Abstract
Congenital adrenal hyperplasia (CAH) describes a group of autosomal recessive disorders where there is impairment of cortisol biosynthesis. CAH due to 21-hydroxylase deficiency accounts for 95% of cases and shows a wide range of clinical severity. Treatment of the classic or severe form of CAH is targeted at replacing cortisol and aldosterone and effectively controlling excess androgen symptoms by using the lowest possible glucocorticoid dose. Treatment of the mild or nonclassic form is targeted at controlling excess androgen symptoms and may or may not involve glucocorticoid therapy. Hydrocortisone is the treatment of choice for children, but there is no consensus on how patients should be treated as adults. Current glucocorticoid therapy is suboptimal because it is often difficult to reduce excess androgen without giving excess glucocorticoid, and patients may experience hypercortisolism, androgen excess, or a combination of these states. Treatment of CAH, especially in the adult patient, remains controversial given the lack of prospective randomized controlled trials comparing treatment regimens. Nevertheless, patients benefit from careful individualized therapy with avoidance of Cushingoid side effects and optimization of reproductive, sexual, and bone health.
The Case
An 18-yr-old female with classic 21-hydroxylase deficiency has achieved her adult height. She was born with ambiguous genitalia. She was diagnosed with 21-hydroxylase deficiency based on a 17-OH-progesterone level of 16,000 ng/dl (483 nmol/liter) at 3 d old and was started on hydrocortisone, fludrocortisone, and salt. Genital surgery was performed at 6 months old. At approximately 2 yr old, she was taken off salt supplementation. She has always been on hydrocortisone thrice daily and fludrocortisone once or twice daily. She had three salt-losing adrenal crises during the first 3 yr of life, and all were associated with febrile illnesses. She had menarche at age 14 yr and has irregular menses. She has no other medical conditions. Her height is 159 cm (25th percentile), weight is 83 kg (95th percentile), with a body mass index (BMI) of 32.8 kg/m2. Her mid-parental height is 168 cm (75th percentile). She will be starting college soon. Her current treatment regimen is 10 mg hydrocortisone first thing in the morning, 5 mg at 1500 h, and 15 mg at 2200 h (15.8 mg/m2·d) and 50 μg fludrocortisone in the morning and 50 μg at 2200 h. Laboratory evaluation was performed at 0800 h before taking morning medication with the following results: 17-hydroxyprogesterone, 1150 ng/dl (34.7 nmol/liter) (<285 ng/dl); androstenedione, 225 ng/dl (7.9 nmol/liter) (30–200 ng/dl); plasma renin activity, 2.0 ng/ml·h (0.2–4.5 ng/ml·h). Pelvic ultrasound was normal.
Background
This young woman has the most severe form of congenital adrenal hyperplasia (CAH), classic salt-losing 21-hydroxylase deficiency. CAH describes a group of autosomal recessive disorders where there is impairment of cortisol biosynthesis. 21-Hydroxylase deficiency accounts for 95% of cases, and the discussion here will be restricted to 21-hydroxylase deficiency. In CAH, there is a wide range of clinical severity (1). In general, there is high concordance between genotype and phenotype where phenotype corresponds to the degree of 21-hydroxylase impairment caused by CYP21A2 mutations (2,3,4,5). In the most severe form, concomitant aldosterone deficiency leads to salt loss. The clinical phenotype is typically classified as classic salt-losing (most severe), classic non-salt-losing (simple-virilizing), or nonclassic (mild or late-onset). Females with classic CAH, whether salt-losing or non-salt-losing, present at birth with ambiguous genitalia due to in utero exposure to excess fetal adrenal androgens, and CAH is the most common cause of ambiguous genitalia. Males with classic CAH do not have abnormalities at birth. Thus, the age at diagnosis in untreated boys varies according to the severity of 21-hydroxylase impairment and the extent of aldosterone deficiency. Neonatal screening programs for classic CAH are common practice in the United States and many other countries; this has led to earlier diagnosis of the affected male. The overall worldwide incidence of classic CAH is one in 15,000 live births of which two thirds are salt-losers (6).
Nonclassic CAH is a mild form of the disease. Although the same gene, CYP21A2, is involved in both the severe and mild forms, genetic mutations typically associated with nonclassic CAH only partially impair 21-hydroxylase activity. Thus, patients with nonclassic CAH do not have cortisol deficiency but instead have manifestations of hyperandrogenism, later in childhood or in early adulthood. Females are born with normal genitalia, and some patients with nonclassic CAH have no apparent clinical symptoms. Treatment is indicated for females with signs of virilization and for children who have early onset of disease and rapid progression of skeletal age (7).
Clinical Considerations
Our patient’s history is typical of a classic CAH female. She was born with ambiguous genitalia and had vaginoplasty and clitoral reduction in infancy. She has experienced adrenal crises with febrile illnesses. She has been followed by a pediatric endocrinologist, and her treatment has been directed toward optimizing growth and pubertal development. She has reached a normal adult height but is shorter than her expected height based on parental stature. She has irregular menses with a BMI of 32.8 kg/m2. Now that she has achieved adult height, treatment should be focused on optimizing fertility and quality of life and minimizing the side effects of glucocorticoid therapy.
In approaching this patient, I would be thinking about the most convenient way to administer glucocorticoid replacement with the least amount of adverse side effects and optimizing her reproductive, sexual, and bone health.
Glucocorticoid Replacement
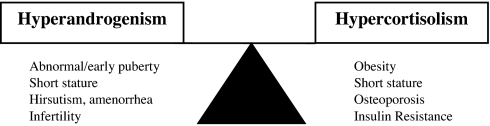
In the treatment of classic CAH, glucocorticoid is given in doses sufficient to suppress adrenal androgen secretion without total suppression of the hypothalamic-pituitary-adrenal axis. At physiological doses, hydrocortisone prevents adrenal insufficiency but does not suppress corticotrophin and androgen production. Thus, it is sometimes quite difficult to reduce excess androgen without giving excess glucocorticoid, and patients often experience hypercortisolism, androgen excess, or a combination of these states (Fig. 1).
Figure 1.
Clinical management of CAH is often a balancing act between two undesirable states: hyperandrogenism and hypercortisolism. If glucocorticoid is administered so as to achieve physiological replacement or prevent adrenal insufficiency, excess adrenal androgen remains. If glucocorticoid is increased to maximize suppression of excess adrenal androgen production, iatrogenic hypercortisolism will occur.
It is generally accepted by pediatricians that hydrocortisone is the first line of treatment, and The Lawson Wilkins Pediatric Endocrine Society and the European Society for Pediatric Endocrinology have stated that the optimal glucocorticoid dosing for children is hydrocortisone 10–15 mg/m2·d divided three times daily (7). However, hydrocortisone twice daily is sometimes used (8). Longer-acting glucocorticoids are generally avoided in children because of potential growth suppression (9), but this requires further study.
Suboptimal management of CAH with current regimens may be due to the nonphysiological timing of glucocorticoid replacement. There is a circadian rhythm in cortisol and 17-hydroxyprogesterone release; concentrations are low at night, begin to rise at 0200 h, peak in the early morning around 0800 h, and then decrease throughout the day (10). No current glucocorticoid therapy is able to simulate the normal cortisol circadian rhythm, and there is no consensus on how to divide the glucocorticoid dosing. In a pharmacokinetic study of the interrelation between cortisol and 17-hydroxyprogesterone in 36 CAH patients, the authors suggest that hydrocortisone should be given with the largest dose in the morning (11). The National Institutes of Health experience is that optimal control of adrenal androgens is achieved by giving the largest glucocorticoid dose at bedtime or to use a reverse circadian pattern of glucocorticoid replacement in an attempt to suppress nighttime corticotrophin secretion, and this was used in our patient. Similarly, other centers have claimed to achieve optimal CAH control by giving the largest glucocorticoid fraction at bedtime (12). Nighttime glucocorticoid also has been recommended for nonclassic CAH (13). There have been no randomized clinical trials comparing different regimens.
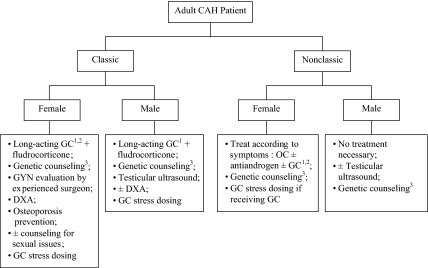
For adults, clinicians may use hydrocortisone, prednisone, prednisolone, dexamethasone, or a combination of treatments. Long-acting glucocorticoids are preferable because they are effective given once or twice daily (Fig. 2). I typically use dexamethasone given once daily at night (250–500 μg/d; most common dose is 375 μg/d). However, a glucocorticoid that is inactivated by placental 11β-hydroxysteroid dehydrogenase type II (hydrocortisone, prednisone, and prednisilone) should be used in the sexually active female who is not on an oral contraceptive. Practice varies worldwide. In a survey in the United Kingdom of 30 teaching centers (14), practitioners used a variety of different regimens to treat CAH adults; hydrocortisone was the most common, followed by dexamethasone and then prednisolone. Sixty percent of practitioners used a reverse circadian pattern of glucocorticoid administration, whereas 16% used a dose based on weight or surface area. Patient-related factors often determine the specific regimen.
Figure 2.
Algorithm outlining the clinical considerations of patients with CAH. GC, Glucocorticoid; OC, oral contraceptive. 1 Long-acting glucocorticoid is preferred, but hydrocortisone should be considered and might be the drug of choice in the older adult when fertility is no longer desired. 2 Dexamethasone should not be used in the sexually active female without concomitant use of oral contraceptive. 3 Genetic counseling should include information regarding maternal dexamethasone for the fetus at risk for classic CAH.
When glucocorticoid therapy is indicated for nonclassic CAH, the same principal applies; the goal is to effectively control excess androgen symptoms by using the lowest possible glucocorticoid dose. Treatment is indicated for children who have early signs of hyperandrogenism and rapid progression of skeletal age and for females with virilization (7). Adult males do not require treatment, and adult females can often be treated with an oral contraceptive and antiandrogen (13) (Fig. 2). The risk of adrenal rest in patients with nonclassic CAH is unknown but is thought to be quite low, and prevention of adrenal rest is not an indication for treatment of the nonclassic patient.
Mineralocorticoid Replacement
Fludrocortisone is given to the classic CAH patient to maintain normal electrolyte and plasma renin activity. The use of fludrocortisone in simple-virilizing CAH is recommended and allows management with lower doses of glucocorticoid (7), but fludrocortisone is not indicated in the treatment of nonclassic CAH. The same daily dose of fludrocortisone given twice daily is more effective than once daily; thus, splitting the dose is the equivalent of a dose increase (personal experience). This should be considered for classic patients. Fludrocortisone has some glucocorticoid activity. Overtreatment should be avoided and may result in hypertension. Salt-losing patients may benefit from supplemental salt intake when greater salt loss is expected, such as with exposure to hot weather or intense exercise.
Laboratory Evaluation
Levels of 17-hydroxyprogesterone, androstenedione, and plasma renin activity are used to evaluate adequacy of therapy in conjunction with clinical signs and symptoms of over- or undertreatment. In general, 17-hydroxyprogesterone should not be normalized because of risk of iatrogenic Cushing syndrome, whereas androstenedione and plasma renin activity are maintained within a range appropriate for a patient’s age and sex. Adrenal androgen concentrations later in the day and after medication will be lower, and target levels for hormones measured in this manner are unknown; thus, hormones are best measured early in the morning and before medication. The target 17-hydroxyprogesterone range suggested for children with CAH is 400-1200 ng/dl (12–36 nmol/liter) (1). Target hormone levels for adults are similar but should be individualized. For example, the young adult female interested in fertility should be maintained at a lower 17-hydroxyprogesterone (<800 ng/dl; 24.2 nmol/liter), whereas the adult male with no evidence of testicular adrenal rest by ultrasound could be maintained at a higher 17-hydroxyprogesterone (<2500 ng/dl; 75.5 nmol/liter). Clinical factors dictate individual management.
Fertility in CAH
Women with CAH have reduced fertility, especially those with salt-losing CAH (15,16,17). There are several potential causes. Hyperandrogenemia due to inadequate glucocorticoid therapy results in anovulation (15). Abnormal gonadotropin dynamics with excess ovarian production of progesterone, 17-hydroxyprogesterone, and androgens may occur (18,19) In utero exposure to excess androgens might have long-term effects on the function of the hypothalamic-pituitary-ovarian axis; this has been shown in animal studies (20). In addition to hormonal factors, structural factors related to genital malformations or suboptimal surgical reconstruction may leave the vaginal introitus inadequate and may contribute to impaired self-image and decreased sexual activity (21,22). In a recent study of 35 classic CAH women, 18 had experienced pain with vaginal penetration, and by questionnaire, overall sexual function was decreased compared with controls (21). Counseling regarding sexual function should be part of the anticipatory guidance of classic CAH females. Genital examination with attention to the adequacy of the vaginal introitus should be performed at an appropriate time for the patient. Vaginal dilatation is sometimes indicated.
Another adverse medical condition associated with CAH is the development of ectopic adrenal tissue or adrenal rest. Adrenal rest grows in response to corticotrophin, and clinically significant adrenal rest is most commonly found in untreated or poorly controlled classic CAH. Adrenal rest is most commonly found in the testes but has been described in the celiac plexus, broad ligaments, and ovaries (23,24). Testicular adrenal rest has been characterized, and these hypoechoic lesions can be easily detected by ultrasound or magnetic resonance imaging (23,25). Most adult CAH men are fertile, but testicular adrenal rest may result in oligoazoospermia or Leydig cell failure (26), and higher-dose glucocorticoid therapy may reverse infertility (27). At least one third of classic CAH males have sonographic evidence of testicular adrenal rest (23,26); the prevalence in women is unknown, and the prevalence in nonclassic CAH is unknown. Ovarian adrenal rest is difficult to visualize; hypoechoic lesions are difficult to distinguish from normal (or polycystic) ovarian tissue. A screening testicular ultrasonography is recommended in males in adolescence or early adulthood (25). A screening pelvic ultrasound is not routinely needed for women but may be useful in the evaluation of menstrual disorders.
Ultrasonic evidence of polycystic ovaries has been studied in CAH. The largest study of 77 female CAH patients (36 adults) found polycystic ovaries in 74% of postpubertal females and 22% of controls (28). However, in this study, female relatives without CAH also had high rates of polycystic ovaries, implicating a cause possibly unrelated to CAH. Similarly, polycystic ovaries were found in two of 13 CAH females, reflecting the general population prevalence (29). Ultrasound features of polycystic ovaries are a common finding in the clinical presentation of the nonclassic CAH female (13).
With appropriate therapy, classic CAH women can become pregnant. Their unaffected female offspring do not have genital virilization, but careful monitoring of androgen levels during gestation is indicated (30). Longer-acting glucocorticoids are often used to regulate menstrual cycles and optimize fertility. However, dexamethasone crosses the placenta and suppresses the fetal adrenal. Thus, dexamethasone should not be used during pregnancy unless the fetus is at risk for classic CAH (i.e. the father is a known carrier) and in utero suppression of the fetal adrenal is desired.
Genetic counseling is indicated for adult CAH patients. CAH is autosomal recessive. Most patients are compound heterozygotes (have two different CYP21A2 mutations), and the expressed phenotype typically corresponds to the less severe mutation (2,3,4,5). Thus, all children of classic CAH patients will inherit a mutated allele associated with classic CAH. Nonclassic CAH patients may carry one classic and one nonclassic mutation or two nonclassic mutations. The probability of a healthy partner being heterozygote for a CYP21A2 mutation is based on population disease frequencies and is one in 63 for a classic mutation and one in 17 for a nonclassic mutation. It is prudent for the partner of a CAH patient to be genotyped. If both parents are known carriers of a classic CAH mutation, they should be counseled regarding the option of maternal dexamethasone therapy. Very early institution of maternal dexamethasone is effective in reducing virilization of the affected female fetus (31). Parents should be fully informed of the potential risks to the mother and fetus and should consider this option before pregnancy.
Bone Mineral Density (BMD)
Chronic glucocorticoid therapy is a known risk factor for osteoporosis. Reports of younger CAH patients failed to show decreased BMD (32,33,34), but studies including older CAH patients report reduced BMD (Table 1) (35,36,37,38,39). Although increased BMI might protect against low BMD, this has not been found in CAH patients. Glucocorticoid overtreatment has been implicated as the cause of decreased BMD in multiple studies (35,36,38).
Table 1.
Summary of BMD studies in adult patients with CAH
| Study | No. of patients | Findings |
|---|---|---|
| Jaaskelainen et al. (35) | 32 (30 classic, 16 female, 16–52 yr) | Patient BMD Z-scores lower than Finnish population mean (lumbar spine, −0.52; P = 0.045; femoral neck, −0.83, P < 0.001); long-term glucocorticoid dose negatively correlated with BMD Z-score; patients receiving longer-acting glucocorticoids had lower BMD than patients receiving hydrocortisone |
| King et al. (36) | 26 classic females (21–71 yr) | Patients BMD Z-scores, T-scores, and lumbar spine measurements significantly lower than controls (unaffected sisters); osteopenia present in 45% salt-losers, 13% simple virilizers, 11% controls; patients with osteopenia had lowest levels of adrenal androgens |
| Sciannamblo et al. (37) | 30 classic (12 female, 16–29 yr) | Whole-body BMD measurements significantly lower than controls (P < 0.03); bone metabolism markers (serum bone-specific alkaline phosphatase and C-terminal telopeptide of type I collagen) higher in CAH patients than controls (P < 0.04) |
| Bachelot et al. (38) | 45 (35 classic, 36 females, 18–47 yr) | Osteopenia present in 52% of salt-losers, 42% of simple virilizers, and 20% of nonclassic patients at femoral neck and 39% of salt-losers, 33% of simple virilizers, and 30% of nonclassic patients at lumbar spine; osteoporosis found in 7%; negative correlation between BMD T-score and hydrocortisone dose |
| Falhammer et al. (39) | 61 females (55 classic, 18–63 yr) | Osteopenia in 48% of patients <30 yr old, 73% of patients ≥30 yr old, and 21% of age-matched controls; patients had lower BMD than controls at all measured sites (P < 0.001); more osteoporotic fractures (vertebrae, wrist, and hip) in patients vs. controls (P = 0.058) |
Osteopenia was defined as T-score between −1 and −2.5 sd; osteoporosis was defined as T-score less than −2.5 sd.
These studies provide compelling evidence that the lowest possible glucocorticoid dose should be used in the treatment of CAH, and lower-dose and/or shorter-acting glucocorticoid may be sufficient in the middle-aged and elderly female when osteoporosis rather than fertility is a main concern. Osteoporosis prophylaxis such as physical activity and calcium and vitamin D supplementation should be implemented at a young age. Screening dual-energy x-ray absorptiometry (DXA) should be performed in CAH adults.
Quality of Life and Psychological Health
CAH patients are expected to have normal cognition and intelligence. Some studies suggest that CAH patients who experienced adrenal crises are at risk for cognitive impairment (40,41). Careful management of adrenal crises with attention to the risk of hypoglycemia and electrolyte imbalance, especially in neonates, would prevent this potential adverse outcome.
In utero exposure to excess androgens may influence the brain. Several studies have shown that classic CAH females, especially salt-losers, have more male-typical childhood play and behavior (42,43) and a more male-typical cognitive pattern than unaffected girls (44,45). In a functional magnetic resonance imaging study, amygdala activation in response to emotional stimuli was significantly greater in CAH females than control females in a pattern similar to control males, suggesting an androgen effect on emotional processing (46). However, the majority of studies have been done in younger patients, and a psychological study of 24 CAH women that included older women (21–71 yr) did not support long-lasting androgen effects (47).
Classic CAH women have more sexual concerns and are less likely to have sexual relations than controls, and women with the salt-losing form usually have worse psychosexual functioning than those with the non-salt-losing form (21,48). Although masculinization of behavior has been shown, gender identity confusion is extremely rare and is not characteristic of the CAH female. CAH females identify themselves as female and do not express dissatisfaction with their female gender identity (49).
Overall favorable quality of life (19,50) and good psychological health (51) have been reported in CAH patients. A study of 45 CAH women revealed high quality of life comparable to age- and education-matched controls (50). However, CAH patients were more often single (66.7 vs. 47.8%), were less sexually active, displayed more negative body image, and had more negative self-image in regard to self-confidence, sociability, and social acceptance. Despite these findings, good physical function, active coping mechanism, and high global satisfaction with life resulted in overall high quality of life. Similarly, in a study of 72 females and 42 males with CAH (age 3–31 yr) and 113 unaffected relatives, there were no significant differences between CAH females and unaffected females on any measure of psychological health (51). Psychological adjustment was not significantly associated with genital virilization or age at genital surgery.
In general, quality of life involves evaluation of physical and psychological state, social relationships, and functional capacity. Our patient, typical of the young adult with classic CAH, is in excellent physical state with high functional capacity. She is at risk for negative body image and might avoid sexual relations. However, she is expected to have an excellent quality of life.
Other Metabolic Effects
Classic CAH patients have adrenomedullary impairment (52). Intraadrenal cortisol is necessary for normal epinephrine secretion and normal adrenal medulla development. In a study of 38 classic CAH children (52), epinephrine and metanephrine concentrations were 40–80% lower than controls. In three salt-losing CAH patients who underwent bilateral adrenalectomy, the adrenal medulla was poorly formed. The clinical implications of epinephrine deficiency are not well understood. However, the combination of cortisol and epinephrine deficiency puts patients at risk for hypoglycemia with illness or prolonged fasting. Exercise is a powerful stimulus of the adrenal medulla, and studies of adrenomedullary reserve in CAH patients using standardized exercise paradigms have shown impaired epinephrine response resulting in defective glucose, insulin, and leptin regulation (53,54,55). Adrenomedullary function has not been studied in nonclassic CAH.
Obesity is common in CAH patients, and normally growing CAH children have been shown to increase their BMI throughout childhood (56). Insulin sensitivity has been found to be lower in patients with both classic and nonclassic CAH compared with BMI-matched controls (57,58). Hyperleptinemia has also been described (54,57). Long-standing adrenomedullary hypofunction may play a role because catecholamines inhibit insulin and leptin secretion (52,57). A recent study of 19 classic CAH adults (age 28.5 ± 3.5 yr) found reduced insulin sensitivity, and also evidence of early arterial disease, an important cardiovascular risk factor (59). Doppler studies of major arteries showed increased intima-media thickness in classic CAH patients compared with age-, sex-, and BMI-matched controls. Long-term cardiovascular risks need further study.
Controversies and Unanswered Questions
CAH constitutes a continuum of clinical challenges that affect patients throughout their lives, beginning with ambiguous genitalia in the newborn. The surgical management of ambiguous genitalia is complex and controversial. Patient advocacy groups have appealed to families to carefully consider whether and when surgery should be done. However, genital surgery between 2 and 6 months of age is considered the standard of practice for the virilized classic CAH female (7). Early institution of maternal dexamethasone prevents virilization in the majority of affected females (31). However, this therapy is also controversial because unaffected at-risk fetuses are treated until definitive diagnosis is available, and the long-term safety remains unknown. Patient education and informed consent is essential in the management of these controversial issues. Uncertainty regarding the method and timing of glucocorticoid administration will remain as long as current therapy remains suboptimal and there are no randomized prospective clinical trials. New ways of delivering hydrocortisone are being studied (10). Unresolved clinical problems that need to be considered in the management of CAH include adult short stature, iatrogenic Cushing syndrome, infertility, osteoporosis, and in the classic female, negative self-image and compromised sexual function.
Returning to the Patient
The patient is a healthy young adult and has achieved her adult height. She has irregular menses and is not sexually active, and her adrenal hormone levels are mildly elevated. Therapy with a long-acting glucocorticoid was recommended to optimize compliance and further suppress adrenal androgen secretion. She was started on 375 μg dexamethasone once daily before bedtime, and fludrocortisone dose remained unchanged. Laboratory evaluation was repeated 6 wk later with the following results: 17-hydroxyprogesterone, 400 ng/dl (12.1 nmol/liter); androstenedione, 90 ng/dl (3.1 nmol/liter) (30–200 ng/dl); and plasma renin activity, 3.2 ng/ml·h (0.2–4.5 ng/ml·h). Although laboratory evaluation was not performed until 6 wk, the patient was educated regarding signs and symptoms of over- or under-treatment. She did not experience weight gain or weight loss, did not have trouble sleeping, had good energy level, had no change in her salt cravings, and had a normal menses. DXA was performed, and Z-score was −0.52 for the anteroposterior spine L1–L4 and −0.7 in the femoral neck. Osteoporosis prophylaxis was discussed with encouragement of physical activity and calcium and vitamin D supplementation. She was referred to a surgeon who performs reconstructive genital surgery for evaluation of her vaginal introitus.
She will be reevaluated in 3 months time. An oral contraceptive will be added to her regimen if irregular menses continue or if contraception is likely needed in the near future. If she is doing well on her new regimen, additional follow-up will occur every 6 months.
CAH-related education will be provided at each encounter. Management of illnesses and the proper use of stress doses of hydrocortisone will be reviewed. All patients with classic CAH and nonclassic patients who are being treated with glucocorticoid should wear medical identification that states adrenal insufficiency. Patients should be instructed to take extra doses of hydrocortisone in addition to their usual glucocorticoid regimen for fever, gastrointestinal illnesses, and significant trauma. An emergency supply of im hydrocortisone should be available, and adult patients should be taught self-administration. Prolonged fasting should be discouraged during illnesses because of potential risk of hypoglycemia, especially in children. Extra glucocorticoid is not indicated for mental stress or sports, but patients should not fast before intense or prolonged exercise.
Conclusions
In utero and lifetime hormonal imbalances lead to the multiple clinical challenges of CAH including ambiguous genitalia in the newborn, management of childhood growth and pubertal development, hypoglycemic adrenal crises, genetic counseling of families and adult patients, optimization of fertility, prevention and treatment of osteoporosis, and ensuring excellent quality of life. The Lawson Wilkins Pediatric Endocrine Society and European Society for Pediatric Endocrinology consensus statement and other published recommendations regarding treatment are mostly based on clinical experience and retrospective reports. Randomized prospective clinical trials are needed to develop new treatment approaches and further address the unresolved clinical problems. Patients with CAH should experience a long life span. Thus, future investigations are needed that focus on the adult and long-term consequences of CAH management.
Footnotes
D.P.M received research funds during 2007–2008 from Phoqus Pharmaceuticals, Ltd. Research funds were also received from the Congenital Adrenal Hyperplasia Research, Education and Support (CARES) Foundation, Inc. D.P.M. is a Commissioned Officer in the U.S. Public Health Service.
Abbreviations: BMD, Bone mineral density; BMI, body mass index; CAH, congenital adrenal hyperplasia; DXA, dual-energy x-ray absorptiometry.
References
- Merke DP, Bornstein SR 2005 Congenital adrenal hyperplasia. Lancet 365:2125–2136 [DOI] [PubMed] [Google Scholar]
- Jaaskelainen J, Levo A, Voutilainen R, Partanen J 1997 Population-wide evaluation of disease manifestation in relation to molecular genotype in steroid 21-hydroxylase (CYP21) deficiency: good correlation in a well defined population. J Clin Endocrinol Metab 82:3293–3297 [DOI] [PubMed] [Google Scholar]
- Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP 2000 Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab 85:1059–1065 [DOI] [PubMed] [Google Scholar]
- Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna MT, Lesser M, New MI, White PC 1992 Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest 90:584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedell A, Thilen A, Ritzen EM, Stengler B, Luthman H 1994 Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J Clin Endocrinol Metab 78:1145–1152 [DOI] [PubMed] [Google Scholar]
- Therrell BL 2001 Newborn screening for congenital adrenal hyperplasia. Endocrinol Metab Clin North Am 30:15–30 [DOI] [PubMed] [Google Scholar]
- Joint LWPES/ESPE CAH Working Group 2002 Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab 87:4048–4053 [DOI] [PubMed] [Google Scholar]
- New MI 1998 Diagnosis and management of congenital adrenal hyperplasia. Annu Rev Med 49:311–328 [DOI] [PubMed] [Google Scholar]
- Bonfig W, Bechtold S, Schmidt H, Knorr D, Schwarz HP 2007 Reduced final height outcome in congenital adrenal hyperplasia under prednisone treatment: deceleration of growth velocity during puberty. J Clin Endocrinol Metab 92:1635–1639 [DOI] [PubMed] [Google Scholar]
- Newell-Price J, Whiteman M, Rostami-Hodjegan A, Darzy K, Shalet S, Tucker GT, Ross RJ 2008 Modified-release hydrocortisone for circadian therapy: a proof-of-principle study in dexamethasone-suppressed normal volunteers. Clin Endocrinol (Oxf) 68:130–135 [DOI] [PubMed] [Google Scholar]
- Charmandari E, Matthews DR, Johnston A, Brook CG, Hindmarsh PC 2001 Serum cortisol and 17-hydroxyprogesterone interrelation in classic 21-hydroxylase deficiency: is current replacement therapy satisfactory? J Clin Endocrinol Metab 86:4679–4685 [DOI] [PubMed] [Google Scholar]
- Rosenfield RL 2002 Serum cortisol and 17-hydroxyprogesterone concentrations in children with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab 87:2993; author reply 2993 [DOI] [PubMed] [Google Scholar]
- Azziz R, Dewailly D, Owerbach D 1994 Nonclassic adrenal hyperplasia: current concepts. J Clin Endocrinol Metab 78:810–815 [DOI] [PubMed] [Google Scholar]
- Ross RJ, Rostami-Hodjegan A 2005 Timing and type of glucocorticoid replacement in adult congenital adrenal hyperplasia. Horm Res 64(Suppl 2):67–70 [DOI] [PubMed] [Google Scholar]
- Mulaikal RM, Migeon CJ, Rock JA 1987 Fertility rates in female patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med 316:178–182 [DOI] [PubMed] [Google Scholar]
- Claahsen-van der Grinten HL, Stikkelbroeck NM, Sweep CG, Hermus AR, Otten BJ 2006 Fertility in patients with congenital adrenal hyperplasia. J Pediatr Endocrinol Metab 19:677–685 [DOI] [PubMed] [Google Scholar]
- Jaaskelainen J, Hippelainen M, Kiekara O, Voutilainen R 2000 Child rate, pregnancy outcome and ovarian function in females with classical 21-hydroxylase deficiency. Acta Obstet Gynecol Scand 79:687–692 [PubMed] [Google Scholar]
- Ghizzoni L, Virdis R, Vottero A, Cappa M, Street ME, Zampolli M, Ibanez L, Bernasconi S 1996 Pituitary-ovarian responses to leuprolide acetate testing in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 81:601–606 [DOI] [PubMed] [Google Scholar]
- Jaaskelainen, Voutilainen R 2000 Long-term outcome of classical 21-hydroxylase deficiency: diagnosis, complications and quality of life. Acta Paediatr 89:183–187 [DOI] [PubMed] [Google Scholar]
- Forsdike RA, Hardy K, Bull L, Stark J, Webber LJ, Stubbs S, Robinson JE, Franks S 2007 Disordered follicle development in ovaries of prenatally androgenized ewes. J Endocrinol 192:421–428 [DOI] [PubMed] [Google Scholar]
- Gastaud F, Bouvattier C, Duranteau L, Brauner R, Thibaud E, Kutten F, Bougneres P 2007 Impaired sexual and reproductive outcomes in women with classical forms of congenital adrenal hyperplasia. J Clin Endocrinol Metab 92:1391–1396 [DOI] [PubMed] [Google Scholar]
- Krege S, Walz KH, Hauffa BP, Korner I, Rubben H 2000 Long-term follow-up of female patients with congenital adrenal hyperplasia from 21-hydroxylase deficiency, with special emphasis on the results of vaginoplasty. BJU Int 86:253–258; discussion 258–259 [DOI] [PubMed] [Google Scholar]
- Avila NA, Premkumar A, Merke DP 1999 Testicular adrenal rest tissue in congenital adrenal hyperplasia: comparison of MR imaging and sonographic findings. Am J Roentgenol 172:1003–1006 [DOI] [PubMed] [Google Scholar]
- Russo G, Paesano P, Taccagni G, Del Maschio A, Chiumello G 1998 Ovarian adrenal-like tissue in congenital adrenal hyperplasia. N Engl J Med 339:853–854 [DOI] [PubMed] [Google Scholar]
- Stikkelbroeck NM, Suliman HM, Otten BJ, Hermus AR, Blickman JG, Jager GJ 2003 Testicular adrenal rest tumours in postpubertal males with congenital adrenal hyperplasia: sonographic and MR features. Eur Radiol 13:1597–1603 [DOI] [PubMed] [Google Scholar]
- Cabrera MS, Vogiatzi MG, New MI 2001 Long term outcome in adult males with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:3070–3078 [DOI] [PubMed] [Google Scholar]
- Claahsen-van der Grinten HL, Otten BJ, Sweep FC, Hermus AR 2007 Repeated successful induction of fertility after replacing hydrocortisone with dexamethasone in a patient with congenital adrenal hyperplasia and testicular adrenal rest tumors. Fertil Steril 88:705–708 [DOI] [PubMed] [Google Scholar]
- Hague WM, Adams J, Rodda C, Brook CG, de Bruyn R, Grant DB, Jacobs HS 1990 The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin Endocrinol (Oxf) 33:501–510 [DOI] [PubMed] [Google Scholar]
- Stikkelbroeck NM, Hermus AR, Schouten D, Suliman HM, Jager GJ, Braat DD, Otten BJ 2004 Prevalence of ovarian adrenal rest tumours and polycystic ovaries in females with congenital adrenal hyperplasia: results of ultrasonography and MR imaging. Eur Radiol 14:1802–1806 [DOI] [PubMed] [Google Scholar]
- Lo JC, Schwitzgebel VM, Tyrrell JB, Fitzgerald PA, Kaplan SL, Conte FA, Grumbach MM 1999 Normal female infants born of mothers with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 84:930–936 [DOI] [PubMed] [Google Scholar]
- New MI, Carlson A, Obeid J, Marshall I, Cabrera MS, Goseco A, Lin-Su K, Putnam AS, Wei JQ, Wilson RC 2001 Prenatal diagnosis for congenital adrenal hyperplasia in 532 pregnancies. J Clin Endocrinol Metab 86:5651–5657 [DOI] [PubMed] [Google Scholar]
- Gussinye M, Carrascosa A, Potau N, Enrubia M, Vicens-Calvet E, Ibanez L, Yeste D 1997 Bone mineral density in prepubertal and in adolescent and young adult patients with the salt-wasting form of congenital adrenal hyperplasia. Pediatrics 100:671–674 [DOI] [PubMed] [Google Scholar]
- Girgis R, Winter JS 1997 The effects of glucocorticoid replacement therapy on growth, bone mineral density, and bone turnover markers in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 82:3926–3929 [DOI] [PubMed] [Google Scholar]
- Mora S, Saggion F, Russo G, Weber G, Bellini A, Prinster C, Chiumello G 1996 Bone density in young patients with congenital adrenal hyperplasia. Bone 18:337–340 [DOI] [PubMed] [Google Scholar]
- Jaaskelainen J, Voutilainen R 1996 Bone mineral density in relation to glucocorticoid substitution therapy in adult patients with 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 45:707–713 [DOI] [PubMed] [Google Scholar]
- King JA, Wisniewski AB, Bankowski BJ, Carson KA, Zacur HA, Migeon CJ 2006 Long-term corticosteroid replacement and bone mineral density in adult women with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab 91:865–869 [DOI] [PubMed] [Google Scholar]
- Sciannamblo M, Russo G, Cuccato D, Chiumello G, Mora S 2006 Reduced bone mineral density and increased bone metabolism rate in young adult patients with 21-hydroxylase deficiency. J Clin Endocrinol Metab 91:4453–4458 [DOI] [PubMed] [Google Scholar]
- Bachelot A, Plu-Bureau G, Thibaud E, Laborde K, Pinto G, Samara D, Nihoul-Fekete C, Kuttenn F, Polak M, Touraine P 2007 Long-term outcome of patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res 67:268–276 [DOI] [PubMed] [Google Scholar]
- Falhammar H, Filipsson H, Holmdahl G, Janson PO, Nordenskjold A, Hagenfeldt K, Thoren M 2007 Fractures and bone mineral density in adult women with 21-hydroxylase deficiency. J Clin Endocrinol Metab 92:4643–4649 [DOI] [PubMed] [Google Scholar]
- Berenbaum SA 2001 Cognitive function in congenital adrenal hyperplasia. Endocrinol Metab Clin North Am 30:173–192 [DOI] [PubMed] [Google Scholar]
- Johannsen TH, Ripa CP, Reinisch JM, Schwartz M, Mortensen EL, Main KM 2006 Impaired cognitive function in women with congenital adrenal hyperplasia. J Clin Endocrinol Metab 91:1376–1381 [DOI] [PubMed] [Google Scholar]
- Berenbaum SA 1999 Effects of early androgens on sex-typed activities and interests in adolescents with congenital adrenal hyperplasia. Horm Behav 35:102–110 [DOI] [PubMed] [Google Scholar]
- Dittmann RW, Kappes MH, Kappes ME, Borger D, Meyer-Bahlburg HF, Stegner H, Willig RH, Wallis H 1990 Congenital adrenal hyperplasia. II. Gender-related behavior and attitudes in female salt-wasting and simple-virilizing patients. Psychoneuroendocrinology 15:421–434 [DOI] [PubMed] [Google Scholar]
- Helleday J, Bartfai A, Ritzen EM, Forsman M 1994 General intelligence and cognitive profile in women with congenital adrenal hyperplasia (CAH). Psychoneuroendocrinology 19:343–356 [DOI] [PubMed] [Google Scholar]
- Kelso WM, Nicholls ME, Warne GL, Zacharin M 2000 Cerebral lateralization and cognitive functioning in patients with congenital adrenal hyperplasia. Neuropsychology 14:370–378 [DOI] [PubMed] [Google Scholar]
- Ernst M, Maheu FS, Schroth E, Hardin J, Golan LG, Cameron J, Allen R, Holzer S, Nelson E, Pine DS, Merke DP 2007 Amygdala function in adolescents with congenital adrenal hyperplasia: a model for the study of early steroid abnormalities. Neuropsychologia 45:2104–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouf MA, Migeon CJ, Carson KA, Petrucci L, Wisniewski AB 2006 Cognitive outcome in adult women affected by congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res 65:142–150 [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Migeon CJ, Malouf MA, Gearhart JP 2004 Psychosexual outcome in women affected by congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Urol 171:2497–2501 [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HF, Dolezal C, Baker SW, Carlson AD, Obeid JS, New MI 2004 Prenatal androgenization affects gender-related behavior but not gender identity in 5–12-year-old girls with congenital adrenal hyperplasia. Arch Sex Behav 33:97–104 [DOI] [PubMed] [Google Scholar]
- Kuhnle U, Bullinger M, Schwarz HP 1995 The quality of life in adult female patients with congenital adrenal hyperplasia: a comprehensive study of the impact of genital malformations and chronic disease on female patients life. Eur J Pediatr 154:708–716 [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Korman Bryk K, Duck SC, Resnick SM 2004 Psychological adjustment in children and adults with congenital adrenal hyperplasia. J Pediatr 144:741–746 [DOI] [PubMed] [Google Scholar]
- Merke DP, Chrousos GP, Eisenhofer G, Weise M, Keil MF, Rogol AD, Van Wyk JJ, Bornstein SR 2000 Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med 343:1362–1368 [DOI] [PubMed] [Google Scholar]
- Green-Golan L, Yates C, Drinkard B, VanRyzin C, Eisenhofer G, Weise M, Merke DP 2007 Patients with classic congenital adrenal hyperplasia have decreased epinephrine reserve and defective glycemic control during prolonged moderate-intensity exercise. J Clin Endocrinol Metab 92:3019–3024 [DOI] [PubMed] [Google Scholar]
- Riepe FG, Krone N, Kruger SN, Sweep FC, Lenders JW, Dotsch J, Monig H, Sippell WG, Partsch CJ 2006 Absence of exercise-induced leptin suppression associated with insufficient epinephrine reserve in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Exp Clin Endocrinol Diabetes 114:105–110 [DOI] [PubMed] [Google Scholar]
- Weise M, Mehlinger SL, Drinkard B, Rawson E, Charmandari E, Hiroi M, Eisenhofer G, Yanovski JA, Chrousos GP, Merke DP 2004 Patients with classic congenital adrenal hyperplasia have decreased epinephrine reserve and defective glucose elevation in response to high-intensity exercise. J Clin Endocrinol Metab 89:591–597 [DOI] [PubMed] [Google Scholar]
- Cornean RE, Hindmarsh PC, Brook CG 1998 Obesity in 21-hydroxylase de-ficient patients. Arch Dis Child 78:261–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E, Weise M, Bornstein SR, Eisenhofer G, Keil MF, Chrousos GP, Merke DP 2002 Children with classic congenital adrenal hyperplasia have elevated serum leptin concentrations and insulin resistance: potential clinical implications. J Clin Endocrinol Metab 87:2114–2120 [DOI] [PubMed] [Google Scholar]
- Speiser PW, Serrat J, New MI, Gertner JM 1992 Insulin insensitivity in adrenal hyperplasia due to nonclassical steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab 75:1421–1424 [DOI] [PubMed] [Google Scholar]
- Sartorato P, Zulian E, Benedini S, Mariniello B, Schiavi F, Bilora F, Pozzan G, Greggio N, Pagnan A, Mantero F, Scaroni C 2007 Cardiovascular risk factors and ultrasound evaluation of intima-media thickness at common carotids, carotid bulbs, and femoral and abdominal aorta arteries in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 92:1015–1018 [DOI] [PubMed] [Google Scholar]