Abstract
Context: Two reports suggested that vitamin D2 is less effective than vitamin D3 in maintaining vitamin D status.
Objective: Our objective was to determine whether vitamin D2 was less effective than vitamin D3 in maintaining serum 25-hydroxyvitamin D levels or increased the catabolism of 25-hydroxyvitamin D3.
Subjects and Design: This was a randomized, placebo-controlled, double-blinded study of healthy adults ages 18–84 yr who received placebo, 1000 IU vitamin D3, 1000 IU vitamin D2, or 500 IU vitamin D2 plus 500 IU vitamin D3 daily for 11 wk at the end of the winter.
Results: Sixty percent of the healthy adults were vitamin D deficient at the start of the study. The circulating levels of 25-hydroxyvitamin D (mean ± sd) increased to the same extent in the groups that received 1000 IU daily as vitamin D2 (baseline 16.9 ± 10.5 ng/ml; 11 wk 26.8 ± 9.6 ng/ml), vitamin D3 (baseline 19.6 ± 11.1 ng/ml; 11 wk 28.9 ± 11.0 ng/ml), or a combination of 500 IU vitamin D2 and 500 IU vitamin D3 (baseline 20.2 ± 10.4 ng/ml; 11 wk 28.4 ± 7.7 ng/ml). The 25-hydroxyvitamin D3 levels did not change in the group that received 1000 IU vitamin D2 daily. The 1000 IU dose of vitamin D2 or vitamin D3 did not raise 25-hydroxyvitamin D levels in vitamin D-deficient subjects above 30 ng/ml.
Conclusion: A 1000 IU dose of vitamin D2 daily was as effective as 1000 IU vitamin D3 in maintaining serum 25-hydroxyvitamin D levels and did not negatively influence serum 25-hydroxyvitamin D3 levels. Therefore, vitamin D2 is equally as effective as vitamin D3 in maintaining 25-hydroxyvitamin D status.
This study finds that vitamin D2 was as effective as vitamin D3 in maintaining serum 25-hydroxyvitamin D levels and did not negatively affect serum 25-hydroxyvitamin D3 levels.
Vitamin D2, which comes from the UV irradiation of ergosterol obtained from yeast, has been the mainstay for the prevention and treatment of vitamin D deficiency in children and adults for more than 80 yr (1,2). As little as 100 IU vitamin D2 was found to be effective in the prevention of rickets (2,3,4). When humans are exposed to sunlight, 7-dehydrocholesterol in the skin absorbs UVB (290–315 nm) radiation resulting in the production of vitamin D3 (1,3). Vitamin D3 is found naturally in cod liver oil and oily fish such as salmon (1,3). Vitamin D3 is also made by irradiating 7-dehydrocholesterol obtained from lanolin from sheep’s wool with UVB radiation (1). Both vitamin D2 and vitamin D3 when ingested undergo metabolism in the liver to form 25-hydroxyvitamin D [25(OH)D; D represents either D2 or D3] and in the kidneys to 1,25-dihydroxyvitamin D (1,3,5,6). Both vitamin D2 and vitamin D3 are available in supplements, but only vitamin D2 is available as a pharmaceutical preparation because its use predated the Food and Drug Administration and, thus, was grandfathered as a pharmaceutical drug. Vitamin D3 was commercially developed in the 1950s and has not been approved as a pharmaceutical agent in the United States but is used in food supplementation and vitamin supplements.
Since the 1930s, vitamin D2 has been considered to be equally as effective as vitamin D3 for bone health (3,7). Recently, it was suggested that vitamin D2 was less effective than vitamin D3 in maintaining serum 25(OH)D levels when given either as 4000 IU/d for 2 wk (8) or as a single dose of 50,000 IU (9). Furthermore, it was observed that when a single dose of 50,000 IU vitamin D2 was given to healthy adults that the serum 25(OH)D levels decreased more rapidly than the placebo group, suggesting that vitamin D2 not only was less effective in maintaining serum 25(OH)D levels but also enhanced the degradation of 25(OH)D3 (9).
These two observations have led to the conclusion that vitamin D2 is approximately 30–50% as effective as vitamin D3 in maintaining serum 25(OH)D in humans (8,9). Our purpose was to evaluate in healthy adults what the effect of ingesting 1000 IU vitamin D2, 1000 IU vitamin D3, or a combination of 500 IU vitamin D2 and 500 IU vitamin D3 daily for 11 wk at the end of the winter had on circulating levels of total 25(OH)D as well as 25(OH)D2 and 25(OH)D3.
Subjects and Methods
Subjects
Healthy, white, African-American, Hispanic, Asian, and Native American adults between the ages of 18 and 84 yr were enrolled in February 2007 after signing a consent form approved by our Institutional Review Board at Boston University Medical Center. We excluded those with chronic liver and kidney disease and those taking medications, including anticonvulsants, glucocorticoids, and barbiturates, that might affect vitamin D metabolism as well as subjects who were taking a vitamin D supplement. Subjects were permitted to take their multivitamin, a majority of which contained 400 IU vitamin D3 (Table 1).
Table 1.
Subject demographics
| Characteristics | Placebo group (n = 14) | D2+D3 group (n = 18) | D3 group (n = 20) | D2 group (n = 16) |
|---|---|---|---|---|
| Age (yr) | ||||
| Mean ± sd | 40.5 ± 11.7 | 35.5 ± 14.6 | 40.0 ± 18.0 | 38.4 ± 12.0 |
| Range | 22–59 | 18–70 | 20–81 | 18–59 |
| Females, n (%) | 11 (78.6) | 13 (72.2) | 13 (65) | 10 (62.5) |
| Males, n (%) | 3 (21.4) | 5 (27.8) | 7 (35) | 6 (37.5) |
| Body mass index | 29.3 | 31.7 | 30.0 | 31.0 |
| Multivitamin, n (%) | 6 (42.9) | 4 (22.2) | 5 (25) | 6 (37.5) |
| Multivitamin-D3, n (%) | 4 (28.6) | 3 (16.7) | 5 (25) | 6 (37.5) |
| Vitamin D supplement intake | 0 | 0 | 0 | 0 |
| Dropout, n (%) | 4 (20) | 2 (10) | 0 (0) | 4 (20) |
| Menopausal status, n (%) | 3 (21.4) | 2 (11.1) | 5 (25) | 3 (18.8) |
| Oral contraceptive pill use, n (%) | 0 (0) | 2 (11.1) | 1 (5) | 0 (0) |
| Compliance (%) | 96.6 | 95.0 | 95.3 | 93.6 |
| Mean initial 25(OH)D ± sd (ng/ml) | 18.6 ± 8.9 | 20.2 ± 10.4 | 19.6 ± 11.1 | 16.9 ± 10.5 |
| Mean final 25(OH)D ± sd (ng/ml) | 18.8 ± 7.9 | 28.4 ± 7.7 | 28.9 ± 11.0 | 26.8 ± 9.6 |
| Mean differences ± sd | 0.2 ± 5.3 | 8.2 ± 7.8a,d | 9.3 ± 7.1b,d | 9.9 ± 3.2c,d |
| 95% CI | −2.6–3.0 | 4.6–11.8 | 6.2–12.7 | 5.2–14.6 |
| Demographics, n (%) | ||||
| Asian | 2 (14.3) | 1 (5.6) | 4 (20) | 1 (6.3) |
| American Indian | 0 (0) | 0 (0) | 0 (0) | 1 (6.3) |
| Black | 6 (42.9) | 9 (50) | 8 (40) | 9 (56.3) |
| Hispanic | 0 (0) | 2 (11.1) | 2 (10) | 1 (6.3) |
| White | 6 (42.9) | 6 (33.3) | 6 (30) | 4 (25) |
CI, Confidence interval.
P = 0.041 for D3+D2 vs. placebo.
P = 0.027 for D3 vs. placebo.
P = 0.023 for D2 vs. placebo.
No statistically significant difference between D3+D2, D3, and D2 (P = 0.957).
Design
Sixty-eight subjects were randomly assigned in a double-blinded fashion to receive daily in a capsule for 11 wk 1) placebo, 2) 1000 IU (25 μg) vitamin D2 (ergocalciferol), 3) 1000 IU (25 μg) vitamin D3 (cholecalciferol), or 4) 500 IU vitamin D2 plus 500 IU vitamin D3. All of the capsules made by Tishcon Corp. (Salisbury, MD) contained lactose (98.75%), magnesium stearate (1.0%), and silicon dioxide (1.25%). All of the products were analyzed in our laboratory by HPLC and found to contain either no vitamin D (placebo) or concentrations within 10% of their specified content. All subjects had blood samples collected at baseline and every week for a total of 11 wk. Each subject was given a dietary questionnaire at baseline to assess multivitamin and milk consumption. Pill compliance (Table 1) was determined by a pill count at each visit.
Analytical methods
Serum 25(OH)D2 and 25(OH)D3 were determined by liquid chromatography tandem mass spectroscopy at Quest Diagnostics Nichols Institute, San Juan Capistrano, CA (10). The detection limit for the assay was 4 ng/ml, and the interassay coefficient of variation was about 10%. Values for serum 25(OH)D2 reported as less than 4 ng/ml were obtained by subtracting 25(OH)D3 from the total 25(OH)D.
Statistical methods
The results are presented as means ± sd. Data were analyzed using mixed-effects regression to perform a repeated-measures analysis of 25(OH)D levels across time and groups. Pairwise comparisons were performed between all treatment groups as well as each treatment group vs. placebo. Interactions between treatment group and time compared the linear change in 25(OH)D over time between the groups. A repeated-measures mixed-effect model also compared the 25(OH)D2 and 25(OH)D3 across visits for each of the treatment groups. Statistical analysis was performed using SAS (SAS Institute, Inc., Cary, NC).
Results
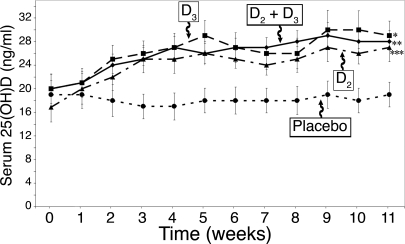
Sixty percent of our healthy adult subjects were vitamin D deficient [25(OH)D < 20 ng/ml], and 87% were insufficient [25(OH)D < 30 ng/ml], even though about 29% took a multivitamin daily that contained 400 IU vitamin D and about 47% drank about 1.2 glasses of milk per day. Adults who received the placebo capsule daily for 3 months demonstrated no significant change in their total 25(OH)D levels during the winter and early spring (Fig. 1). Adults who ingested 1000 IU vitamin D2/d gradually increased their total 25(OH)D levels from 16.9 ± 10.5 ng/ml to 25.8 ± 6.6 ng/ml during the first 6 wk and then remained stable (Fig. 1). Adults who ingested 1000 IU vitamin D3 had a baseline 25(OH)D of 19.6 ± 11.1 ng/ml that was statistically no different from the baselines of either the placebo group or the groups that took 1000 IU vitamin D2/d or 500 IU vitamin D2 plus 500 IU vitamin D3/d (P = 0.79). The vitamin D3 group increased their serum 25(OH)D levels similar to that of the group that ingested vitamin D2. The 25(OH)D levels in the vitamin D3 group began to plateau by wk 6 and was 28.9 ± 11.0 ng/ml at the end of the study, which was not statistically different from the vitamin D2 group (26.8 ± 9.6 ng/ml) (Fig. 1).
Figure 1.
Mean (± sem) serum 25(OH)D levels after oral administration of vitamin D2 and/or vitamin D3. Healthy adults recruited at the end of the winter received placebo (•; n = 14), 1000 IU vitamin D3 (D3, ▪; n = 20), 1000 IU vitamin D2 (D2, ▴; n = 16), or 500 IU vitamin D2 and 500 IU vitamin D3 [D2 and D3, ♦; n = 18) daily for 11 wk. The total 25(OH)D levels are demonstrated over time. *, P = 0.027 comparing 25(OH)D over time between vitamin D3 and placebo; **, P = 0.041 comparing 25(OH)D over time between 500 IU vitamin D3 plus 500 IU vitamin D2 and placebo; ***, P = 0.023 comparing 25(OH)D over time between vitamin D2 and placebo.
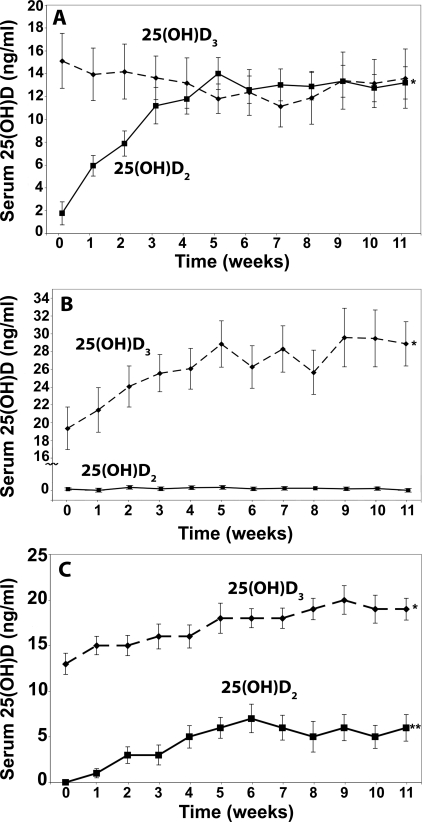
To determine whether vitamin D2 ingestion had any effect on circulating levels of 25(OH)D3, we determined 25(OH)D2 and 25(OH)D3 in the samples. The 25(OH)D2 levels increased from undetectable (<4 ng/ml) to 14 ± 5.3 ng/ml by wk 6 and remained at approximately 14 ng/ml for the ensuing 5 wk in the group that received 1000 IU vitamin D2 (Fig. 2A). The baseline 25(OH)D3 level in the same subjects was 15.1 ± 9.8 ng/ml and did not significantly change during the entire study and was 13.6 ± 10.2 ng/ml at the end of the study (P = 0.14) (Fig. 2A). Similarly, the group that received vitamin D3 showed no significant change in the serum 25(OH)D2 throughout the study (P = 0.33) (Fig. 2B).
Figure 2.
Effect of vitamin D2 or vitamin D3 on serum 25(OH)D2 and 25(OH)D3 levels. Serum levels of 25(OH)D2 (▪) and serum 25(OH)D3 (♦) were measured in healthy subjects receiving 1000 IU vitamin D2 (A), 1000 IU vitamin D3 (B), or 500 IU vitamin D2 plus 500 IU vitamin D3 (C) daily for 11 wk. Results are presented as means ± sem over time. *, P < 0.0001 comparing 25(OH)D2 between baseline and 11 wk (A); *, P < 0.0001 comparing 25(OH)D3 between baseline and 11 wk (B); *, P = 0.0014 comparing between 25(OH)D3 and placebo group (C); **, P = 0.0031 comparing 25(OH)D2 and placebo group (C). Note serum 25(OH)D2 levels less than 4 ng/ml were obtained by subtracting the total 25(OH)D3 from the total 25(OH)D levels.
To determine further whether vitamin D2 interfered with vitamin D3 metabolism, we gave one group of subjects 500 IU vitamin D2 mixed with 500 IU vitamin D3. The rise in the total 25(OH)D was identical to that observed for the groups who received either 1000 IU vitamin D2 or 1000 IU vitamin D3 daily, and the total 25(OH)D levels at the end of the study were no different in all three groups (P = 0.957) (Fig. 1). An analysis of the 25(OH)D2 and 25(OH)D3 also demonstrated a comparable increase in both levels in the group that received the combination of 500 IU vitamin D2 (5.7 ± 4.5 ng/ml) and 500 IU vitamin D3 (6.1 ± 4.3 ng/ml) (Fig. 2C).
Discussion
Many multivitamin preparations and some foods are fortified with vitamin D2. Two recent observations have raised questions as to whether vitamin D2 should be used either as a pharmaceutical agent or as a supplement because it appeared that vitamin D2 not only was less effective than vitamin D3 in maintaining 25(OH)D levels (8,9) but that it also had a negative effect on 25(OH)D status (9). There has also been concern that vitamin D2 may not be bioequivalent to vitamin D3 in maintaining bone health (11).
The Food and Nutrition Board has recommended that adults up to the age of 50 yr require 200 IU vitamin D/d, whereas adults 51–70 yr and 71 yr and older require 400 and 600 IU/d, respectively (12). However, many experts now agree that in the absence of adequate sun exposure, at least 1000 IU vitamin D/d is required to maintain 25(OH)D in the sufficient range (1,13).
Because the placebo group did not demonstrate any change in their circulating levels of 25(OH)D, there was little influence of environmental sun exposure or dietary or supplemental vitamin D intake on their vitamin D status. Subjects who received 1000 IU vitamin D2 or 1000 IU vitamin D3 daily gradually increased blood levels of 25(OH)D to the same levels throughout the study. The increase from baseline in the total 25(OH)D levels at the end of the study was 9.3 ng/ml for the vitamin D3 group, 9.9 ng/ml for the vitamin D2 group, and 8.2 ng/ml for the vitamin D2 plus vitamin D3 group, which is consistent with the observation that serum 25(OH)D levels increased by 1 ng/ml for every 100 IU vitamin D3 (14). However, the 25(OH)D levels did not rise above 30 ng/ml, which is now considered to be the vitamin D-sufficient range, suggesting that more than 1000 IU vitamin D2 or vitamin D3 is necessary to maintain serum 25(OH)D levels above 30 ng/ml when the sun provides no vitamin D3.
Armas et al. (9) reported that a single dose of 50,000 IU vitamin D2 was less effective than 50,000 IU vitamin D3 in maintaining serum 25(OH)D levels over the ensuing 30 d in the summer. Furthermore, when compared with the group that received placebo, the group that received 50,000 IU vitamin D2 had a significant reduction in serum 25(OH)D at the end of the study. We did not observe any negative influence of vitamin D2 on either total 25(OH)D or 25(OH)D3 levels (Figs. 1 and 2). The maintenance of the serum 25(OH)D3 levels observed in this report (Fig. 1) was most likely due to the release of vitamin D3 stored in the body fat because skin synthesis of vitamin D3 does not occur during the winter in Boston (1). It is possible that a single pharmacological dose of vitamin D2 enhanced the destruction of both vitamin D2 and vitamin D3 and their 25-hydroxy derivatives. However, when 50,000 IU vitamin D2 was given weekly for 8 wk (15) or twice a week for 5 wk (16), there was on average a 100% increase in serum 25(OH)D levels (15) and a significant increase in bone mineral density in both the hip and spine (16). Thus, vitamin D2 when given in pharmacological doses is effective in maintaining serum 25(OH)D levels and is beneficial for skeletal health (16). Why Trang et al. (8) observed that the daily dosing of 4000 IU vitamin D3 for 2 wk was 1.7 times more effective in raising blood levels of 25(OH)D (increased 9.0 ± 2 ng/ml) than 4000 IU vitamin D2/d (increased 4.2 ± 2 ng/ml) is unclear at this time. The rise in serum 25(OH)D3 was only about 20% of what would have been expected for a 4000 IU dose, i.e. 40 ng/ml. This may be due to their ethanol formulation. This could also be due to the short time course because we observed that 25(OH)D levels did not begin to plateau until 6 wk. When vitamin D2 was combined with vitamin D3, there was no significant difference in the rise in 25(OH)D (Fig. 1). Furthermore, the group that received 1000 IU vitamin D2 had no significant change in the level of 25(OH)D3, suggesting that vitamin D2 at least at 1000 IU/d had no influence on the catabolism of vitamin D3 or 25(OH)D3. Thus, 1000 IU vitamin D2/d is as effective as vitamin D3 in maintaining 25(OH)D status. These observations are consistent with those of Markestad et al. (17) and Rapuri et al. (18) who observed that vitamin D2 and vitamin D3 contributed equally to serum 25(OH)D levels in mothers and their neonates and elderly women, respectively. Furthermore, the concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 were reported to be proportional to the distribution of 25(OH)D2 and 25(OH)D3 (19,20), implying that the 25(OH)D-1-hydroxylase (CYP27B-1) recognized 25(OH)D2 equally as well as 25(OH)D3. Therefore, collectively, these data and our results suggest that vitamin D2 is as effective as vitamin D3 in sustaining both 25(OH)D and 1,25(OH)2D levels (19,20) and improving bone health (16). More studies are needed to determine whether the carrier (i.e. ethanol vs. oil vs. lactose) that vitamin D2 and vitamin D3 are dissolved in influence either their bioavailability or catabolism. Our observations also suggest that 1000 IU vitamin D2 or vitamin D3 is required to sustain blood levels of 25(OH)D above a mean of 20 ng/ml but was insufficient in raising the levels above a mean of 30 ng/ml.
Acknowledgments
We thank Lindsey Minion, Bernadette Folly, Thomas Peeples, Sr., Horace Shearer, Johanna Gusman, Tarma Johnson, and Deborah Lancaster for their help with the study; Tim Heeren for his statistical acumen; and Donna Gendron and Lorrie MacKay for their secretarial assistance.
Footnotes
This work was supported by National Institutes of Health Grant M01RR00533 and The Beverage Institute for Health and Wellness, a Division of The Coca-Cola Co., Atlanta, GA.
Disclosure Statement: M.F.H. is on the Speaker’s Bureau for Merck, Proctor and Gamble, and Eli Lilly and a consultant for Amgen, Novartis, Quest Diagnostics, Proctor and Gamble, and Merck. R.M.B., T.C.C., E.K.K., A.Y., D.B., W.S., A.A., and A.D.T. have nothing to declare. R.R. is Medical Director of Quest Diagnostics/Nichols Institute and has equity interests in Quest Diagnostics/Nichols Institute.
First Published Online December 18, 2007
Abbreviation: 25(OH)D, 25-Hydroxyvitamin D.
References
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Eliot MM, Park EA 1938 Rickets. In: Brennemann’s practice of pediatrics. Vol. 1. Hagerstown, MD: WF Prior; 1–110 [Google Scholar]
- Holick MF 2006 Resurrection of vitamin D deficiency and rickets. J Clin Invest 116:2062–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeans PC 1950 Vitamin D. JAMA 143:177–181 [DOI] [PubMed] [Google Scholar]
- Dusso AS, Brown AJ, Slatopolsky 2005 Vitamin D. Am J Physiol Renal Physiol 289:F8–F28 [DOI] [PubMed] [Google Scholar]
- DeLuca H 2004 Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80(Suppl):1689S–1696S [DOI] [PubMed] [Google Scholar]
- Selye, H 1932 On the stimulation of new bone-formation with parathyroid extract and irradiated ergosterol. Endocrinology 16:547–588 [Google Scholar]
- Trang HM, Cole DEC, Rubin LA, Pierratos A, Siu S, Vieth R 1998 Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nut 68:854–858 [DOI] [PubMed] [Google Scholar]
- Armas LAG, Hollis B, Heaney RP 2004 Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89:5387–5391 [DOI] [PubMed] [Google Scholar]
- Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE 2005 Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90:3215–3224 [DOI] [PubMed] [Google Scholar]
- Glendenning P 2002 Vitamin D deficiency and multicultural Australia. Med J Aust 176:242–243 [DOI] [PubMed] [Google Scholar]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board, Institute of Medicine 1999 Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D and fluoride. Washington, DC: National Academy Press [Google Scholar]
- Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland C, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman Aw, Scragg R, Whiting SJ, Willett WC, Zittermann A 2007 The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 85:649–650 [DOI] [PubMed] [Google Scholar]
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ 2003 Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77:204–210 [DOI] [PubMed] [Google Scholar]
- Malabanan A, Veronikis IE, Holick MF 1998 Redefining vitamin D insufficiency. Lancet 351:805–806 [DOI] [PubMed] [Google Scholar]
- Adams JS, Kantrorovich V, Wu C, Javanbakht M, Hollis BW 1999 Resolution of vitamin D insufficiency in osteopenic patients results in rapid recovery of bone mineral density. J Clin Endocrinol Metab 84:2729–2730 [DOI] [PubMed] [Google Scholar]
- Markestad T, Aksnes L, Ulstein M, Aarskog D 1984 25-Hydroxyvitamin D and 1,25-dihydroxyvitamin D of D2 and D3 origin in maternal and umbilical cord serum after vitamin D2 supplementation in human pregnancy. Am J Clin Nutr 40:1057–1063 [DOI] [PubMed] [Google Scholar]
- Rapuri PB, Gallagher JC, Haynatzki G 2004 Effect of vitamins D2 and D3 supplement use on serum 25(OH)D concentration in elderly women in summer and winter. Calcif Tissue Int 74:150–156 [DOI] [PubMed] [Google Scholar]
- Clemens TL, Zhou XY, Myles M, Endres D, Lindsay R 1986 Serum vitamin D2 and vitamin D3 metabolite concentrations and absorption of vitamin D2 in elderly subjects. J Clin Endocrinol Metab 63:656–660 [DOI] [PubMed] [Google Scholar]
- Hartwell D, Hassager C, Christiansen C 1987 Effect of vitamin D2 and vitamin D3 on the serum concentrations of 1,25(OH)2D2 in normal subjects. Acta Endocrinol (Copenh) 115:378–384 [DOI] [PubMed] [Google Scholar]