Abstract
Context: Studies on the regulation of LH secretion by sex steroids in men are conflicting.
Objective: Our aims were to determine the relative contributions of testosterone (T) and estradiol (E2) to LH regulation and localize their sites of negative feedback.
Design: This was a prospective study with three arms.
Setting: The study was conducted at a General Clinical Research Center.
Patients or Other Participants: Twenty-two normal (NL) men and 11 men with GnRH deficiency due to idiopathic hypogonadotropic hypogonadism (IHH) participated.
Intervention: Medical castration and inhibition of aromatase were achieved using high-dose ketoconazole (KC) for 7 d with 1) no sex steroid add-back; 2) T enanthate 125 mg im starting on d 4; or 3) E2 patch 37.5 μg/d starting on d 4. Blood sampling was performed every 10 min for 12 h at baseline, overnight on d 3–4 and d 6–7.
Main Outcome Measures: Mean LH levels, LH pulse amplitude, and GnRH pulse frequency were assessed at baseline, d 3–4, and d 6–7.
Results: In NL men, KC caused a 3-fold increase in mean LH on d 3–4, which was stable on d 6–7 with no add-back. Addition of T reduced LH levels (34.6 ± 3.9 to 17.4 ± 3.6 IU/liter, P < 0.05) by slowing GnRH pulse frequency (13.3 ± 0.4 to 6.7 ± 1.0 pulses/12 h, P < 0.005). LH amplitude increased (6.9 ± 1.0 to 12.1 ± 1.4 IU/liter, P < 0.005). E2 add-back suppressed LH levels (36.4 ± 5.6 to 19.0 ± 2.4 IU/liter, P < 0.005), by slowing GnRH pulse frequency (11.4 ± 0.2 to 8.6 ± 0.4 pulses/12 h, P < 0.05) and had no impact on LH pulse amplitude. In IHH men, restoring normal T levels caused no suppression of mean LH levels or LH amplitude. E2 add-back normalized mean LH levels and decreased LH amplitude from 14.7 ± 1.7 to 12 ± 1.5 IU/liter (P < 0.05).
Conclusions: 1) T and E2 have independent effects on LH. 2) Inhibition of LH by T requires aromatization for its pituitary, but not hypothalamic effects. 3) E2 negative feedback on LH occurs at the hypothalamus.
This study examining sex hormone regulation of LH in men suggests that testosterone and estradiol have independent effects on LH secretion; inhibition of LH by testosterone requires aromatization for its pituitary but not hypothalamic effects; and estrogen has dual sites of feedback with predominant hypothalamic effects.
Under physiological conditions, serum LH levels in men are kept within a tight range, reflecting the balance between stimulation by GnRH and inhibition by gonadal sex steroids. However, questions remain about the precise mechanisms of the sex steroid component to LH regulation, specifically the relative contributions of testosterone (T) and estradiol (E2), the degree to which aromatization to E2 is important in mediating T’s effects, and the respective sites of feedback of these hormones. Previous work by our group (1,2) and others (3) suggested that T and E2 were equally effective at suppressing endogenous LH levels in healthy men (Table 1). However, the estrogen replacement regimens in these studies resulted in supraphysiological E2 levels. Similarly, some investigators have reported that aromatization is needed for T negative feedback at the pituitary (4), whereas others have suggested that T’s restraint of LH secretion is largely conditional on its aromatization at both hypothalamic and pituitary levels (3). In contrast, other studies provide evidence for an E2-independent effect of T by demonstrating elevated LH levels in patients with androgen insensitivity (5) and in healthy men after administration of anti-androgens (6,7,8). Similarly, previous work from our group showed that the increase in LH levels in healthy men in response to selective E2 suppression with an aromatase inhibitor is only one third of that seen when both T and E2 are suppressed to castrate levels (9).
Table 1.
Summary of key studies on sex steroid regulation of LH secretion in men
| Ref. | Steroid regimen | Subjects | Mean LH | LH amp | LH frequency |
|---|---|---|---|---|---|
| Finkelstein et al. (1) | T 15 mg/d × 4 d | Normal men GnRH-deficient men | ↓58% ↓28% | ↓ ↓ | ↓ EC |
| Finkelstein et al. (2) | E2 90 μg/d × 4 d | Normal men GnRH-deficient men | ↓52% ↓42% | ↓ ↓ | ↓ EC |
| Schnorr et al. (3) | KC with T patch 7.5 mg/d, KC with E2 patch 50 μg/d, KC with T patch plus AI | Normal men | ↑ 3-fold after KC; ↓ to BL with T or E2 No change after T plus AI | Not assessed | Not assessed |
| Bagatell et al. (4) | T 15 mg/d × 3 d E290 μg/d × 3 d DHT 500 μg/d × 3 d | GnRH-deficient men | ↓40% ↓55% no change | Not assessed | EC |
| Santen et al. (24) | E2 3.5 μg/h × 6 h T 600 μg/h × 6 h | Normal men | ↓21% ↓18% | ↓ ↑ | No Δ ↓ |
AI, Aromatase inhibitor; EC, experimentally controlled.
A number of issues have hindered clinical investigation in this field and contribute to the discrepancies between studies. First, use of pharmacological sex steroid regimens precludes conclusions about the relative importance of T and E2 for LH regulation under normal physiological conditions. Second, the aromatase inhibitor used in some studies, testolactone, was subsequently shown to have additional anti-androgenic activity (10). Third, in the intact male, gonadotropin secretion reflects the integrated response of both the hypothalamus and pituitary. Some investigators have assumed that any change in LH pulse frequency reflects an exclusively hypothalamic site of negative feedback and, conversely, that all changes in LH pulse amplitude are determined solely by alterations in pituitary sensitivity to GnRH. However, there is actually a linear relationship between the amount of GnRH secreted per pulse and the amplitude of the ensuing pituitary LH response (11,12). Therefore, it follows that any change in LH amplitude could, in fact, reflect either a pituitary and/or a hypothalamic effect. In the human, it is not possible to measure GnRH to help distinguish hypothalamic from pituitary effects because of its confinement to the hypophyseal-portal circulation and short half-life.
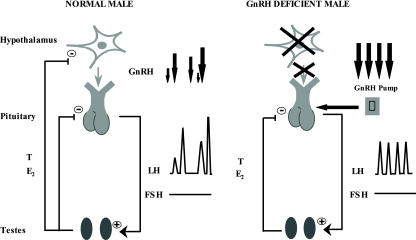
Accurate localization of the sites of T and E2 feedback in the human thus requires a complementary model in which the dose and frequency of GnRH can be experimentally controlled. Men with idiopathic hypogonadotropic hypogonadism (IHH), who lack endogenous hypothalamic GnRH secretion and whose pituitary-gonadal axis can be normalized with long-term pulsatile GnRH replacement (13), provide such a model (Fig. 1). Because the dose and frequency of exogenous GnRH therapy can be experimentally controlled, this model in effect represents a hypothalamic clamp. Therefore, any effects of altering gonadal steroid levels on gonadotropin secretion in such GnRH-deficient men can only reflect a pituitary site of action. In contrast, in normal men with an intact hypothalamic-pituitary gonadal axis, gonadal steroids can modulate gonadotropin secretion by pituitary and/or hypothalamic effects (Fig. 1). Thus, using the tandem study of these two human models, a hypothalamic site of action of sex steroids can be inferred whenever there is a difference in the gonadotropin responses of normal and GnRH-deficient men to alterations in their sex steroid milieu.
Figure 1.
Schematic of the hypothalamic-pituitary gonadal axis in normal and GnRH-deficient men. In normal men, T and E2 can exert negative feedback at both the hypothalamus and pituitary. In contrast, men with congenital GnRH deficiency lack endogenous GnRH secretion. However, their pituitary-gonadal axis can be normalized with pulsatile GnRH therapy. Because the dose and frequency of exogenous GnRH are experimentally controlled, this model represents a hypothalamic clamp. Therefore, any effect on gonadotropin secretion of altering gonadal steroids in such GnRH-deficient men can reflect only a pituitary site of action. Thus, a hypothalamic site of action of sex steroids can be inferred whenever there is a difference in the gonadotropin responses of normal and GnRH-deficient men to alterations in their sex steroid milieu.
Thus, the aims of this study were to 1) determine the relative contributions of T and E2 to LH regulation in men, 2) assess the degree to which aromatization mediates T’s effects on LH, and 3) localize the precise sites of T and E2 negative feedback.
Subjects and Methods
Subjects
Healthy normal men (NL men)
Thirty-one healthy NL men (age 30.1 ± 7 yr) were enrolled in the study. All study subjects met the following criteria: 1) normal pubertal development, sexual function, and general health; 2) normal physical examination including a testicular volume of at least 15 ml; 3) normal renal and hepatic function; 4) normal serum levels of T, E2, LH, FSH, TSH, and prolactin; and 5) normal semen analysis according to World Health Organization criteria (14).
GnRH-deficient men
Thirteen men (35.8 ± 9.2 yr) with congenital IHH participated in the study. The diagnosis of IHH was based on the following criteria: 1) failure to go through puberty by age 18 yr, 2) serum T less than or equal to 100 ng/dl (3.5 nmol/liter) in association with inappropriately low gonadotropin levels, 3) absence of normal endogenous gonadotropin pulsations during a 12- to 24-h period of frequent blood sampling, 4) otherwise normal reserve testing of anterior pituitary function, and 5) a normal hypothalamic-pituitary region by magnetic resonance imaging. At the time of study participation, all subjects had normalized their serum T, LH, and FSH for at least 3 months with pulsatile GnRH therapy delivered at 2-h intervals (13), which they continued to receive throughout the study.
The study was approved by the Human Research Committee at Massachusetts General Hospital, and all subjects provided written informed consent before the initiation of any study related procedures.
Study design
Subjects were studied using an ablation and replacement model. A detailed baseline (BL) neuroendocrine evaluation was performed in the presence of normal sex steroids. It was repeated on d 3–4 of acute sex steroid ablation and again on d 6–7 with or without selective sex steroid add-back from d 4–7. Eleven NL and three GnRH-deficient men were withdrawn from the study because of adverse events (abnormal liver function tests, skin rash, or nausea). Therefore, 20 NL and 11 GnRH-deficient subjects completed the protocol. One NL volunteer participated in two arms and five GnRH-deficient men participated in more than one arm of the study. In each case, the interval between consecutive studies was at least 3 months.
All subjects were admitted to the General Clinical Research Center at Massachusetts General Hospital at 1800 h, and commencing at 2000 h, blood was drawn every 10 min for 12 h for their BL evaluation. Medical castration and inhibition of aromatase activity were then induced using high-dose ketoconazole (KC), 1 g loading dose followed by 400 mg four times daily for 7 d. KC is a potent inhibitor of C17–20 lyase, the rate-limiting step in androgen biosynthesis (15), and we (9,16) and others (3) have previously shown that this regimen suppresses T levels to the castrate range within 24 h. At high doses, KC also inhibits both cortisol biosynthesis and aromatase activity (17,18). For this reason, we gave study participants dexamethasone (0.5 mg twice daily) for the duration of the study, having first shown that this dose does not suppress gonadotropin or testosterone secretion in men (9).
After completing the BL evaluation, subjects participated in one or more of the following regimens.
1) Sex steroid ablation alone (T−, E2−)
Nine NL and five GnRH-deficient men received KC for 7 d and underwent repeat overnight frequent-sampling studies on d 3–4 and d 6–7.
2) Sex steroid ablation with T add-back (T+, E2−)
Six NL and GnRH-deficient men received the same KC regimen but were replaced with T for d 4–7 administered as a single im injection of T enanthate 125 mg after completing the second frequent-sampling study. The fact that KC inhibits aromatase meant that concomitant administration of an aromatase inhibitor was not necessary to prevent endogenous E2 levels from increasing in response to T add-back.
3) Sex steroid ablation with E2 add-back (T−, E2+)
Seven NL and seven IHH men received KC for 7 d with selective add-back of E2 on d 4–7 administered as a transdermal patch at a dose of 37.5 μg/d.
All samples were assayed for LH and free α-subunit (FAS). T and E2 levels were determined from a study pool composed of equal aliquots of each sample drawn at 10-min intervals. For every frequent-sampling study, each of the 10-min LH and FAS results was averaged to give a mean, and this single mean value from each subject was then used for statistical analysis.
Pulsatile LH and FAS secretion were analyzed using a modified Santen and Bardin (19) method as previously validated by our group (20). Because we have shown FAS to be a better marker of GnRH secretion at fast pulse frequencies, FAS was used to analyze pulse frequency (20).
Hormone assays
Serum LH concentrations were determined by microparticle enzyme immunoassay using an automated Abbott AxSYM system (Abbott Laboratories, Chicago, IL). The Second International Reference Preparation was used as the reference standard. The assay sensitivity for LH was 1.6 mIU/ml with an intraassay coefficient of variation (CV) of less than 7% and an interassay CV of less than 7.4%. FAS concentrations were determined by a previously described monoclonal antibody RIA, using highly purified α-subunit of human chorionic gonadotropin as standard. The cross-reactivity of LH in the FAS assay was 0.7%, compatible with the known contamination of the human LH standard with FAS (21). Serum T concentrations were measured using the DPC Coat-A-Count RIA kit (Diagnostics Products Corp., Los Angeles, CA), which has an intra- and interassay CV of less than 10%. E2 was measured by RIA using hexane ethylacetate extraction and LH-20 chromatography (Endocrine Sciences, Calabasas Hills, CA). This E2 assay has a sensitivity of 5 pg/ml (18 pmol/liter) and, based on a male serum pool, has an intraassay CV of 4.9% and an interassay CV of 15%; the normal range for adult men in this assay is 8–35 pg/ml.
Statistical methods
Data are presented as mean ± sem unless otherwise stated. Respective pair-wise comparisons between BL, d 3–4, and d 6–7 were performed using two tailed t tests. When assay results were below the level of detection, the level of detection was used for statistical analysis, and a P value < 0.05 was considered statistically significant.
Results
Normal men
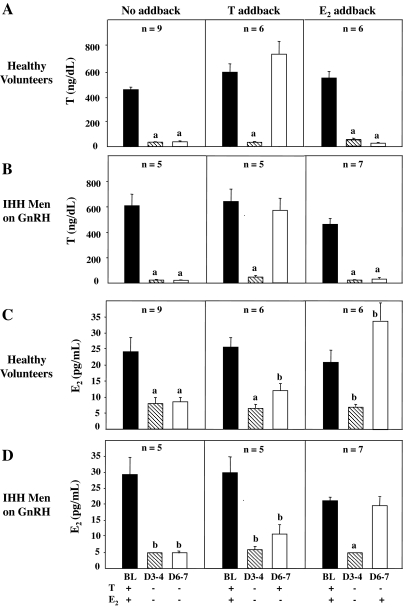
The KC regimen employed was successful in creating the desired sex steroid milieu (Fig. 2, A and C). In all three study arms, T levels decreased from the physiological range at BL to castrate levels on d 3–4 of KC administration (P < 0.005) (Fig. 2A). In subjects receiving no sex steroid add-back, T and E2 levels on d 6–7 remained suppressed at 37 ± 8 ng/dl, P < 0.005 (Fig. 2A) and 9 ± 1 pg/ml (P < 0.005) (Fig. 2C), respectively. T add-back restored T levels on d 6–7 (728 ± 99 ng/dl) to BL values (Fig. 2A) without any significant increase in E2 levels (11 ± 2 pg/ml) (Fig. 2C). Similarly, E2 administration restored E2 levels on d 6–7 to the normal range (34 ± 6 pg/ml), although levels were slightly higher than BL concentrations of 21 ± 4 pg/ml (P < 0.05) (Fig. 2C).
Figure 2.
Serum T and E2 levels in healthy male volunteers (A and C) and GnRH-deficient men on GnRH therapy (B and D) during medical castration for 7 d with either no sex steroid add-back or replacement with T or E2 from d 4–7. Values represent the pool of samples drawn every 10 min for 12 h. a, Significantly different from BL, P < 0.005; b, significantly different from BL, P < 0.05.
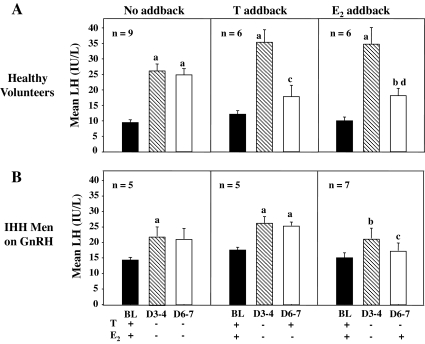
In NL men receiving no sex steroid add-back, mean LH levels increased from 9.5 ± 0.9 to 26.2 ± 2.1 IU/liter on d 3–4 (P < 0.005) and remained stable at 25.0 ± 2.0 on d 6–7 (Fig. 3A). After T replacement, mean LH levels decreased from 34.6 ± 3.9 to 17.4 ± 3.6 IU/liter (P < 0.05), such that levels on d 6–7 were not significantly different from BL (Fig. 3A). E2 add-back caused LH levels to decrease from 36.4 ± 5.6 to 19.0 ± 2.4 IU/liter (P < 0.005); however, values on d 6–7 remained significantly higher than BL concentrations of 10.5 ± 1·3 IU/liter (P < 0.05) (Fig. 3A).
Figure 3.
Mean LH levels in healthy male volunteers (A) and GnRH-deficient men on GnRH therapy (B) during medical castration for 7 d with either no sex steroid add-back or replacement with T or E2 from d 4–7. Values represent the mean of samples drawn every 10 min for 12 h. a, Significant difference from BL, P < 0.005; b, significant difference from BL, P < 0.05; c, significant difference between d 3–4 and d 6–7, P < 0.05; d, significant difference between d 3–4 and d 6–7, P < 0.005.
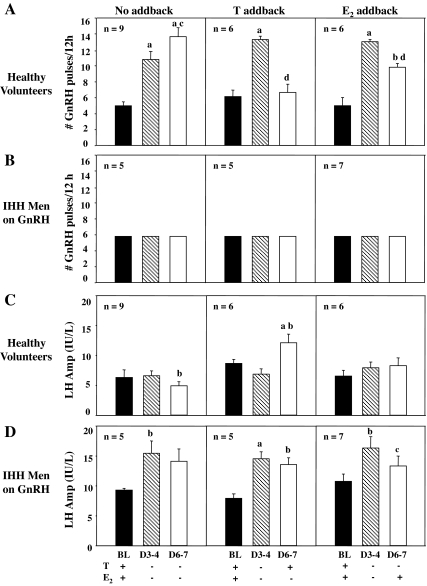
In NL subjects receiving no sex steroid add-back, GnRH pulse frequency increased from 5.1 ± 0.5 pulses/12 h at BL to 10.6 ± 0.8 on d 3–4 (P < 0.005) and 14.3 ± 0.9 on d 6–7 (P < 0.0005) (Fig. 4A). Administration of T slowed GnRH pulse frequency to that seen at BL (Fig. 4A). E2 administration also slowed pulse frequency, but the number of pulses on d 6–7 remained significantly higher than BL (8.6 ± 0.4 vs. 4.4 ± 0.9, P < 0.05) (Fig. 4A).
Figure 4.
GnRH pulse frequency and LH pulse amplitude in healthy male volunteers (A and C) and IHH men on GnRH therapy maintained at a constant 2 h frequency (B and D) during medical castration for 7 d with either no sex steroid add-back or replacement with T or E2 from d 4–7. a, Significant difference from BL, P < 0.005; b, significant difference from BL, P < 0.05; c, significant difference between d 3–4 and d 6–7, P < 0.05; d, significant difference between d 3–4 and d 6–7, P < 0.005.
In subjects receiving no sex steroid add-back, LH pulse amplitude on d 3–4 was unchanged from that at BL (6.6 ± 0.8 vs. 6.3 ± 1.3 IU/liter, respectively), but levels on d 6–7 were significantly lower than d 3–4 (4.9 ± 0.7 vs. 6.6 ± 0.8 IU/liter, P < 0.05) (Fig. 4C). Administration of T was associated with an increase in LH amplitude from 6.9 ± 1.0 IU/liter on d 3–4 to 12.1 ± 1.4 IU/liter on d 6–7 (P < 0.005) (Fig. 4C). No significant changes in LH pulse amplitude were seen with E2 add-back (7.9 ± 1.0 on d 3–4 vs. 8.3 ± 1.3 IU/liter on d 6–7) (Fig. 4C).
GnRH-deficient men
As in the NL men, the desired hormonal milieu was achieved in the GnRH-deficient subjects with sustained suppression of T (Fig. 2B) and E2 levels (Fig. 2D) in those receiving no add-back. Restoration of physiological T levels on d 6–7 (574 ± 95 ng/dl) with T replacement (Fig. 2B) occurred without a significant increase in E2 levels (Fig. 2D). Similarly, E2 administration led to normalization of E2 levels on d 6–7 (23.0 ± 4.4 IU/liter) (Fig. 2D). In GnRH-deficient men who received no add-back, mean LH levels increased from 14.4 ± 0.9 to 22.2 ± 2.9 IU/liter (P < 0.05) on d 3–4, and remained stable at 21.1 ± 3.4 IU/liter on d 6–7 (Fig. 3B). In striking contrast to the response of the NL men, administration of T to the GnRH-deficient subjects caused no suppression of either mean LH levels (Fig. 3B) or LH pulse amplitude (Fig. 4D) on d 6–7 when their GnRH pulse frequency was maintained constant at 2 h (Fig. 4B). E2 replacement, on the other hand, normalized mean LH levels (Fig. 3B) and LH pulse amplitude on d 6–7 (Fig. 4D).
Discussion
Our tandem study of normal and GnRH-deficient men provides unique insights to the understanding of LH regulation in the human male. Using the experimental model of biochemical castration and selective sex steroid replacement, our data demonstrate that T and E2 have independent effects on LH secretion. The demonstration that T can slow GnRH pulse frequency in normal men while E2 levels remain suppressed indicates that T feedback at the hypothalamus can occur via a direct androgen effect that does not require aromatization. In contrast, the failure of T add-back to suppress either mean LH levels or LH amplitude in the GnRH-deficient men who are maintained at a constant dose and frequency of GnRH administration indicates that T’s negative feedback at the pituitary is mediated by aromatization to E2. These data also confirm previous studies indicating that E2 has dual sites of negative feedback (22,23). However, the demonstration that E2 lowers LH levels in the intact male by slowing pulse frequency without any effect on pulse amplitude adds to our existing knowledge by suggesting that the dominant site of E2 feedback is, in fact, at the hypothalamus.
One of the challenges to studying endogenous gonadotropin feedback mechanisms in humans is developing an experimental model capable of faithfully recreating a normal sex steroid milieu. A major strength of this study is that we were successful in this regard. KC was effective in reducing T and E2 to castrate levels. The T add-back regimen we used restored T concentrations to BL values in both normal and GnRH-deficient men. In keeping with the previous demonstration that KC inhibits aromatase activity (17,18), we saw no significant increase in E2 levels after T add-back. Similarly, the dose of transdermal estrogen we used restored E2 levels to BL values in the IHH men; E2 levels in the normal men after E2 add-back, although slightly higher than BL, were still within the physiological range at 34 pg/ml. In contrast, other groups have failed to achieve adequate E2 suppression using a KC regimen similar to ours except for omission of the loading dose (3). Similarly, previous studies by our group (2) and others (3) used estrogen replacement regimens that resulted in supraphysiological E2 levels.
In previous studies, we showed that the increase in LH in response to selective E2 suppression was significantly lower than that seen after suppression of both T and E2, suggesting that T has both direct effects on LH mediated through the androgen receptor as well as indirect effects mediated by aromatization (9). However, in that study, we were unable to determine whether it was the hypothalamic or pituitary effects of T that required aromatization. The demonstration by Bagatell and colleagues (4) that the nonaromatizable androgen dihydrotestosterone causes no suppression of LH levels in GnRH-deficient men undergoing GnRH administration suggested that aromatization is needed for T’s negative feedback at the pituitary. Based on the demonstration that addition of an aromatase inhibitor completely blocks the inhibition of LH secretion by T in men, Schnorr et al. (3) suggested that T’s restraint of LH secretion is largely conditional on its in vivo aromatization at both the hypothalamic and pituitary level. The present study demonstrates that T can normalize GnRH pulse frequency in healthy men when E2 levels remain suppressed, thereby indicating that T’s feedback at the hypothalamus is a direct androgen effect. In contrast, the failure of T add-back to suppress either mean LH levels or LH amplitude in GnRH-deficient men maintained at a constant GnRH pulse frequency indicates that T’s negative feedback at the pituitary requires aromatization as previously suggested (4).
These data confirm previous work from our group, which using an aromatase inhibitor to cause selective suppression of E2 in normal and GnRH-deficient men, showed that E2 has both hypothalamic and pituitary sites of negative feedback in the male (22). Using a human ablation and replacement model, the present study confirms a hypothalamic site of action of E2 by demonstrating a decrease in GnRH pulse frequency in normal men in response to selective physiological E2 add-back, whereas the suppression of mean LH levels and LH pulse amplitude in GnRH-deficient men provide evidence for a pituitary site of negative feedback. The failure of several previous studies to show any impact of exogenous estrogen administration on LH pulse frequency in normal men (24,25,26) is likely due to the suboptimal 20-min sampling paradigm for LH that was employed resulting in erroneous estimations. An additional strength of the present study is the use of FAS secretion rather than LH to analyze changes in GnRH pulse frequency. We previously demonstrated the superiority of this marker of GnRH secretion over LH at fast GnRH pulse frequencies (20). Had we relied on LH as a surrogate of GnRH pulsatility in response to medical castration, we would have picked up 40% fewer pulses on d 3 of sex steroid ablation and failed to show a suppressive effect of E2 on GnRH pulse frequency as a result.
Had this study been confined to healthy men, the demonstration that E2 reduces mean LH levels by lowering LH pulse frequency without any apparent changes in LH pulse amplitude could have led one to conclude that E2 feedback occurs exclusively at the hypothalamus. However, previous work from our group has shown that there is an inverse relationship between GnRH pulse frequency and LH amplitude (27). Therefore, if the dominant effect of E2 is to slow pulse frequency, an additional suppressive effect of E2 at the pituitary level might be masked by the tendency for LH amplitude to increase at slower frequencies. An alternative possibility is that E2 suppresses the amplitude of GnRH-induced LH pulses to such a degree that some LH pulses are no longer detectable, leading to the erroneous conclusion that LH pulse frequency is actually reduced.
In the present study, the inclusion of GnRH-deficient men maintained on a fixed dose and frequency of exogenous GnRH allowed a pituitary site of action to be identified based on the suppression of both mean LH levels and LH amplitude after E2 add-back. Our conclusion from the present study that the hypothalamus is the dominant site of E2 feedback on LH in the male is supported by the following observations. First, selective suppression of E2 levels in healthy men causes a marked increase in GnRH pulse frequency, despite a concomitant increase in endogenous T levels that would normally serve to restrain the GnRH pulse generator (22). Second, administration of antiestrogens to normal men also causes an increase in LH pulse frequency (28,29,30,31,32).
The demonstration in this study that circulating E2 can modify GnRH secretion in the presence of castrate T levels (the major substrate for central aromatase activity) provides indirect evidence that estrogen effects at the hypothalamus are not dependent on central aromatization. Two recent studies also support this hypothesis. The first takes advantage of an experiment of nature, namely men with congenital aromatase deficiency. In this unique human model of both peripheral and central estrogen deficiency, normalization of E2 levels with transdermal estrogen reduces LH pulse frequency, LH pulse amplitude, and GnRH-stimulated LH secretion (23). These data provide evidence not only for dual sites of E2 negative feedback but also highlight the importance of circulating, as opposed to locally generated, estrogen in mediating this effect. One could argue that this genetic model has the limitation that congenital estrogen deficiency may have interfered with maturation of the hypothalamic-pituitary gonadal axis. However, using a clever experimental paradigm, Raven et al. (33) examined the importance of circulating vs. centrally generated estrogens in healthy adult males by first suppressing aromatase activity with letrozole and then restoring physiological E2 levels with transdermal estrogen. Data from this experimental model confirm that restoring physiological concentrations of circulating E2 can normalize gonadotropin secretion in men receiving an aromatase inhibitor. These data therefore argue against proposed models of gonadotropin feedback, which emphasize a greater role for in situ estrogen formation within the brain than for circulating E2 concentrations in regulating LH secretion (3).
This human investigative model employing sex steroid ablation and selective physiological sex steroid add-back in healthy and GnRH-deficient men provides novel insights into the study of LH regulation in men. These data suggest a model of sex steroid feedback whereby 1) T and E2 have independent effects on LH secretion, 2) inhibition of LH by T requires aromatization for its pituitary but not its hypothalamic effects, and 3) E2 has dual sites of feedback, but its predominant effect is at the hypothalamus.
Footnotes
This work was supported by National Institutes of Health Grants R01 HD15788-15, DK07028-24, and M01-RR-01066 and by the National Center for Research Resources, General Clinical Research Centers Program.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 11, 2007
Abbreviations: BL, Baseline; CV, coefficient of variation; E2, estradiol; FAS, free α-subunit; IHH, idiopathic hypogonadotropic hypogonadism; KC, ketoconazole; NL men, normal men; T, testosterone.
References
- Finkelstein JS, Whitcomb R, O’Dea LS, Longcope C, Schoenfeld DA, Crowley Jr WF 1991 Sex steroid control of gonadotropin secretion in the human male. I. Effects of testosterone administration in normal and GnRH deficient men. J Clin Endocrinol Metab 73:609–620 [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, O’Dea LS, Whitcomb R, Crowley Jr WF 1991 Sex steroid control of gonadotropin secretion in the human male. II. Effects of estradiol administration in normal and GnRH deficient men. J Clin Endocrinol Metab 73:621–628 [DOI] [PubMed] [Google Scholar]
- Schnorr JA, Bray MJ, Veldhuis JD 2001 Aromatization mediates testosterone’s short-term feedback restraint of 24-hour endogenously driven and acute exogenous gonadotropin-releasing hormone-stimulated luteinizing hormone and follicle-stimulating hormone secretion in men. J Clin Endocrinol Metab 86:2600–2606 [DOI] [PubMed] [Google Scholar]
- Bagatell CJ, Dahl KD, Bremner WJ 1994 The direct pituitary effect of testosterone to inhibit gonadotropin secretion in men is partially mediated by aromatization to estradiol. J Androl 15:15–21 [PubMed] [Google Scholar]
- Boyar RM, Moore RJ, Rosner W, Aiman J, Chipman J, Madden JD, Marks JF, Griffin JE 1978 Studies of gonadotropin-gonadal dynamics in patients with androgen insensitivity. J Clin Endocrinol Metab 47:1116–1122 [DOI] [PubMed] [Google Scholar]
- Gooren L, Spinder T, Spijkstra JJ, van Kessel H, Smals A, Rao BR, Hoogslag M 1987 Sex steroids and pulsatile luteinizing hormone release in men. Studies in estrogen-treated agonadal subjects and eugonadal subjects treated with a novel nonsteroidal antiandrogen. J Clin Endocrinol Metab 65:929–936 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Davis MR, Rogol AD, Johnson ML, Veldhuis JD 1988 Acute androgen receptor blockade increases luteinizing hormone secretory activity in men. J Clin Endocrinol Metab 67:1149–1155 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Urban RJ, Dufau ML 1992 Evidence that androgen negative feedback regulates hypothalamic gonadotropin-releasing hormone impulse strength and the burst-like secretion of biologically active luteinizing hormone in men. J Clin Endocrinol Metab 74:1227–1235 [DOI] [PubMed] [Google Scholar]
- Hayes FJ, DeCruz S, Seminara SB, Boepple PA, Crowley Jr WF 2001 Differential regulation of gonadotropin secretion by testosterone in the human male: absence of a negative feedback effect of testosterone on follicle stimulating hormone secretion. J Clin Endocrinol Metab 86:53–58 [DOI] [PubMed] [Google Scholar]
- Vigersky RA, Mozingo D, Eil C, Purohit V, Bruton J 1982 The antiandrogenic effects of δ1-testolactone (Teslac) in vivo in rats and in vitro in human cultured fibroblasts, rat mammary carcinoma cells, and rat prostate cytosol. Endocrinology 110:214–219 [DOI] [PubMed] [Google Scholar]
- Levine JE, Pau KYF, Ramirez VD, Jackson GL 1982 Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology 111:1449–1455 [DOI] [PubMed] [Google Scholar]
- Spratt DI, Finkelstein JS, Badger TM, Butler JP, Crowley WF 1986 Bio- and immunoreactive luteinizing hormone responses to low dose of gonadotropin-releasing hormone (GnRH): dose response curves in GnRH deficient men. J Clin Endocrinol Metab 63:143–150 [DOI] [PubMed] [Google Scholar]
- Hoffman AR, Crowley Jr WF 1982 Induction of puberty in men by longterm pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med 307:1237–1241 [DOI] [PubMed] [Google Scholar]
- World Health Organization 1992 WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 3rd ed. Cambridge, UK: Cambridge University Press [Google Scholar]
- Sonino N 1987 The use of ketoconazole as an inhibitor of steroid production. N Engl J Med 317:812–818 [DOI] [PubMed] [Google Scholar]
- Hayes FJ, Pitteloud N, DeCruz S, Crowley Jr WF, Boepple PA 2001 Importance of Inhibin B in the regulation of FSH secretion in the human male. J Clin Endocrinol Metab 86:5541–5546 [DOI] [PubMed] [Google Scholar]
- Wouters W, De Coster R, Goeminne N, Beerens D, van Dun J 1998 Aromatase inhibition by the antifungal ketoconazole. J Steroid Biochem 30:387–389 [DOI] [PubMed] [Google Scholar]
- Weber MM, Will A, Adelmann B, Engelhardt D 1991 Effect of ketoconazole on human ovarian C17,20-desmolase and aromatase. J Steroid Biochem Mol Biol 38:213–218 [DOI] [PubMed] [Google Scholar]
- Santen RJ, Bardin CW 1973 Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest 52:2617–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE 1999 Free α-subunit is superior to LH as a marker of gonadotropin-releasing hormone at fast frequencies despite desensitization. J Clin Endocrinol Metab 84:1028–1036 [DOI] [PubMed] [Google Scholar]
- Whitcomb RW, Sangha JS, Schneyer AL, Crowley Jr WF 1988 Improved measurement of free α-subunit of glycoprotein hormones by assay with use of a monoclonal antibody. Clin Chem 34:2022–2025 [PubMed] [Google Scholar]
- Hayes FJ, Seminara SB, DeCruz S, Boepple PA, Crowley Jr WF 2000 Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab 85:3027–3035 [DOI] [PubMed] [Google Scholar]
- Rochira V, Zirilli L, Genazani AD, Balestrieri A, Aranda C, Fabre B, Antunez P, Diazzi C, Carani C, Maffei L 2006 Hypothalamic-pituitary-gonadal axis in two men with aromatase deficiency: evidence that circulating estrogens are required at the hypothalamic level for the integrity of gonadotropin negative feedback. Eur J Endocrinol 155:513–522 [DOI] [PubMed] [Google Scholar]
- Santen RJ 1975 Is aromatization of testosterone to estradiol required for inhibition of luteinizing hormone secretion in men? J Clin Invest 56:1555–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters SJ, Janick JJ, Loriaux DL, Sherins RJ 1979 Studies on the role of sex steroids in the feedback control of gonadotropin concentrations in men. II. Use of the estrogen antagonist, clomiphene citrate. J Clin Endocrinol Metab 48:222–227 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Rogol AD, Samojlik E, Ertel NH 1984 Role of endogenous opiates in the expression of negative feedback actions of androgen and estrogen on pulsatile properties of luteinizing hormone secretion in man. J Clin Invest 74:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dea LS, Finkelstein JS, Schoenfeld DA, Butler JP, Crowley Jr WF 1989 Interpulse interval of GnRH stimulation independently modulates LH secretion. Am J Physiol 256:E510–E515 [DOI] [PubMed] [Google Scholar]
- Boyar RM, Perlow M, Kapen S, Lefkowitz G, Weitzman E, Hellman L 1973 The effect of clomiphene citrate on the 24-hour LH secretory pattern in normal men. J Clin Endocrinol Metab 36:561–567 [DOI] [PubMed] [Google Scholar]
- Santen RJ, Ruby EB 1979 Enhanced frequency and magnitude of episodic luteinizing hormone-releasing hormone discharge as a hypothalamic mechanism for increased luteinizing hormone secretion. J Clin Endocrinol Metab 48:315–319 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Troen P 1985 Evidence for a role of endogenous estrogen in the hypothalamic control of gonadotropin secretion in men. J Clin Endocrinol Metab 61:842–845 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Dufau ML 1987 Estradiol modulates the pulsatile secretion of biologically active luteinizing hormone in man. J Clin Invest 80:631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijkstra JJ, Spinder T, Gooren L, Van Kessel H 1988 Divergent effects of the antiestrogen tamoxifen and of estrogens on luteinizing hormone (LH) pulse frequency, but not on basal LH levels and LH pulse amplitude in men. J Clin Endocrinol Metab 66:355–360 [DOI] [PubMed] [Google Scholar]
- Raven G, de Jong FH, Kaufman JM, de Ronde W 2006 In men, peripheral estradiol levels directly reflect the action of estrogens at the hypothalamo-pituitary level to inhibit gonadotropin secretion. J Clin Endocrinol Metab 91:3324–3328 [DOI] [PubMed] [Google Scholar]