Abstract
The possible contribution of Na+-Ca2+ exchange to the triggering of Ca2+ release from the sarcoplasmic reticulum in ventricular cells remains unresolved. To gain insight into this issue, we measured the “trigger flux” of Ca2+ crossing the cell membrane in rabbit ventricular myocytes with Ca2+ release disabled pharmacologically. Under conditions that promote Ca2+ entry via Na+-Ca2+ exchange, internal [Na+] (10 mM), and positive membrane potential, the Ca2+ trigger flux (measured using a fluorescent Ca2+ indicator) was much greater than the Ca2+ flux through the L-type Ca2+ channel, indicating a significant contribution from Na+-Ca2+ exchange to the trigger flux. The difference between total trigger flux and flux through L-type Ca2+ channels was assessed by whole-cell patch-clamp recordings of Ca2+ current and complementary experiments in which internal [Na+] was reduced. However, Ca2+ entry via Na+-Ca2+ exchange measured in the absence of L-type Ca2+ current was considerably smaller than the amount inferred from the trigger flux measurements. From these results, we surmise that openings of L-type Ca2+ channels increase [Ca2+] near Na+-Ca2+ exchanger molecules and activate this protein. These results help to resolve seemingly contradictory results obtained previously and have implications for our understanding of the triggering of Ca2+ release in heart cells under various conditions.
Contraction in cardiac muscle cells results from an increase in intracellular Ca2+ concentration ([Ca2+]i) from a diastolic level of roughly 100 nM to a peak of ∼1 μM. The source of most of this Ca2+ is release from the sarcoplasmic reticulum (SR), which occurs as a collection of microscopic release events known as Ca2+ sparks (1). Ca2+ sparks have been shown to be triggered primarily by Ca2+ ions crossing the cell membrane through L-type Ca2+ channels (2).
As the heart cell relaxes, the electrogenic Na+-Ca2+ exchanger (NCX) removes Ca2+ from the cell. This protein imports three Na+ ions for every Ca2+ ion that exits (3). Depending on cellular transmembrane potential (Vm) and the relative concentrations of Na+ and Ca2+ inside and outside the cell, NCX can also work in “reverse mode,” in which Ca2+ is imported and Na+ is extruded. The issue of whether Ca2+ entry via this pathway can contribute to the trigger for Ca2+ release has remained controversial because of apparently contradictory data from different groups. On the one hand, blockade Ca2+ entry due to other pathways has led to the conclusion that Ca2+ entry via NCX is ineffective at triggering release (4–6). On the other hand, some investigators have demonstrated dramatically increased Ca2+ release at positive potentials when internal Na+ is present compared with when it is absent (7–9). Because NCX is presumably working in reverse mode under these conditions, this extra release has been ascribed to the actions of the exchanger.
One possible confounding factor in these experiments is the SR Ca2+ load, which strongly influences the quantity of Ca2+ released (10). Because changes in intracellular [Na+] affect the balance of Ca2+ across the cell membrane, measurements of SR Ca2+ release with and without reverse-mode NCX might be performed at different SR Ca2+ loads. To avoid this potential complication, we measured the Vm dependence of Ca2+ flux crossing the cell membrane with SR release disabled. Our results, which indicate that reverse mode NCX can augment the transmembrane Ca2+ flux substantially, suggest a resolution to the apparently contradictory results obtained in previous studies.
Experiments were performed on isolated rabbit ventricular myocytes incubated with ryanodine and thapsigargin (1 μM each) to prevent SR Ca2+ release. Cells were voltage-clamped in whole-cell mode and loaded with fluo-3. The pipette solution contained 120 mM Cs+ to block most outward K+ currents and either 10 mM or 0 mM Na+. Cells were held at −80 mV, a slow ramp (500 ms) to −50 mV was applied to inactivate Na+ current, and then cells were depolarized to a range of test potentials. Confocal line scan recordings of fluorescence (F) were made concurrently. This protocol therefore allowed simultaneous measurement of L-type Ca2+ current (ICa) and the total transmembrane flux of Ca2+, assessed by increases in fluorescence (ΔF/F0).
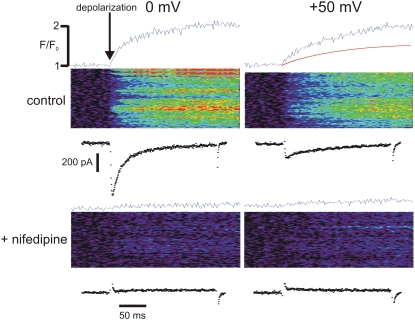
Examples of patch-clamp and line-scan recordings are shown in Fig. 1. A depolarization to 0 mV (top left) induced both a large inward ICa and a sizable ΔF/F0. A depolarization to +50 mV (top right) caused a much smaller current, but still a substantial ΔF/F0. This probably occurred because Ca2+ was also entering via reverse mode NCX at this potential.
FIGURE 1.
Average ΔF/F0, spatiotemporal changes in F, and ICa measured at different membrane potentials, before and after adding 40 μM nifedipine. SR Ca2+ release was disabled and pipette [Na+] was 10 mM in all cases.
We used two methods to assess the relative contributions of these pathways. First, we added nifedipine (40 μM) to block L-type Ca2+ current. ΔF/F0 was essentially zero at 0 mV (bottom left), a potential below the NCX reversal potential under these conditions (+27 mV). At +50 mV, an increase in F was observed (bottom right); however, this was much less than that seen with both inward NCX and ICa present. We also integrated the ICa recorded at 0 mV and scaled the result to approximate the ΔF/F0 time course at that Vm. When ICa at +50 mV was integrated, and the same scaling factor was applied, the estimated ΔF/F0 due to ICa was only 51% of the total increase observed (top right; red trace). Thus, the Ca2+ trigger flux at +50 mV under control conditions is much greater than a simple sum of fluxes due to ICa and reverse-mode NCX measured in the absence of ICa.
Fig. 2 shows summary data from experiments such as that shown in Fig. 1. Peak ICa and trigger flux, approximated as the maximal rate of rise of F (dF/dtmax; see Supplementary Material, Fig. S1), before and after adding nifedipine, are plotted together on a normalized scale for comparison. Peak ICa and dF/dtmax match one another below +20 mV, but the latter is greater at more positive Vm, consistent with Ca2+ entry via reverse mode NCX at these potentials. We considered whether imperfect selectivity of the L-type Ca2+ channel, by underestimating Ca2+ entry via ICa at potentials such as +50 mV, could cause the divergence between the two curves. Even after correcting for this, however, the trigger flux at +50 mV assessed by dF/dtmax is still ∼2.5 times the calculated Ca2+ influx through ICa (see Supplementary Material Fig. S2).
FIGURE 2.
Summary data from experiments such as that shown in Fig. 1. Estimated Ca2+ trigger flux (dF/dtmax; red circles) is greater than peak ICa (black squares) only at Vm above +30 mV. dF/dtmax measured after blocking ICa (green triangles) is too small to account for this difference.
To verify that NCX contributed to the surprisingly large trigger flux observed at positive Vm, we performed additional experiments with 0 mM Na+ in the patch pipette. Fig. 3 compares dF/dtmax measured with 0 mM [Na+] (black squares) and 10 mM [Na+] (red circles). Plots are normalized for comparison. At Vm >+20 mV, dF/dtmax is relatively much larger with [Na+] in the pipette, confirming that reverse-mode NCX augments the trigger flux at these voltages. It is striking that the flux of Ca2+ at +50 mV in the presence of internal [Na+] is more than twice that measured in its absence.
FIGURE 3.
dF/dtmax as a function of Vm, with (red circles; n = 7) and without (black squares; n = 3) 10 mM [Na+] in the pipette.
Overall, these results suggest that the Ca2+ “trigger flux” at positive Vm is much greater than that estimated from a simple summation of the fluxes through ICa and reverse mode NCX measured in the absence of ICa. The most likely explanation for this finding is that Ca2+ entering through L-type channels activates the catalytic Ca2+-binding site on NCX (11,12), thereby causing an increase of Ca2+ influx on the exchanger. Indeed, early studies on the function of cardiac NCX suggested that this could be the case (13). Reverse-mode NCX after L-type channel block is less than in control conditions because this catalysis does not occur, and, consistent with this idea, large differences in Ca2+ fluxes tend to shrink at very positive Vm (e.g., +80 mV), probably because smaller ICa leads to a reduction in NCX catalysis. This hypothesis therefore provides an explanation for the contradictory results mentioned earlier. More important, the data presented suggest that NCX may contribute substantially to Ca2+ entry at Vm corresponding to the early action potential plateau.
These results are similar to observations recently made by Viatchenko-Karpinski et al. in rat myocytes (14). These authors, however, only observed nonlinear summation of ICa and reverse mode NCX after β-adrenergic stimulation. The fact that we observe such an augmentation of the trigger flux under control conditions is consistent with the greater NCX currents recorded in larger mammals and suggests that NCX may have a larger role in triggering Ca2+ release in such species. This species difference also suggests that experiments such as those performed here may provide insight into spatial relationships between L-type Ca2+ channels and Na+-Ca2+ exchangers under a range of conditions.
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Acknowledgments
Supported by the National Institutes of Health grants No. HL076230 and No. HL62690.
Editor: Michael Edidin.
References
- 1.Cheng, H., W. J. Lederer, and M. B. Cannell. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744. [DOI] [PubMed] [Google Scholar]
- 2.Cannell, M. B., H. Cheng, and W. J. Lederer. 1995. The control of calcium release in heart muscle. Science. 268:1045–1049. [DOI] [PubMed] [Google Scholar]
- 3.Bridge, J. H., J. R. Smolley, and K. W. Spitzer. 1990. The relationship between charge movements associated with ICa and INa-Ca in cardiac myocytes. Science. 248:376–378. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard, R. A., R. B. Clark, and W. R. Giles. 1993. Role of sodium-calcium exchange in activation of contraction in rat ventricle. J. Physiol. 472:391–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sham, J. S., L. Cleemann, and M. Morad. 1992. Gating of the cardiac Ca2+ release channel: the role of Na+ current and Na+-Ca2+ exchange. Science. 255:850–853. [DOI] [PubMed] [Google Scholar]
- 6.Sipido, K. R., M. Maes, and F. Van de Werf. 1997. Low efficiency of Ca2+ entry through the Na+-Ca2+ exchanger as trigger for Ca2+ release from the sarcoplasmic reticulum. A comparison between L-type Ca2+ current and reverse-mode Na+-Ca2+ exchange. Circ. Res. 81:1034–1044. [DOI] [PubMed] [Google Scholar]
- 7.Litwin, S. E., J. Li, and J. H. Bridge. 1998. Na-Ca exchange and the trigger for sarcoplasmic reticulum Ca release: studies in adult rabbit ventricular myocytes. Biophys. J. 75:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su, Z., K. Sugishita, M. Ritter, F. Li, K. W. Spitzer, and W. H. Barry. 2001. The sodium pump modulates the influence of INa on [Ca2+]i transients in mouse ventricular myocytes. Biophys. J. 80:1230–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wasserstrom, J. A., and A.-M. Vites. 1996. The role of Na+-Ca2+ exchange in activation of excitation- contraction coupling in rat ventricular myocytes. J. Physiol. 493:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shannon, T. R., K. S. Ginsburg, and D. M. Bers. 2000. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys. J. 78:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilgemann, D. W., A. Collins, and S. Matsuoka. 1992. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J. Gen. Physiol. 100:933–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miura, Y., and J. Kimura. 1989. Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca. J. Gen. Physiol. 93:1129–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haworth, R. A., and A. B. Goknur. 1991. Control of the Na-Ca exchanger in isolated heart-cells. 2. Beat-dependent activation in normal-cells by intracellular calcium. Circ. Res. 69:1514–1524. [DOI] [PubMed] [Google Scholar]
- 14.Viatchenko-Karpinski, S., D. Terentyev, L. A. Jenkins, L. O. Lutherer, and S. Gyorke. 2005. Synergistic interactions between Ca2+ entries through L-type Ca2+ channels and Na+-Ca2+ exchanger in normal and failing heart. J. Physiol. 567:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]