Abstract
Context: Evidence for an association between alcohol consumption and activity of the hypothalamic-pituitary-adrenal (HPA) axis is inconclusive.
Objective: Our objective was to assess the relationship between indices of alcohol consumption and salivary cortisol concentration.
Design: This was a cross-sectional study of alcohol consumption and cortisol secretion from phase 7 (2002–2004) of the Whitehall II study.
Setting: An occupational cohort originally recruited in 1985–1987 was included in the study.
Participants: A total of 2693 men and 977 women had information on cortisol levels and alcohol consumption.
Outcome Measures: Saliva samples were taken on waking, waking + 0.5, 2.5, 8, and 12 h, and bedtime for the assessment of cortisol.
Results: In men there was a positive association between cortisol and units of alcohol intake per week (3% increase in cortisol per unit of alcohol consumed; P = 0.010). The slope of cortisol decline over the day in heavy drinkers was reduced (heavy drinkers β = −0.155, moderate drinkers β = −0.151), indicating reduced control of the HPA axis in heavy drinkers. In women the cortisol awakening response was greater in heavy drinkers 14.15 nmol/liter (9.12–19.17) compared with moderate drinkers 8.69 nmol/liter (7.72–9.67) (P = 0.037).
Conclusions: This study suggests that alcohol consumption is associated with activation of the HPA axis. These results are not due to alcohol consumption on the day, suggesting chronic changes of the HPA axis in heavy drinking groups.
This study suggests an association between different indices of alcohol intake and daily release of cortisol. Heavy drinkers have greater amounts of the hormone, indicating reduced control and chronic changes of the hypothalamic-pituitary-adrenal axis.
Excess cortisol levels, a product of the hypothalamic-pituitary-adrenal (HPA) axis, have been shown to be detrimental to health. They are related to hypertension, impairment of immune function, and alteration of metabolism (1,2,3). Given the recent evidence that the HPA axis may be associated with this broad range of morbidities (4), understanding predictors of HPA axis function assumes increasing importance. The behavioral determinants of HPA axis activity are poorly understood. Recent evidence demonstrates that cortisol secretion is associated with smoking behavior (5), but the association with alcohol consumption in community populations is not well examined.
The majority of evidence from animal studies suggest a direct effect of alcohol on HPA axis activity (6,7). The function of the HPA axis in alcohol-dependant people and those going through the withdrawal process has been altered (8,9,10,11), but extrapolation from these studies to the general population is difficult. Few studies have looked at the relationship between alcohol consumption and endocrine function in human populations (12). Alcohol consumption is often included as a covariate in analysis when examining the function of the HPA axis (13), but not investigated directly, or it is measured poorly.
We are aware of two epidemiological studies examining the function of the HPA axis in relation to one measure of alcohol consumption in general population samples. The first, in an apparently healthy male working population, found elevated cortisol levels in heavy drinkers (14). The second study also suggests increased HPA axis activity in heavy drinking groups (15), but plasma cortisol was measured once, and covariates were not fully accounted for in the analysis.
Cortisol, a marker of HPA axis activity, can be measured in urine, blood, or saliva (16). The HPA axis has a diurnal rhythm; therefore, repeated measures are needed. The use of salivary cortisol allows measurement in a naturalistic setting (17,18), with minimal inconvenience for participants. It allows measurement of the activity of the HPA axis capturing the cortisol awakening response (CAR) and levels over the day.
To our knowledge the assessment of the relationship between alcohol consumption and salivary cortisol levels in a naturalistic setting has not been reported. Our study has enough power to investigate different indices of alcohol consumption and account for relevant covariates. In this paper we examine the relation between alcohol consumption and two markers of cortisol secretion (CAR and slope of decline in levels over the day). Furthermore, we examine whether these relationships are independent of a wide number of covariates.
Subjects and Methods
Participants
Data reported here are from phase 7 (2002–2004) of the Whitehall II study. The Whitehall II cohort initially recruited 10,308 participants between 1985 and 1988 (phase 1) from 20 London based civil service departments. The number participating at phase 7 was 6941. Of those participants that attended the screening clinic and were asked to collect saliva samples, 90.1% (n = 4609) returned samples. Details of the clinical assessment and cohort profile have been reported elsewhere (19). Ethical approval for the Whitehall II study was obtained from the University College London Medical School committee on the ethics of human research. Informed consent was gained from every participant.
Cortisol analysis
To collect a saliva sample, participants used a device called a Salivette (Sarstedt, Leicester, UK). Participants were recruited by a face-to-face interview at the end of their clinical assessment; they were asked to complete saliva sampling the following day and mail samples back. The participants were instructed to provide six samples at waking, waking + 30min, waking + 2.5 h, waking + 8 h, waking + 12, h and bedtime. An instruction booklet also recorded information on wake time, smoking, alcohol consumption, and stressful events on the day of sampling. Returned samples were stored at −80 C until assay. Salivette devices were centrifuged at 3000 rpm for 5 min, resulting in a clear supernatant of low viscosity. Salivary cortisol levels were measured using a commercial immunoassay with chemiluminescence detection (IBL Hamburg, Hamburg, Germany). The lower concentration limit of this assay is 0.44 nmol/liter; intraassay and interassay coefficients of variance were less than 8%. Any sample over 50 nmol/liter was repeated.
Alcohol consumption
The frequency of drinking alcohol was assessed in a self-completed questionnaire by asking “In the last 12 months have you taken an alcoholic drink?”, with six response options: “twice a day or more”; “daily or almost daily”; “once or twice a week”; “once or twice a month”; “special occasions only”; or “No.”
Mean alcohol intake was calculated from questionnaire answers to “In the last seven days, how many drinks of each of the following have you had?”; a separate response box for spirits (measures), wine (glasses), and beer (pints) was provided. This was calculated into equivalent units; in the United Kingdom for standard measures, 1 U alcohol is equivalent to 8 g ethanol. Alcohol consumption on the day of sampling was assessed from the response to “Did you drink alcoholic beverages today?”: yes or no.
Heavy drinking was defined using a cutoff of more than or equal to 28 U for men and more than or equal to 21 U for women per week. Problem drinking was assessed using the cutting down, annoyance by criticism, guilty feeling, and eye-openers (CAGE) questionnaire (20), which is a tool for detection of problem drinkers, not a clinical diagnostic tool (21). It includes four questions relating to drinking behavior. Each question has “yes” or “no” options. Persons stating “yes” for two or more questions were classified as “problem drinkers.”
Covariates
Job grade was determined if still in the civil service, or based on their last employment grade if they left the service. For this analysis, civil service grades were collapsed into three categories (22).
Income was determined by “What is the current yearly amount you receive from the above sources added together?”, with eight possible answers ranging from “less than £9,999′” to “more than £70,000′.” Low income was defined as earning less than £15,000 a year.
Wealth was determined by the question “If you sold all your assets your household owns for example [list of assets] and cashed in savings and investments and paid off all your debts (including your mortgage) how much money do you think you would have?” Low wealth defined as having less than £100,000. Further details on the definitions of income and wealth can be found in the study by Martikainen et al. (23).
Financial insecurity was assessed by “Thinking of the next ten years how financially secure do you feel?”, with secure, fairly secure, fairly insecure, and insecure the possible answers. Persons answering fairly insecure and insecure were classed as feeling financially insecure, as in the study by Ferrie et al. (24).
Smoking status was categorized as either a current, ex, or never smoker. If any nicotine replacement therapy was listed, they were assigned a smoker (n = 4). In terms of healthy eating, participants were classed as having a poor diet if they reported less than once a week consumption of fruit or vegetables.
Systolic and diastolic blood pressure (BP) was measured at the clinical screening after a 5-min rest in a sitting position, using an Omron HEM 907 (Omron Healthcare, Inc., Bannockburn, IL). The measurement was taken twice, and the average was used in this analysis. Sleep disturbance was assessed with the scale described by Jenkins et al. (25), consisting of four items regarding sleep quality over the past month. There were six response options ranging from zero (not at all) to five (22–31 d). The total was summed to give a score between zero and 20. Body mass index (BMI) was calculated from measures of height and weight taken at the clinical screening calculated as weight (kg)/height (m)2. Depression was measured using the 30-item General Health Questionnaire (GHQ) depression subscale. This consists of four items, all items scored on a Likert scale from zero to three. Respondents scoring three or more were assigned “GHQ depression cases.” This is not a clinical definition of depression, but one validated to be used for the 30-item GHQ (26). Stress on the day of sample collection was assessed with the questions “We’d like to know if this was a typical day for you, compared with your usual workdays (or weekends), in terms of how busy, pressured, or stressed you felt.” Options were “Today was typical/greater/lower in terms of my workload or stress level.” Participants were asked about the most stressful event and if it made them feel “not at all stressed,” “somewhat stressed,” “moderately stressed,” “very stressed,” or “the most stressed I have ever felt.” Participants were classified as having a stressful experience if they were “very stressed” or “the most stressed I have ever felt.”
Statistical analysis
From the samples returned, 168 individual samples were not taken by participants (0.55% of the number expected). During laboratory analysis, 1002 individual samples were not assayed for technical reasons. Missing cortisol data were imputed for participants missing samples at times 3, 4, or 5 [number imputed for sample 3 (n = 16), 4 (n = 37), and 5 (n = 36)] using the average of the previous and subsequent sample. The analyses were repeated with and without imputation. No statistical differences were observed. The results using imputation are presented here. Outliers were assessed for each time using a box plot [time 1 (n = 4), time 2 (n = 5), time 3 (n = 5), time 4 (n = 4), time 5 (n = 4), and time 6 (n = 3)] and removed from analysis. Any participants taking sample 1 more than 10 min after waking were excluded from analysis (n = 634); this is the commonly used cutoff when investigating daytime cortisol levels because the CAR is already substantially under way (27). The distribution of all alcohol consumption measures and if participants followed the protocol correctly were assessed. Those taking the first sample more than 10 min after waking were more likely to score on the CAGE questionnaire P < 0.05 for men and women. Participants taking medications (e.g. glucocorticoids such as prednisolone) affecting cortisol levels were removed from the analysis (n = 236). For this analysis the sample was restricted to those respondents who had data on cortisol levels, alcohol consumption, and covariates included.
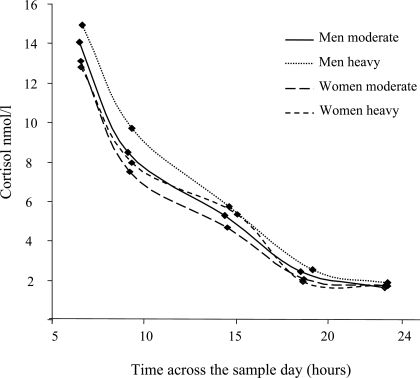
The cortisol data were not normally distributed, therefore, all data were log transformed for analysis. The CAR was calculated by subtracting cortisol at time 1 from cortisol at time 2. The slope of the decline in cortisol levels over the day is calculated by regressing cortisol values on time after waking samples were taken for samples 1 and 3–6. To ensure the CAR does not obscure the slope calculation, sample 2 is not included. Figure 1 illustrates the unadjusted cortisol levels in men and women by time of day samples taken for samples 1 and 3–6. The mixed models procedure in SPSS (SPSS, Inc., Chicago, IL) was used, cortisol and time each sample taken are nested within person. Covariates are then added as between-person variables, interaction terms between time samples taken over the day and covariates are entered into the model and used to assess if significant differences in rate of cortisol decline over the day exist between groups. The slope of cortisol decline over the day is a negative coefficient, therefore, the lower (more negative) the slope, the more rapid the decline in cortisol levels, whereas slope values closer to zero reflect flatter diurnal rhythms (28,29,30). Men and women were analyzed separately as is common for assessment of alcohol consumption.
Figure 1.
Unadjusted mean cortisol (nmol/liter) by heavy drinking category in men (n = 2167) and women (n = 738) by mean time of day each sample was taken. The markers on the lines represent the average cortisol concentration and average time sample taken for samples 1 and 3–6.
The main analysis focused on the relationship between cortisol and frequency of consumption, weekly alcohol consumption, and CAGE score. These were analyzed using univariate ANOVA. Linear regression was performed on weekly unit consumption and CAGE score. All analyses were initially run adjusting for age only (model 1), then an indicator of whether alcohol was consumed on the day of saliva sample collection (model 2). A third included BMI and smoking status (model 3). The final model included sleep disturbance, depressive symptoms, last employment grade, financial insecurity, stressful event on the day, and if it was a typical day (model 4). Assessment of the CAR was also adjusted for wake-up time in all models. To keep tables brief, only models 1 and 4 are displayed. Adjustment for multiple comparisons was made using Bonferroni correction; the statistical package used in all analyses was SPSS version 14.0.
Results
Participants’ characteristics are shown in Table 1. A total of 2693 men and 977 women with valid data were available for analysis. Men reported a higher frequency of drinking, consumed more alcohol per week, had higher CAGE score, and had higher BP and mean cortisol production over the day than women. Greater proportions of women were in the lowest social position groups, suffered from depression (GHQ defined), and had sleep problems.
Table 1.
Participants’ characteristics for men and women who had data available for indices of alcohol consumption and cortisol secretion
| Men | Women | |
|---|---|---|
| No. with valid data | 2693 | 977 |
| Mean age (sd)a | 60.89 yr (5.85) | 61.36 yr (6.06) |
| Social position (%) | ||
| Lowest employment gradea | 3.3 | 26.2 |
| Lowest incomea | 11.8 | 44.1 |
| Lowest wealtha | 5.4 | 14.8 |
| Physical characteristics | ||
| Smokers (%)a | 10.2 | 9.4 |
| Less than once a week eat fresh fruit/vegetable (%) | 1.8 | 1.5 |
| Mean systolic BP (sd)a | 128.29 mm Hg (15.85) | 126.88 mm Hg (18.33) |
| Mean diastolic BP (sd)a | 74.74 mm Hg (10.23) | 73.35 mm Hg (10.69) |
| Mean sleep disturbance score (sd)a | 5.00 (4.17) | 6.56 (4.91) |
| Mean BMI (sd)a | 26.6 kg/m2 (3.86) | 27.29 kg/m2 (5.61) |
| Psychological characteristics | ||
| Financial insecurity (%)a | 8.5 | 15.4 |
| Mean depression score (sd)a,b | 0.81 (1.69) | 1.04 (1.82) |
| Very stressful experience on the day of sampling (%)a | 5.4 | 11.2 |
| More busy or stressed than usual (%)a | 12.6 | 17.3 |
| Alcohol consumption | ||
| Frequency (%)a | ||
| Twice a day or more | 6.5 | 2.1 |
| Daily or almost daily | 44.6 | 29.2 |
| Once or twice a week | 32.3 | 27.4 |
| Once or twice a month | 7.5 | 11.0 |
| Special occasions only | 6.0 | 21.6 |
| No | 4.4 | 8.6 |
| CAGE score (%)a | ||
| 0 | 71.1 | 82.8 |
| 1 | 17.7 | 11.4 |
| 2 | 7.7 | 4.6 |
| 3 | 3.0 | 0.9 |
| 4 | 0.5 | 0.2 |
| Problem drinking (CAGE 2+) (%)a | 11.2 | 5.8 |
| Mean weekly alcohol intake (sd)a | 113.01 g (110.17) | 48.50 g (60.27) |
| Drinking ≥ 28/21 U (%)a | 12.8 | 3.7 |
A significant (P ≤ 0.05) difference between men and women (χ2 test used for categorical variables and ANOVA for continuous variables).
Defined using the GHQ depression subscale.
We assessed the distribution of potential confounders across the categories of frequency of drinking (data not shown). In men, less frequent alcohol consumers were in the lowest employment grade, wealth, and income groups, felt more financially insecure, (P < 0.001 for all), had higher depressive symptoms (P = 0.004), and consumed less fruit and vegetables (P = 0.006). In men, more frequent drinkers were older (P = 0.002), had higher systolic and diastolic BP (P = 0.002 and P < 0.001, respectively), and contained a higher proportion of smokers (P = 0.001). Frequency of drinking was not associated with sleep disturbance, BMI, stressful event on the day, or if it was a typical day. In women, trends across frequency of consumption mirror those in men for measures of social position, financial insecurity, and age (P < 0.001 for all). In addition, in women, more frequent drinkers had higher BMI (P < 0.001), were more likely to be smokers (P = 0.001), and report a poor diet (P = 0.035). Frequency of drinking was not associated with sleep disturbance, BP, depressive symptoms, very stressful event on the day, or if it was a typical day.
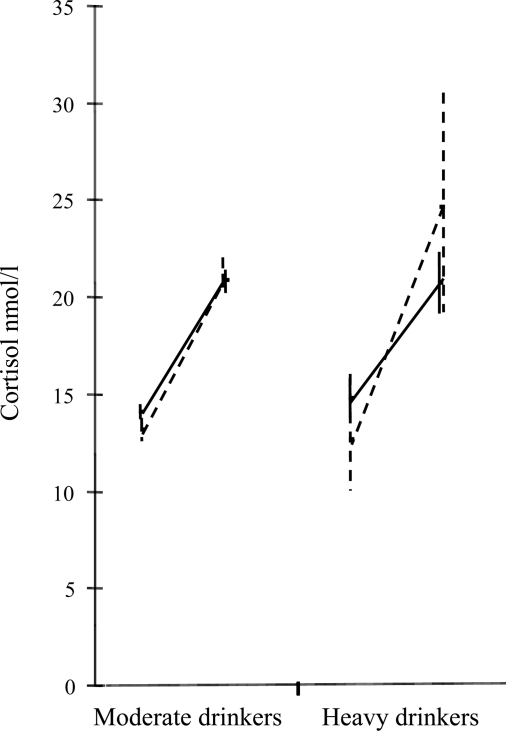
The CAR was assessed (Table 2), and no relationships were apparent for men. Interestingly, the nondrinkers had a larger CAR, however, these were nonsignificant relationships. In women an array of effects were apparent; heavy drinking was associated with a larger CAR in fully adjusted models. The CAR in heavy compared with moderate drinkers for men and women is further illustrated by Table 3 and Fig. 2.
Table 2.
CAR nmol/liter (plus 95% confidence interval) by frequency of alcohol consumption, weekly alcohol intake, and CAGE score in men (n = 2167) and women (n = 738), adjusted for covariates
| Men
|
Women
|
|||
|---|---|---|---|---|
| Model 1 | Model 4 | Model 1 | Model 4 | |
| Frequency (past 12 months) | ||||
| Twice a day or more | 4.97 (3.06–6.88) | 5.24 (3.25–7.23) | 12.23 (5.31–19.15) | 13.07 (5.92–20.21) |
| Daily or almost daily | 6.99 (6.25–7.74) | 7.22 (6.41–8.03) | 8.33 (6.54–10.12) | 8.84 (6.76–10.93) |
| Once or twice a week | 8.44 (7.59–9.30) | 8.27 (7.38–9.16) | 8.82 (7.06–10.59) | 8.78 (7.01–10.56) |
| Once or twice a month | 8.26 (6.49–40.03) | 7.85 (6.01–9.69) | 9.54 (6.64–12.44) | 9.22 (6.24–12.19) |
| Special occasions only | 7.15 (5.12–9.18) | 6.84 (4.70–8.97) | 8.96 (6.90–11.02) | 8.51 (6.26–10.77) |
| No | 10.02 (7.09–12.96) | 9.96 (6.68–12.71) | 9.255 (5.82–12.69) | 9.03 (5.39–12.67) |
| P value for ANOVA | 0.005 | 0.084 | 0.911 | 0.910 |
| Heavy drinkinga | ||||
| Moderate consumption | 7.75 (7.20–8.26) | 7.70 (7.17–8.23) | 8.65 (7.65–9.70) | 8.69 (7.72–9.67) |
| Heavy consumption | 6.42 (4.91–7.72) | 6.42 (5.01–7.83) | 14.80 (9.76–19.80) | 14.15 (9.12–19.17) |
| P value for ANOVA | 0.085 | 0.098 | 0.015 | 0.037 |
| U/wk | ||||
| % Change in cortisol for 1 U increase in alcohol | −25 (−60 to 10) | −3 (−44 to 37) | 37 (−96 to 171) | 89 (−72 to 251) |
| P value for linear trend | 0.164 | 0.874 | 0.583 | 0.278 |
| CAGE | ||||
| <2 | 7.57 (7.04–8.10) | 7.58 (7.04–8.11) | 8.96 (7.80–9.94) | 8.88 (7.85–9.92) |
| ≥2 | 7.33 (5.83–8.82) | 7.25 (5.72–8.77) | 12.10 (7.89–16.42) | 11.97 (7.61–16.32) |
| P value for ANOVA | 0.749 | 0.691 | 0.150 | 0.178 |
| Linear regression percent change for one point increase | 27 (−599 to 653) | 172 (−478 to 821) | −14 (−505 to 476) | 4 (−499 to 619) |
| P value for linear trend | 0.932 | 0.604 | 0.954 | 0.890 |
Model 1 includes age and waking time. Model 4 includes age, waking time, alcohol consumption on the day, BMI, smoking status, depression scale, sleep disturbance, financial insecurity, last known employment grade, very stressful experience, and typical day.
Moderate consumption for men is less than 28 U/wk, heavy is more than or equal to 28 U. Moderate consumption for women is less than 21 U/wk, heavy is more than or equal to 21 U.
Table 3.
Mean levels of cortisol (nmol/liter) (plus 95% confidence interval) for samples 1 and 2 by drinking category in men (n = 2167) and women (n = 738), adjusted for covariates
| Cortisol sample | Moderate drinkers | Heavy drinkers | |
|---|---|---|---|
| Men | Sample 1 | 14.07 (13.69–14.46) | 14.75 (13.72–15.86) |
| Sample 2 | 20.84 (20.25–21.44) | 20.70 (19.18–22.33) | |
| Women | Sample 1 | 13.17 (12.58–13.78) | 12.68 (10.03–16.02) |
| Sample 2 | 21.07 (20.15–22.04) | 24.25 (19.25–30.56) |
Figure 2.
FIG. 2. CAR nmol/liter (plus 95% confidence interval) by heavy drinking category, in men (n = 2167) and women (n = 738), adjusted for covariates, adjusted for age, waking time, alcohol consumption on the day, BMI, smoking status, depression scale, sleep disturbance, financial insecurity, last known employment grade, very stressful experience, and typical day. Men are the solid line and women the dashed line.
The slope of cortisol release was calculated and is displayed in Table 4 for men. A significant relationship exists for heavy drinkers; in the fully adjusted model, heavy drinkers have a flatter slope than moderate drinkers (slopes are closer to zero). The slope of secretion in women is displayed in Table 5. Different rates of decline in cortisol are apparent between the frequency of alcohol consumption groups and these remain significant in the fully adjusted model. There is an indication of flatter slopes of secretion in heavy drinking groups, but this is not significant.
Table 4.
Slope of cortisol decline over the day (β) (calculated as log of cortisol level regressed onto time sample was taken), and intercept by frequency of alcohol consumption, weekly alcohol intake, and CAGE score in men (n = 2141), adjusted for covariates
| Model 1
|
Model 4
|
|||
|---|---|---|---|---|
| β | Intercept | β | Intercept | |
| Frequency (past 12 months) | ||||
| Twice a day or more | −0.043 | 1.90 | −0.146 | 2.91 |
| Daily or almost daily | −0.156 | 2.56 | −0.165 | 3.32 |
| Once or twice a week | −0.115 | 2.92 | −0.138 | 3.16 |
| Once or twice a month | −0.069 | 1.96 | −0.065 | 1.36 |
| Special occasions only | −0.178 | 3.41 | −0.225 | 3.36 |
| No | −0.118 | 3.57 | −0.143 | 3.64 |
| P value for difference in slope | 0.136 | 0.586 | ||
| Heavy drinkinga | ||||
| Moderate consumption | −0.125 | 2.77 | −0.155 | 3.16 |
| Heavy consumption | −0.120 | 2.64 | −0.151 | 3.12 |
| P value for difference in slope between the groups | 0.865 | 0.784 | ||
| P value for difference in slope using units of consumption a week | 0.033 | 0.011 | ||
| CAGE | ||||
| <2 | −0.126 | 2.74 | −0.138 | 3.08 |
| ≥2 | −0.125 | 2.66 | −0.222 | 3.27 |
| P value for difference in slope between the groups | 0.677 | 0.764 | ||
| P value for difference in slope for CAGE score | 0.161 | 0.519 | ||
Model 1 includes age and waking time. Model 4 includes age, waking time, alcohol consumption on the day, BMI, smoking status, depression scale, sleep disturbance, financial insecurity, last known employment grade, very stressful experience, and typical day.
Moderate consumption is less than 28 U/wk and heavy more than or equal to 28 U.
Table 5.
Slope of cortisol decline over the day, and intercept by frequency of alcohol consumption, weekly alcohol intake, and CAGE score in women (n = 724), adjusted for covariates
| Model 1
|
Model 4
|
|||
|---|---|---|---|---|
| β | Intercept | β | Intercept | |
| Frequency (past 12 months) | ||||
| Twice a day or more | −0.004 | 2.73 | −0.124 | 1.94 |
| Daily or almost daily | −0.210 | 3.08 | −0.245 | 3.37 |
| Once or twice a week | −0.112 | 2.54 | −0.126 | 2.57 |
| Once or twice a month | −0.205 | 3.08 | −0.195 | 3.14 |
| Special occasions only | −0.089 | 2.52 | −0.102 | 2.54 |
| No | −0.082 | 2.47 | −0.129 | 2.35 |
| P value for difference in slope | 0.014 | 0.013 | ||
| Heavy drinkinga | ||||
| Moderate consumption | −0.144 | 2.90 | −0.167 | 2.70 |
| Heavy consumption | −0.077 | −0.05 | −0.015 | −0.956 |
| P value for difference in slope between the groups | 0.573 | 0.774 | ||
| P value for difference in slope using units of consumption a week | 0.244 | 0.195 | ||
| CAGE | ||||
| <2 | −0.174 | 2.96 | −0.193 | 3.12 |
| ≥2 | −0.063 | 0.36 | −0.057 | 1.50 |
| P value for difference in slope between the groups | 0.489 | 0.741 | ||
| P value for difference in slope for CAGE score | 0.551 | 0.641 | ||
Model 1 includes age and waking time. Model 4 includes age, waking time, alcohol consumption on the day, BMI, smoking status, depression scale, sleep disturbance, financial insecurity, last known employment grade, very stressful experience, and typical day.
Moderate consumption is less than 21 U/wk, and heavy is more than or equal to 21 U.
Discussion
The principal aim of the study was to examine the relationship between alcohol consumption and cortisol secretion. The results indicate that, in a large community dwelling population, alcohol consumption has a positive relationship with cortisol release over the day. An increased number of alcohol units consumed per week and heavy drinking are associated with increased cortisol levels. The slope of cortisol decline was flatter in heavy drinkers; this indicates reduced inhibitory control of the HPA axis, as described by Thayer et al. (14). A greater array of effects was apparent in women than in men. However, the proportion of heavy and problem drinking women in this cohort is small, and this finding deserves further investigation. This is one of few studies examining the relation between alcohol and cortisol in a large community dwelling population.
Our findings accord with Thayer (14) and Gianoulakis (15) et al. The results described in our study are robust to adjustment and are found in a much larger sample than previously described. We were able to investigate a number of different measures of alcohol consumption, and explore the diurnal rhythm of cortisol secretion. Mediators and confounders were adjusted for, and the results remain statistically significant. Of the four measures of alcohol consumption investigated, the strongest predictor of increased cortisol levels in men was units of alcohol consumed per week. This is in accordance with other studies investigating endocrine function and alcohol consumption (31). In men in this cohort, there is little evidence that frequency of consumption per se is detrimental to the function of the HPA axis, but the volume of consumption and heavy drinking are related to poorer endocrine function. The findings in women suggest that all three measures of alcohol consumption are associated with a wider array of effects on the HPA axis, and affect the CAR and slope differently. This could indicate that drinking behavior has different effects on men and women. This has not been described previously, but due to the small number of heavy and problem female drinkers in this cohort, it deserves wider investigation.
The biological basis the association described is not clear. A direct acute effect of alcohol on the HPA axis is theoretically possible, as in animal studies (7), but our finding of a flatter slope after considering the effect of alcohol consumption on the day of sampling and an alteration in the CAR in women suggest that additional mechanisms are at play. Excess alcohol consumption damages liver function and may, therefore, reduce the bodies’ ability to metabolize cortisol (or other active substances of the HPA axis); this would result in altered endocrine function. Impaired inhibitory control of the HPA axis in heavy drinking groups has been the proposed mechanism (14). Our results are consistent with other studies in men in that alcohol consumption is associated with a change in endocrine function (12).
Although the effects of alcohol consumption on cortisol secretion in this cohort were relatively small, acute differences in cortisol concentration of this magnitude have had a detrimental effect on insulin and glucose function (2). Previously, alcohol intake has predicted the development of type 2 diabetes (31,32,33). Our findings may provide a mechanism for these associations because increased cortisol is related to insulin resistance (34). The analysis of heavy drinking provides some support for the hypothesis that heavy drinking has a more detrimental effect on health than regular drinking, and that the physiological responses to different drinking patterns vary.
The advantages and disadvantages of the current analyses need to be stated. The number of participants with valid data on cortisol levels was 39% of phase 1 of the Whitehall II study. However, of those still participating at phase 7 and asked to complete cortisol collection, 90% returned the samples; there were no differences between participants and those who refused by age, gender, or last known employment grade. This is a cross-sectional study, and salivary cortisol has been measured at one phase only; therefore, the direction of causation cannot be determined. Increased stress can increase cortisol levels and influence a person’s health behaviors; however, adjustment for concurrent indicators of stress and alcohol consumption and for items (sleep disturbance, depression, financial insecurity, and last known employment grade) that may represent or be associated with chronic stress failed to explain fully the associations observed. The use of a self-report measure of problem drinking is a limitation, however, the CAGE questionnaire has had high validity (35,36). Adherence to the protocol was assessed by a self-reported measure (time of taking samples); it is possible that some people who followed the protocol incorrectly might have been included in the analysis. It is also possible, although unlikely, that increased cortisol levels encourage the adoption of detrimental alcohol consumption behaviors. The Whitehall II study was set up as an occupational cohort. At this phase it now represents an older age group (55–72 yr) and, therefore, may not be fully representative of the general population. In our analysis the results are significant, even with the relatively small variation in drinking behavior; this would suggest that the effects within the general population would be greater.
In summary, this study suggests that there is a positive relationship between different indices of alcohol intake and daily release of cortisol. The mechanisms mediating these relationships are not fully elucidated but appear to be independent of the potential confounders and mediators examined. This is the first study of alcohol consumption and diurnal cortisol secretion in a naturalistic setting. This study confirms laboratory work and smaller studies that describe alteration of the HPA axis in heavy drinkers.
Acknowledgments
We thank all participants involved in the Whitehall II study and the Whitehall study team.
Footnotes
The Whitehall II study has been supported by grants from: the Medical Research Council; Economic and Social Research Council; British Heart Foundation, Health and Safety Executive; Department of Health; National Heart Lung and Blood Institute (HL36310); United States National Institutes of Health, National Institute on Aging (AG13196); United States National Institutes of Health, Agency for Health Care Policy Research (HS06516); and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health. M.M. is supported by a Medical Research Council Research Professorship.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 11, 2007
Abbreviations: BMI, Body mass index; BP, blood pressure; CAGE, cutting down, annoyance by criticism, guilty feeling, and eye-openers; CAR, cortisol awakening response; GHQ, General Health Questionnaire; HPA, hypothalamic-pituitary-adrenal.
References
- Plat L, Leproult R, L’Hermite-Baleriaux M, Fery F, Mockel J, Polonsky KS, Van Cauter E 1999 Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab 84:3082–3092 [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E 1999 Impact of sleep debt on metabolic and endocrine function. Lancet 354:1435–1439 [DOI] [PubMed] [Google Scholar]
- Whitworth JA, Williamson PM, Mangos G, Kelly JJ 2005 Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag 1:291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelli F, Giustina A 2000 Glucocorticoid-induced osteoporosis. Trends Endocrinol Metab 11:79–85 [DOI] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M 2007 The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab 92:819–824 [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW 2000 Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res 24:1836–1849 [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, Rivier C 1998 Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcohol Clin Exp Res 22(5 Suppl):243S–247S [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M 2003 Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol 38:189–193 [DOI] [PubMed] [Google Scholar]
- Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G 1996 An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology 21:263–275 [DOI] [PubMed] [Google Scholar]
- Bernardy NC, King AC, Parsons OA, Lovallo WR 1996 Altered cortisol response in sober alcoholics: an examination of contributing factors. Alcohol 13:493–498 [DOI] [PubMed] [Google Scholar]
- Groote VR, Meinders AE 1996 On the mechanism of alcohol-induced pseudo-Cushing’s syndrome. Endocr Rev 17:262–268 [DOI] [PubMed] [Google Scholar]
- Adler RA 1992 Clinical review 33: clinically important effects of alcohol on endocrine function. J Clin Endocrinol Metab 74:957–960 [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht SR, Brydon L, Wardle J 2004 Central adiposity and cortisol responses to waking in middle-aged men and women. Int J Obes Relat Metab Disord 28:1168–1173 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hall M, Sollers III JJ, Fischer JE 2006 Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int J Psychophysiol 59:244–250 [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Brown T 2003 Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary β-endorphin as a function of alcohol intake, age, and gender. Alcohol Clin Exp Res 27:410–423 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C 2004 Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology 29:983–992 [DOI] [PubMed] [Google Scholar]
- Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschbaum C, Hellhammer DH, Stone AA 1997 Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology 22:89–105 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH 1989 Salivary cortisol in psychobiological research: an overview. Neuropsychobiology 22:150–169 [DOI] [PubMed] [Google Scholar]
- Marmot M, Brunner E 2005 Cohort profile: the Whitehall II study. Int J Epidemiol 34:251–256 [DOI] [PubMed] [Google Scholar]
- Ewing JA 1984 Detecting alcoholism. The CAGE questionnaire. JAMA 252:1905–1907 [DOI] [PubMed] [Google Scholar]
- Ewing JA 1998 Screening for alcoholism using CAGE. Cut down, Annoyed, Guilty, Eye opener. JAMA 280:1904–1905 [DOI] [PubMed] [Google Scholar]
- Kuper H, Marmot M 2003 Job strain, job demands, decision latitude, and risk of coronary heart disease within the Whitehall II study. J Epidemiol Community Health 57:147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen P, Adda J, Ferrie JE, Davey SG, Marmot M 2003 Effects of income and wealth on GHQ depression and poor self rated health in white collar women and men in the Whitehall II study. J Epidemiol Community Health 57:718–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrie JE, Shipley MJ, Stansfeld SA, Smith GD, Marmot M 2003 Future uncertainty and socioeconomic inequalities in health: the Whitehall II study. Soc Sci Med 57:637–646 [DOI] [PubMed] [Google Scholar]
- Jenkins CD, Stanton BA, Niemcryk SJ, Rose RM 1988 A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol 41:313–321 [DOI] [PubMed] [Google Scholar]
- Stansfeld SA, Head J, Marmot MG 1998 Explaining social class differences in depression and well-being. Soc Psychiatry Psychiatr Epidemiol 33:1–9 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C 2003 Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med 65:313–319 [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL 2005 Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology 30:92–100 [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T 2006 Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med 68:41–50 [DOI] [PubMed] [Google Scholar]
- Matthews K, Schwartz J, Cohen S, Seeman T 2006 Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med 68:657–661 [DOI] [PubMed] [Google Scholar]
- Holbrook TL, Barrett-Connor E, Wingard DL 1990 A prospective population-based study of alcohol use and non-insulin-dependent diabetes mellitus. Am J Epidemiol 132:902–909 [DOI] [PubMed] [Google Scholar]
- Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG 1995 Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ 310:560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC 1995 Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ 310:555–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmond R 2003 Stress induced disturbances of the HPA axis: a pathway to type 2 diabetes? Med Sci Monit 9:RA35–RA39 [PubMed] [Google Scholar]
- Bernadt MW, Mumford J, Taylor C, Smith B, Murray RM 1982 Comparison of questionnaire and laboratory tests in the detection of excessive drinking and alcoholism. Lancet 1:325–328 [DOI] [PubMed] [Google Scholar]
- Mayfield D, McLeod G, Hall P 1974 The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 131:1121–1123 [DOI] [PubMed] [Google Scholar]