Abstract
Elevated serum low-density lipoprotein (LDL) is a risk factor for atherosclerotic disorders. However, prominent atherosclerosis, which has been observed in LDL receptor (LDLR)-knockout mice, has diminished the significance of LDLR as a cause of atherosclerosis, while elaborate studies have focused on the receptors for denatured LDL. Here we report that native LDL (nLDL) activates vascular endothelial growth factor (VEGF) receptor 1 (VEGFR1) but not VEGFR2 through LDLR and is as potent as VEGF in macrophage migration. Binding and co-endocytosis of VEGFR1 and LDLR were enhanced by nLDL, which is concomitant with ubiquitination-mediated degradation of VEGFR1. We propose that LDLR-mediated use of VEGFR1 by nLDL could be a potential therapeutic target in atherosclerotic disorders.
Keywords: VEGF receptor 1, LDL receptor, macrophage migration, atherosclerosis
Introduction
Accumulating evidence indicates that serum low-density lipoprotein (LDL) is a crucial risk factor for atherosclerotic cardiovascular disorders. Therapeutic reduction in the serum LDL level can suppress the onset and recurrence of these disorders, and eventually decrease the mortality rate (Baigent et al, 2005). Mutations in the native LDL (nLDL) receptor (LDLR) have been reported in familial hypercholesterolaemia (Brown & Goldstein, 1986). Interestingly, however, in both familial hypercholesterolaemia patients and LDLR-knockout mice (Ishibashi et al, 1994), paradoxical progression of atherosclerosis has been observed, suggesting the possible existence of other functional receptors for LDL. Receptors for modified versions of LDL such as oxidized LDL, which include the scavenger receptors (SR)-A, CD36 and FcγRII-B2, have been extensively studied (Moore & Freeman, 2006). Macrophages in the atherosclerotic plaque are morphologically and functionally transformed by oxidized LDL but not nLDL (Steinberg et al, 1989). However, nLDL induces macrophage migration by unknown mechanisms (Hara et al, 1992), whereas little attention has been paid to LDLR.
Microvessels in atherosclerotic plaques greatly contribute to the progression and stability of atherosclerosis (Moulton et al, 2003). It is well known that vascular endothelial growth factor (VEGF) has a central role in promoting vascularization. VEGF has two specific receptors, VEGFR1 and VEGFR2, with tyrosine kinase activity. Both are expressed in endothelial cells, VEGFR2 exclusively but VEGFR1 is also exceptionally expressed in macrophages (Shibuya, 2006). It has been reported that VEGFR1 antibody has an inhibitory effect on atherosclerotic plaque formation in apoE−/− hypercholesterolaemic mice, suggesting that VEGFR1 promotes atherosclerosis (Luttun et al, 2002).
Here, we report a previously unrecognized linkage between LDLR and VEGFR1.
Results
Native LDL induces VEGFR1 endocytosis
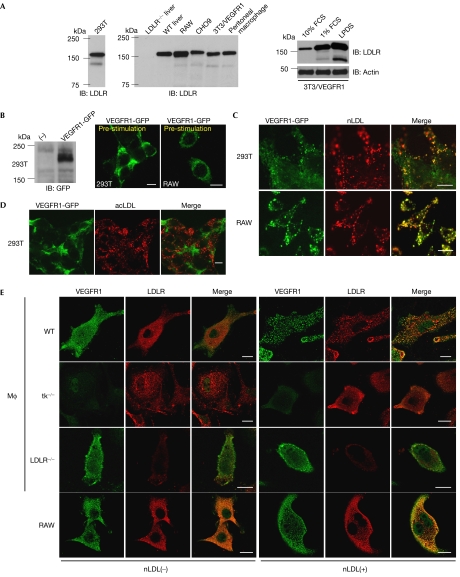
Because of sterol-mediated transcriptional downregulation, LDLR is thought to be upregulated by lipoprotein-deficient serum (LPDS) or serum starvation (Brown & Goldstein, 1986). We found that LDLR was expressed in a variety of cells when deprived of serum (Fig 1A; supplementary Fig 1A online). When nLDL labelled with DiI (1,1′-dioctadecyl-3,3,3′,3′ -tetramethylindocarbocyanine perchlorate) was applied to 293T cells transiently transfected with VEGFR1 tagged with green fluorescent protein (GFP; VEGFR1-GFP) and subsequently starved of serum for 8 h, VEGFR1 was internalized and colocalized with DiI-nLDL in a VEGF-independent manner (Fig 1B,C). A similar phenomenon was observed in RAW cells (Fig 1B,C), a macrophage cell line that has been shown to express endogenous VEGFR1 (Matsumoto et al, 2002). The concentration of nLDL that gave a significant number of VEGFR1-GFP endocytic vesicles was at least 10 μg/ml, which was comparable with that of VEGF on a molar basis in the same assay (Crouse et al, 1985; Kobayashi et al, 2004; supplementary Fig 1B online, also see legend). Interestingly, denatured LDL such as acetylated LDL (DiI-acLDL) failed to induce VEGFR1 endocytosis (Fig 1D). Given that acLDL uses receptors that cannot bind to LDL, we suppose that nLDL could stimulate specific co-endocytosis of its own receptor LDLR and VEGFR1.
Figure 1.
Native low-density lipoprotein induces endocytosis of VEGFR1. (A) Immunoblotting (IB) of whole-cell lysates (WCLs; 30 μg each) from human 293T cells (left) with human LDLR antibody and those from rodent cells with mouse LDLR antibody, including the liver of LDLR−/− and WT mice, RAW, CHO9, NIH3T3 cells overexpressing VEGFR1 (3T3/VEGFR1) and peritoneal macrophages (middle). Cell lines were cultured in 1% FCS for 24 h before protein extraction. WCLs from 3T3/VEGFR1 cultured in 10% and 1% serum, or LPDS were also subjected to anti-LDLR and anti-actin immunoblotting (right). (B) WCLs of 293T cells transfected with the VEGFR1-GFP expression vector or mock (−) were immunoblotted with GFP antibody (left). Membrane localization of VEGFR1-GFP transfected into 293T or RAW cells before nLDL stimulation (right). Scale bars, 10 μm. (C,D) 293T or RAW cells transfected with VEGFR1-GFP (green) were incubated with 10 μg/ml DiI-nLDL (red) (C) or 10 μg/ml DiI-acLDL (red) (D) for 30 min. Note the merged images with nLDL but not acLDL. Scale bars, 10 μm. (E) Peritoneal macrophages (MΦ) derived from WT, tk−/−, LDLR−/− and RAW cells were immunostained with VEGFR1 and LDLR antibodies before (−) and after (+) stimulation by nLDL at 100 μg/ml. acLDL, acetylated LDL; GFP, green fluorescent protein; LDL, low-density lipoprotein; LDLR, LDL receptor; LPDS, lipoprotein-deficient serum; nLDL, native LDL; VEGFR, vascular endothelial growth factor receptor; WT, wild type.
Native LDL stimulates recruitment of VEGFR1 to LDLR
Transient co-transfection of CHO9 cells with VEGFR1-GFP and LDLR tagged with Flag (LDLR-Flag) showed nLDL-stimulated endocytosis and colocalization of both receptors (supplementary Fig 1C,D online). This was blocked by SU5416, a VEGFR tyrosine kinase inhibitor. VEGF induced endocytosis of VEGFR1, but not LDLR, suggesting that the co-endocytosis is governed by nLDL and is VEGFR1-dependent. Clathrin has been shown to mediate LDLR endocytosis (Brown & Goldstein, 1986). However, we repeatedly failed to show nLDL-stimulated colocalization of VEGFR1 and clathrin (supplementary Fig 2A online). These results indicate a clathrin-independent pathway for LDLR endocytosis.
We established a RAW cell line that stably overexpressed the Flag-tagged human LDLR (RAW/LDLR-Flag). Stimulation by nLDL changed its subcellular localization from the membrane to the internalized vesicles (supplementary Fig 2B online). We also observed that some of the cells showed endocytic vesicles even before nLDL stimulation (data not shown). VEGFR1 was detected in the anti-Flag immunoprecipitates from RAW/LDLR-Flag cells. We pulled down VEGFR1 by using the ability of VEGFR1 to bind efficiently to a heparin column and used the heparin-bound VEGFR1 as a size control.
To test whether the co-endocytosis of LDLR and VEGFR1 is dependent on LDLR, we transiently transfected VEGFR1-GFP into skin fibroblasts derived from wild-type or LDLR−/− mice (Ishibashi et al, 1994) and stimulated them with nLDL. As shown in supplementary Figure 2C online, endocytosis was observed in wild-type but not LDLR−/− cells. We further examined the co-endocytosis of endogenous LDLR and VEGFR1 in RAW cells and peritoneal macrophages derived from wild-type, LDLR−/− and tk−/− mice (Hiratsuka et al, 1998), in which the intracellular domain of VEGFR1 was genetically deleted (Fig 1E). Consistent with the results shown above, we found that co-endocytosis is dependent on both LDLR and the VEGFR1 tyrosine kinase domain.
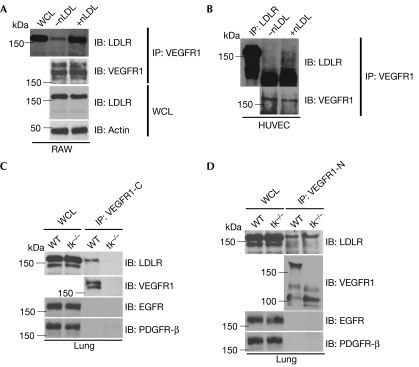
Binding of endogenous LDLR to VEGFR1 in the anti-VEGFR1 immunoprecipitates from RAW cells was detected and increased 3.5-fold (average of three independent experiments) with nLDL stimulation (Fig 2A). To test whether VEGFR1 in endothelial cells also interacts with LDLR, we stimulated human umbilical vein endothelial cells with nLDL. Enhanced binding between the receptors was observed (Fig 2B). To confirm further the interaction between the two receptors of endogenous origin, we immunoprecipitated VEGFR1 from the whole mouse lung, as the lung is one of the organs that express VEGFR1 most abundantly (Shibuya, 2006). The antibody used for immunoprecipitation recognizes only the intracellular domain of VEGFR1. The immunoprecipitates contained endogenous LDLR when the lung was derived from wild-type but not tk−/− mice (Fig 2C). This indicates that the interaction is specific, and the observed band is not a nonspecifically precipitated LDLR. To test whether the binding between LDLR and VEGFR1 is mediated by the intracellular or extracellular portion of each receptor, we tried another antibody that recognizes only the extracellular domain of VEGFR1. As LDLR was immunoprecipitated with VEGFR1 in both wild-type and tk−/− cells with a similar efficiency, we assume that the binding might be mediated by the extracellular domains (Fig 2D). In both VEGFR1 antibodies, the immunoprecipitates did not contain nonspecific membrane proteins such as epidermal growth factor receptor (EGFR) or platelet-derived growth factor receptor; (PDGFR; Fig 2C,D).
Figure 2.
Enhanced recruitment of the low-density lipoprotein receptor with VEGFR1 by native LDL stimulation. (A) Whole-cell lysates (WCLs) and anti-VEGFR1 immunoprecipitates (IP) from RAW cells, with (+nLDL) or without (−nLDL) stimulation by nLDL, were immunoblotted (IB) with LDLR, VEGFR1 and actin antibodies. (B) Anti-LDLR IP and anti-VEGFR1 IP from HUVEC, with (+nLDL) or without (−nLDL) stimulation by nLDL, were immunoblotted with LDLR or VEGFR1 antibody. (C,D) WCLs (60 μg each) and IPs by VEGFR (C) carboxy-terminal antibody (VEGFR1-C) or (D) amino-terminal antibody (VEGFR1-N) from lung lysates of WT or tk−/− mice were immunoblotted with LDLR, EGFR, PDGFR-β and the corresponding VEGFR1 antibody. EGFR, epidermal growth factor receptor; HUVEC, human umbilical vein endothelial cells; LDL, low-density lipoprotein; LDLR, LDL receptor; nLDL, native LDL; PDGFR, platelet-derived growth factor receptor; VEGFR, vascular endothelial growth factor receptor; WCL, whole-cell lysate; WT, wild type.
Native LDL induces VEGFR1 autophosphorylation
As cells that express VEGFR1 at levels high enough for experimentally controlled molecular analysis are unavailable, we used NIH3T3 cells that stably overexpressed VEGFR1 (3T3/VEGFR1) and that were previously shown to have VEGF-dependent endocytosis of VEGFR1 (Kobayashi et al, 2004). We initially confirmed that endocytosis of LDLR takes place in these cells by an 125I-LDL internalization assay, as shown in supplementary Fig 3 online. Both nLDL and VEGF induced degradation of VEGFR1 (by 45% and 50% within 20 min, respectively) but not LDLR in a time-dependent manner (supplementary Fig 4A,B online). As ErbB2 receptor tyrosine kinase was internalized and degraded by heat-shock protein 90 (Hsp90) inhibitor geldanamycin (Lerdrup et al, 2006), we examined the effects of the Hsp90 inhibitors on VEGFR1 (supplementary Fig 5A–D online). The inhibitors induced time-dependent degradation of VEGFR1 by more than 90% in 2 h. Geldanamycin also induced endocytosis of VEGFR1 in NIH3T3 cells transiently transfected with VEGFR1-GFP. Moreover, the geldanamycin-induced degradation of the endogenous VEGFR1 was evident in RAW cells. However, neither endocytosis (data not shown) nor protein degradation was observed in LDLR in the presence of geldanamycin.
To gain an insight into the mechanism of nLDL-induced degradation of VEGFR1, 3T3/VEGFR1 cells transfected with ubiquitin-Flag expression vectors were treated with VEGF, geldanamycin or nLDL. In all cases, the degradation of VEGFR1 was accompanied by ubiquitination of VEGFR1 (supplementary Fig 6A online). As the VEGF- or nLDL-induced degradation was inhibited by MG132 or bafilomycin, both proteasomes and lysosomes could be the site for degradation (supplementary Fig 6B online).
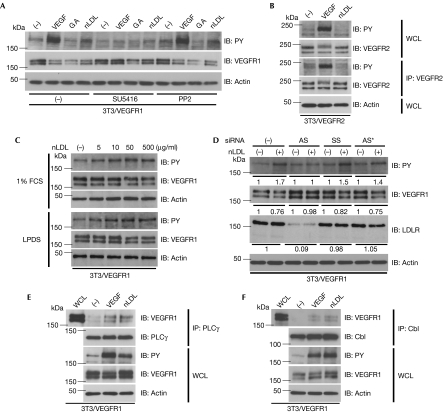
Intriguingly, nLDL induced autophosphorylation of VEGFR1, but not VEGFR2, in 3T3 cells that overexpress either of the receptors (Fig 3A,B). The phosphorylation was similarly observed with both commercially available nLDL and nLDL freshly prepared from volunteers (data not shown). The minimum dose of nLDL required for VEGFR1 phosphorylation is 5–10 μg/ml (Fig 3C). VEGFR1 has the ability to bind heparin. To eliminate the possibility that VEGFR1 could be activated by nLDL bound to heparin, which might have been contaminated during the preparation of nLDL, heparinase treatment experiments were carried out. The result was negative (supplementary Fig 6C online). The intensity of the VEGFR1 autophosphorylation by nLDL was approximately 20–30% of that by VEGF. As VEGFR1 could be transphosphorylated by Src family tyrosine kinases, such as Fyn or Yes (Mary et al, 2002), when it is stimulated by VEGF, we pretreated the cells with the VEGFR inhibitor SU5416 or the Src inhibitor PP2. SU5416 suppressed VEGFR1 phosphorylation by nLDL or VEGF, but PP2 failed to do so (Fig 3A). Anti-LDLR short interfering RNA (siRNA) experiments were carried out to test the LDLR dependency for VEGFR1 activation. As shown in Fig 3D, knockdown of LDLR expression levels by more than 90% abrogated nLDL-induced VEGFR1 autophosphorylation and its degradation, whereas control RNAs did not. Therefore, we suppose that phosphorylation of VEGFR by nLDL is specific for VEGFR1 and mediated by LDLR. VEGFR1 has major autophosphorylation sites, such as Tyr 1169, which is responsible for cell migration (Cunningham et al, 1997), and minor ones, such as Tyr 1333 that we have shown to be essential for receptor endocytosis with recruitment of the c-Cbl–CD2AP complex (Kobayashi et al, 2004). Both anti-phospholipase C γ and anti-c-Cbl immunoprecipitates from 3T3/VEGFR1 cells stimulated by nLDL contained autophosphorylated VEGFR1 (Fig 3E,F). These results indicate that nLDL and VEGF use the same molecular mechanism for endocytosis and protein degradation, and that nLDL might regulate some biological functions of VEGF.
Figure 3.
Native low-density lipoprotein induces autophosphorylation of VEGFR1. (A) 3T3/VEGFR1 cells untreated (−) or pretreated with a VEGFR tyrosine kinase inhibitor SU5416 at 1 μM or Src inhibitor PP2 at 2 μM were stimulated by VEGF, GA or nLDL for 10 min. WCLs were immunoblotted (IB) with phosphotyrosine (PY), VEGFR1 and actin antibody. (B) WCLs or anti-VEGFR2 immunoprecipitates (IP) from 3T3/VEGFR2 cells stimulated with VEGF or nLDL were immunoblotted with PY, VEGFR2 and actin antibody. (C) WCLs from 3T3/VEGFR1 cells stimulated by the indicated concentration of nLDL after culture in 1% FCS or LPDS for 24 h were subjected to anti-PY, anti-VEGFR1 and anti-actin immunoblotting. (D) 3T3/VEGFR1 cells pretreated with mock (−), anti-LDLR siRNAs (antisense/sense: AS) or control siRNAs (sense/sense: SS or irrelevant siRNA against Nox1: AS*) at 40 nM were stimulated by nLDL for 10 min and then subjected to anti-PY, anti-VEGFR1, anti-LDLR and anti-actin immunoblotting. Relative average values of the band intensities from three independent experiments are shown below the western blot panels. (E,F) WCLs and (E) anti-PLCγ or (F) anti-c-Cbl IP from 3T3/VEGFR1 cells stimulated with VEGF or nLDL were immunoblotted with VEGFR1, PY, PLCγ (E), c-Cbl (F) and actin antibody. 3T3/VEGFR1, NIH3T3 cells that overexpress VEGFR1; GA, geldanamycin; LPDS, lipoprotein-deficient serum; nLDL, native LDL; PLC, phospholipase C; siRNA, short interfering RNA; VEGFR, vascular endothelial growth factor receptor; WCL, whole-cell lysate.
nLDL accelerates macrophage migration through VEGFR1
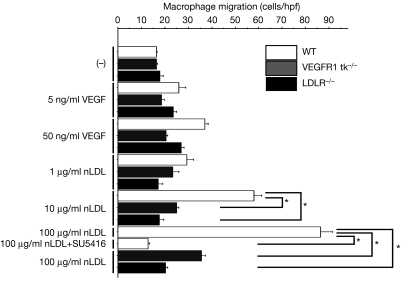
As reported previously (Hara et al, 1992; Shibuya, 2006), peritoneal macrophage migration can be promoted by both VEGF and nLDL (Fig 4). To eliminate the possibility that commercially available nLDL might be oxidized to some extent and thus could stimulate expression of the chemokine MCP1 (also known as chemokine ligand 2 (CCL2); Berliner et al, 1992), we treated the cells with MCP1-blocking antibody. Cells stimulated by MCP1, added to the culture, showed migrating ability that was blocked by the antibody. However, the antibody had no effect on the cells stimulated by nLDL (supplementary Fig 7 online). Interestingly, the nLDL-induced macrophage migration was repressed by SU5416 (Fig 4). This is consistent with the results shown in Fig 3E. The requirement for nLDL-induced migration by the tyrosine kinase activity of VEGFR1 was further confirmed by the inability of peritoneal macrophages from tk−/− mice to migrate. nLDL-induced migration was totally absent in macrophages from LDLR−/− mice, supporting our conclusion that nLDL activates VEGFR1 through LDLR.
Figure 4.
Native low-density lipoprotein-induced macrophage migration is VEGFR1-dependent. VEGF or nLDL at the indicated concentrations was added to the lower chambers. Data were corrected for background intensities with no stimulation in WT mice and expressed as means±s.d. *P<0.001. Assays were carried out in triplicate. hpf, high-power field; nLDL, native LDL; LDLR, LDL receptor; VEGFR, vascular endothelial growth factor receptor; WT, wild type.
Discussion
The sorting proteins involved in LDLR endocytosis, such as autosomal recessive hypercholesterolaemia (ARH; Garuti et al, 2005), exert their function by binding to the NPXY motif found in the intracellular domain of LDLR. A mutation in the tyrosine residue within the motif abrogates receptor clustering in clathrin-coated pits. However, a recent study showed that LDLR can be internalized independently of this motif and dependently on the ligand very low density lipoprotein (VLDL) (Michaely et al, 2007). VEGFR1 has the conserved Tyr 1053 followed by NPD, which might act as the NPXY motif. Although Tyr 1053 is not a major autophosphorylation site in the detailed phosphotyrosine mapping analysis (Ito et al, 1998), the possibility that it is one of the minor sites cannot be completely excluded.
It still remains to be explained how VEGFR1 is activated by nLDL. Ligand-independent activation of receptor tyrosine kinase by another receptor is known in EGFR (Saito et al, 2001). In this case, platelet-derived growth factor (PDGF) transactivates EGFR in a superoxide-dependent or cytoplasmic Src tyrosine kinase-dependent manner with heterodimeric formation of PDGFR and EGFR. However, we repeatedly observed VEGFR1 activation and colocalization of LDLR and VEGFR1 even in the presence of PP2 or N-acetyl-cysteine (data not shown). Enzyme-linked immunosorbent assay of nLDL and fragment proteins containing the extracellular domain of VEGFR1 fused to the Fc portion of the immunoglobulin failed to prove direct binding between the two (data not shown). VEGF was not upregulated in nLDL-treated cells, thus negating the possibility of VEGFR1 activation by paracrine mechanisms. As the interaction of both receptors outside of the cells seems to be important for VEGFR1 activation by nLDL (Fig 2), we assume that nLDL-bound LDLR directly induces a conformational setting of VEGFR1 that might mimic the VEGF-bound and activated state.
VEGF expression is switched on and off depending on the biological circumstances, such as hypoxia, whereas nLDL is constantly and abundantly present in the serum. Given the VEGFR1 activation by nLDL in the absence of VEGF, our findings indicate that nLDL not only is the source of denatured LDL that activates scavenger receptors but also by itself could be a cause of atherosclerosis through VEGFR1.
Methods
Molecular reagents. Antibodies and chemicals are listed in supplementary Table 1 online.
Plasmid construction. The GFP-tagged wild-type VEGFR1 expression vector has been described previously (Kobayashi et al, 2004). The full-length human LDLR complementary DNA was isolated from a human placenta cDNA library (Shibuya, 2006), sequenced and subcloned into pCMV-Tag4B expression vector.
Cell culture and transfections. NIH3T3 cells that overexpress VEGFR1 (3T3/VEGFR1) and VEGFR2 (3T3/VEGFR2) were described previously (Kobayashi et al, 2004). 293T, RAW264.7, CHO9 and NIH3T3 cells and skin fibroblasts isolated from wild-type and LDLR−/− mice were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. LPDS was prepared as described previously (Goldstein et al, 1983). The combination of transfection reagents, cells and primers for siRNA experiments are listed in supplementary Table 1 online.
Western blotting and immunoprecipitation analysis. 3T3/VEGFR1 cells cultured for 16–24 h in 1% FCS-containing DMEM were stimulated with 100 ng/ml VEGF or 500 μg/ml nLDL. Immunoprecipitation and western blotting were carried out as described previously (Okamoto et al, 2006) with the indicated antibodies (supplementary Table 1 online). For immunoprecipitation assay with lung lysates, 1% NP-40 was used instead of 1% Triton X-100. The intensity of bands in western blotting was quantified by Scion Image.
Immunofluorescence. 293T and RAW264.7, and CHO9 cells were transfected with VEGFR1-GFP and/or LDLR-Flag expression vectors. At 24 h after the transfection, the cells were starved of serum for 8 h before being stimulated for 15 min at 37°C with Dil-nLDL at 10 μg/ml, Dil-acLDL at 10 μg/ml, nLDL at 1, 10 or 100 μg/ml, or VEGF at 100 ng/ml. Peritoneal macrophages from mice were starved of serum for 24 h in 1% FCS before nLDL stimulation. They were then immunostained with the indicated antibody (supplementary Table 1 online) as described previously (Kobayashi et al, 2004). Fluorescent images were obtained by using Zeiss confocal laser scan microscopy (Zeiss, Oberkochen, Germany).
Ubiquitination assay. 3T3/VEGFR1 cells were transfected with a Flag-ubiquitin expression vector. At 24 h after transfection, the cells were treated with trypsin and replated to collagen-coated culture dishes with 1% FCS-containing media for 20 h. Cells stimulated by 100 ng/ml VEGF, 10 μM geldanamycin or 500 μg/ml nLDL were lysed in the ubiquitination assay buffer (50 mM NaCl, 10 mM Tris (pH 7.4), 5 mM EDTA, 50 mM NaF, 1% NP-40, 100 U/ml aprotinin, 10 mM N-ethylmaleimide and 50 μM LLnL).
Macrophage migration assay. The Boyden-chamber cell migration assay was carried out as described previously (Hiratsuka et al, 1998) with or without VEGF or nLDL at the indicated concentrations. In the statistical analyses, two-sided P-values of <0.05 were considered statistically significant. Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figures and Tables
Supplementary Legends
Acknowledgments
We thank B. Levene for correction of English in the manuscript. This study was partly supported by Grants-in-Aid for Scientific Research from the Japanese government (no. 12147210) to Y.M.
References
- Baigent C et al. , Cholesterol Treatment Trialists' (CTT) Collaborators (2005) Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366: 1267–1278 [DOI] [PubMed] [Google Scholar]
- Berliner JA, Territo M, Navab M, Andalibi A, Parhami F, Liao F, Kim J, Estworthy S, Lusis AJ, Fogelman AM (1992) Minimally modified lipoproteins in diabetes. Diabetes 41(Suppl 2): 74–76 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL (1986) A receptor-mediated passway for cholesterol homeostasis. Science 232: 34–47 [DOI] [PubMed] [Google Scholar]
- Crouse JR, Parks JS, Schey HM, Kahl FR (1985) Studies of low density lipoprotein molecular weight in human beings with coronary artery disease. J Lipid Res 26: 566–574 [PubMed] [Google Scholar]
- Cunningham SA, Arrate MP, Brock TA, Waxham MN (1997) Interactions of FLT-1 and KDR with phospholipase Cγ: identification of the phosphotyrosine binding sites. Biochem Biophys Res Commun 240: 635–639 [DOI] [PubMed] [Google Scholar]
- Garuti R, Jones C, Li WP, Michaely P, Herz J, Gerard RD, Cohen JC, Hobbs HH (2005) The modular adaptor protein autosomal recessive hypercholesterolemia (ARH) promotes low density lipoprotein receptor clustering into clathrin-coated pits. J Biol Chem 280: 40996–41004 [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Basu SK, Brown MS (1983) Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol 98: 241–260 [DOI] [PubMed] [Google Scholar]
- Hara S, Nagano Y, Sasada M, Kita T (1992) Probucol pretreatment enhances the chemotaxis of mouse peritoneal macrophages. Arterioscler Thromb 12: 593–600 [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M (1998) Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA 95: 9349–9354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK (1994) Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest 93: 1885–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Wernstedt C, Engstrom U, Claesson-Welsh L (1998) Identification of vascular endothelial growth factor receptor-1 tyrosine phosphorylation sites and binding of SH2 domain-containing molecules. J Biol Chem 273: 23410–23418 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Sawano A, Nojima Y, Shibuya M, Maru Y (2004) The c-Cbl/CD2AP complex regulates VEGF-induced endocytosis and degradation of Flt-1 (VEGFR-1). FASEB J 18: 929–931 [DOI] [PubMed] [Google Scholar]
- Lerdrup M, Hommelgaard AM, Grandal M, van Deurs B (2006) Geldanamycin stimulates internalization of ErbB2 in a proteasome-dependent way. J Cell Sci 119: 85–95 [DOI] [PubMed] [Google Scholar]
- Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B (2002) Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 8: 831–840 [DOI] [PubMed] [Google Scholar]
- Mary TC, Jing W, Donald JF (2002) Src kinase becomes preferentially associated with the VEGFR, KDR/Flk-1, following VEGF stimulation of vascular endothelial cells. BMC Biochem 3: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Tanaka K, Hirata G, Hanada M, Matsuda S, Shuto T, Iwamoto Y (2002) Possible involvement of the vascular endothelial growth factor-Flt-1-focal adhesion kinase pathway in chemotaxis and the cell proliferation of osteoclast precursor cells in arthritic joints. J Immunol 168: 5824–5831 [DOI] [PubMed] [Google Scholar]
- Michaely P, Zhao Z, Wei-Ping Li, Garuti R, Huang LJ, Hobbs HH, Cohen JC (2007) Identification of a VLDL-induced, FDNPVY-independent internalization mechanism for the LDLR. EMBO J 26: 3273–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KJ, Freeman MW (2006) Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol 26: 1702–1711 [DOI] [PubMed] [Google Scholar]
- Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J (2003) Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA 100: 4736–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto A, Iwamoto Y, Maru Y (2006) Oxidative stress-responsive transcription factor ATF3 potentially mediates diabetic angiopathy. Mol Cell Biol 26: 1087–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Haendeler J, Hojo Y, Yamamoto K, Berk BC (2001) Receptor heterodimerization: essential mechanism for platelet-derived growth factor-induced epidermal growth factor receptor transactivation. Mol Cell Biol 21: 6387–6394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M (2006) Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol 39: 469–478 [DOI] [PubMed] [Google Scholar]
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL (1989) Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 320: 915–924 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Tables
Supplementary Legends