrapolate to U.S. patients. It also clouds the association between TSH and malignancy, given the unknown urinary iodine status.
In our study, we had a larger number of DTC cases (n = 241) because it was a cohort undergoing thyroid surgery instead of a cohort undergoing FNA. Of the 843 patients undergoing surgery, 241 (29%) were DTC vs. approximately 5% of FNA patients. The 87% incidence of PTC and 7% incidence of FTC were similar to the distribution found by the Surveillance, Epidemiology, and End Results program (1). In addition, unique to this study, final pathology was available thus confirming or refuting any benign or malignant FNAs.
In our analysis, TSH was an independent predictor of DTC on univariate and multivariable analysis. This predictive value persisted when TSH was subdivided into ranges. In the subset of patients with FNA cytology suspicious for malignancy and not diagnostic for malignancy, there was a trend toward increased rate of DTC with higher TSH level. Statistical significance was not shown because the absolute number of patients was small (n = 18). Management of patients with FNA suspicious for malignancy is not as straightforward as management when aspirates are clearly benign or malignant (27,28,29,30). Larger sample sizes are needed, but if high TSH level is predictive of DTC when aspirates are suspicious for malignancy, TSH could play a key role in determining optimal surgical intervention.
It was previously believed that the low rate of malignancy associated with TSH below normal range was secondary to either autonomous functioning nodules having a lower malignancy rate or gradual thyroid failure secondary to autoimmune thyroid disease associated with higher malignancy rate (26). The low cancer incidence in hyperfunctioning nodules is attributed to the constitutive activating mutations of TSH receptors driving the cAMP pathway through Gαs and very rarely the cancer associated Ras-dependent MAPK pathway through Gβγ and phosphatidylinositol 3-kinase-γ (31). Although autonomous functioning nodules are less likely to have malignancy because of this selective pathway activation, this does not explain the escalating cancer risk within euthyroid and hypothyroid TSH range. The escalating risk in patients with TSH in the upper end of normal or frankly elevated is also unlikely to be solely due to antithyroid antibody levels because there was no difference in malignancy rate between TSH range of 1.40–2.49 vs. 2.50–4.99 mIU/liter. A limitation of this study is the lack of thyroid antibodies drawn preoperatively, and thus, this assumption is based on previous population studies revealing a rise in the prevalence of thyroid antibodies starting at a TSH of 2 or 2.5 mIU/liter (12). With hyperfunctioning nodules and positive antithyroid antibodies unable to fully explain the pattern of elevated thyroid cancer risk with higher TSH, our data suggest TSH itself is the unifying variable.
In addition to preoperative TSH being predictive of DTC, male gender, younger age, and smaller nodule size were independent predictors of malignancy. This is not because men and younger patients have a higher absolute number of malignancies but because older patients and females have an increased likelihood of surgery for benign reasons such as resection of benign multinodular goiter with compressive symptoms. Because women and older patients tend to have a higher TSH (9) and women and older patients were overrepresented in the subgroup undergoing surgery for benign conditions, one may have anticipated higher TSH would correlate with benign disease, not malignancy. The fact that in this patient population, higher TSH significantly correlated with increased risk of malignancy is strong evidence that TSH receptor stimulation is likely involved in the pathogenesis of thyroid cancer. Further population studies with large sample sizes are needed to confirm this hypothesis.
A subset analysis of tumors less than 1 cm in diameter was performed. In this subset the escalating risk of DTC with higher TSH persisted until TSH was 5.00 mIU/liter or greater. There was a low incidence of microcarcinomas in patients with TSH above the upper end of normal (two of eight). In general, the data on incidental microcarcinomas are more difficult to interpret for the following reasons. First, it is possible not all benign nodules under 1 cm were recorded. Small incidental cysts or benign nodules may not have been deemed clinically relevant, and thus, the total number of benign tumors less than 1 cm may not be accurate. Second, it is possible not all microcarcinomas were detected. Patients undergoing surgery for a 100-g goiter with compressive symptoms may not have had the same pathological inspection as those undergoing a lobectomy for an aspirate suspicious for malignancy. Third, more than half of the surgical patients underwent unilateral resection, and in these cases, the remaining lobe was not examined for microcarcinomas. Despite the limitations of the subset analysis of microcarcinomas, there was a pattern of escalating cancer risk with higher TSH level. This pattern stopped at a TSH of 4.99 mIU/liter, but otherwise it was consistent with the compiled data on DTC. It is plausible this discrepancy with microcarcinomas when TSH is 5.00 mIU/liter or greater is due to selection bias and limitations of study design. However, given the previous data on TSH as a growth factor, it is also not implausible DTC growth is accelerated in patients with overt hypothyroidism in which case the cancer is detected as a larger tumor. Further analysis of TSH and microcarcinomas is warranted.
Shown for the first time in this analysis, higher TSH was associated with not only incidence of DTC but also advanced stage of DTC. Although all of the mean TSH levels fall within the accepted normal range (0.4–4.99 mIU/liter), the mean TSH for benign thyroid disease was 1.6 ± 0.1 vs. 2.1 ± 0.2 mIU/liter for stage I and II disease vs. 4.9 ± 1.5 mIU/liter for stage III and IV disease. Although the absolute number with advanced DTC was small (35 of 239), this escalating risk of advanced disease with higher TSH level suggests TSH is involved in the pathogenesis of thyroid cancer.
TSH is a known thyroid growth factor and, based on our analysis, may play a central role in the development and progression of thyroid cancer. Within normal range TSH, a TSH level above the population mean had an increased risk of malignancy relative to a TSH level below the mean. In addition, unique to this study, stage III and IV DTC was associated with a significantly higher mean TSH than stage I and II DTC. Further understanding of the role of TSH in DTC may help with the prevention, diagnosis, and management of thyroid cancer.
Acknowledgments
We thank Anna Bargren and Yi-Wei Zhang for their involvement in data collection.
Footnotes
The Academic Oncologist K12 Training Grant through the University of Wisconsin Paul P. Carbone Comprehensive Cancer Center supports the research of M.R.H. J.C.J. is supported through the Veterans Affair Merit Review Programs and the National Institutes of Health (NIH). H.C. has several sources of funding through the NIH.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 26, 2007
Abbreviations: DTC, Differentiated thyroid cancer; FNA, fine-needle aspiration; FTC, follicular thyroid cancer; PTC, papillary thyroid cancer.
References
- Davies L, Welch HG 2006 Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- The American Cancer Society 2007 Detailed guide: thyroid cancer. What are the key statistics about thyroid cancer? Retrieved 10/2/2007, from http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_thyroid_cancer_43.asp?sitearea= [Google Scholar]
- Stocker DJ, Burch HB 2003 Thyroid cancer yield in patients with Graves’ disease. Minerva Endocrinol 28:205–212 [PubMed] [Google Scholar]
- Shi Y, Zou M, Farid NR 1993 Expression of thyrotropin receptor gene in thyroid carcinoma is associated with good prognosis. Clin Endocrinol (Oxf) 39:269–274 [DOI] [PubMed] [Google Scholar]
- Derwahl M, Broecker M, Kraiem Z 1998 Thyrotropin may not be the dominant growth factor in benign and malignant thyroid tumors. J Clin Endocrinol Metab 84:829–834 [DOI] [PubMed] [Google Scholar]
- Mazzaferri EL 2000 Thyroid cancer and Graves’ disease: the controversy 10 years later. Endocr Pract 6:221–225 [DOI] [PubMed] [Google Scholar]
- Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis M, Maxon HR, Sherman SI 2006 Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 16:1229–1242 [DOI] [PubMed] [Google Scholar]
- Braga M, Ringel MD, Cooper DS 2001 Sudden enlargement of local recurrent thyroid tumor after recombinant TSH administration. J Clin Endocrinol Metab 86:5148–5151 [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Flanders DW, Harmon WH, Gunter EW, Spencer CA, Braverman LE 2002 Serum TSH, T4 and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- Kratzsch J, Fiedler GM, Leichtle A, Brugel M, Buchbinder S, Otto L, Sabri O, Matthes G, Thiery J 2005 New reference intervals for thyrotropin and thyroid hormones based on national academy of clinical biochemistry criteria and regular ultrasonography of the thyroid. Clin Chem 51:1480–1486 [DOI] [PubMed] [Google Scholar]
- Canaris GJ, Manowitz NR, Mayor G, Ridgway C 2000 The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- Vanderpump MPJ, Tunbridge WMG, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F, Young ET 1995 The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 43:55–68 [DOI] [PubMed] [Google Scholar]
- Matsuo K, Friedman E, Gejman PV, Fagin JA 1993 The thyrotropin receptor (TSH-R) is not an oncogene for thyroid tumors: structural studies of the TSH-R and the α-subunit of Gs in human thyroid neoplasms. J Clin Endocrinol Metab 76:1446–1451 [DOI] [PubMed] [Google Scholar]
- Satta MA, DeRosa G, Testa A, Maussier ML, Valenza V, Rabitti C, Saletrich I, D’Ugo D, Picciocchi A 1993 Thyroid cancer in suppressed contralateral lobe of patients with hot thyroid nodule. Eur J Cancer 29A:1190–1192 [DOI] [PubMed] [Google Scholar]
- Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP 1999 Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery 126:1070–1077 [DOI] [PubMed] [Google Scholar]
- Hales IB, McElduff A, Crummer P, Clifton-Bligh P, Delbridge L, Hoschl R, Poole A, Reeve TS, Wilmshurst E, Wiseman J 1992 Does Graves’ disease or thyrotoxicosis affect the prognosis of thyroid cancer? J Clin Endocrinol Metab 75:886–889 [DOI] [PubMed] [Google Scholar]
- Yano Y, Shibuya H, Kitagawa W, Nagahama M, Sugino K, Ito K, Ito K 2007 Recent outcome of Graves’ disease patients with papillary thyroid cancer. Eur J Endocrinol 157:325–329 [DOI] [PubMed] [Google Scholar]
- Pellegriti G, Belfiore A, Giuffrida D, Lupo L, Vigneri R 1998 Outcome of differentiated thyroid cancer in Graves’ patients. J Clin Endocrinol Metab 83:2805–2809 [DOI] [PubMed] [Google Scholar]
- Filetti S, Belfiore A, Amir SM, Daniels GH, Ippolito O, Vigneri R, Ingbar SH 1988 The role of thyroid-stimulating antibodies of Graves’ disease in differentiated thyroid cancer. N Engl J Med 318:753–759 [DOI] [PubMed] [Google Scholar]
- Papini E, Petrucci L, Guglielmi R, Panunzi, Rinaldi R, Bacci V, Crescenzi A, Nardi F, Fubbrini R, Pacella CM 1998 Long-term changes in nodular goiter: a five-year prospective randomized trial of levothyroxine suppressive therapy for benign cold thyroid nodules. J Clin Endocrinol Metab 83:780–783 [DOI] [PubMed] [Google Scholar]
- Zelmanovitz F, Genro S, Gross JL 1998 Suppressive therapy with levothyroxine for solitary thyroid nodules: a double blind controlled clinical study and cumulative meta-analyses. J Clin Endocrinol Metab 83:3881–3885 [DOI] [PubMed] [Google Scholar]
- Vermiglio F, Presti VPL, Violi MA, Moleti M, Castagna MG, Finocchiaro MD, Mattina F, Mandolfino M, Zimbaro G, Trimarchi F 2003 Changes in both size and cytologic features of thyroid nodules after levothyroxine treatment. Clin Endocrinol (Oxf) 59:347–353 [DOI] [PubMed] [Google Scholar]
- Gharib H 2004 Changing trends in thyroid practice: understanding nodular thyroid disease. Endocr Pract 10:31–39 [DOI] [PubMed] [Google Scholar]
- Kumar H, Daykin J, Holder R, Watkinson JC, Sheppard MC, Franklyn JA 1999 Gender, clinical findings, and serum thyrotropin measurements in the prediction of thyroid neoplasia in 1005 patients presenting with thyroid enlargement and investigated by fine-needle aspiration cytology. Thyroid 9:1105–1109 [DOI] [PubMed] [Google Scholar]
- Lim AKP, Daykin J, Holder R, Sheppard MC, Franklyn JA 1998 Measurement of serum TSH in the investigation of patients presenting with thyroid enlargement. QJM 91:687–689 [DOI] [PubMed] [Google Scholar]
- Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA 2006 Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab 91:4295–4301 [DOI] [PubMed] [Google Scholar]
- Haymart MR, Greenblatt DY, Elson DF, Chen H The role of intraoperative frozen section if suspicious for papillary thyroid cancer. Thyroid, in press [DOI] [PubMed] [Google Scholar]
- Chen H, Zeiger MA, Clark DP, Westra WH, Udelsman R 1997 Papillary carcinoma of the thyroid: can operative management be based solely on fine-needle aspiration? J Am Coll Surg 184:605–610 [PubMed] [Google Scholar]
- Callcut RA, Selvaggi SM, Mack E, Ozgul O, Warner T, Chen H 2004 The utility of frozen section evaluation for follicular thyroid lesions. Ann Surg Oncol 11:94–98 [DOI] [PubMed] [Google Scholar]
- Udelsman R, Westra WH, Donovan PI, Sohn TA, Cameron JL 2001 Randomized prospective evaluation of frozen-section analysis for follicular neoplasms of the thyroid. Ann Surg 233:716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Villard JA, Wicker R, Crespo P, Russo D, Filetti S, Gutkind JS, Sarasin A, Suárez HG 2000 Role of the cAMP and MAPK pathways in the transformation of mouse 3T3 fibroblasts by a TSHR gene constitutively activated by point mutation. Oncogene 19:4896–4905 [DOI] [PubMed] [Google Scholar]