Abstract
Hypoferremia is more prevalent in obese than nonobese adults, but the reason for this phenomenon is unknown. To elucidate the role dietary factors play in obesity-related hypoferremia, the intake of heme and nonheme iron and the intake of other dietary factors known to affect iron absorption were compared cross-sectionally from April 2002 to December 2003 in a convenience sample of 207 obese and 177 nonobese adults. Subjects completed 7-day food records, underwent phlebotomy for serum iron measurement, and had body composition assessed by dual-energy x-ray absorptiometry, during a 21-month period. Data were analyzed by analysis of covariance and multiple linear regression. Serum iron (mean±standard deviation) was significantly lower in obese than nonobese individuals (72.0±61.7 vs 85.3±58.1 µg/dL [12.888±11.0443 vs 15.2687±10.3999 µmol/L]; P<0.001). The obese cohort reported consuming more animal protein (63.6±34.5 vs 55.7±32.5 g/day; P<0.001) and more heme iron (3.6±2.8 vs 2.7±2.6 mg/day; P<0.001). Groups did not differ, however, in total daily iron consumption, including supplements. Obese subjects reported consuming less vitamin C (77.2±94.9 vs 91.8±89.5 mg/day; P=0.01), which may increase absorption of nonheme iron, and less calcium (766.2±665.0 vs 849.0±627.2 mg/day; P=0.038), which may decrease nonheme iron absorption, than nonobese subjects. Groups did not significantly differ in intake of other dietary factors that can impact absorption of iron, including phytic acid, oxalic acid, eggs, coffee, tea, zinc, vegetable protein, or copper. After accounting for demographic covariates and dietary factors expected to affect iron absorption, fat mass (P=0.007) remained a statistically significant negative predictor of serum iron. This cross-sectional, exploratory study suggests that obesity-related hypoferremia is not associated with differences in reported intake of heme and nonheme iron or intake of dietary factors that can affect iron absorption.
Several studies in children and adults have shown that obesity is associated with low serum iron concentrations (1–3) and a greater prevalence of iron deficiency (4). However, the pathophysiological mechanisms leading to hypoferremia among obese individuals are unknown. One proposed mechanism is an iron-poor diet (5). However, a difference in iron intake between obese and nonobese subjects has not been shown among US adults.
Humans obtain dietary iron in two forms: nonheme and heme. Nonheme iron is found mainly in enriched cereals and pasta, beans, and dark green leafy vegetables, while heme iron is derived primarily from hemoglobin and myoglobin in animal protein sources (6,7). Separate pathways are involved in the absorption of nonheme-associated and heme-associated iron (6). Although uptake of heme iron by enterocytes is affected little by consumption of other foods, absorption of nonheme iron is relatively inefficient, and can be altered substantially by co-consumption of certain dietary elements (8). Factors known to enhance nonheme iron absorption include animal protein, copper, and vitamin C. Factors known to inhibit nonheme iron absorption include vegetable protein, phytic acid, oxalic acid, zinc, calcium, eggs, tea, and coffee.
No prior study has examined the foods selected by obese and nonobese adults to determine whether dietary factors might account for the greater prevalence of hypoferremia observed among obese individuals. Therefore, the association between fat mass and hypoferremia was investigated by comparing differences in heme and nonheme dietary iron intake and by the intake of dietary factors that affect heme and nonheme iron absorption. It was hypothesized that the hypoferremia of obesity can be explained by differences in food choices that would result in lower iron intake and increased intake of dietary factors that could inhibit iron absorption.
METHODS
Study Design
This cross-sectional study was conducted using baseline data from a randomized clinical trial examining the effects of calcium supplementation on body weight, body composition, and comorbid conditions (9). From April 2002 to December 2003, healthy overweight adults, defined as having body mass index (BMI; calculated as kg/m2) ≥25.0, and healthy normal weight adults (BMI of 18.0 to 24.9) were recruited. Eligibility criteria for subjects included age older than 18 years, being in good general health, not regularly taking most prescription or any anorexiant medications, and having stable weight within 3% over past 2 months. The study was approved by the National Institutes of Health Intramural Clinical Research Review Board. Each participant gave written consent for protocol participation and received compensation.
Subjects reported after a 10-hour overnight fast to undergo evaluation. Subjects were weighed in hospital gowns using a digital scale (Life Measurement Instruments, Concord, CA) that was calibrated with a known weight before each subject’s measurement. Height was measured using a stadiometer (Holtain Ltd, Crymych, UK). Race was self-reported. Whole-body dual-energy x-ray absorptiometry for estimation of fat mass (Delphi A software, version 11.2 [2004], LX 20; Beckman, Bedford, MA) was performed in 369 subjects. Socioeconomic status was determined by Hollingshead score (10). Fasting blood was obtained for iron, hemoglobin, transferrin, and ferritin. Serum iron was measured using an iron ferrozine complex method with sensitivity 5 µg/dL (0.895 µmol/L), and serum transferrin was measured using a turbidimetric method with sensitivity 70 mg/dL (0.7 g/L), both using a Beckman 20 LX analyzer (Beckman Coulter, Inc, Fullerton, CA). Transferrin saturation was calculated as (serum iron [µg/dL]/transferrin [mg/dL])×71.2. Hypoferremia was defined as a serum iron <50 µg/dL (<8.95 µmol/L) based on laboratory normative data. Serum ferritin was measured by immunometric assay with the IMMULITE 2000 analyzer, sensitivity 0.4 ng/mL (0.8988 pmol; /LEURO/DPC, Los Angeles, CA). Hemoglobin was measured using standard methods.
Dietary Assessment
Dietary iron intake was assessed by 7-day food records. Subjects were given written instructions to record all foods and beverages consumed over 7 consecutive days. Food records were reviewed in person with subjects by a registered dietitian to maximize accuracy and completeness and were analyzed for dietary iron intake using the Nutrition Data System for Research software (version 2005, Nutrition Coordinating Center, University of Minnesota, Minneapolis). A further analysis using the Nutrition Data System for Research version 2005 software placed foods consumed into 166 different food subgroups in order to separate foods into heme and nonheme ironcontaining foods. A dietary iron content value was assigned to each food group based on the USDA National Nutrient Database for Standard Reference Release No. 18 (11) and was used to measure heme and nonheme dietary intake. While food records are considered the gold standard for dietary intake assessment, iron from supplements is not measured by the food-record method. Therefore, a second measure of iron intake was employed: the Diet History Questionnaire, a validated, 36-page booklet listing 124 separate food items developed by the National Cancer Institute for use in epidemiological research to assess total diet over a year-long period and, therefore, reflect seasonal differences in iron intake (12). In addition to dietary iron intake, estimated iron intake from iron and multivitamin supplements was determined from reports on Diet History Questionnaires. Estimated iron intake from multivitamin supplements was determined by assigning an average value to the amount of iron in multivitamins. Dietary iron intake from food records was added to supplemental iron intake from Diet History Questionnaires to estimate total daily iron intake. Diet History Questionnaires were analyzed using Diet*Calc Analysis Program (version 1.3.2, June 2003, National Cancer Institute, Applied Research Program, Silver Spring, MD). Average total macronutrient and micronutrient intakes from food records were also calculated from 7-day food records for each individual. Macronutrient and micronutrient totals from individual food records were used to determine intake of substances known to affect iron absorption positively: animal protein, copper, vitamin C; or negatively: coffee, tea, calcium, eggs, zinc, oxalic acid, phytic acid, and vegetable protein (8,13,14).
Statistical Analysis
Two primary analyses were conducted. Laboratory and dietary intake results from obese and nonobese subjects were compared by analysis of covariance, accounting for sex, race, age, and socioeconomic status. A multiple linear regression analysis combined data from all subjects to determine whether dietary factors could explain the relationship between serum iron concentrations and fat mass. Data were analyzed using StatView (version 5.01, 1998, SAS Institute Inc, Cary, NC) and SPSS for Windows (version 14.0, 2005, SPSS Inc, Chicago, IL). Unless otherwise indicated, data are reported as adjusted mean±standard deviation. P<0.05 were considered significant.
RESULTS AND DISCUSSION
Two-hundred and seven obese and 178 nonobese adults were enrolled (Table). Data from 10 subjects were excluded for reporting implausible energy intake of <600 calories or >3,500 calories per day for women and <800 calories or >4,200 calories per day for men (12), or for incomplete dietary records. Obese and nonobese subjects did not differ substantially in their demographic characteristics, other than BMI.
Table.
Clinical characteristics of obese and nonobese adults with completed 7-day food records and fasting laboratory values (n=384)
| Obese (n=207) | Nonobese (n=177) | P value | |
|---|---|---|---|
| % | |||
| Female sex | 78 | 72 | 0.19 |
| Race | 0.026 | ||
| White | 57 | 66 | |
| Black | 36 | 24 | |
| Other | 7 | 10 | |
| mean±standard deviation | |||
| Age (y) | 38.6±9.8 | 37.2±11.8 | 0.21 |
| Body mass indexa | 37.4±5.9 | 25.8±2.8 | <0.05 |
| Body fat (kg) | 43.7±15.5 | 22.8±8.6 | <0.05 |
| Serum iron (µg/dL)b | 72.0±61.7 | 85.3±58.1 | <0.001 |
| Transferrin saturation (%) | 20.7±17.6 | 23.3±16.6 | <0.05 |
| Hemoglobin (g/dL)c | 13.7±2.7 | 13.8±2.5 | 0.38 |
| Ferritin (µg/L)d | 95.4±135.1 | 80.8±144.8 | 0.10 |
| Energy intake (kcal/d) | 2,251.5±878.8 | 2,028.4±828.9 | <0.001 |
| Dietary heme iron (mg/d) | 3.6±2.8 | 2.7±2.6 | <0.001 |
| Dietary nonheme iron (mg/d) | 14.0±13.6 | 13.5±12.9 | 0.56 |
| Total iron intake including supplements (mg/d) | 18.2±14.4 | 16.9±12.8 | 0.10 |
Calculated as kg/m2.
To convert µg/dL iron to µmol/L iron, multiply µg/dL by 0.179.
To convert g/dL hemoglobin to g/L hemoglobin, multiply g/dL by 10.0.
To convert µg/L ferritin to pmol/L ferritin, multiply µg/L by 2.247.
Hypoferremia was significantly more prevalent in obese compared to nonobese adults (25.1% [95% confidence interval: 10.1% to 20.6%] vs 14.6% [95% confidence interval: 19.7% to 31.5%]; P<0.05), and mean serum iron was lower among obese subjects (72.0±61.7 vs 85.3±58.1 µg/dL [12.888±11.0443 vs 15.2687±10.3999 µmol/L]; P<0.001). Results are in accord with most previous studies (1–3). Further, transferrin saturation (20.7%±17.6% vs 23.3%±16.6%; P=0.012) was lower among obese compared with nonobese subjects (Table). There was no difference in hemoglobin or ferritin concentrations between obese and nonobese groups.
Mean total daily dietary iron intake, including supplements containing iron, was not significantly different between obese and nonobese subjects (P=0.10). The obese cohort, though, consumed more heme iron (3.6±2.8 vs 2.7±2.6 mg/day; P<0.001) than the nonobese cohort (Table).
Obese subjects reported consuming less vitamin C (77.2±94.9 vs 91.8±89.5 mg/day; P=0.01) and calcium (766.2±665.0 vs 848.9±627.2 mg/day; P=0.04) than nonobese subjects (Figure), and more animal protein (63.5±34.5 vs 55.7±32.6 g/day; P<0.001) than nonobese subjects. Groups did not differ, however, in mean daily intake of copper, vegetable protein, phytic acid, oxalic acid, zinc, eggs, tea, or coffee (Figure).
Figure.
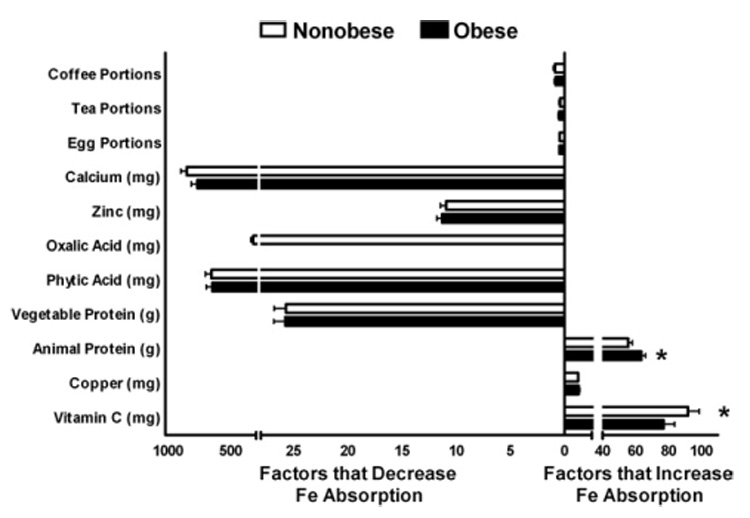
Total dietary intake among obese and nonobese adults of factors known to enhance and inhibit heme and nonheme iron absorption.
After accounting for demographic covariates, a multiple linear regression analysis, which included variables known to affect iron absorption, showed that fat mass (β=−.330; P<0.05) remained a significant negative predictor of serum iron concentration. Other than percentage of calories from vegetable protein, which was a negative predictor for serum iron (P=0.004), none of the remaining factors known to affect dietary iron absorption were significant predictors of serum iron in this model.
Although there were small but significant differences between obese and nonobese study participants in consumption of dietary heme iron, vitamin C, and calcium, these differences were not associated with the inverse relationship observed between adiposity and serum iron. In addition, obese subjects reported consuming less vitamin C than nonobese subjects. The enhancing effect of vitamin C on iron absorption is due to both its reducing and chelating properties (15). An acidic environment in the stomach can solubilize dietary iron and has been shown to be an efficient enhancer of nonheme iron absorption in humans (15,16). Vitamin C intake, however, did not predict serum iron in the multiple regression model. Consistent with a lack of clinical impact on serum iron from dietary vitamin C, is a prior study that found addition of 2,000 mg/day of vitamin C to the diet for 2 years did not significantly alter iron stores (16). Dietary intake of calcium was also lower in obese compared with nonobese subjects. Calcium has been shown to diminish iron absorption, by inhibiting the transfer of heme and nonheme iron into mucosal cells, and by interfering with degradation of phytic acid (8). Because calcium intake was lower in the obese group, dietary calcium intake could not, however, help explain the hypoferremia of obesity.
One previously proposed cause of hypoferremia among the obese is a deficient iron store due to a greater iron requirement in obese adults because of their larger blood volume. Because obesity is considered a chronic inflammatory state, inflammatory-mediated sequestration of iron in the reticuloendothelial system, with resultant hypoferremia, despite adequate or even increased iron stores could also play a role in the hypoferremia of obesity (17).
The health consequences of hypoferremia may have important clinical implications in adults. In one study, presence of hypoferremia was found to impair aerobic adaptation among untrained women (18). No longitudinal studies have been performed, however, to determine whether hypoferremia precedes development of obesity or is the result of the obese state.
Limitations to this study include the fact that 7-day food records were self-recorded and may not necessarily reflect habitual iron intake. Obese subjects are known to underreport dietary intake to a greater extent compared to lean subjects (19–21), which may have affected results. However, if obese subjects underreported iron intake, this would bias the results toward underestimating the available dietary iron and make dietary factors less likely to be an explanation for obesity-related hypoferremia. In addition, other factors that can affect iron absorption, including heat treatment of foods, which denatures the cysteine groups in heme iron, decreasing iron absorption (22,23), and use of nonprescription antacids were not accounted for. Finally, menstrual blood flow, which contributes to serum iron concentrations, was not included as a covariate.
CONCLUSION
Etiology of the hypoferremia of obesity remains uncertain. The current cross-sectional, exploratory study suggests that differences in intake of heme and nonheme iron, or of dietary factors known to affect iron absorption, are not associated with lower serum iron concentrations found in obese adults. Additional studies investigating other factors that may be associated with the hypoferremia of obesity are warranted.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, grant HD-000641 from the National Institute of Child Health and Human Development, National Institutes of Health (J.A.Y.) and Y2-OD-2067 (to J.A.Y.) from the Office of Dietary Supplements, National Institutes of Health, Department of Health and Human Services.
Preliminary results presented at the North American Association for the Study of Obesity. Boston, MA, 2006.
N. Sebring, B. Denkinger, and J. Yanovski are Commissioned Officers in the US Public Health Service, Department of Health and Human Services.
References
- 1.Pinhas-Hamiel O, Newfield RS, Koren I, Agmon A, Lilos P, Phillip M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord. 2003;27:416–418. doi: 10.1038/sj.ijo.0802224. [DOI] [PubMed] [Google Scholar]
- 2.Micozzi MS, Albanes D, Stevens RG. Relation of body size and composition to clinical biochemical and hematologic indices in US men and women. Am J Clin Nutr. 1989;50:1276–1281. doi: 10.1093/ajcn/50.6.1276. [DOI] [PubMed] [Google Scholar]
- 3.Rossi E, Bulsara MK, Olynyk JK, Cullen DJ, Summerville L, Powell LW. Effect of hemochromatosis genotype and lifestyle factors on iron and red cell indices in a community population. Clin Chem. 2001;47:202–208. [PubMed] [Google Scholar]
- 4.Lecube A, Carrera A, Losada E, Hernandez C, Simo R, Mesa J. Iron deficiency in obese postmenopausal women. Obesity (Silver Spring) 2006;14:1724–1730. doi: 10.1038/oby.2006.198. [DOI] [PubMed] [Google Scholar]
- 5.Seltzer CC, Mayer J. Serum iron and iron-binding capacity in adolescents. II. Comparison of obese and nonobese subjects. Am J Clin Nutr. 1963;13:354–361. doi: 10.1093/ajcn/13.6.354. [DOI] [PubMed] [Google Scholar]
- 6.Food and Nutrition Board, Institute of Medicine, editor. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2000) Washington, DC: National Academies Press; 2001. Iron; pp. 290–393. [PubMed] [Google Scholar]
- 7.Syed BA, Sargent PJ, Farnaud S, Evans RW. An overview of molecular aspects of iron metabolism. Hemoglobin. 2006;30:69–80. doi: 10.1080/03630260500455318. [DOI] [PubMed] [Google Scholar]
- 8.Food and Nutrition Board, Institute of Medicine, editor. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc in Iron. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- 9.US National Institutes of Health, Clinical Research Studies. [Accessed January 6, 2006];Supplemental calcium in overweight people, NCT00030238. Available at: http://www.clinicaltrials.gov/ct/show/NCT00030238.
- 10.Hollingshead AB. Hollingshead two factor index of social position. In: Miller DC, editor. Handbook of Research Design and Social Measurement (1957) Newbury Park, CA: Sage Publications; 1991. pp. 351–359. [Google Scholar]
- 11.Gebhardt SE, Lemar LE, Cutrufelli RL, Haytowitz DB, Howe JC, Pehrsson PR, Stup MA, Exler J, Holcomb GT, Thomas RG, Showell BA, Holden JM. USDA National Nutrient Database for Standard Reference, Release No. 18. Washington, DC: US Department of Agriculture; 2005. [Google Scholar]
- 12.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America’s Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 13.Groff JL, Hunt SM. Advanced Nutrition and Human Metabolism. 2nd ed. St Paul, MN: West Publishing Company; 1995. [Google Scholar]
- 14.Hurrell RF, Reddy MB, Juillerat M, Cook JD. Meat protein fractions enhance nonheme iron absorption in humans. J Nutr. 2006;136:2808–2812. doi: 10.1093/jn/136.11.2808. [DOI] [PubMed] [Google Scholar]
- 15.Teucher B, Olivares M, Cori H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403–419. doi: 10.1024/0300-9831.74.6.403. [DOI] [PubMed] [Google Scholar]
- 16.Cook JD, Reddy MB. Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet. Am J Clin Nutr. 2001;73:93–98. doi: 10.1093/ajcn/73.1.93. [DOI] [PubMed] [Google Scholar]
- 17.Yanoff LB, Menzie CM, Denkinger B, Sebring NG, McHugh T, Remaley AT, Yanovski JA. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes (Lond) 2007;31:1412–1419. doi: 10.1038/sj.ijo.0803625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brownlie TT, Utermohlen V, Hinton PS, Giordano C, Haas JD. Marginal iron deficiency without anemia impairs aerobic adaptation among previously untrained women. Am J Clin Nutr. 2002;75:734–742. doi: 10.1093/ajcn/75.4.734. [DOI] [PubMed] [Google Scholar]
- 19.Hassapidou M, Fotiadou E, Maglara E, Papadopoulou SK. Energy intake, diet composition, energy expenditure, and body fatness of adolescents in northern Greece. Obesity (Silver Spring) 2006;14:855–862. doi: 10.1038/oby.2006.99. [DOI] [PubMed] [Google Scholar]
- 20.Johansson L, Solvoll K, Bjorneboe GE, Drevon CA. Under- and over-reporting of energy intake related to weight status and lifestyle in a nationwide sample. Am J Clin Nutr. 1998;68:266–274. doi: 10.1093/ajcn/68.2.266. [DOI] [PubMed] [Google Scholar]
- 21.Macdiarmid JI, Blundell JE. Dietary under-reporting: What people say about recording their food intake. Eur J Clin Nutr. 1997;51:199–200. doi: 10.1038/sj.ejcn.1600380. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Torres C, Leets I, Taylor P, Ramirez J, del Valle Camacho M, Layrisse M. Heme, ferritin and vegetable iron absorption in humans from meals denatured of heme iron during the cooking of beef. J Nutr. 1986;116:1720–1725. doi: 10.1093/jn/116.9.1720. [DOI] [PubMed] [Google Scholar]
- 23.Baech SB, Hansen M, Bukhave K, Kristensen L, Jensen M, Sorensen SS, Purslow PP, Skibsted LH, Sandstrom B. Increasing the cooking temperature of meat does not affect nonheme iron absorption from a phytate-rich meal in women. J Nutr. 2003;133:94–97. doi: 10.1093/jn/133.1.94. [DOI] [PubMed] [Google Scholar]


