Abstract
Priapism, abnormally prolonged penile erection in the absence of sexual excitation, is associated with ischemia-mediated erectile tissue damage and subsequent erectile dysfunction. It is common among males with sickle cell disease (SCD), and SCD transgenic mice are an accepted model of the disorder. Current strategies to manage priapism suffer from a poor fundamental understanding of the molecular mechanisms underlying the disorder. Here we report that mice lacking adenosine deaminase (ADA), an enzyme necessary for the breakdown of adenosine, displayed unexpected priapic activity. ADA enzyme therapy successfully corrected the priapic activity both in vivo and in vitro, suggesting that it was dependent on elevated adenosine levels. Further genetic and pharmacologic evidence demonstrated that A2B adenosine receptor–mediated (A2BR-mediated) cAMP and cGMP induction was required for elevated adenosine–induced prolonged penile erection. Finally, priapic activity in SCD transgenic mice was also caused by elevated adenosine levels and A2BR activation. Thus, we have shown that excessive adenosine accumulation in the penis contributes to priapism through increased A2BR signaling in both Ada–/– and SCD transgenic mice. These findings provide insight regarding the molecular basis of priapism and suggest that strategies to either reduce adenosine or block A2BR activation may prove beneficial in the treatment of this disorder.
Introduction
Priapism is a condition of persistent penile erection in the absence of sexual excitation (1, 2). This condition is common among males with sickle cell disease (SCD), 40% of whom display priapism (3, 4). The disorder is a urological emergency requiring prompt and accurate diagnosis and treatment because it is associated with erectile tissue damage and erectile disability (3–6). However, effective treatment and preventive approaches to limit abnormal erection tendencies are lacking due to poor understanding of specific factors and signaling pathways involved in priapism.
It has become clear that priapism and erectile dysfunction are vascular diseases involving the interaction of multiple cell types, including neuronal, endothelial, and vascular smooth muscle cells. To fully understand the interplay among these cells it will be necessary to decipher the intercellular signaling pathways involved. This is a major reason why animal models, in which complex cellular interactions can be studied, will play an important role in studying this disease. For example, the SCD transgenic mouse is a well-accepted animal model that displays features of priapism similar to those seen in humans (7–9). However, the molecular mechanisms contributing to priapism in this mouse model are not well understood. In recent years, numerous studies have focused on the functional role of NO in priapism and erectile dysfunction (10, 11). Unexpectedly, a recent report indicates that eNOS-deficient mice and eNOS/neuronal NOS double-deficient mice display priapic activity characterized with a pronounced erectile response to electrical stimulation of the cavernous nerve (12). This surprising finding suggests that factors other than NO may contribute to priapism.
Adenosine shares multiple features with NO, making it an excellent candidate for contributing to priapism. First, both are well-known potent vasodilators and neurotransmitters. Second, both have a very short half life (<10 s) (13). Third, both induce cyclic nucleotide second messengers and penile erection (14–19). Specifically, adenosine functions through G protein–coupled receptors to modulate adenylyl cyclase and the synthesis of cAMP. NO functions through guanylyl cyclase to induce the synthesis of cGMP. Finally, adenosine-mediated cAMP induction and NO-mediated cGMP induction are capable of inducing protein kinase A and protein kinase G, respectively, resulting in decreased calcium/calmodulin-dependent myosin light chain phosphorylation and enhanced smooth muscle relaxation (14). Earlier studies in multiple animal species, including humans (20), showed that intracavernous injection of adenosine resulted in tumescence and penile erection (14–19). Theophylline, an adenosine receptor antagonist, inhibited adenosine-induced penile tumescence (21). These findings suggest that adenosine may contribute to penile erection through the activation of adenosine receptors. Consistent with these earlier reports, a recent study demonstrates that erectile dysfunction in men may, in some cases, be caused by endothelial A2B adenosine receptor (A2BR) dysfunction (22). However, the role of adenosine signaling in priapism is unknown.
Here we report the analysis of priapism in 2 independent mouse genetic models, Ada–/– mice, which lack adenosine deaminase (ADA), and SCD transgenic mice. In each case we provide evidence that elevated adenosine via A2BR signaling contributes to priapism. Ada–/– mice serve as a useful mouse model to study the consequences of enhanced adenosine receptor signaling, whereas SCD transgenic mice are a well-accepted mouse model of priapism. Thus, an unexpected priapic phenotype associated with Ada–/– mice led us to identify what we believe to be a previously unrecognized pathophysiological role for increased adenosine coupled with A2BR signaling in priapism of SCD transgenic mice. Our findings suggest that this signaling pathway may contribute to priapism in general and may represent an important novel therapeutic target in the treatment of priapism.
Results
Ada–/– mice exhibit priapic activity with spontaneous prolonged penile erection.
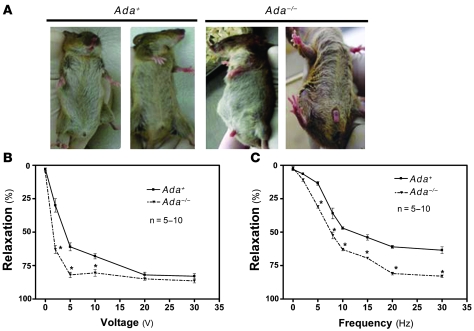
Although ADA deficiency is a lethal condition in humans and mice, it is possible to prolong life indefinitely with the use of polyethylene glycol–ADA (PEG-ADA) enzyme therapy, a treatment that reduces, but does not eliminate, the accumulation of adenosine (23). During the course of maintaining our production colony of Ada–/– mice, we noticed that Ada–/– male breeders (kept alive by weekly injection with PEG-ADA) frequently presented with prolonged penile erections lasting up to 72 hours (Figure 1A). The penile erections were typically noticed at the time of the scheduled weekly PEG-ADA injection. We know from previous studies that the lowest levels of circulating adenosine are achieved during the first day following PEG-ADA injection (24, 25). Adenosine levels rise continually during the subsequent days preceding the next PEG-ADA injection. Thus, adenosine levels were highest just prior to the scheduled weekly injection of PEG-ADA, when the priapism was observed (23, 26). Priapic erections were never observed in wild-type male mice (Figure 1A). This finding suggests that Ada–/– mice may be a unique and valuable genetic animal model of priapism characterized by spontaneous prolonged penile erection.
Figure 1. Ada–/– mice display spontaneous prolonged penile erection associated with increased CCS relaxation in response to nerve stimulation.
(A) Ada–/– mice exhibited prolonged penile erections, lasting 8–72 hours. Priapic erections were never observed in control Ada+ male mice. (B and C) Ada–/– mice displayed hypersensitivity to EFS in both dosage-dependent (B) and frequency-dependent (C) manners. Data are means ± SEM. *P < 0.05, Ada–/– versus Ada+. n = 5–10.
Prolonged penile erection in Ada–/– mice is associated with increased corpus cavernosal smooth muscle relaxation in response to nerve stimulation.
To determine whether the potent and prolonged penile erection in Ada–/– mice is associated with increased corpus cavernosal smooth muscle relaxation, we comparatively evaluated the relaxation of phenylephrine-precontracted isolated corpus cavernosal strips (CCSs) of Ada–/– and control Ada+ mice (see Methods) in response to nerve stimulation (27, 28). Corpus cavernosal smooth muscle is under sympathetic influence to maintain a contracted state resulting in a flaccid penis (29). To emulate this situation, contraction was induced by treatment with the α-adrenergic receptor agonist phenylephrine (10 μM). The maximum phenylephrine-induced contraction was reached within 10–20 s of application to CCSs from Ada+ and Ada–/– mice. Electrical field stimulation (EFS) is commonly used to stimulate the nerves that innervate the smooth muscle cells of the corpus cavernosum to mediate smooth muscle relaxation, corresponding to normal physiological penile erection (12, 27, 28, 30, 31). In our studies, EFS induced relaxation of CCSs from both Ada+ and Ada–/– mice in a voltage- and frequency-dependent manner (Figure 1, B and C). For Ada+ mice, relaxation increased incrementally over a standard voltage range, with a maximal response at 20 V and 30 Hz. However, Ada–/– mice showed increased sensitivity to EFS and achieved maximal relaxation with 5 V and 30 Hz stimulation (Figure 1, B and C). These results suggest that the spontaneous prolonged penile erection observed in Ada–/– mice is associated with enhanced corpus cavernosal smooth muscle relaxation in response to nerve stimulation.
Prolonged penile erection in Ada–/– mice depends on pronounced accumulation of adenosine in the penis.
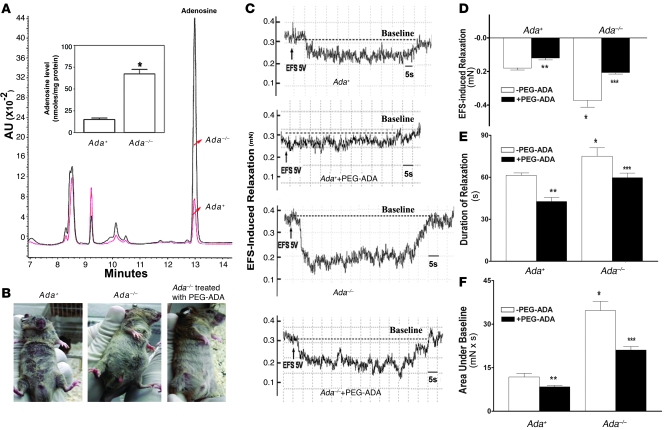
We hypothesized that the increased penile erection and corpus cavernosal smooth muscle relaxation in response to nerve stimulation in Ada–/– mice were the result of elevated concentrations of adenosine. To test this hypothesis, adenosine levels were quantified in penile tissue from Ada+ and Ada–/– mice (23, 26, 32). We found that Ada–/– mice exhibited a marked increase in adenosine concentrations in the penis (Figure 2A). To determine the critical role of elevated adenosine in priapic activity in Ada–/– mice, we injected PEG-ADA into mice that presented with priapic activity. We found that the prolonged penile erections observed in Ada–/– mice were quickly corrected by intraperitoneal injection of a high dose of PEG-ADA (Figure 2B). This in vivo observation provided the clue that elevated adenosine in the penes of Ada–/– mice was responsible for the spontaneous prolonged penile erections.
Figure 2. Priapic activity seen in Ada–/– mice is dependent on elevated adenosine in the penis.
(A) Adenosine levels were elevated in the penes of Ada–/– mice. Inset bar graph shows the average adenosine levels from 3 Ada–/– mice and 3 wild-type mice. Data are means ± SEM (n = 3). *P < 0.005 versus Ada+. (B) The prolonged penile erection in Ada–/– mice was corrected by intraperitoneal injection of PEG-ADA. n = 3–5. (C) Representative recordings of EFS-induced CCS relaxation (5 V and 30 Hz) using 10 μM phenylephrine–precontracted CCSs of Ada+ mice and Ada–/– mice treated with or without PEG-ADA. (D–F) Average EFS-induced relaxation from the phenylephrine-precontracted CCSs of Ada+ mice, Ada–/– mice, and Ada–/– mice treated with PEG-ADA. EFS-induced changes in the force of CCS relaxation (D), the duration of relaxation (E), and the combination of force and duration (area under baseline; F). Data are means ± SEM (n = 5–6). *P < 0.05 versus Ada+; **P < 0.05 versus untreated Ada+; ***P < 0.05 versus untreated Ada–/–.
Elevated adenosine in Ada–/– mice contributes to increased CCS relaxation.
To further confirm our in vivo observation, we functionally tested the effect of PEG-ADA on EFS-induced relaxation of CCSs from Ada–/– mice. This is a commonly used and well-accepted functional assay of penile vascular responses in multiple species (12, 27, 28, 30, 31). CCSs of Ada–/– mice achieved maximal relaxation with 5 V and 30 Hz stimulation (Figure 1, B and C). Therefore, we chose to stimulate CCSs of both Ada+ and Ada–/– mice at 5 V and 30 Hz for 60 s in the presence or absence of PEG-ADA. Specifically, a 60-s EFS at 5 V and 30 Hz evoked substantial and prolonged relaxation of CCSs from Ada–/– mice compared with those from Ada+ mice (Figure 2, C–E). A significantly greater area under baseline was observed that was due to the prolonged and substantial relaxation of CCSs of Ada–/– mice (Figure 2F). Moreover, we found that treating Ada–/– mouse CCSs with PEG-ADA significantly reduced the force and duration of relaxation as well as the area under baseline when compared with untreated CCSs (Figure 2, C–F). Taken together, these results indicate that elevated adenosine levels contribute to the prolonged and substantial penile vascular relaxation and erection associated with Ada–/– mice.
Inhibition of ADA activity in wild-type mice induces potent penile CCS relaxation.
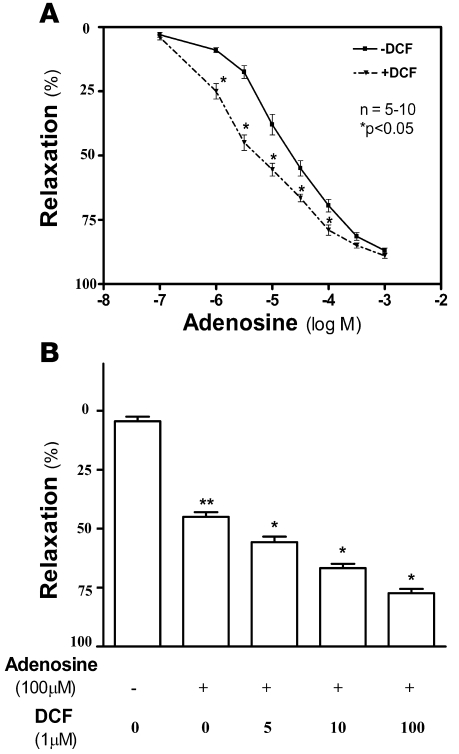
To directly assess the potential role of elevated adenosine in priapism, we measured the extent of smooth muscle relaxation in CCSs from wild-type mice following treatment with adenosine in the presence or absence of deoxycoformycin (DCF; a potent ADA inhibitor). We found that adenosine induced CCS relaxation in a dose-dependent manner (Figure 3A), consistent with earlier studies in other species (22). In addition, we found that the combination of DCF and adenosine significantly increased relaxation compared with adenosine alone (Figure 3B). These pharmacological findings with coincubation of adenosine and DCF support our in vivo and in vitro findings that high concentrations of adenosine in the penis may contribute to priapism associated with Ada–/– mice.
Figure 3. Inhibition of ADA activity in wild-type mice induces potent penile CCS relaxation.
(A and B) CCSs of wild-type mice were treated with different concentrations of adenosine in the presence or absence of DCF (5 μM; A) or with 100 μM adenosine in the presence of different concentrations of DCF (0–100 μM; B). The resulting adenosine-induced CCS relaxation was monitored by force transducer. Data are means ± SEM. *P < 0.05 versus adenosine alone; **P < 0.05 versus untreated.
Adenosine-induced corpus cavernosal smooth muscle relaxation requires A2BR activation.
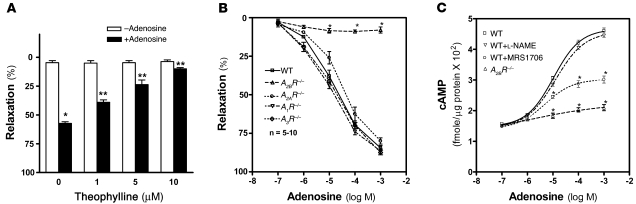
To determine whether adenosine-mediated corpus cavernosal relaxation and prolonged penile erection occurs via adenosine receptor signaling, we measured adenosine-induced CCS relaxation in the presence of theophylline, a general adenosine receptor antagonist. Treatment with theophylline significantly inhibited adenosine-mediated CCS relaxation in a dose-dependent manner (Figure 4A), suggesting that adenosine-mediated corpus cavernosal smooth muscle relaxation occurs via adenosine receptor activation.
Figure 4. A2BR signaling is required for adenosine-mediated CCS relaxation.
(A) The extent of adenosine-induced CCS relaxation in wild-type mice with theophylline treatment was measured by a force transducer. Data are means ± SEM (n = 5). *P < 0.05 versus untreated; **P < 0.05 versus adenosine alone. (B) Adenosine-induced CCS relaxation in wild-type, A1R–/–, A2AR–/–, A2BR–/–, and A3R–/– mice was measured by a force transducer. Data are means ± SEM (n = 5–10). *P < 0.005 versus wild-type. (C) Adenosine-mediated cAMP production of CCSs from wild-type and A2BR–/– mice. Some of the CCSs from wild-type mice were treated with l-NAME or MRS1706. Data are means ± SEM (n = 6–7). *P < 0.05 versus wild-type treated with adenosine alone.
Next, to identify which adenosine receptor is essential for adenosine-mediated penile vascular smooth muscle relaxation, we measured CCS relaxation in adenosine receptor–deficient mice in response to different dosages of adenosine. We found that A1R–/–, A2AR–/–, A3R–/–, and wild-type mice showed a dose-dependent increase in relaxation following treatment with adenosine. In contrast, the adenosine-mediated relaxation was completely absent in CCSs from A2BR–/– mice (Figure 4B). These results provide strong genetic evidence that the A2BR is required for CCS relaxation in response to adenosine.
To determine the signaling components functioning downstream of A2BR, we measured the cAMP levels in response to adenosine in the cultured isolated CCSs of both wild-type and A2BR–/– mice. Adenosine induced cAMP levels in a dose-dependent manner in wild-type but not A2BR–/– cultured CCSs (Figure 4C). Similarly, we found that the adenosine-mediated induction of cAMP in wild-type CCSs was inhibited by MRS1706, an A2BR antagonist, but not by NOS inhibitor l-nitroarginine methyl ester (l-NAME; Figure 4C). Thus, both genetic and pharmacological studies demonstrated that adenosine acted via A2BR activation to induce cAMP levels in CCSs and that the effect did not require NO signaling.
Adenosine acts directly on corpus cavernosal smooth muscle cells and leads to cAMP induction via A2BR activation.
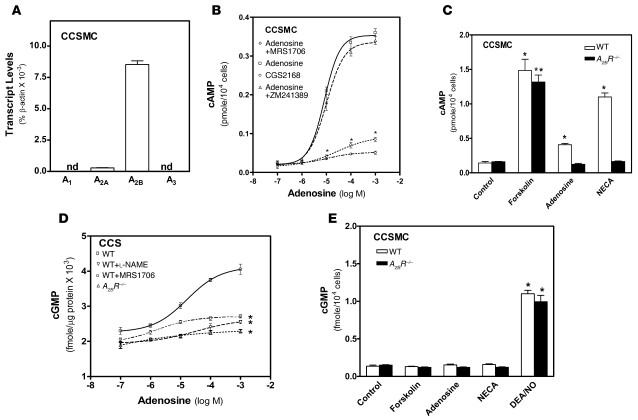
Next, in an effort to decipher specific cell types involved with A2BR signaling in the penis, we purified and cultured corpus cavernosal smooth muscle cells (CCSMCs), the key cell type in the regulation of penile vascular tone (33), from both wild-type and A2BR–/– mice. Quantitative RT-PCR showed that the major adenosine receptor expressed in wild-type CCSMCs was the A2BR (Figure 5A), strongly supporting our intact CCS culture findings that the A2BR is essential for adenosine-induced cAMP production and vascular smooth muscle relaxation (Figure 5, A–C). Additionally, we showed that adenosine-mediated cAMP induction in CCSMCs was blocked by the A2BR antagonist MRS1706, but not by the A2AR antagonist ZM241389. Similarly, the A2AR agonist CGS21680 failed to induce cAMP production in CCSMCs (Figure 5B). Consistent with these results, both adenosine and 5′-N-ethylcarboxamidoadenosine (NECA; a potent and broad-spectrum adenosine receptor agonist) failed to induce cAMP production in CCSMCs of A2BR–/– mice. In contrast, these agents induced cAMP in wild-type CCSMCs. However, forskolin, an adenyl cyclase stimulator, was capable of inducing cAMP production in CCSMCs of both wild-type and A2BR–/– mice (Figure 5C). These results confirm our pharmacological findings that A2BR signaling is required for adenosine-mediated stimulation of cAMP production in CCSMCs.
Figure 5. Adenosine stimulates an increase in both cAMP and cGMP in CCSMCs via A2BR activation.
(A) Adenosine receptor mRNA expression profile in purified primary CCSMCs were determine by quantitative real-time RT-PCR. nd, not determined. (B) cAMP levels of CCSMCs from wild-type mouse penes in the presence of different concentrations of adenosine with or without specific adenosine receptor agonists or antagonists. Data are means ± SEM. *P < 0.05 versus adenosine alone. (C) cAMP levels of CCSMCs from wild-type and A2BR–/– mice treated with adenosine, NECA, or forskolin. Data are means ± SEM (n = 4). *P < 0.05 versus untreated wild-type; **P < 0.05 versus untreated A2BR–/–. (D) cGMP levels in CCSs of wild-type and A2BR–/– mice treated with l-NAME or MRS1706. Data are means ± SEM (n = 6–7). *P < 0.05 versus wild-type treated with adenosine alone. (E) cGMP levels in CCSMCs of wild-type and A2BR–/– mice treated with the indicated compounds. Data are means ± SEM. *P < 0.05 versus respective control.
Adenosine stimulates cGMP accumulation in CCSMCs through A2BR signaling.
NO-mediated cGMP induction is a well-known signaling pathway involved in penile vascular relaxation (34). However, whether adenosine is capable of contributing to NO-mediated cGMP production in penile tissue is unknown. To test this possibility, we conducted genetic and pharmacological studies using CCSs. Unexpectedly, we found that cGMP production was also induced by adenosine in a dose-dependent manner (Figure 5D). However, the induction of cGMP production by adenosine was absent in A2BR–/– mice and was blocked by MRS1706 and l-NAME (Figure 5D). These results indicate that adenosine-mediated cGMP production in penile tissue requires A2BR activation.
Next we explored the potential role of NO signaling in adenosine-mediated cGMP induction using CCSs. We found that adenosine-mediated cGMP induction was completely blocked by l-NAME, a well-known NOS inhibitor (Figure 5D). This finding suggests that adenosine signaling may promote vascular smooth muscle relaxation in part by stimulating nonmuscle cells to produce NO, which diffuses to CCSMCs to activate guanylyl cyclase and cause cGMP accumulation. To test this hypothesis, we measured cGMP levels in CCSMCs of wild-type and A2BR–/– mice in the presence of different agents. Adenosine, NECA, and forskolin had no effect on cGMP levels in CCSMCs of either wild-type or A2BR–/– mice (Figure 5E). In contrast, 2-(N,N-diethylamino)-diazenolate-2-oxide (DEA/NO; a potent NO donor) induced cGMP production in CCSMCs of both wild-type and A2BR–/– mice. These results suggest that adenosine-mediated cGMP induction in CCSMCs occurs via A2BR-mediated NO synthesis and release from nonmuscle cells. Taken together, these findings indicate that adenosine contributes to vascular relaxation by inducing both cAMP and cGMP production via A2BR activation.
Elevated adenosine increases cAMP and cGMP production via enhanced A2BR signaling in Ada–/– mice.
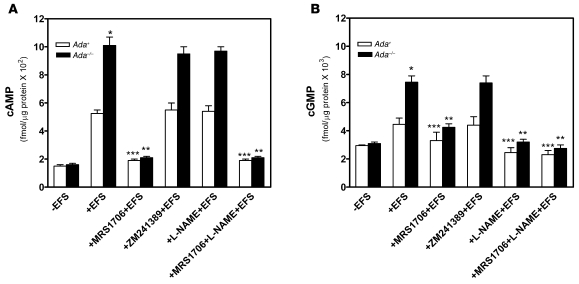
The essential role of A2BR activation in adenosine-mediated penile vascular relaxation in wild-type mice led us to speculate that elevated adenosine in Ada–/– mice may lead to enhanced adenosine signaling via A2BR activation. To test this possibility, we measured levels of cAMP and cGMP in CCSs of Ada+ and Ada–/– mice in response to EFS stimulation. EFS increased cAMP and cGMP levels in CCSs of both Ada+ and Ada–/– mice (Figure 6, A and B). However, the concentrations of cAMP and cGMP in CCSs of Ada–/– mice were significantly higher than those observed in CCSs of Ada+ mice. As expected, increased cAMP levels were completely blocked by MRS1706, but not by ZM241389 or l-NAME, in CCSs of either Ada–/– or Ada+ mice (Figure 6A). These findings indicate that EFS-mediated cAMP induction occurs via A2BR activation in both Ada+ and Ada–/– mice. Similarly, we found that EFS-mediated induction of cGMP in CCSs of Ada+ and Ada–/– mice was significantly inhibited by MRS1706, but not by ZM241389 (Figure 6B). l-NAME completely blocked cGMP induction in CCSs of both Ada+ and Ada–/– mice, indicating that EFS-mediated cGMP induction in CCSs of Ada–/– mice is also caused by enhanced NO signaling via A2BR activation. Taken together, these results suggest that the priapism seen in Ada–/– mice is associated with an increase in both cAMP and cGMP levels that results from adenosine-mediated A2BR activation.
Figure 6. EFS leads to increased cAMP and cGMP production via A2BR in Ada–/– mice.
(A and B) EFS-mediated cAMP (A) and cGMP (B) production were measured in phenylephrine-precontracted CCSs of Ada–/– and Ada+ mice with or without MRS1706, ZM241389, and/or l-NAME treatment. Data are means ± SEM (n = 4). *P < 0.05, versus wild-type with EFS; **P < 0.05 versus Ada–/– with EFS alone; ***P < 0.05 versus wild-type with EFS alone.
Ada–/– mice develop penile vascular damage and fibrosis.
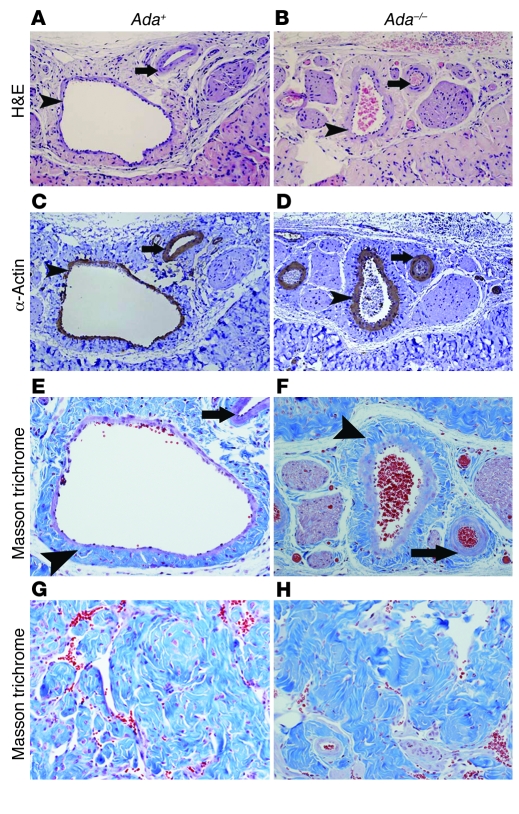
Vascular damage and fibrosis are major complications of priapism (35). Here we also observed penile vascular damage and fibrosis in Ada–/– mice subsequent to prolonged penile erection. H&E staining and anti–α-SMA immunostaining demonstrated extensive endothelial damage, including marked intimal thickening with smooth muscle hypertrophy and endothelial swelling (Figure 7, B and D, arrowheads) in the deep dorsal vein in Ada–/– mice after 48- to 72-hour priapic episodes. Parallel arteries showed muscular hypertrophy of the vascular wall (Figure 7, B and D, arrows). Extensive fibrosis with extension into the intima was also seen in the deep dorsal vein (Figure 7, E and F). Because of the thickening of the intima and fibrotic changes of the vessels, the lumens of both veins and arteries in corpus spongiosum were significantly narrowed (Figure 7, A–F), leading to the more rigid and less flexible vessels in the Ada–/– mice. As an end result of ischemic/hypoxic damage, substantial fibrosis with sclerotic changes was also observed in the corpus cavernosum, accompanied by loss of cellularity, compared with that of Ada+ mice (Figure 7, G and H). Taken together, these results demonstrate that Ada–/– mice develop significant vascular damage and subsequent tissue fibrosis after priapism, which is a major complication of priapism in humans.
Figure 7. Ada–/– mice develop penile vascular damage and fibrosis subsequent to priapism.
(A–D) Histological examination of the vascular structures in the corpus spongiosum. (A and B) H&E staining. (C and D) Anti–α-SMA immunohistochemical staining. Arrowheads indicate intimal thickening with smooth muscle hypertrophy of the vascular wall in the deep dorsal vein; arrows indicate muscular hypertrophy of the arterial vascular wall. (E–H) Fibrosis in corpus spongiosum (E and F) and corpus cavernosum (G and H) visualized by Masson trichrome staining. Arrowheads denote the extensive fibrosis with extension into the intima of the deep dorsal vein; arrows indicate fibrosis around the lumen of the artery. Original magnification, ×100 (A–D); ×200 (E–H).
Increased adenosine contributes to priapic activity in SCD transgenic mice via A2BR signaling.
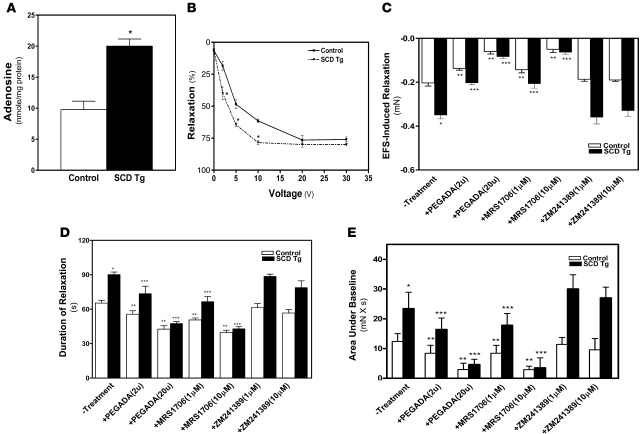
To assess the general significance of high adenosine levels in the pathophysiology of priapism, we investigated the potential contribution of excess adenosine to the priapism associated with SCD transgenic mice (7–9, 12, 36). The adenosine levels in the penes of SCD transgenic mice were significantly higher than those of controls (Figure 8A), which suggests that accumulated adenosine in the penis may contribute to priapic activity in these mice. As with Ada–/– mice, we found that CCSs of SCD transgenic mice were more sensitive to EFS than those of the controls: maximum relaxation was reached at 10 V in SCD transgenic mice and 20 V in controls (Figure 8B). To determine whether the increased adenosine contributed to prolonged and potent penile vascular relaxation (i.e., priapic activity) in SCD transgenic mice, we tested the effect of PEG-ADA on EFS-induced vascular relaxation in CCSs of SCD transgenic mice. Treatment of CCSs from SCD transgenic mice with PEG-ADA significantly reduced the force and duration of relaxation as well as area under baseline compared with untreated CCSs (Figure 8, C–E). These results were very similar to the effect of PEG-ADA on priapic activity in Ada–/– mice and suggest a general contributory role of elevated adenosine in priapism.
Figure 8. Priapic activity in SCD transgenic mice is dependent on elevated adenosine in penis and A2BR signaling.
(A) Adenosine levels were elevated in the penes of SCD transgenic mice. Data are means ± SEM (n = 5–6). *P < 0.005 versus control. (B) Increased CCS relaxation by EFS in SCD transgenic mice. Data are means ± SEM (n = 5). P < 0.05, SCD transgenic versus control. (C–E) Prolonged penile erection in SCD transgenic mice is due to A2BR signaling. EFS-induced relaxation in CCSs (C), duration of relaxation (D), and the combination of force and duration (area under baseline; E) were measured by force transducer in mice left untreated or treated with PEG-ADA, MRS1706, or ZM241389. Data are means ± SEM (n = 5). *P < 0.05 versus control; **P < 0.05, versus untreated SCD transgenic; ***P < 0.05 versus untreated control.
Next, to test whether elevated adenosine–mediated priapic activity in SCD transgenic mice occurs via A2BR signaling, as seen in Ada–/– mice, we measured the EFS-induced relaxation of CCSs of both control and SCD transgenic mice in response to EFS in the presence or absence of A2BR or A2AR antagonists. We found that MRS1706, but not ZM241389, reduced the magnitude and duration of EFS-induced contraction of CCSs from SCD transgenic mice compared with untreated CCSs (Figure 8, C–E), which indicates that A2BR signaling is critical for priapism in SCD transgenic mice. Taken together, these findings suggest that increased adenosine, via A2BR signaling, may contribute to priapic activity in SCD transgenic mice.
Discussion
Here we report that male Ada–/– mice displayed features of priapism seen in humans, including spontaneous prolonged penile erection and increased CCS relaxation following neurostimulation with subsequent penile vascular damage and fibrosis. In addition, we demonstrated that reducing the accumulation of adenosine by ADA enzyme therapy relieved spontaneous prolonged penile erection and CCS relaxation both in vivo and in vitro. Moreover, the analysis of 4 adenosine receptor–deficient mice revealed that the A2BR is essential for adenosine-dependent penile vascular smooth muscle relaxation and erection and that upregulated A2BR signaling contributes to priapic activity in Ada–/– mice. Finally, we found that priapic activity in SCD transgenic mice was also due to elevated adenosine signaling via A2BR, suggesting a general contributory role of adenosine and A2BR signaling in priapism. Thus, although a role for adenosine signaling in priapism was initially suggested by an unexpected phenotype associated with Ada–/– mice, this hypothesis was confirmed and extended by analysis of SCD transgenic mice, a well-accepted animal model of priapism. Overall, our analysis of priapism in Ada–/– and SCD transgenic mice provides strong support for the concept that elevated adenosine via A2BR signaling contributes to priapism and that this signaling pathway represents a potentially important therapeutic target for the treatment of priapism.
Penile vascular tone is a key regulator of penile erection. In recent years, NO signaling has received considerable attention for its role in normal (10, 34) and abnormal penile vascular regulation (11). The focus on NO was initiated by the unexpected observation by cardiologists that hypertensive patients, when treated with phosphodiesterase-5A (PDE5) inhibitors, frequently displayed persistent penile erections. These unexpected observations during clinical trials of PDE5 inhibitors drew attention to the potential role of NO in penile vascular regulation. However, studies of the role of adenosine signaling in penile vascular function are very limited. Earlier studies in several animal species, including humans (20), showed that intracavernous injection of adenosine resulted in tumescence and penile erection (14–19). These findings suggest that adenosine may contribute to penile erection. Faria et al. recently reported that corpus cavernosal tissue from patients with erectile dysfunction is partially resistant to adenosine-mediated relaxation due to A2BR dysfunction (22). However, to our knowledge, a role for adenosine signaling in priapism has not previously been described. One possible reason is that adenosine has a very short half life and fails to accumulate at a high level for a prolonged time period through local injection. Reminiscent of the unexpected effects of PDE5 inhibitors on penile vascular relaxation in hypertensive patients, we surprisingly discovered prolonged penile erections in Ada–/– mice that were characterized by excessively elevated levels of adenosine in the penis. This finding led us to hypothesize that persistent elevated adenosine may contribute to priapism. This hypothesis is supported by our finding that priapic activity in SCD transgenic mice was also due to elevated adenosine. Thus, an unexpected priapic phenotype associated with Ada–/– mice led us to identify what we believe to be a previously unrecognized pathophysiological role for increased adenosine via A2BR signaling in priapism in SCD transgenic mice. Our findings suggest that this signaling pathway may contribute to priapism in general.
The elevation of adenosine in Ada–/– mice is a direct metabolic consequence of the enzyme deficiency. In this case the accumulation of adenosine in Ada–/– mice contributed to spontaneous prolonged penile erection. Given that adenosine accumulates under hypoxic conditions and that priapism is frequently seen in SCD patients when they are under hypoxic stress (37), we speculate that hypoxia-mediated enhanced adenosine signaling may contribute to the priapism associated with SCD. We found that adenosine levels were elevated in the penes of SCD transgenic mice and that this correlated with enhanced penile vascular smooth muscle relaxation induced by EFS. Removal of adenosine by treatment with PEG-ADA reversed the enhanced relaxation, which suggests that elevated adenosine levels in the penes of SCD transgenic mice contribute to the priapic phenotype. Based on these findings, we speculate that in humans with SCD, penile tissue hypoxia (perhaps resulting from hemolytic crisis) leads to an increased level of local adenosine, which contributes to prolonged penile erection through A2BR activation. Prolonged penile erection will result in ischemia and endovascular damage in the penis and further enhance production of adenosine locally. The resulting increase in local adenosine level will lead to even longer and more potent penile erection. Thus, hypoxia-induced adenosine accumulation, prolonged penile erection, and ischemic vascular damage act in a detrimental cycle, eventually leading to priapism. If not corrected, it will result in penile fibrosis and erectile dysfunction, which is one of the most common and serious complications of priapism seen in humans. It is possible that therapeutic intervention with PEG-ADA enzyme therapy, as we have shown here with SCD transgenic mice, may interrupt this detrimental cycle and allow for penile detumescence.
Approximately 40% of male patients with SCD develop priapism (2, 35). A commonly assumed mechanism for priapism in these individuals is venous occlusion (2, 35, 38). According to this hypothesis, the decrease in oxygen tension that accompanies normal erection promotes erythrocyte sickling, leading to venous occlusion and obstruction of the deep dorsal vein. This condition is believed to result in locally severe ischemia leading to a low-flow ischemic type of priapism characteristic of SCD. However, this classic paradigm of venous occlusion has been challenged by several recent studies in favor of mechanisms that entail altered regulation of penile erection. In particular, recent research indicates that dysregulated NO signaling may lead to priapism. Champion et al. found decreased PDE5 expression and activity in penes of genetically altered mouse models lacking eNOS and transgenic SCD mice, all of which display a priapism phenotype (12). These observations imply that pathological changes in PDE5 may contribute to priapism. Here we presented evidence in support of what we believe to be a novel explanation for the priapism associated with SCD transgenic mice and Ada–/– mice. In particular, we showed that increased adenosine in the penes of SCD transgenic mice and Ada–/– mice contributed to priapism via A2BR activation and increased penile vascular relaxation. Based on the fact that adenosine is highly induced under ischemic and hypoxic conditions and the report by Lin et al. that PDE5 is downregulated in ischemic CCSMCs in vitro (39), it is possible that in addition to its potent direct vascular relaxation effect, increased adenosine caused by ischemia may be a potential factor downregulating PDE5 in priapism associated with SCD transgenic or eNOS-deficient mice.
Adenosine is a purine-signaling nucleoside that is highly induced under hypoxia conditions. Once produced, adenosine can engage specific G protein–coupled receptors on the surface of cells. Four adenosine receptors have been identified: A1R, A2AR, A2BR, and A3R (40, 41). Among the 4 adenosine receptors, the A2BR has the lowest affinity for adenosine (41). It is likely that A2BRs are only engaged under pathological conditions, such as hypoxia, that contribute to elevated adenosine levels. This view is consistent with our present results in Ada–/– and SCD transgenic mice, where we have shown that pathological conditions for each mice included elevated levels of penile adenosine. Thus, the concentrations of adenosine achieved in the penes of these mutant mice are sufficient to activate the A2BR. It will be interesting to test whether A2BR antagonists will be useful for the treatment of priapism in humans in the future by using these animal models of priapism for preclinical studies.
Adenosine-mediated intracellular signaling pathways play an essential role in processes such as cell differentiation, proliferation, survival, and apoptosis as well as vascular relaxation (42, 43). More recent studies demonstrate that adenosine-mediated vascular relaxation may couple to NO signaling in the mouse aorta (44). Consistent with these studies, we showed here that adenosine was capable of inducing cAMP accumulation through A2BR activation in penile organ culture and CCSMCs. In addition, we found that adenosine also induced cGMP via A2BR signaling in intact penile organ culture but not in purified CCSMCs. These results suggest that adenosine may contribute to cavernosal smooth muscle relaxation, at least in part, by stimulating neighboring nonmuscle cells to produce NO that diffuses to CCSMCs and activates guanylyl cyclase. Endothelial cells and neuronal cells are likely candidates to synthesize and release NO in response to adenosine via A2BR activation in the penis. More studies are needed to define the cell types that release NO in response to adenosine in penis.
To our knowledge, there is no report of priapism in ADA–/– children. However, the extensive use of PEG-ADA enzyme therapy from the time of diagnosis may prevent the appearance of adenosine-dependent priapism in humans. In this regard, it is interesting to note that numerous nonimmune phenotypes were described for ADA–/– infants prior to the generalized use of enzyme therapy in the early 1990s (24, 45, 46). During these years before the use of enzyme therapy, ADA–/– children usually died within the first 2 years of life and did not live to an age at which the issue of priapism would be addressed. Although ADA deficiency was originally and primarily associated with an immunodeficiency phenotype (41), it is now well accepted that ADA deficiency is a general model for studying consequences of enhanced adenosine receptor signaling. This is especially true for Ada–/– mice because it is possible to experimentally regulate endogenous adenosine levels by varying the dose of PEG-ADA therapy. Thus, Ada–/– mice not only show immunodeficiency, the major symptom of ADA–/– humans, but they also have malformations and malfunctions of multiple organs, including liver, small intestine, kidneys, bone, and cartilage (23). In some cases phenotypic consequences of ADA deficiency were initially discovered in Ada–/– mice and only subsequently recognized in ADA–/– children (46). Thus, we may need to pay close attention to ADA–/– males to determine whether they also present with priapism.
In summary, we unexpectedly observed spontaneous prolonged penile erections in Ada–/– mice. Subsequent analysis of these mice and SCD transgenic mice revealed that their priapism was caused by enhanced A2BR signaling associated with increased penile adenosine levels. We believe these studies provide significant new insight concerning the potential pathological role of adenosine signaling in priapism and penile fibrosis. Continued efforts to understand the contribution of adenosine signaling to priapism in Ada–/– mice and SCD transgenic mice are likely to identify targets for adenosine-based therapeutics to treat priapism in humans.
Methods
Mice.
Ada–/– mice were generated and genotyped as previously described (25, 26, 32). Ada–/– mice were on a mixed background of 129/sV, C57BL/6, and FVB/N strains. Control mice, designated Ada+, were littermates that were either wild-type (+/+) or heterozygotes (+/–) for the null Ada allele. Heterozygous mice do not display a phenotype. All phenotypic comparisons were performed among littermates. A1R–/– mice were obtained from J. Schnermann (NIDDK, NIH, Bethesda, Maryland, USA); A2AR–/– mice were obtained from J.-F. Chen (Boston University School of Medicine, Boston, Massachusetts, USA); A2BR–/– mice were generated in our laboratory; and A3R–/– mice were obtained from M. Jacobson (Merck Research Laboratories, West Point, Philadelphia, USA). All adenosine receptor–deficient mice were backcrossed at least 10 generations onto the C57BL/6 background and were genotyped according to established protocols (47–50). SCD transgenic mice, expressing exclusively human sickle hemoglobin, were purchased from The Jackson Laboratory (7, 8). All mice were maintained and housed in accordance with NIH guidelines and with the approval of the Animal Care and Use Committee at the University of Texas Health Science Center at Houston.
ADA enzyme therapy.
PEG-ADA was generated by the covalent modification of purified bovine ADA with activated PEG as described previously (51–53). Ada–/– mice were identified at birth by screening for ADA enzyme activity in the blood as described previously (23, 51–53). Ada–/– mice were maintained on ADA enzyme therapy from postnatal day 2. The mice were maintained with ADA enzyme therapy for at least 8 weeks to allow them to reach reproductive maturity.
Quantification of penile adenosine levels.
Mice were anesthetized, and the penes were rapidly removed and frozen in liquid nitrogen. Adenine nucleosides were extracted from frozen penes using 0.4 N perchloric acid, and adenosine was separated and quantified using reverse-phase HPLC as described previously (26, 32).
Physiological function experiments.
The tunica albuginea was cut longitudinally, starting at the most proximal point of the corpus cavernosum toward the penile shaft, and the erectile tissue was partially dissected free from the tunica. One strip of tissue (0.3 × 0.3 × 3 mm) was obtained from each corpus cavernosum. The contractility of each isolated CCS was measured using an isometric force transducer (AD Instruments Inc.). The strips were mounted in a thermostatically controlled tissue bath containing aerated standard Krebs solution (119 mM NaCl, 4.7 mM KCl, 24 mM NaHCO3, 1.2 mM KH2PO4, 2.5 mM CaCl2, 1.2 mM MgSO4, 0.023 mM EDTA, and 11 mM glucose; 5 ml volume, 95% O2 and 5% CO2, 37°C) and stretched to a resting tension of 0.1 mN. The contractile responses of the strips were analyzed by adding 10 μM phenylephrine to the bath, and force changes were recorded in response to drug application and to EFS. The particular EFS parameters of 0–30 V and 30 Hz, with a pulse width of approximately 0.5 ms, are commonly used in mice. Available evidence indicates that these electrical field strength parameters stimulate only neurons, not muscle, and thus are thought to mimic normal penile erection (27, 28, 31). The concentrations of cAMP and cGMP in CCSs were subsequently determined (see below).
In vitro corpus cavernosum tissue culture.
CCSs were prepared as described above. The isolated CCSs were then immersed in standard Krebs solution (pH 7.4) bubbled with 95% O2 at 37°C. After 30 min equilibration, strips were exposed to different concentrations of adenosine in the presence or absence of various adenosine receptor agonists or antagonists or l-NAME. After 10 min, the CCSs were removed and immediately frozen in liquid nitrogen for later determination of cAMP and cGMP levels (see below). In a separate series of experiments, CCSs were exposed to phenylephrine (10 μM) with or without treatment with adenosine or l-NAME (10 min).
Isolation of primary CCSMCs.
CCSs were isolated as described above, washed in PBS, and minced into 12-mm3 pieces. Segments were incubated in 5–10 ml enzyme solutions containing 0.02% collagenase A (0.272 U/mg protein; Roche) and 0.5% elastase (3.73 U/mg protein; Cell Systems) in a 75-mm flask at 37°C for 6 hours. Enzymatic digestion was terminated by adding 10 ml DMEM supplemented with 10% FCS. Afterward, the suspension was filtered through a 40-μm nylon mesh to separate single cells and centrifuged at 200 g for 10 min. Cell pellets were resuspended and cultivated for 14 days in 75-cm2 cell culture flasks using 10 ml supplemented vascular smooth muscle cell growth medium as described previously (33), including antibiotics and 10% FCS. Vascular smooth muscle cells typically accounted for approximately 95% of the cell culture as determined by α-SMA immunostaining.
Measurement of cAMP and cGMP in CCSs and CCSMCs.
Quantitative assays for cAMP and cGMP were performed using a commercial enzyme immunoassay kit (Amersham Biosciences). For penile cAMP and cGMP content, frozen cavernosal tissue was homogenized in 6% trichloroacetic acid (1 ml trichloroacetic acid per 100 mg tissue), centrifuged, and extracted with water-saturated diethyl ether (12). In studies to determine changes in cAMP and cGMP levels in response to drug treatment or neurostimulation, CCSs excluded from previous in vitro contraction studies were snap-frozen in liquid nitrogen immediately after drug treatment or nerve stimulation. Similarly, CCSMCs were cultured and treated with a series of drugs, and 10 min later cellular cAMP and cGMP levels were measured as described above.
Real-time RT-PCR analysis.
Total RNA was isolated from CCSMCs using TRIzol reagent (Invitrogen). RNase-free DNase (Invitrogen) was used to eliminate genomic DNA contamination. Transcript levels were quantified using real-time quantitative RT-PCR. Adenosine receptor and β-actin transcripts were analyzed using Taqman probes or SYBER green on a Smart Cycler (Cepheid), with primer sequences and conditions as previously described (51, 52).
Histological analysis.
Mice were anesthetized, and the penes were isolated and pressure-infused with 4% paraformaldehyde in PBS and fixed overnight at 4°C. Fixed penes were rinsed in PBS, dehydrated through graded ethanol washes, and embedded in paraffin. The histological studies including tissue sectioning and staining were performed according to the standard protocols. Specifically, 5-μm sections were obtained on slides, and routine H&E and Masson trichrome staining were performed according to the manufacturer’s instructions (Shardon-Lipshaw). To evaluate the expression of SMA, the 5-μm sections of formalin-fixed, paraffin-embedded tissue were immunostained using the labeled streptavidin-biotin complex system (1:500; Vector Laboratories) and the primary antibody anti-SMA (1:700; Dako Corp.).
Statistics.
All values are expressed as means ± SEM. Data were analyzed for statistical significance by Student’s t test using GraphPad Prism software. A P value less than 0.05 was considered significant.
Acknowledgments
This work was supported by NIH grants DK077748 (to Y. Xia), DK46207 (to R.E. Kellems), and HL070952 (to M.R. Blackburn).
Footnotes
Nonstandard abbreviations used: A2BR, A2B adenosine receptor; ADA, adenosine deaminase; CCS, corpus cavernosal strip; CCSMC, corpus cavernosal smooth muscle cell; DCF, deoxycoformycin; EFS, electrical field stimulation; l-NAME, l-nitroarginine methyl ester; NECA, 5′-N-ethylcarboxamidoadenosine; PEG, polyethylene glycol; SCD, sickle cell disease.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:1491–1501 (2008). doi:10.1172/JCI33467
References
- 1.Burnett A.L. Priapism pathophysiology: clues to prevention. Int. J. Impot. Res. 2003;15(Suppl. 5):S80–S85. doi: 10.1038/sj.ijir.3901077. [DOI] [PubMed] [Google Scholar]
- 2.Burnett A.L. Therapy insight: Priapism associated with hematologic dyscrasias. Nat. Clin. Pract. Urol. 2005;2:449–456. doi: 10.1038/ncpuro0277. [DOI] [PubMed] [Google Scholar]
- 3.Burnett A.L. Erectile dysfunction. J. Urol. 2006;175:S25–S31. doi: 10.1016/S0022-5347(05)00309-5. [DOI] [PubMed] [Google Scholar]
- 4.Burnett A.L.1998Erectile dysfunction: a practical approach for primary care. Geriatrics. 5334–35, 39–40, 46–38. . [PubMed] [Google Scholar]
- 5.Burnett A.L., Allen R.P., Tempany C.M., Dover G.J., Brendler C.B. Evaluation of erectile function in men with sickle cell disease. Urology. 1995;45:657–663. doi: 10.1016/S0090-4295(99)80059-4. [DOI] [PubMed] [Google Scholar]
- 6.Bochinski D.J., Dean R.C., Lue T.F. Erectile dysfunction and priapism. Nat. Clin. Pract. Urol. 2004;1:49–53; quiz 53. doi: 10.1038/ncpuro0022. [DOI] [PubMed] [Google Scholar]
- 7.Paszty C. Transgenic and gene knock-out mouse models of sickle cell anemia and the thalassemias. Curr. Opin. Hematol. 1997;4:88–93. doi: 10.1097/00062752-199704020-00003. [DOI] [PubMed] [Google Scholar]
- 8.Paszty C., et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 9.Ryan T.M., Ciavatta D.J., Townes T.M. Knockout-transgenic mouse model of sickle cell disease. Science. 1997;278:873–876. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 10.Burnett A.L., Lowenstein C.J., Bredt D.S., Chang T.S., Snyder S.H. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 11.Lue T.F. Erectile dysfunction. N. Engl. J. Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 12.Champion H.C., Bivalacqua T.J., Takimoto E., Kass D.A., Burnett A.L. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1661–1666. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patole S., Lee J., Buettner P., Whitehall J. Improved oxygenation following adenosine infusion in persistent pulmonary hypertension of the newborn. Biol. Neonate. 1998;74:345–350. doi: 10.1159/000014052. [DOI] [PubMed] [Google Scholar]
- 14.Lin C.S., Lin G., Lue T.F. Cyclic nucleotide signaling in cavernous smooth muscle. J. Sex Med. 2005;2:478–491. doi: 10.1111/j.1743-6109.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 15.Yonezawa A., Sakurada S., Furukawa K., Kimura Y. Adenosine and adenosine triphosphate [In Japanese.]. Nippon Rinsho. 2002;60(Suppl. 6):52–56. [PubMed] [Google Scholar]
- 16.Noto T., Inoue H., Mochida H., Kikkawa K. Role of adenosine and P2 receptors in the penile tumescence in anesthetized dogs. Eur. J. Pharmacol. 2001;425:51–55. doi: 10.1016/S0014-2999(01)01167-0. [DOI] [PubMed] [Google Scholar]
- 17.Filippi S., et al. Functional adenosine receptors in human corpora cavernosa. Int. J. Androl. 2000;23:210–217. doi: 10.1046/j.1365-2605.2000.00232.x. [DOI] [PubMed] [Google Scholar]
- 18.Shalev M., Staerman F., Allain H., Lobel B., Saiag B. Stimulation of P2y purinoceptors induces, via nitric oxide production, endothelium-dependent relaxation of human isolated corpus cavernosum. J. Urol. 1999;161:955–959. doi: 10.1016/S0022-5347(01)61828-7. [DOI] [PubMed] [Google Scholar]
- 19.Sharifzadeh M., Zarrindast M.R., Samini M. Effects of adenosine analogues on apomorphine-induced penile erection in rats. Gen. Pharmacol. 1995;26:1785–1790. doi: 10.1016/0306-3623(95)00114-x. [DOI] [PubMed] [Google Scholar]
- 20.Kilic S., Salih M., Anafarta K., Baltaci S., Kosar A. Adenosine: a new agent in the diagnosis of impotence. Int. J. Impot. Res. 1994;6:191–198. [PubMed] [Google Scholar]
- 21.Chiang P.H., et al. Adenosine modulation of neurotransmission in penile erection. Br. J. Clin. Pharmacol. 1994;38:357–362. doi: 10.1111/j.1365-2125.1994.tb04366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faria M., Magalhaes-Cardoso T., Lafuente-de-Carvalho J.M., Correia-de-Sa P. Corpus cavernosum from men with vasculogenic impotence is partially resistant to adenosine relaxation due to endothelial A(2B) receptor dysfunction. J. Pharmacol. Exp. Ther. 2006;319:405–413. doi: 10.1124/jpet.106.107821. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn M.R., et al. The use of enzyme therapy to regulate the metabolic and phenotypic consequences of adenosine deaminase deficiency in mice. Differential impact on pulmonary and immunologic abnormalities. J. Biol. Chem. 2000;275:32114–32121. doi: 10.1074/jbc.M005153200. [DOI] [PubMed] [Google Scholar]
- 24.Hershfield M.S. PEG-ADA replacement therapy for adenosine deaminase deficiency: an update after 8.5 years. Clin. Immunol. Immunopathol. 1995;76:S228–S232. doi: 10.1016/S0090-1229(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 25.Blackburn M.R., Kellems R.E. Adenosine deaminase deficiency: metabolic basis of immune deficiency and pulmonary inflammation. Adv. Immunol. 2005;86:1–41. doi: 10.1016/S0065-2776(04)86001-2. [DOI] [PubMed] [Google Scholar]
- 26.Blackburn M.R., Datta S.K., Wakamiya M., Vartabedian B.S., Kellems R.E. Metabolic and immunologic consequences of limited adenosine deaminase expression in mice. J. Biol. Chem. 1996;271:15203–15210. doi: 10.1074/jbc.271.25.15203. [DOI] [PubMed] [Google Scholar]
- 27.Werner M.E., Zvara P., Meredith A.L., Aldrich R.W., Nelson M.T. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J. Physiol. (Lond.) 2005;567:545–556. doi: 10.1113/jphysiol.2005.093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedlund P., et al. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2349–2354. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnett A.L. Neurophysiology of erectile function: androgenic effects. J. Androl. 2003;24:S2–S5. doi: 10.1002/j.1939-4640.2003.tb02741.x. [DOI] [PubMed] [Google Scholar]
- 30.Filippi S., Amerini S., Maggi M., Natali A., Ledda F. Studies on the mechanisms involved in the ATP-induced relaxation in human and rabbit corpus cavernosum. J. Urol. 1999;161:326–331. doi: 10.1016/S0022-5347(01)62140-2. [DOI] [PubMed] [Google Scholar]
- 31.Muneer A., et al. Investigation of cavernosal smooth muscle dysfunction in low flow priapism using an in vitro model. Int. J. Impot. Res. 2004;17:10–18. doi: 10.1038/sj.ijir.3901231. [DOI] [PubMed] [Google Scholar]
- 32.Blackburn M.R., Datta S.K., Kellems R.E. Adenosine deaminase-deficient mice generated using a two-stage genetic engineering strategy exhibit a combined immunodeficiency. J. Biol. Chem. 1998;273:5093–5100. doi: 10.1074/jbc.273.9.5093. [DOI] [PubMed] [Google Scholar]
- 33.Pilatz A., et al. 2005Isolation of primary endothelial and stromal cell cultures of the corpus cavernosum penis for basic research and tissue engineering. Eur. Urol. 47710–718; discussion 718–719. . 10.1016/j.eururo.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 34.Burnett A.L. Role of nitric oxide in the physiology of erection. Biol. Reprod. 1995;52:485–489. doi: 10.1095/biolreprod52.3.485. [DOI] [PubMed] [Google Scholar]
- 35.Burnett A.L. Pathophysiology of priapism: dysregulatory erection physiology thesis. J. Urol. 2003;170:26–34. doi: 10.1097/01.ju.0000046303.22757.f2. [DOI] [PubMed] [Google Scholar]
- 36.Hsu L., Diwan B., Ward J.M., Noguchi C.T. Pathology of “Berkeley” sickle-cell mice includes gallstones and priapism. Blood. 2006;107:3414–3415. doi: 10.1182/blood-2005-11-4500. [DOI] [PubMed] [Google Scholar]
- 37.Nolan V.G., Wyszynski D.F., Farrer L.A., Steinberg M.H. Hemolysis-associated priapism in sickle cell disease. Blood. 2005;106:3264–3267. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keoghane S.R., Sullivan M.E., Miller M.A. The aetiology, pathogenesis and management of priapism. BJU Int. 2002;90:149–154. doi: 10.1046/j.1464-410X.2002.02825.x. [DOI] [PubMed] [Google Scholar]
- 39.Lin G., Xin Z.C., Lue T.F., Lin C.S.2003Up and down-regulation of phosphodiesterase-5 as related to tachyphylaxis and priapism. J. Urol. 170S15–S18; discussion S19. . 10.1097/01.ju.0000075500.11519.e8 [DOI] [PubMed] [Google Scholar]
- 40.Fredholm B.B., Ijzerman A.P., Jacobson K.A., Klotz K.N., Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 41.Hershfield M.S. New insights into adenosine-receptor-mediated immunosuppression and the role of adenosine in causing the immunodeficiency associated with adenosine deaminase deficiency. Eur. J. Immunol. 2005;35:25–30. doi: 10.1002/eji.200425738. [DOI] [PubMed] [Google Scholar]
- 42.Schulte G., Fredholm B.B. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003;15:813–827. doi: 10.1016/S0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson K.A., Gao Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ansari H.R., Nadeem A., Talukder M.A.H., Sakhalkar S., Mustafa S.J. Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H719–H725. doi: 10.1152/ajpheart.00593.2006. [DOI] [PubMed] [Google Scholar]
- 45.Hershfield M.S. PEG-ADA: an alternative to haploidentical bone marrow transplantation and an adjunct to gene therapy for adenosine deaminase deficiency. Hum. Mutat. 1995;5:107–112. doi: 10.1002/humu.1380050202. [DOI] [PubMed] [Google Scholar]
- 46.Migchielsen A.A., et al. Adenosine-deaminase-deficient mice die perinatally and exhibit liver-cell degeneration, atelectasis and small intestinal cell death. Nat. Genet. 1995;10:279–287. doi: 10.1038/ng0795-279. [DOI] [PubMed] [Google Scholar]
- 47.Sun D., et al. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nemeth Z.H., et al. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007;21:2379–2388. doi: 10.1096/fj.07-8213com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J.F., et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salvatore C.A., et al. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J. Biol. Chem. 2000;275:4429–4434. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- 51.Chunn J.L., et al. Partially adenosine deaminase-deficient mice develop pulmonary fibrosis in association with adenosine elevations. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L579–L587. doi: 10.1152/ajplung.00258.2005. [DOI] [PubMed] [Google Scholar]
- 52.Chunn J.L., et al. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J. Immunol. 2005;175:1937–1946. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- 53.Chunn J.L., et al. Adenosine-dependent airway inflammation and hyperresponsiveness in partially adenosine deaminase-deficient mice. . J. Immunol. 2001;167:4676–4685. doi: 10.4049/jimmunol.167.8.4676. [DOI] [PubMed] [Google Scholar]