Abstract
Context: Short-term aerobic exercise training can improve whole-body insulin sensitivity in humans with type 2 diabetes mellitus; however, the contributions of peripheral and hepatic tissues to these improvements are not known.
Objective: Our objective was to determine the effect of 7-d aerobic exercise training on peripheral and hepatic insulin sensitivity during isoglycemic/hyperinsulinemic clamp conditions.
Design: Subjects were randomly assigned to one of two groups. The energy balance group consumed an isocaloric diet consisting of 50% carbohydrate, 30% fat, and 20% protein for 15 d. The energy balance plus exercise group consumed a similar diet over the 15 d and performed 50-min of treadmill walking at 70% of maximum oxygen consumption maximum during the second 7 d of the 15-d study period. Each subject underwent an initial isoglycemic/hyperinsulinemic clamp after 1-wk dietary control and a second clamp after completing the study.
Setting: The study was performed at Ohio State University’s General Clinical Research Center.
Participants: There were 18 obese, mildly diabetic humans included in the study.
Intervention: Aerobic exercise training was performed for 7 d.
Main Outcome Measures: Whole-body, peripheral, and hepatic insulin sensitivity were measured.
Results: Exercise training did not have an impact on peripheral glucose uptake or endogenous glucose production during the basal state or low-dose insulin. Likewise, it did not alter endogenous glucose production during high-dose insulin. However, 1-wk of exercise training increased both whole-body (P < 0.05) and peripheral insulin sensitivity (P < 0.0001) during high-dose insulin.
Conclusion: Improvements to whole body insulin sensitivity after short-term aerobic exercise training are due to gains in peripheral, not heptic insulin sensitivity.
Aerobic exercise training for seven days in obese patients with mild diabetes mellitus augments whole-body insulin sensitivity, peripheral glucose uptake, and peripheral insulin sensitivity. Because hepatic insulin sensitivity was not affected, a lengthier training duration may be required to improve this as well.
Type 2 diabetes mellitus (DM) is a metabolic disease characterized by insulin resistance, relative β-cell dysfunction, and resultant hyperglycemia that can contribute to the development of both microvascular and macrovascular disease. Despite an apparently strong genetic influence on the development of DM, environmental factors such as obesity and physical inactivity can hasten this process in humans (1,2,3), while interventions designed to target these factors can improve insulin sensitivity and reduce the risk of developing DM (4).
It has been shown consistently that the increase in skeletal muscle glucose uptake seen in response to hyperinsulinemia is lower in humans with DM than in non-DM controls (5,6,7). Furthermore, hepatic glucose production during the postabsorptive state is greater in those with DM compared with controls (8,9,10,11), and the inhibitory response to insulin is diminished. Together, these consequences of insulin resistance in muscle and liver contribute to hyperglycemia and glucose intolerance.
Aerobic exercise training is a nonpharmacological treatment for DM that can improve whole-body insulin sensitivity and glucose tolerance. Previous research has indicated that plasma glucose levels can be lowered during aerobic exercise due to increased contraction-mediated glucose uptake (12,13), and whole-body insulin sensitivity improved during glucose clamp conditions. Specifically, a single bout of exhaustive exercise by humans with DM can reduce hepatic glucose production during both the postabsorptive state, and during insulin infusion (14), and longer training durations can reduce hepatic glucose production during hyperinsulinemia even after gains to peripheral insulin sensitivity have been lost (15). Similar to its effects on the liver, exercise training can improve skeletal muscle insulin sensitivity, which also contributes to improved whole-body insulin sensitivity. Previous studies have indicated that regularly performed aerobic exercise can increase glucose uptake at a given insulin level (6,16) and that this alteration may in part be mediated by changes in pathways downstream of the insulin receptor that ultimately result in greater insulin-induced translocation of glucose transporter 4 (Glut4) to the cell surface (17,18), and greater nonoxidative glucose disposal (19).
Potential caveats of studies that use prolonged training durations to study the effect of exercise on insulin sensitivity include changes in body fat levels or aerobic power, which themselves can improve the insulin resistant state. Because of this, some investigators have used training protocols lasting 7–10 d (6,20), which can better determine the independent effect of exercise on insulin sensitivity. Results of those studies indicate that exercise training, in the absence of changes in body fat levels and aerobic power, improves glucose tolerance and whole-body insulin sensitivity. However, it is not known if these changes are due to improved insulin regulation of glucose uptake by muscle, glucose production by the liver, or a combination of these events. Therefore, the purpose of this study was to determine the effect of 7-d aerobic exercise training on peripheral glucose uptake (PGU) and endogenous glucose production (EGP) in obese humans with mild DM during the postabsorptive state and during isoglycemic/hyperinsulinemic clamp conditions.
Subjects and Methods
Subjects
Before enrollment, all subjects were informed of the risks associated with participation, and provided written consent. The Ohio State University’s Institutional Review Board for human subject research approved the methods of this study. An a priori power calculation based on the work of Devlin et al. (14) indicated that nine subjects per group were required for significant F ratios. Inclusion criteria included the following: males and nonpregnant females who had been previously sedentary for 6 months or more, aged 30–60 yr, have DM as determined from oral glucose tolerance test (OGTT) responses, and have a glycosylated hemoglobin (HbA1c) value of 7.5% or less. All subjects were in apparently good overall health as determined by a physician. Of the 13 women that participated, 10 were postmenopausal. None of these subjects reported being on estrogen replacement therapy.
Exclusion criteria
Exclusion criteria included uncontrolled hypertension, an adverse cardiac or blood pressure response to exercise during the stress test, or a self-reported history of neuropathy, nephropathy, retinopathy, or musculoskeletal conditions that would make exercise difficult or dangerous. At the outset of the study, all subjects discontinued their diabetes-related medications, which included diet therapy only (n = 3), sulfonylurea (n = 3), metformin (n = 11), and a combination of sulfonylurea and metformin (n = 1). Individuals prescribed insulin or thiazolidinediones (because of the half-life length) were not eligible to participate.
Study overview
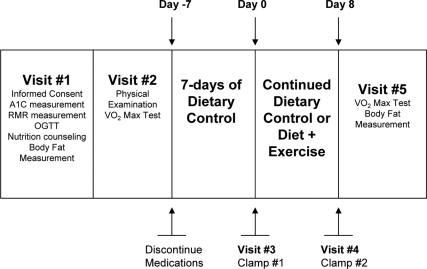
The purpose of this study was to determine the effect of 7-d aerobic exercise training on EGP and PGU in humans with DM. Subjects were randomly assigned to one of two groups: energy balance only (EB), or energy balance plus 7-d aerobic exercise training (EBE). Initial participation required each subject to undergo a battery of tests at The Ohio State University’s General Clinical Research Center (GCRC) characterizing their DM (Fig. 1). Next, all subjects discontinued their diabetic medications, and began 7-d dietary control, followed by the preintervention isoglycemic clamp. This clamp was followed by 7 more days of dietary control for subjects in both groups, while the EBE group performed the prescribed exercise during the second week. After the intervention period, each subject underwent a second isoglycemic clamp within 24 h of the final exercise bout. A final visit was made to the GCRC within 3 d of the second clamp for a follow-up measurement of body fat levels and maximal (max) oxygen consumption (VO2).
Figure 1.
Schematic representation of the study.
OGTT
Not less than 1 wk before the preintervention clamp, each subject underwent a 75-g OGTT. Samples were collected for the determination of plasma glucose and insulin at −15, 0, 15, 30, and 120-min time points of the OGTT. A 120-min plasma glucose concentration more than 199 mg/dl classified the subject as diabetic. Blood samples were also used to measure HbA1c. A value of 7.5% or less was necessary to participate in the study.
Body fat measurement
Body fat percentage was measured using bioelectric impedance analysis according to the equation of Segal et al. (21).
Resting metabolic rate (RMR) measurement and dietary control
RMR was measured using indirect calorimetry over a 30-min period while the subject rested comfortably in a hospital bed. Attainment of RMR was defined as VO2 rates that differed by less than 2% over a 5-min period. Final estimation of daily energy expenditure, and thus the initial estimation of caloric needs of each subject were increased by 30% to account for daily activity and postprandial thermogenesis. This level was then modified slightly throughout the study on a per subject basis as necessary to maintain body weight as close to the baseline measure as possible.
Beginning 1 wk before the first clamp, subjects began consuming an isocaloric diet, determined by the RMR measurement, consisting of a target of 50% carbohydrate, 30% fat, and 20% protein that was prepared by the GCRC metabolic kitchen staff. Counseling and specific directions were provided by a registered dietitian to ensure and monitor compliance. The use of all nutritional supplements was discontinued at the outset of dietary control. During exercise training (d 1–7), daily caloric intake was adjusted, for the EBE group on a per subject basis, to account for the estimated energy expenditure during exercise. Each exercise session generated a caloric expenditure of approximately 400 kcal.
VO2 max/electrocardiogram testing
Each VO2 max test was performed with 12-lead electrocardiogram monitoring. This test was performed not less than 1 wk before the preintervention clamp. The Balke 3.0 protocol was used, with the modification that each stage of the test lasted 3 min to allow VO2 to reach a steady state. Exercise prescriptions were then made for the EBE group by determining the workload that solicited 70% of the individually measured VO2 max.
Isoglycemic/hyperinsulinemic clamp studies
Before each clamp, subjects were admitted to the GCRC after a 12-h fast. Twenty gauge needles were inserted into the antecubital and contralateral vein for infusions and periodical blood draws, and a heating pad was kept over the drawing vein for the collection of arterialized blood samples.
After blood samples were drawn at min 0, subjects were infused with a priming dose of HPLC-purified 3-3H glucose as described previously (22,23). This was followed by the continuous infusion of the tracer at a rate of 0.25 μCi/min for the remainder of the first 2-h phase of the clamp. Plasma glucose values during the final 30 min of this first phase of the clamp, taken every 5 min, were averaged to give the plasma glucose level at which the subject would be clamped.
The second and third 2-h phases of the clamp were characterized by the primed (24), continuous infusion of insulin (Humulin; Eli Lilly and Co., Indianapolis, IN) at 20 and 40 mU/m2·min, respectively. These infusion rates were chosen for study because they would not be expected to completely suppress EGP in this population, allowing the measurement of hepatic insulin action. The tracer infusion rate was reduced to 50 and 25% of basal 20 and 40 min, respectively, after beginning insulin infusion, and maintained for the remainder of the clamp. Plasma glucose was monitored using a bedside plasma glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH), and maintained at isoglycemia by the variable infusion of 20% (wt/vol) dextrose, which was labeled with 3-3H glucose to maintain specific activity (25).
Calculations
After the determination of plasma 3-3H specific activity, Steele’s nonsteady-state equation (26) was used to quantify rates of appearance and disappearance of glucose. EGP was calculated by subtracting the rate of exogenous glucose infusion from the total rate of appearance. Whole-body insulin sensitivity was calculated by expressing the glucose infusion rate per average insulin level, whereas insulin sensitivity of peripheral and hepatic tissues are expressed as the rate of isotopically determined PGU and EGP, respectively, during the final 30 min of each clamp phase per average insulin level during the respective period.
Biochemical analyses
Insulin, glucagon, and cortisol (Diagnostic Products Corp., Los Angeles, CA) were analyzed using commercially available RIA kits. Plasma glucose (Yellow Springs Instruments), lactate (State University of New York at Buffalo, Buffalo, NY), glycerol (Sigma Diagnostics, St. Louis, MO), and free fatty acids (Wako Diagnostics, Wako, TX) were measured using enzymatic assays. Plasma radioactivity was determined by liquid scintillation counting (LS6500; Beckman Instruments, Fullerton, CA) as described previously (27).
Exercise training
All exercise sessions were supervised to ensure that they were performed as prescribed. One subject performed only six of the seven exercise sessions, making compliance greater than 98%. The training protocol mimicked that used by Rogers et al. (20), which improved insulin action in 7 d without altering VO2 max or body fat percentage. On d 1–3 and 5–7, subjects performed 2 × 25 min bouts of aerobic exercise (treadmill walking) with a 10-min break between bouts. The intensity of the exercise was 70% VO2 max. Day four of exercise required subjects to exercise for 60 min at 60% of their age-predicted max heart rate (220-age), a lower intensity than the other 6-d exercise. Subjects were provided with a heart rate monitor on d 4, allowing easy measurement of heart rate during this bout.
Statistical analysis
ANOVA (group × time × insulin infusion rate during the clamp) with repeated measures was used for analysis of all data from the clamps. Body composition and VO2 max data were analyzed using ANOVA (group × time) with repeated measures, whereas RMR and HbA1c data were analyzed using the unpaired Student’s t test. With significant F ratios, post hoc comparisons were made as appropriate, and statistical significance was P < 0.05. Data are presented as mean ± sem unless stated otherwise.
Results
Subject characteristics
Baseline subject characteristics are presented in Table 1. A total of 18 obese men and women with DM successfully completed the study. One subject from the EBE group was unable to complete the study because of an inability to keep the drawing line patent during the second clamp (none of this subject’s data are included in the analysis). Of the variables presented, no differences were observed between the EB and EBE groups.
Table 1.
Baseline characteristics for 18 subjects that completed the study
| Variable | EB | EBE |
|---|---|---|
| Sample size (M/F) | 9 (3/6) | 9 (2/7) |
| Race (CA/AA) | 5/4 | 4/5 |
| Age (yr) | 50.9 ± 3.2 | 48.4 ± 8.4 |
| Height (cm) | 164.6 ± 9.6 | 168.5 ± 8.2 |
| Weight (kg) | 87.1 ± 17.3 | 99.5 ± 15.0 |
| BMI (kg/m2) | 32.0 ± 5.3 | 34.9 ± 3.1 |
| Body fat (%) | 39.5 ± 7.0 | 43.0 ± 6.1 |
| HbA1c (%) | 6.7 ± 0.6 | 6.5 ± 0.4 |
| VO2 max (ml/kg·min) | 20.4 ± 4.5 | 22.6 ± 3.4 |
Values are presented as mean ± sd. No differences were detected between groups for any of the outcomes listed (P > 0.05). AA, African-American; BMI, body mass index; CA, Caucasian; F, female; M, male.
OGTT glucose and insulin responses
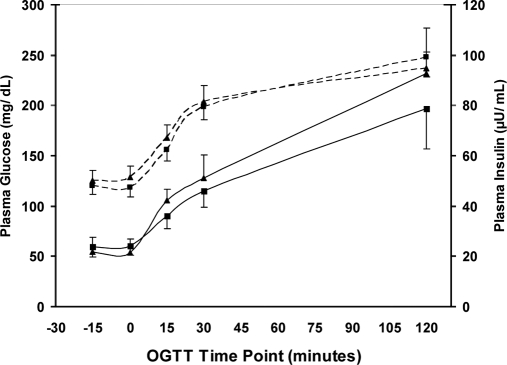
A 75-g OGTT was performed on each subject during the first visit to determine glucose tolerance. As expected, there was a significant increase in plasma glucose values (Fig. 2) over 2 h in both the EB and EBE groups (P < 0.05), whereas no differences in plasma glucose were observed between groups at any time point. Plasma insulin levels also increased steadily in response to the OGTT, reaching significance by the 30-min mark in each group (P < 0.05). No differences in plasma insulin were observed between groups.
Figure 2.
Plasma glucose (dotted line) and insulin (solid line) responses over a 2-h period to a 75-g oral glucose challenge in EB (▴) and EBE (▪) groups. No differences were detected between groups for either glucose or insulin at any of the indicated time points (P > 0.05).
RMR and caloric intake
RMR was not different between the EB (1628 ± 112; kcal/24 h) and EBE groups (1707 ± 77). Caloric intake of the EB group during the first week of the study (i.e. the week leading up to the first isoglycemic clamp) averaged 2267 ± 164 (kcal/d), and 2411 ± 125 in the EBE group. During the second week of the study, subjects in the EB group consumed 2293 ± 179, whereas those in the EBE group consumed 2834 ± 167 kcal/d (P < 0.04). The percentage of calories from carbohydrate, fat, and protein was not different between groups.
Body fat levels
No differences in body fat percentage were observed between the EB (39.5 ± 2.3%) and EBE groups (43.0 ± 2.0%) at baseline, or after the intervention (38.9 ± 2.4 vs. 42.6 ± 2.0%).
VO2 max
In total, eight subjects from the exercise group and six subjects from the control group had complete pre-post VO2 max data. VO2 max was not different at baseline between the EB (20.4 ± 1.7; ml/kg·min) and EBE groups (22.6 ± 1.2), and it was not different between groups (21.5 ± 1.9 vs. 22.1 ± 1.2) after the intervention.
Fasting plasma glucose values
Average fasting plasma glucose level was 131 ± 12 (mg/dl) for the EB group before the baseline clamp and 131 ± 12 mg/dl for the EBE group. Before the post-intervention clamp, fasting plasma glucose averaged 123 ± 11 and 128 ± 17 mg/dl in the EB and EBE groups, respectively. No differences were observed between groups for either clamp.
Clamp hormonal responses
Steady-state insulin values are presented in Table 2. Despite an increase in plasma insulin during each insulin infusion period of the clamp, no differences were observed between groups. As expected, the average plasma glucagon level (Table 2) was significantly (P < 0.01) lower during insulin infusion (i.e. at min 210 and 330 of the clamp) and lower during the high dose of insulin than the low dose (P < 0.03). No differences were observed between the EB and EBE groups at any time period. Despite the modest decline in plasma cortisol between time point zero and each subsequent time point (P < 0.0001 for each), no differences were observed between the EB and EBE groups (Table 2).
Table 2.
Measured values of selected hormones and substrates during the isoglycemic clamp
| Outcome variable | Group | Time point of the study | Time point of the clamp (min)
|
|||
|---|---|---|---|---|---|---|
| 0 | 90 | 210 | 330 | |||
| Plasma insulin (μU/ml) | EB | Pre | 16 ± 4 | 34 ± 4 | 69 ± 5 | |
| Post | 15 ± 4 | 32 ± 3 | 64 ± 3 | |||
| EBE | Pre | 15 ± 2 | 38 ± 2 | 65 ± 4 | ||
| Post | 13 ± 2 | 30 ± 2 | 59 ± 2 | |||
| Plasma cortisol (μg/dl) | EB | Pre | 21 ± 4 | 11 ± 1 | 12 ± 3 | 11 ± 2 |
| Post | 16 ± 2 | 10 ± 1 | 10 ± 3 | 9 ± 2 | ||
| EBE | Pre | 16 ± 2 | 10 ± 2 | 13 ± 2 | 13 ± 2 | |
| Post | 17 ± 2 | 9 ± 2 | 11 ± 2 | 12 ± 2 | ||
| Plasma glucagon (pg/ml) | EB | Pre | 58 ± 9 | 43 ± 6 | 37 ± 6 | 32 ± 5 |
| Post | 52 ± 8 | 39 ± 4 | 42 ± 7 | 35 ± 7 | ||
| EBE | Pre | 56 ± 7 | 50 ± 5 | 43 ± 3 | 41 ± 4 | |
| Post | 58 ± 3 | 49 ± 4 | 48 ± 4 | 42 ± 5 | ||
| Plasma glycerol (μm/liter) | EB | Pre | 308 ± 63 | 170 ± 19 | 143 ± 19 | 120 ± 26 |
| Post | 224 ± 44 | 220 ± 42 | 144 ± 36 | 129 ± 38 | ||
| EBE | Pre | 214 ± 40 | 178 ± 28 | 122 ± 9 | 129 ± 18 | |
| Post | 187 ± 25 | 148 ± 12 | 128 ± 11 | 71 ± 12 | ||
| Plasma FFA (mmol/liter) | EB | Pre | 0.53 ± 0.04 | 0.53 ± 0.05 | 0.19 ± 0.05 | 0.11 ± 0.03 |
| Post | 0.56 ± 0.08 | 0.50 ± 0.05 | 0.23 ± 0.05 | 0.10 ± 0.03 | ||
| EBE | Pre | 0.60 ± 0.05 | 0.56 ± 0.05 | 0.18 ± 0.03 | 0.08 ± 0.01 | |
| Post | 0.60 ± 0.05 | 0.55 ± 0.04 | 0.22 ± 0.02 | 0.08 ± 0.01 | ||
| Plasma lactate (μm/liter) | EB | Pre | 947 ± 226 | 580 ± 55 | 562 ± 52 | 570 ± 94 |
| Post | 551 ± 55 | 513 ± 34 | 480 ± 36 | 515 ± 65 | ||
| EBE | Pre | 620 ± 51 | 440 ± 36 | 474 ± 55 | 473 ± 62 | |
| Post | 604 ± 89 | 611 ± 79 | 542 ± 72 | 460 ± 46 | ||
| Glucose-specific activity (dpm/mg) | EB | Pre | 2254 ± 134 | 2273 ± 118 | 2297 ± 150 | |
| Post | 2356 ± 131 | 2334 ± 150 | 2382 ± 197 | |||
| EBE | Pre | 2237 ± 86 | 2198 ± 111 | 2209 ± 113 | ||
| Post | 2273 ± 202 | 2107 ± 127 | 2053 ± 67 | |||
No differences were observed between groups at any time point (P > 0.05). Pre, Before intervention; Post, after intervention.
Clamp substrate responses
Plasma lactate levels (Table 2) were significantly higher during the 0-min sampling point compared with the 90, 210, and 330-min points (P < 0.01 for each), although no differences were observed between groups. As expected, plasma glycerol levels (Table 2) were significantly lower during insulin infusion compared with both the 0 and 90-min time points of the clamp (P < 0.04 for each). However, there were no differences in plasma glycerol detected between the EB and EBE groups at any of these time points. Similar to glycerol, plasma unesterified free fatty acid (FFA) levels (Table 2) declined (P < 0.0001) with the infusion of insulin, and there was no difference between groups.
Measurement of PGU and EGP
Clamp glucose levels
The steady-state glucose level in the EB group averaged 128 ± 1, 137 ± 1, and 135 ± 1 mg/dl, and in the EBE group averaged 119 ± 1, 131 ± 1, and 123 ± 1 mg/dl during the postabsorptive, and low-dose and high-dose periods of insulin infusion. During the post-intervention clamp, plasma glucose levels in the EB group averaged 121 ± 1, 131 ± 1, and 119 ± 1 mg/dl, and in the EBE group averaged 116 ± 1, 129 ± 1, and 124 ± 1 mg/dl during the same respective time periods.
Glucose-specific activity
Glucose-specific activity levels are presented in Table 2. Glucose-specific activity was not different between the EB and EBE groups at any time point, and remained unchanged throughout the entire clamp (P > 0.05).
Whole-body insulin sensitivity
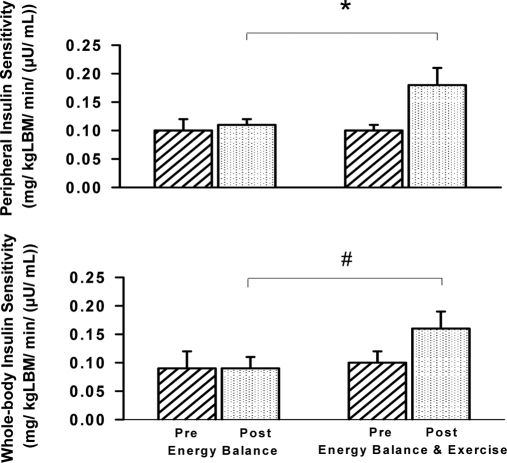
Whole-body insulin sensitivity (Fig. 3) is expressed as the exogenous glucose infusion rate (mg/kg·min) per unit of insulin, and during the preintervention clamp averaged 0.12 ± 0.02 and 0.09 ± 0.03 for the EB group, and 0.10 ± 0.01 and 0.10 ± 0.02 for the EBE group during the low-dose and high-dose insulin periods, respectively. During the post-intervention clamp, these respective values remained similar in the EB group (0.12 ± 0.02 and group 0.09 ± 0.02) and in the EBE group during the low dose insulin period (0.12 ± 0.01). However, whole body insulin sensitivity increased to 0.16 ± 0.03 during the high dose insulin period after exercise (P < 0.05).
Figure 3.
Peripheral (top panel) and whole-body (bottom panel) insulin sensitivity before (Pre) and after (Post) intervention during the high-insulin infusion period. *, The EBE group compared with the EB group after intervention (P < 0.0001). #, The EBE group compared with the EB group after intervention (P < 0.05).
PGU
PGU is expressed as the rate of isotopically determined disappearance of glucose (mg/min) per kg lean body mass (LBM). PGU during the preintervention clamp averaged 3.0 ± 0.2, 4.2 ± 0.8, and 6.4 ± 1.1 for the EB group, and 3.0 ± 0.2, 4.0 ± 0.4, and 6.3 ± 0.6 for the EBE group during the postabsorptive period, and the low and high-dose insulin periods, respectively. Although PGU did not change during these periods in the EB group during the post-intervention clamp (3.0 ± 0.2, 4.2 ± 0.6, and 6.8 ± 1.1), and did not change in the EBE group during the postabsorptive or low-dose insulin period (3.4 ± 0.4 and 4.0 ± 0.4), exercise training increased PGU to 10.0 ± 1.2 during the high-dose insulin period (P < 0.05).
Peripheral insulin sensitivity
Peripheral insulin sensitivity (Fig. 3) is expressed as the rate of PGU (mg/kg LBM·min) per unit of insulin. Values for the EB group during the preintervention clamp averaged 0.12 ± 0.02 and 0.09 ± 0.02, and for the EBE group averaged 0.11 ± 0.01 and 0.10 ± 0.01 during the low-dose and high-dose insulin periods, respectively. During the post-intervention clamp, these respective values did not change in the EB group (0.13 ± 0.02 and 0.11 ± 0.01) or in the EBE group during the low-dose insulin period (0.13 ± 0.01). In a pattern similar to whole-body insulin sensitivity, peripheral insulin sensitivity increased to 0.18 ± 0.03 for the EBE group during the high-dose insulin period (P < 0.0001).
EGP
EGP is expressed as the rate of isotopically determined endogenous glucose appearance (mg/min) per kg LBM. EGP during the preintervention clamp averaged 3.0 ± 0.2, 0.8 ± 0.3, and 0.5 ± 0.2 for the EB group, and 3.0 ± 0.2, 0.6 ± 0.2, and 1.0 ± 0.4 for the EBE group during the postabsorptive period, and the low and high-dose insulin periods, respectively. EGP did not change during these periods in the EB group during the post-intervention clamp (3.1 ± 0.3, 0.8 ± 0.3, and 1.3 ± 0.4), and did not in the EBE groups either (3.4 ± 0.4, 0.6 ± 0.2, and 1.9 ± 0.5).
Hepatic insulin sensitivity
Hepatic insulin resistance was calculated as the rate of EGP (mg/kg LBM·min) per unit of insulin. This value was then inverted to provide a measure of hepatic insulin sensitivity. Insulin sensitivity values for the EB group during the preintervention clamp averaged 41.7 ± 13.9 and 142.9 ± 61.2 during the low and high-dose insulin periods, respectively, and were similar (62.5 ± 15.6 and 62.5 ± 27.3) in the EBE group during the same periods. During the post-intervention clamp, hepatic insulin sensitivity for the EB group was 40.0 ± 12.8 and 50.0 ± 15.0 during the low and high-dose insulin periods, respectively, and similar (50.0 ± 15.0 and 31.3 ± 11.7) in the EBE group during the same periods. No differences in hepatic insulin sensitivity were observed between the EB and EBE groups, and no changes over the study period were observed in either group (P > 0.05).
Discussion
This study assessed the effects of 7-d aerobic exercise on EGP and PGU in obese humans with mild DM. The major finding of this study is that although 7-d exercise training did not affect EGP or hepatic insulin sensitivity, it did augment whole-body insulin sensitivity, PGU, and peripheral insulin sensitivity during hyperinsulinemia. These data indicate that improved glucose tolerance and whole-body insulin sensitivity after short-term aerobic exercise training that have been reported previously (6,20) are due primarily to changes in insulin-mediated PGU, not insulin-mediated suppression of EGP.
Previous studies have investigated the effect of aerobic exercise training on PGU and EGP in obese humans with DM, observing improved glucose tolerance and improved insulin sensitivity. Although those studies demonstrate the efficacy of aerobic exercise to improve whole-body insulin sensitivity, the experimental design often produces a loss of body fat or gains in aerobic power (i.e. VO2 max), each of which can improve insulin sensitivity independent of exercise training. Therefore, a number of studies have used exercise-training interventions that are from 1–10 d in duration to reduce the likelihood that such changes would occur, permitting a more accurate determination of the independent effect of exercise on whole-body glucose metabolism.
Previous research by Devlin et al. (14) showed that a single bout of exhaustive aerobic exercise in humans with DM improved peripheral insulin sensitivity during euglycemic clamp conditions, and other studies have used longer exercise training durations to determine the independent effect of aerobic exercise training on glucose tolerance and insulin sensitivity. Rogers et al. (20) reported that 7-d aerobic exercise training, using a training protocol similar to the one used in our study, improved glucose tolerance and insulin action in humans with mild DM. Similarly, Arciero et al. (6) showed that 10-d aerobic exercise training in humans with impaired glucose tolerance or “mild” DM increased whole-body glucose disposal during hyperglycemic clamp conditions. Although each of these studies (6,20) has furthered our understanding of the independent effect of aerobic exercise training on whole-body glucose tolerance and insulin sensitivity in humans with DM, they do not provide information on the relative contributions of changes to peripheral and hepatic tissues that are primarily responsible for improved whole-body glucose metabolism in response to exercise training.
Our results indicate that 7-d aerobic exercise training resulted in greater rates of PGU and increased peripheral insulin sensitivity during physiologically relevant hyperinsulinemia (i.e. insulin levels of ∼65 μU/ml). In contrast, with the lack of an extensive literature base examining the effect of aerobic exercise training on factors that regulate EGP, much has been done to improve our understanding of how aerobic exercise training affects factors that regulate PGU. PGU, which occurs primarily in skeletal muscle, is determined by the interaction of three factors: the delivery of glucose to the skeletal muscle (i.e. blood flow); transport of glucose into the cell; and rate of glucose phosphorylation after it has entered the cell. Although this investigation clearly does not attempt to determine the mechanisms responsible for changes in PGU that were observed, it appears that any of these factors, either singly or interactively, contributed to the observed increase in insulin-mediated PGU. Previous research has indicated that endothelial dysfunction is present in DM (28), although only a few investigations have examined the effect of exercise training on blood flow during resting conditions in DM. Sakamoto et al. (29) demonstrated that exercise training improved nitric oxide-mediated vasodilation, whereas other studies (30,31) have indicated that exercise training durations of 12–24 wk can prevent endothelial dysfunction from developing in streptozotocin-induced DM. Therefore, it is plausible that improved insulin-mediated endothelial function could contribute to improved muscle insulin sensitivity, although it is not clear whether such changes could occur after our relatively short training duration.
More thoroughly characterized are the adaptations of short-term exercise training on glucose transport into the muscle and subsequent phosphorylation. Ren et al. (19) showed that 2-d aerobic exercise training in healthy rats can increase insulin-stimulated hexokinase activity and Glut4 protein expression, and double both insulin-stimulated Glut4 translocation and glycogen synthesis. Studies in humans have similarly shown that 7-d aerobic exercise training can increase Glut4 protein expression and glycogen synthesis in those with and without DM (17,32) Thus, it is likely that a combination of these factors contributed to the improved peripheral insulin sensitivity we observed.
Previous research has demonstrated that aerobic exercise training improves hepatic insulin sensitivity during the postabsorptive state and during hyperinsulinemia. Rodnick et al. (16) showed that highly trained runners had lower rates of EGP during insulin infusion compared with otherwise healthy nonrunners, while Bogardus et al. (33) observed that 12-wk caloric restriction plus exercise in humans with insulin resistance reduced EGP during postabsorptive insulin levels and during hyperinsulinemia. In response to a single bout of exhaustive exercise in humans with DM, Devlin et al. (14) observed reduced EGP during the postabsorptive state and during insulin infusion similar to the high infusion dose used in our study. Our results suggest that 7-d aerobic exercise training did not have a significant impact on EGP in the presence of postabsorptive insulin levels, and it did not affect EGP during hyperinsulinemia. This finding is unexpected considering the number of studies using a similar intensity of exercise that have reported improved EGP during both the postabsorptive state and hyperinsulinemia. However, exhaustive exercise such as that used by Devlin et al. (14) appears to have different metabolic consequences compared with steady-state endurance exercise similar to that performed by subjects in our study. Camacho et al. (34) showed that steady-state endurance exercise resulted in an increase in hepatic AMP levels, resulting in a 2-fold increase in AMP-activated protein kinase (AMPK) phosphorylation in hepatocytes, whereas exhaustive exercise further increased AMP levels, resulting in a 7-fold increase in AMPK phosphorylation. Because of the considerable role that AMPK activation (i.e. phosphorylation) can have in the insulin resistant state (35), we postulate that steady-state endurance exercise may improve hepatic insulin sensitivity by chronic, low-level activation of AMPK that did not occur to a great enough extent in our subjects to bring about changes in hepatic glucose metabolism.
Another relevant consideration when interpreting our results is the mode of delivery of insulin into the body. During iv insulin infusion, the actions of insulin on hepatic glucose metabolism are primarily limited to those involving the indirect pathway (36). This pathway involves the suppression of glucagon release by the pancreas, FFA, and glycerol release by adipose tissue, amino acid release by muscle, and an increase in hepatic lactate production (37,38). That our exercise intervention did not bring about changes to hepatic insulin sensitivity is likely due to the absence of changes to any of these hormonal/substrate cues during insulin infusion. However, we cannot exclude the possibility that 7-d exercise training does improve the direct action of insulin on EGP, which is considered the dominant pathway of in vivo insulin action on the liver (39). This idea is supported by the work of Black et al. (40), who showed modest improvements to hepatic insulin sensitivity after a similar 1-wk intervention during iv glucose infusion (i.e. allowing for endogenous insulin release) in obese “at risk” humans that did not have DM. Thus, although reduced EGP may ensue after 7-d aerobic exercise training in response to an oral or iv glucose challenge due to endogenous insulin release, its contribution to improved glucose tolerance in obese humans with DM during these conditions is likely minimal. Considering the magnitude of the change in peripheral insulin sensitivity observed in the current study and the absence of a measured change in hepatic insulin sensitivity, improved whole-body glucose tolerance and insulin sensitivity that have been reported after 7- to 10-d aerobic exercise training (6,20) are likely caused primarily by improved peripheral insulin sensitivity.
In conclusion, this investigation demonstrates that 7-d aerobic exercise training improves whole-body insulin sensitivity in obese humans with mild DM. Furthermore, this improvement is caused primarily by gains of insulin sensitivity in peripheral tissues, and thus it is likely that a lengthier training duration is required to improve hepatic insulin sensitivity in obese humans with DM.
Acknowledgments
We thank all of the professionals from the Ohio State University Medical Center who lent their expertise to this project, including Sonny Edwards, Rodd Reinhart, Dr. Don Chalupa, Bob Fudge, Dr. Nicole Leenders, Janet Buffer, Ginger Childs, Candyce Walchesky, Lillian Perry, Lynnell Chandler, and especially Dr. Kwame Osei and the Ohio State University endocrinology fellows. We also thank Drs. Alan Cherrington and Dale Edgerton (Vanderbilt University Department of Molecular Physiology and Biophysics) for their critical review of this manuscript. Finally, but most importantly, we would like to thank the participants for their compliance with the study.
Footnotes
This work was supported by the International Journal of Sports Medicine (W.M.S. is Co-Editor-in-Chief) and The Ohio State University’s General Clinical Research Center (National Institutes of Health Grant M01RR00034).
Portions of this study were presented at the 67th Annual American Diabetes Association Conference, Chicago, Illinois, June 2007.
Disclosure Statement: The authors have nothing to declare.
First Published Online December 11, 2007
Abbreviations: AMPK, AMP-activated protein kinase; DM, diabetes mellitus; EB, energy balance only; EBE, energy balance plus 7-d aerobic exercise training; EGP, endogenous glucose production; FFA, unesterified free fatty acid; GCRC, General Clinical Research Center; Glut4, glucose transporter 4; HbA1c, glycosylated hemoglobin; LBM, lean body mass; max, maximum; OGTT, oral glucose tolerance test; PGU, peripheral glucose uptake; RMR, resting metabolic rate; VO2, oxygen consumption.
References
- Ohlson L, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, Bjorntorp P, Tibblin G 1985 The influence of body fat distribution on the incidence of diabetes. Diabetes 34:1055–1058 [DOI] [PubMed] [Google Scholar]
- Wei M, Gibbons L, Mitchell T, Kampert J, Lee C, Blair S 1999 The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med 130:89–96 [DOI] [PubMed] [Google Scholar]
- Sesso H, Paffenbarger R, Lee I 2000 Physical activity and coronary heart disease in men: The Harvard Alumni Health Study. Circulation 102:975–980 [DOI] [PubMed] [Google Scholar]
- Knowler DC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group 2002 Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K, Beck-Nielsen H, Kolterman O, Mandarino L 1988 Decreased activation of skeletal muscle glycogen synthase by mixed-meal ingestion in NIDDM. Diabetes 37:436–440 [DOI] [PubMed] [Google Scholar]
- Arciero P, Vukovich M, Holloszy J, Racette S, Hohrt W 1999 Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol 86:1930–1935 [DOI] [PubMed] [Google Scholar]
- Nagasaka S, Tokuyama K, Kusaka I, Hayashi H, Rokkaku K, Nakamura T, Kawakami A, Higashiyama M, Ishikawa S, Saito T 1999 Endogenous glucose production and glucose effectiveness in type 2 diabetic subjects derived from stable-labeled minimal model approach. Diabetes 48:1054–1060 [DOI] [PubMed] [Google Scholar]
- Magnusson I, Rothman D, Katz L, Shulman R, Shulman G 1992 Increased rate of gluconeogenesis in type II diabetes mellitus. J Clin Invest 90:1323–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L, Giaccari A, Barzilai N, Howard K, Sebel G, Hu M 1993 Mechanism by which hyperglycemia inhibits hepatic glucose production in conscious rats. J Clin Invest 92:1126–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldelli A, Baldi S, Pettiti M, Toschi E, Camastra S, Natali A, Landau B, Ferrannini E 2000 Influence of obesity and type 2 diabetes on gluconeogenesis and glucose output in humans. Diabetes 49:1367–1373 [DOI] [PubMed] [Google Scholar]
- Paquot N, Scheen A, Dirlewanger M, Lefebvre P, Tappy L 2002 Hepatic insulin resistance in obese non-diabetic subjects and in type 2 diabetic patients. Obes Res 10:129–134 [DOI] [PubMed] [Google Scholar]
- Minuk H, Vranic M, Marliss E, Hanna A, Albisser A, Zinman B 1981 Glucoregulatory and metabolic response to exercise in obese noninsulin-dependent diabetes. Am J Physiol 240:E458–E464 [DOI] [PubMed] [Google Scholar]
- Giacca A, Groenewoud Y, Tsui E, McClean P, Zinman B 1998 Glucose production, utilization, and cycling in response to moderate exercise in obese subjects with type 2 diabetes and mild hyperglycemia. Diabetes 47:1763–1770 [DOI] [PubMed] [Google Scholar]
- Devlin JT, Hirshman M, Horton ED, Horton ES 1987 Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes 36:434–439 [DOI] [PubMed] [Google Scholar]
- Segal K, Edano A, Abalos A, Albu J, Blando L, Tomas M, Pi-Sunyer F 1991 Effect of exercise training on insulin sensitivity and glucose metabolism in lean, obese, and diabetic men. J Appl Physiol 71:2402–2411 [DOI] [PubMed] [Google Scholar]
- Rodnick K, Haskell W, Swislocki A, Foley J, Reaven G 1987 Improved insulin action in muscle, liver, and adipose tissue in physically trained human subjects. Am J Physiol 253(5 Pt 1):E489–E495 [DOI] [PubMed] [Google Scholar]
- Dela F, Ploug T, Handberg A, Petersen L, Larsen J, Mikines K, Galbo H 1994 Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes 43:862–865 [DOI] [PubMed] [Google Scholar]
- Goodyear L, Hirshman M, Valyou P, Horton, E 1992 Glucose transporter number, function, and subcellular distribution in rat skeletal muscle after exercise training. Diabetes 41:1081–1090 [DOI] [PubMed] [Google Scholar]
- Ren J, Semenkovich C, Gulve E, Gao J, Holloszy J 1994 Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem 269:14396–14401 [PubMed] [Google Scholar]
- Rogers M, Yamamoto C, King D, Hagberg J, Ehsani A, Holloszy J 1988 Improvement in glucose tolerance after 1 wk of exercise in patients with mild NIDDM. Diabetes Care 11:613–618 [DOI] [PubMed] [Google Scholar]
- Segal K, Van Loan M, Fitzgerald P, Hodgdon J, Van Itallie T 1988 Lean body mass estimation by bioelectrical impedance analysis: a four-site cross validation study. Am J Clin Nutr 47:7–14 [DOI] [PubMed] [Google Scholar]
- Hother-Nielsen O, Beck-Nielsen H 1990 On the determination of basal glucose production rates in patients with type 2 (non-insulin-dependent) diabetes mellitus using primed-continuous 3-3H-glucose infusion. Diabetologia 33:603–610 [DOI] [PubMed] [Google Scholar]
- Harper R, Ennis C, Heaney A, Sheridan B, Gormley M, Atkinson A, Johnston G, Bell P 1995 A comparison of the effects of low- and conventional-dose thiazide diuretic on insulin action in hypertensive patients with NIDDM. Diabetologia 38:853–859 [DOI] [PubMed] [Google Scholar]
- DeFronzo R, Tobin J, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Neely R, Rooney D, Atkinson A, Sheridan B, Ennis C, Trimble E, Bell P 1990 Underestimation of glucose turnover determined using [6-3H] glucose tracer in non-steady state. Diabetologia 33:681–687 [DOI] [PubMed] [Google Scholar]
- Steele R 1959 Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82:420–430 [DOI] [PubMed] [Google Scholar]
- Angus D, Febbraio M, Hargreaves M 2002 Plasma glucose kinetics during prolonged exercise in trained humans when fed carbohydrate. Am J Physiol Endocrinol Metab 283:E573–E577 [DOI] [PubMed] [Google Scholar]
- Williams S, Cusco J, Roddy M, Johnstone M, Creager M 1996 Impaired nitric-oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 27:567–574 [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Minami K, Niwa Y, Ohnaka M, Nakaya Y, Mizuno A, Kuwajima M, Shima K 1998 Effect of exercise training and food restriction on endothelium-dependent relaxation in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous NIDDM. Diabetes 47:82–86 [DOI] [PubMed] [Google Scholar]
- Amatyakul S, Chakraphan D, Chotipaibulpan S, Patumraj S 2006 Role of exercise training on pulpal blood flow in diabetic rats. Clin Hemorheol Microcirc 34:295–301 [PubMed] [Google Scholar]
- Chakraphan D, Sridulyakul P, Thipakorn B, Bunnag S, Huxley V, Patumraj S 2005 Attenuation of endothelial dysfunction by exercise training in STZ-induced diabetic rats. Clin Hemorheol Microcirc 32:217–226 [PubMed] [Google Scholar]
- Kraniou G, Cameron-Smith D, Hargreaves M 2004 Effect of short term training on GLUT-4 mRNA and protein expression in human skeletal muscle. Exp Physiol 89:559–563 [DOI] [PubMed] [Google Scholar]
- Bogardus C, Ravussin E, Robbins D, Wolfe R, Horton E, Sims E 1984 Effects of physical training and diet therapy on carbohydrate metabolism in patients with glucose intolerance and non-insulin dependent diabetes mellitus. Diabetes 33:311–318 [DOI] [PubMed] [Google Scholar]
- Camacho R, Donahue P, James F, Berglund E, Wasserman D 2006 Energy state of the liver during short-term and exhaustive exercise in C57BL/6J mice. Am J Physiol Endocrinol Metab 290:E405–E408 [DOI] [PubMed] [Google Scholar]
- Iglesias M, Ye J, Frangioudakis G, Saha A, Tomas E, Ruderman N, Cooney G, Kraegen E 2002 AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes [Erratum (2003) 52:223–224] 51:2886–2894 [DOI] [PubMed] [Google Scholar]
- Cherrington AD 1999 Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 48:1198–1214 [DOI] [PubMed] [Google Scholar]
- Sindelar D, Balcom J, Chu C, Neal D, Cherrington A 1996 A comparison of the effects of selective increases in peripheral or portal insulin on hepatic glucose production in the conscious dog. Diabetes 45:1594–1604 [DOI] [PubMed] [Google Scholar]
- Sindelar D, Chu C, Rohlie M, Neal D, Swift L, Cherrington A 1997 The role of fatty acids in mediating the effects of peripheral insulin on hepatic glucose production in the conscious dog. Diabetes 46:187–196 [DOI] [PubMed] [Google Scholar]
- Edgerton D, Lautz M, Scott M, Everett C, Stettler K, Neal D, Chu C, Cherrington A 2006 Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest 118:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black SE, Mitchell E, Freedson PS, Chipkin SR, Braun B 2005 Improved insulin action following short-term exercise training: role of energy and carbohydrate balance. J Appl Physiol 99:2285–2293 [DOI] [PubMed] [Google Scholar]