Abstract
Tripeptidyl peptidase II (TPPII) is a multifunctional and evolutionarily conserved protease. In the mammalian hypothalamus, TPPII has a proposed anti-satiety role affected by degradation of the satiety hormone cholecystokinin 8. Here, we show that TPPII also regulates the metabolic homoeostasis of Caenorhabditis elegans; TPPII RNA interference (RNAi) decreases worm fat stores. However, this occurs independently of feeding behaviour and seems to be a function within fat-storing tissues. In mammalian cell culture, TPPII stimulates adipogenesis and TPPII RNAi blocks adipogenesis. The pro-adipogenic action of TPPII seems to be independent of protease function, as catalytically inactive TPPII also increases adipogenesis. Mice that were homozygous for an insertion in the Tpp2 locus were embryonic lethal. However, Tpp2 heterozygous mutants were lean compared with wild-type littermates, although food intake was normal. These findings indicate that TPPII has central and peripheral roles in regulating metabolism and that TPPII actions in fat-storing tissues might be an ancient function carried out in a protease-independent manner.
Keywords: adipose, obesity, satiety, evolution, adipogenesis
Introduction
Understanding the mechanisms that underlie metabolism promises the identification of new targets for drugs to treat the growing number of people with lipodystrophy, obesity and diabetes (Campbell & Dhand, 2000). Caenorhabditis elegans is a potential model to help uncover the processes that govern metabolic physiology. Genes that regulate fat formation, including the transcription factors CCAAT/enhancer-binding protein (C/EBP) and sterol regulatory element-binding protein, as well as lipogenic and lipolytic enzymes have related roles in worms and mammals (Ashrafi et al, 2003; McKay et al, 2003). In addition, some of the genes and pathways that control feeding behaviour and satiety, such as serotonin, insulin and tubby, are also conserved (Kimura et al, 1997; Sze et al, 2000; Mukhopadhyay et al, 2005; Mak et al, 2006).
Tripeptidyl peptidase II (TPPII) is a well-conserved and large exopeptidase with an uncommon structure. The TPPII protease domain, located at the amino terminus, has high similarity to other proteases; TPPII also has a conserved, unique and unusually long carboxy-terminal extension of unknown function (Balow et al, 1986; Tomkinson, 1994). TPPII is expressed in many cells and has various intracellular proteolytic actions including peptide processing for antigen presentation, Shigella-induced macrophage apoptosis and protein turnover (Geier et al, 1999; Hilbi et al, 2000; Seifert et al, 2003). TPPII also seems to have extracellular actions in appetite control, acting in the hypothalamus to degrade the satiety peptide cholecystokinin 8 (CCK8), which transduces its satiety effects through the CCK receptors CCK1R and CCK2R (Clerc et al, 2006; Dufresne et al, 2006). The anti-satiety role for TPPII has been supported by in vivo pharmacological studies in rodents (Rose et al, 1996). Here, we report our analysis of worm and mammalian TPPII.
RNA interference (RNAi) of worm TPPII decreased C. elegans fat stores. This reduction was not secondary to changes in feeding behaviour but instead seemed to be due to TPPII function in the intestine, the site of worm fat storage. The idea that TPPII might regulate fat cell biology is supported by mammalian cell culture data, which show that TPPII is required for adipogenesis. Expression of either wild-type or catalytically inactive TPPII stimulated adipogenesis, indicating that this role is protease independent. Heterozygous Tpp2 mutant mice have reduced fat depots, while eating the same amount as control littermates. Thus, in addition to its neuroproteolytic function in mammalian satiety, our data indicate that TPPII also has a role in fat-storing tissues per se.
Results
RNA interference of TPPII reduces worm fat stores
To identify the genes that control C. elegans metabolism, we carried out injection RNAi for hundreds of genes, assessing metabolic abnormalities by staining the worms with Nile Red, a dye the fluorescence of which correlates with fat content (McKay et al, 2003). Although the vast majority of worms had no fat-related phenotypes, we found that adult TPPII (F21H12.6) RNAi-treated worms, which otherwise seemed healthy, fertile and with no obvious morphological defects, had decreased fat stores based on decreased opacity and reduced Nile Red staining (Fig 1A,B). Previous studies indicate that the reduced fat adult phenotype is uncommon and specific to genes involved in fat biology (Ashrafi et al, 2003).
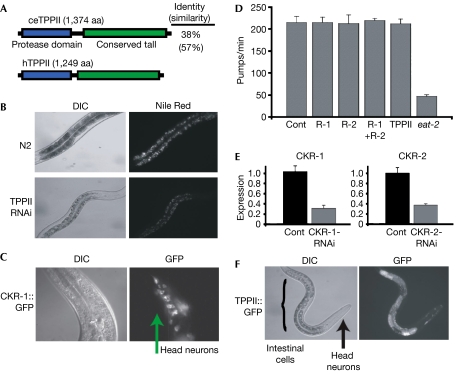
Figure 1.
Analysis of Caenorhabditis elegans TPPII and cholecystokinin signalling. (A) Alignment of worm (ce) and human (h) TPPII. (B) TPPII double-stranded RNA was injected into N2 (wild-type) worms. F1 progeny were examined with DIC microscopy, which showed that TPPII-RNAi worms were paler, indicating decreased fat stores. The TPPII-RNAi and control worms were stained with Nile Red, a lipid-specific dye the emission intensity of which correlates with fat content, and photographed under fluorescence microscopy. Worms are at the same developmental stage. (C) CKR-1∷GFP transgenic worms were examined and the fluorescence pattern indicated that CKR-1 is expressed in a subset of neurons (arrow). (D) Analysis of feeding behaviour (pumps/min) following CKR-1 (R-1), CKR-2 (R-2), CKR-1+CKR-2 (R-1+R-2) and TPPII RNAi in an rrf-3, neural RNAi sensitized, background. The pumping rate of eat-2 mutant worms was measured as a positive control (Cont) for altered feeding behaviour. n=10, three replicates. (E) qPCR analysis of CKR-1 expression in CKR-1-RNAi (left panel) and CKR-2 expression in CKR-2-RNAi (right panel) worms and control (Cont). (F) A TPPII∷GFP transgene was expressed in the intestinal fat-storing cells (bracket) and a few head neurons (arrow). Error bars indicate s.d. CKR, cholecystokinin receptor; DIC, differential interference contrast; qPCR, quantitative PCR; RNAi, RNA interference; TPP, tripeptidyl peptidase.
Analysis of worm cholecystokinin receptors
In mammals, TPPII can degrade the satiety hormone CCK8, which affects appetite by binding to and transducing signals through two cell-surface receptors, CCK1R and CCK2R (Rose et al, 1996; Dufresne et al, 2006). So one possibility for the observed lean phenotype is that TPPII RNAi increased CCK levels thereby reducing feeding and hence fat accumulation. The C. elegans genome contains two genes with high identity to mammalian CCK receptors: T23B3.4 (49% similarity to CCK1R) and Y39A3B.5 (67% similarity to CCK2R). We were unable to identify a clear CCK homologue owing to myriad neuropeptides present in the C. elegans genome (Pierce et al, 2001; Li, 2005). To analyse expression of the CCKRs, we made green fluorescent protein (GFP) fusions: multiple independent CKR-2∷GFP transgenic worms had no detectable GFP expression (data not shown), whereas the CKR-1∷GFP transgenics showed GFP expression in several nerve ring neurons (Fig 1C). We carried out RNAi against the CCK receptors both in N2 (wild-type) worms and in rrf-3 mutant worms that are more susceptible to RNAi effects in neurons, which are often refractory to RNAi (Simmer et al, 2002). RNAi against the CKRs, either individually or together, resulted in no obvious morphological defects, no changes in fat accumulation and also did not affect various aspects of worm feeding in either N2 or rrf-3 worms (Fig 1D, data not shown). Quantitative PCR (qPCR) analysis indicated that the RNAi reduced CKR levels; however, it remained possible that enough receptor protein was present to convey the CCK signals (Fig 1E). So we checked several parameters of worm feeding in the TPPII RNAi worms, but all assessed feeding behaviours were normal in both the N2 and rrf-3 backgrounds, even though the worms had reduced fat accumulation (Fig 1D, data not shown). So it was possible that TPPII regulates worm fat storage independently of central satiety control.
TPPII is expressed in fat-storing cells
To evaluate where TPPII might act, we generated transgenic worms containing a TPPII∷GFP fusion. We consistently and specifically found GFP fluorescence in the intestine and a few nerve ring neurons in all TPPII transgenic lines (Fig 1F). As the intestine is the worm fat-storing organ, expression of TPPII there raised the possibility that TPPII might function within the intestinal fat-storing cells to affect fat accumulation rather than, or in addition to, act centrally to regulate satiety.
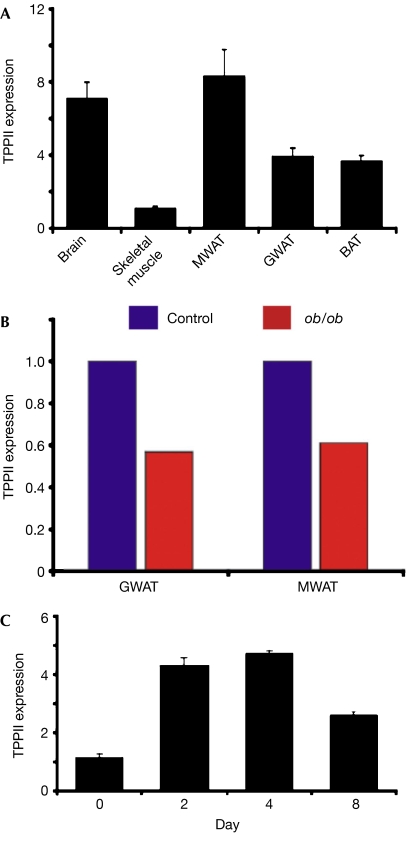
To evaluate whether TPPII might also be expressed in mammalian adipose depots, we extracted RNA from mouse mesenteric and gonadal white adipose tissue (WAT) and brown adipose tissue, and then evaluated TPPII levels with qPCR. We detected TPPII expression in all examined fat tissues and in skeletal muscle and brain, which acted as positive controls (Fig 2A; Facchinetti et al, 1998). To determine whether TPPII expression in adipose depots was affected by obesity, we assessed TPPII expression in control and genetically obese ob/ob littermates (Zhang et al, 1994). TPPII levels were lower in obese WAT (Fig 2B). We also found that TPPII expression increased during adipogenesis of 3T3-L1 cells (Fig 2C), which convert from preadipocytes to fat-storing cells that express adipocyte markers (Green & Kehinde, 1975; Cornelius et al, 1994). Therefore, TPPII expression responds dynamically to metabolic cues in murine models.
Figure 2.
TPPII expression in mammalian fat. (A) qPCR analysis of TPPII expression in the brain, skeletal muscle and adipose depots of mice. (B) qPCR analysis of TPPII expression in GWAT and MWAT collected from control and ob/ob littermate mice. (C) qPCR analysis of TPPII expression during 3T3-L1 adipogenesis. Error bars indicate s.d. BAT, brown adipose tissue; GWAT, gonadal white adipose tissue; MWAT, mesenteric white adipose tissue; qPCR, quantitative PCR; TPP, tripeptidyl peptidase.
TPPII is required for mammalian adipogenesis
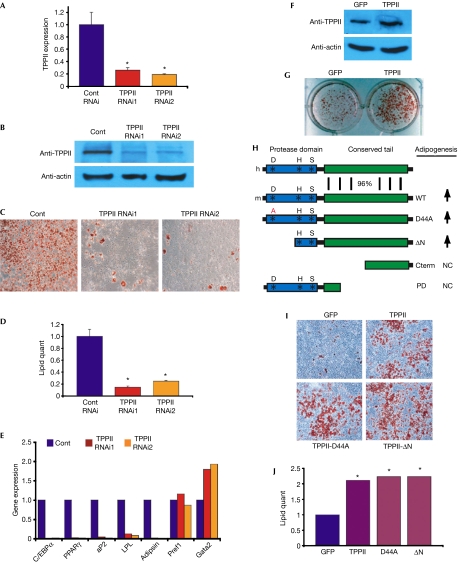
As TPPII is expressed in mouse fat and 3T3-L1s, it might act within adipose tissues to regulate mammalian fat accumulation. To test this possibility, we generated 3T3-L1s expressing either control RNAi or two RNAi constructs targeting different regions of TPPII that reduced TPPII messenger RNA and protein expression (Fig 3A,B). The TPPII RNAi cell lines seemed healthy, had normal fibroblastic morphology and had an equivalent rate of cell proliferation and viability as the control cells (data not shown). We cultured the control and TPPII RNAi cell lines to confluence and incubated them in adipogenic conditions. On the basis of morphology, Oil Red O staining and lipid quantification, it was concluded that TPPII RNAi blocked mammalian adipogenesis (Fig 3C,D). TPPII RNAi also significantly reduced expression of the adipogenic transcription factors C/EBPα and peroxisome proliferator-activated receptor γ and the adipocyte differentiation markers adipsin, aP2 and lipoprotein lipase (Fig 3E). However, other genes, such as the preadipocyte markers Pref1 and Gata2, were unchanged or increased compared with the control, showing that there is not general downregulation of transcription in the RNAi cells (Fig 3E). These data indicate that TPPII is required for 3T3-L1 adipogenesis.
Figure 3.
TPPII is necessary and sufficient for mammalian adipogenesis. (A,B) 3T3-L1 cells expressing control RNAi, TPPII RNAi1 or TPPII RNAi2 were analysed by (A) real-time PCR and (B) western blots for TPPII expression. (C) 3T3-L1 cells expressing control RNAi (Cont), TPPII RNAi1 or TPPII RNAi2 were adipogenically induced and then stained with the fat-specific dye Oil Red O (red) to visualize lipid content. (D) Lipid content of cells in (C) was quantified. (E) Molecular analysis of 3T3-L1 cells in (C,D). C/EBPα and PPARγ are adipogenic transcription factors; adipsin, aP2 and lipoprotein lipase (LPL) are expressed in differentiated adipocytes. Pref1 and Gata2 are preadipocyte markers. (F) Western blot analysis showing that TPPII virus increases TPPII protein levels in NIH3T3 cells. (G) NIH3T3s infected with GFP or TPPII virus were adipogenically induced. Lipid accumulation was visualized with Oil Red O staining. (H) Alignment of human (h) and mouse (m) TPPII, highlighting the marked structural conservation in the unusual carboxy-terminal extension. Diagram of the TPPII-Asp44Ala (D44A) and TPPII-ΔN catalytic mutants forms of TPPII and the C-terminal (Cterm) and protease domain (PD) constructs. Arrows indicate increased adipogenesis. (I) NIH3T3 cells were infected with viruses expressing GFP, TPPII or two catalytic TPPII mutants, TPPII-Asp44Ala or TPPII-ΔN, and adipogenically induced. Lipid accumulation was assessed with Oil Red O staining. (J) Fat content of cells shown in (I) was quantified. Error bars indicate s.d.; *P<0.05. C/EBP, CCAAT/enhancer-binding protein; GFP, green fluorescent protein; NC, no change; PPAR, peroxisome proliferator-activated receptor; TPP, tripeptidyl peptidase.
TPPII stimulates mammalian adipogenesis
To evaluate possible TPPII adipogenic sufficiency, we infected NIH3T3s, which are relatively resistant to adipogenesis, with a control GFP virus or one encoding TPPII that modestly increased TPPII protein levels (Fig 3F). We incubated the GFP and TPPII cells in adipogenic conditions and assessed adipogenesis. We found that TPPII increased NIH3T3 adipogenesis (Fig 3G–J). TPPII also stimulated 3T3-L1 adipogenesis (data not shown).
The TPPII canonical proteolytic catalytic triad is located in the N terminus (Fig 3H). Notably, TPPII has a long C-terminal tail that is conserved from worms to mammals, supporting the idea that this region might have a functional role (Figs 1A, 3H). To analyse the domains of TPPII that might underlie its proadipogenic actions, we generated two TPPII catalytic mutants, TPPII-Asp44Ala, mutating a crucial residue in the catalytic triad, and TPPII-ΔN, deleting the N-terminal aspartic-acid-containing protease domain while retaining the conserved noncatalytic C-terminal extension (Fig 3H). Biochemical studies showed that these mutants should cripple TPPII catalytic activity (Hilbi et al, 2002). We expressed GFP, TPPII, TPPII-Asp44Ala or TPPII-ΔN in NIH3T3 cells and examined adipogenesis, finding that TPPII-Asp44Ala and TPPII-ΔN stimulated adipogenesis to the same extent as wild-type TPPII (Fig 3I,J). Deletion mutants that contained either the amino-half of TPPII (including the protease domain) or just the carboxyl portion did not increase lipid accumulation (Fig 3H, data not shown). Although other interpretations are possible, such as altered tertiary structure, neomorphism or interaction with endogenous TPPII protein, these data support the idea that TPPII can stimulate fat formation in a protease-independent manner.
Tpp2 heterozygous mice are lean and insulin sensitive
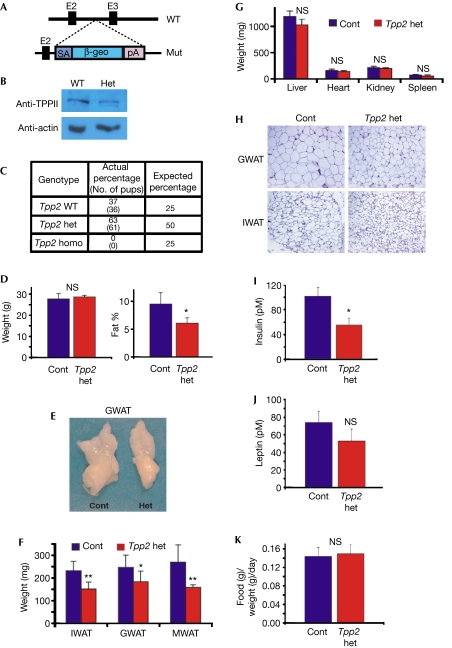
Next, we generated mice from embryonic stem cells with a β-geo-encoding gene-trap inserted in the second intron of the 30 exon-containing Tpp2 locus (Fig 4A). Molecular analyses showed that the Tpp2 locus was appropriately disrupted and western blots indicated that TPPII protein levels were reduced in Tpp2 heterozygous WAT (Fig 4B, data not shown). To examine a possible role of TPPII in adipose tissues, we intercrossed Tpp2 heterozygotes to obtain Tpp2 homozygotes; however, none were viable, supporting the idea that Tpp2 nullizygosity—that is, lacking both copies of a gene—is embryonic lethal (Fig 4C). Further studies indicated that Tpp2 homozygous mutants die before embryonic day 9.0 (E9.0; data not shown). As the formation of the embryonic fat anlagen is not appreciable until approximately E13.5–14.0, the early lethality precluded the analysis of fat biology in the null background.
Figure 4.
Tpp2 heterozygous mutant mice are lean. (A) Schema of the wild-type (WT) Tpp2 locus and the mutant (Mut) Tpp2 allele. Splice acceptor (SA) and the lacZ-neomycin selectable marker (β-geo) cassette were inserted and disrupt gene expression from the third exon (E3). (B) Western blot showing reduction of TPPII protein in GWAT from Tpp2 heterozygous mouse compared with wild-type control. (C) Genomic DNA was extracted from pups of multiple Tpp2 heterozygous (het) intercrosses and then genotyped for the presence of the wild-type and mutant Tpp2 alleles. The data indicate that Tpp2 homozygous (homo) mutant mice die in utero. (D) Average body weight (left panel) and average body fat percentage (right panel) of 16-week-old Tpp2 heterozygous mice (n=15) and control (Cont) littermates (n=14). (E) Photograph of representative gonadal WAT of control and Tpp2 heterozygous littermates. (F) Average weights of IWAT, GWAT and MWAT from Tpp2 heterozygous mice (n=5) and wild-type littermate controls (n=7). (G) Average weights of liver, heart, spleen and kidney isolated from the mice in (F). (H) Histological sections of control and Tpp2 heterozygote GWAT and IWAT. (I,J) Plasma of control (n=5) and Tpp2 heterozygous (n=5) mice were analysed for insulin (I) and leptin (J) levels. (K) Food intake of wild-type (n=6) and Tpp2 heterozygous mice (n=5) was measured daily for 1 week, averaged and then plotted. Error bars indicate s.d.; *P<0.05; **P<0.01; NS, not significant by t-test. GWAT, gonadal white adipose tissue; IWAT, inguinal white adipose tissue; MWAT, mesenteric white adipose tissue; TPP, tripeptidyl peptidase.
We therefore examined Tpp2 heterozygotes fed normal chow for potential fat phenotypes. Although we found no significant difference in weight, we observed a significant reduction in body fat percentage in the Tpp2 heterozygotes compared with control littermates (Fig 4D). We explanted and analysed various fat depots and found that the inguinal, gonadal and mesenteric heterozygous WAT was smaller and weighed significantly less than the control littermate WAT (Fig 4E,F). Analysis of other organs such as liver, heart, kidney and spleen showed no difference in appearance or weight (Fig 4G). Histology indicated that Tpp2+/− adipocytes were smaller than control adipocytes (Fig 4H). Insulin is a key metabolic regulatory hormone, and adipocytes are intimately involved in metabolism and insulin sensitivity (Kahn & Flier, 2000). To assess the potential effects of Tpp2 heterozygosity on metabolism, we measured plasma insulin levels, which were significantly decreased in the Tpp2 heterozygotes, indicating increased insulin sensitivity (Fig 4I). However, leptin levels, which regulate feeding behaviour, approximated controls (Fig 4J).
As TPPII has been proposed to degrade the CCK8 satiety hormone, lowering TPPII levels might result in increased CCK8, which could in turn cause appetite suppression. To evaluate the possibility that Tpp2+/− mutants had reduced food intake accounting for the lower body fat percentage, we monitored food intake for 1 week. However, we found no significant difference in the amount of food consumed by the Tpp2 heterozygotes compared with control littermates, indicating that the reduced adiposity was not secondary to reduced caloric intake (Fig 4K).
Discussion
Energy homeostasis is an essential feature of higher organisms and is orchestrated at several levels (Spiegelman & Flier, 2001). In the hypothalamus, TPPII regulates mammalian feeding behaviour by degrading the satiety hormone CCK8 (Rose et al, 1996). We found that TPPII is expressed in worm fat-storing cells and mammalian adipocytes and regulates fat storage in C. elegans and mice in the absence of changes in feeding behaviour. The observation that TPPII regulates mammalian cell culture adipogenesis, when altered feeding behaviour is eliminated as a confounding variable, further indicates a function in adipose tissues.
TPPII contains a C-terminal tail of unknown function; however, its striking conservation from worms to humans suggests a potential role (Tomkinson, 1994). Our data indicate that the pro-adipogenic actions of TPPII might be independent of its proteolytic ability, which is central to the anti-satiety role, and instead might involve the C-terminal tail. This dual function of a protease involved in metabolism is not unprecedented. For example, the serine-protease dipeptidyl peptidase IV (DPPIV) acts in protease-dependent and protease-independent ways. DPPIV antagonists are a new class of diabetes therapeutics that act by inhibiting the DPPIV-dependent degradation of Gata-like protein 1 (Glp1; Drucker, 2006). DPPIV also binds to nonproteolytic substrates such as collagen and fibronectin, in a region of the protein that is distinct from the catalytic site (Piazza et al, 1989; Loster et al, 1995). The findings that TPPII has an unexpected role in fat biology provide a basis for mechanistic studies that have both basic and clinical relevance and, given the functional conservation, might provide insight into homology and convergence.
TPPII is a highly conserved, versatile protease with reported roles in antigen presentation, protein turnover (that is, a proteasome-like function) and satiety regulation. In addition to its proposed central role in satiety through the degradation of CCK8, our data indicate that TPPII also has a peripheral role in adipose tissues and that this function is conserved from worms to mammalian models. Thus, TPPII seems to regulate at least two aspects of metabolic homoeostasis, affecting both feeding behaviour and adipose biology per se. This dual role for TPPII might make it an appropriate target for drug therapies: inhibiting TPPII centrally should increase feelings of satiety, whereas inhibiting TPPII peripherally might decrease adiposity.
Methods
Worm studies. We generated and injected the indicated double-stranded RNAs (dsRNAs) as described previously (McKay et al, 2003). For the screen, dsRNA for 500 genes was injected and worms were examined for a change in Nile Red staining. Less than 10% of the tested genes resulted in a fat-storage defect. For Nile Red staining, worms were grown on plates with 1 ng/ml Nile Red and photographed with epifluorescence (rhodamine channel). GFP transgenic worms were generated as described previously (McKay et al, 2003). Several independent lines, in several experiments, were established for each construct (TPPII∷GFP and CKR-1∷GFP) and a similar expression pattern was observed among all lines. Feeding behaviours were as described, and pumping rates, scored as in McKay et al (2003), were an average of ten different worms independently counted three times.
Mouse studies. Mice were housed in a 12:12 light/dark cycle. Chow (4%, Teklad, Madison, WI, USA) and water were provided ad libitum. Total body fat mass was measured by Bruker NMR spectroscopy. Food intake was recorded daily for 1 week at 15 weeks of age. After embedding, 8 μm sections of fat tissues were stained with haematoxylin and eosin. ob/ob mice and C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All animals were maintained under the guidelines of the UT Southwestern Medical Center Animal Care and Use Committee according to National Institutes of Health guidelines.
Cell culture and reverse transcription–PCR. Experimental details are listed in the supplementary information online.
Supplementary information is available at EMBO Reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Z. Salo and K. Griffin (Santa Cruz Biotechnology) for technical assistance and members of the Graff lab. This work was supported by awards to J.M.G. from the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney.
COMPETING INTEREST STATEMENT J.M.G., R.M.M. and J.P.M. have shares of Reata Pharmaceuticals, a biotechnology company directed towards finding cures for cancer, inflammation and neurodegenerative diseases.
References
- Ashrafi K, Chang F, Watts J, Fraser A, Kamath R, Ahringer J, Ruvkun G (2003) Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature 421: 268–272 [DOI] [PubMed] [Google Scholar]
- Balow R, Tomkinson B, Ragnarsson U, Zetterqvist O (1986) Purification, substrate specificity, and classification of tripeptidyl peptidase II. J Biol Chem 261: 2409–2417 [PubMed] [Google Scholar]
- Campbell P, Dhand R (2000) Obesity. Nature 404: 631–671 [Google Scholar]
- Clerc P et al. 2006. Involvement of cholecystokinin 2 receptor in food intake regulation: hyperphagia and increased fat deposition in cholecystokinin 2 receptor-deficient mice. Endocrinology 148: 1039–1049 [DOI] [PubMed] [Google Scholar]
- Cornelius P, MacDougald OA, Lane MD (1994) Regulation of adipocyte development. Annu Rev Nutr 14: 99–129 [DOI] [PubMed] [Google Scholar]
- Drucker D (2006) Incretin-based therapies: a clinical need filled by unique metabolic effects. Diabetes Educ 32: 65S–71S [DOI] [PubMed] [Google Scholar]
- Dufresne M, Seva C, Fourmy D (2006) Cholecystokinin and gastrin receptors. Physiol Rev 86: 805–847 [DOI] [PubMed] [Google Scholar]
- Facchinetti P, Rose C, Rostaing P, Triller A, Schwartz J-C (1998) Immunolocalization of tripeptidyl peptidase II, a cholecystokinin-inactivating enzyme, in rat brain. Neuroscience 88: 1225–1240 [DOI] [PubMed] [Google Scholar]
- Geier E, Pfeifer G, Wilm M, Lucchiari-Hartz M, Baumeister W, Eichmann K, Niedermann G (1999) A giant protease with potential to substitute for some functions of the proteasome. Science 283: 978–981 [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O (1975) An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5: 19–27 [DOI] [PubMed] [Google Scholar]
- Hilbi H, Puro R, Zychlinsky A (2000) Tripeptidyl peptidase II promotes maturation of caspase-1 in Shigella flexneri-induced macrophage apoptosis. Infect Immun 68: 5502–5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbi H, Jozsa E, Tomkinson B (2002) Identification of the catalytic triad in tripeptidyl-peptidase II through site-directed mutagenesis. Biochim Biophys Acta 1601: 149–154 [DOI] [PubMed] [Google Scholar]
- Kahn B, Flier J (2000) Obesity and insulin resistance. J Clin Invest 106: 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Tissenbaum H, Liu Y, Ruvkun G (1997) daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277: 942–946 [DOI] [PubMed] [Google Scholar]
- Li C (2005) The ever-expanding neuropeptide gene families in the nematode Caenorhabditis elegans. Parasitology 131: S109–S127 [DOI] [PubMed] [Google Scholar]
- Loster K, Zeilinger K, Schuppan D, Reutter W (1995) The cysteine-rich region of dipeptidyl peptidase IV (CD 26) is the collagen-binding site. Biochem Biophys Res Commun 217: 341–348 [DOI] [PubMed] [Google Scholar]
- Mak H, Nelson L, Basson M, Johnson C, Ruvkun G (2006) Polygenic control of Caenorhabditis elegans fat storage. Nat Genet 38: 363–368 [DOI] [PubMed] [Google Scholar]
- McKay RM, McKay JP, Avery L, Graff JM (2003) C. elegans: a model for exploring the genetics of fat storage. Dev Cell 4: 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Deplancke B, Walhout A, Tissenbaum H (2005) C. elegans tubby regulates life span and fat storage by two independent mechanisms. Cell Metab 2: 35–42 [DOI] [PubMed] [Google Scholar]
- Piazza G, Callanan H, Mowery J, Hixson D (1989) Evidence for a role of dipeptidyl peptidase IV in fibronectin-mediated interactions of hepatocytes with extracellular matrix. Biochem J 262: 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce S et al. (2001) Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev 15: 672–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C, Vargas F, Facchinetti P, Bourgeat P, Bambal R, Bishop P, Chan S, Moore A, Ganellin C, Schwartz J-C (1996) Characterization and inhibition of a cholecystokinin-inactivating serine peptidase. Nature 380: 403–409 [DOI] [PubMed] [Google Scholar]
- Seifert U, Maranon C, Shmueli A, Desoutter J, Wesoloski L, Janek K, Henklin P, Diescher S, Andrieu M (2003) An essential role for tripeptidyl peptidase in the generation of an MHC class I epitope. Nat Immunol 4: 375–379 [DOI] [PubMed] [Google Scholar]
- Simmer F, Tijsterman M, Parrish S, Koushika S, Nonet M, Fire A, Ahringer J, Plasterk R (2002) Loss of putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr Biol 12: 1317–1319 [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS (2001) Obesity and the regulation of energy balance. Cell 104: 531–543 [DOI] [PubMed] [Google Scholar]
- Sze J, Victor M, Loer C, Shi Y, Ruvkun G (2000) Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403: 560–564 [DOI] [PubMed] [Google Scholar]
- Tomkinson B (1994) Characterization of cDNA for murine tripeptidyl-peptidase II reveals alternative splicing. Biochem J 304: 517–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information