Abstract
Background and purpose:
Increased angiotensin II levels and insulin resistance coexist at the early stages of cardiomyopathies. To determine whether angiotensin II increases insulin resistance in cardiomyocytes, we studied the effect of angiotensin II on basal and insulin-stimulated transport rate of energy substrates in immortalized cardiomyocytes (HL-1 cells).
Experimental approach:
Glucose and palmitic acid uptakes were measured using [3H]2-deoxy-D-glucose and [14C]palmitic acid, respectively, in cells exposed or not exposed to angiotensin II (100 nM), angiotensin II plus irbesartan or PD123319, type 1 and 2 receptor antagonists, or PD98059, an inhibitor of ERK1/2 activation. Cell viability, DNA, protein synthesis and surface area were evaluated by the MTT test, [3H]thymydine, [3H]leucine and morphometric analysis, respectively. Type 1 receptor levels were measured by western blot analysis.
Key results:
Basal uptakes of glucose and palmitic acid by HL-1 cells (0.37±0.07 and 7.31±0.22 pmol per 104cells per min, respectively) were both stimulated by 100 nM insulin (+91 and +64%, respectively). Cells exposed to angiotensin II remained viable and did not show signs of hypertrophy. In these conditions, the basal palmitic acid uptake of the cells increased (11.41±0.46 pmol per 104 cells per min) and insulin failed to stimulate the uptake of glucose and fatty acids. Changes in the rate of uptake of energy substrates were prevented or significantly reduced by irbesartan or PD98059.
Conclusions and implications:
Angiotensin II is a candidate for increasing insulin resistance in cardiomyocytes. Our results suggest a further mechanism for the cardiovascular protection offered by the angiotensin II type 1 receptor blockers.
Keywords: HL-1 cells, glucose uptake, fatty acid uptake, angiotensin II type 1 receptor blockers, insulin resistance, hypertrophy
Introduction
There is increasing evidence that alterations in energy substrate transport and utilization by cardiac myocytes represent a primary cause of the pathogenesis of heart diseases including diabetic cardiomyopathy (Fang et al., 2004).
Physiologically, about 80% of the energy required by a healthy adult heart is supplied by the intracellular oxidation of fatty acids, whereas the remaining 20% depends on glucose use. The relative percentage of each of these fuelling substrates is finely regulated by hormone levels in the microenvironment and this includes the concentration of insulin. In fact, insulin not only helps to maintain the correct myocardial energy supply by increasing types 4 and 1 glucose transporter (GLUT4 and GLUT1) activities (Opie, 2004), but also controls the intracellular availability of fatty acids. Recent evidence demonstrates that insulin regulates the docking towards plasma membrane of a translocase (the FAT/CD36 (fatty acid transporter, insulin-sensitive)) that works in addition to simple diffusion and to other carrier-mediated transports (Luiken et al., 2002a, 2002b). While the signalling cascade involving the inositol triphosphate kinase pathway has been recognized as part of the insulin-regulated GLUT4 and FAT/CD36 trafficking, less is known about the subsequent common and/or distinct signalling steps involved (Luiken et al., 2004).
In the heart of insulin-resistant animals, the relative percentage of available intracellular glucose and fatty acids changes. In particular, the FAT/CD36 stabilizes at the plasma membrane and a lower amount of GLUT4 molecules is able to move towards plasmalemma (Bonen et al., 2002; Coort et al., 2004) resulting in an increased intracellular availability of fatty acids. While this condition from one side may saturate the mitochondrial β-oxidation, enhancing tissue fatty acid storage (Stanley et al., 1997), it also can further reduce the glucose entering the myocardial cells. On the whole, this vicious cycle exacerbates insulin resistance further by reducing the glucose and lactate uptake and utilization.
The effects of angiotensin II on the insulin sensitivity of glucose uptake are not consistent and depend on cell type (Izawa et al., 2005; Juan et al., 2005). Also, its direct effect on the basal and insulin-stimulated glucose and fatty acid transport rate has not been elucidated in single cardiomyocytes. However, angiotensin II type 1 receptor (AT1) blockers are widely used, clinically; they represent a first-line approach in reducing the cardiovascular risk in diabetic patients (Schmieder, 2005), but their role in ameliorating insulin resistance is not proved (Lerch et al., 1998). These drugs have consistently been reported to be neutral with regard to insulin sensitivity (Lerch et al., 1998), but have proven effectiveness in preventing diabetic electrophysiological remodelling, probably by counteracting the dysregulation of energy substrate supplementation of cardiomyocytes (Raimondi et al., 2004).
HL-1 cells are an immortalized cardiac cell line that retain a differentiated morphology (Sartiani et al., 2002; White et al., 2004; Fukuda et al., 2005; Clarke et al., 2006) and have been used previously to study hormone-dependent glucose and fatty acid metabolism (Palanivel et al., 2006). Moreover, HL-1 cells express on their surface several hormone receptors including the AT1 receptor (Filipeanu et al., 2004). For all these reasons, HL-1 cells represent an excellent model for studying the effects of angiotensin II on cardiomyocyte metabolism.
We measured the basal and insulin-stimulated glucose and fatty acid uptake in HL-1 cells cultured in the absence or in the presence of angiotensin II, a condition mimicking a pathological microenvironment.
Methods
HL-1 cells were cultured as described previously (Sartiani et al., 2002). Briefly, cells were grown as a monolayer (37 °C, 5% CO2) in culture flasks, dishes and plates precoated with 2 μg cm−2 fibronectin dissolved in a 0.02% gelatin solution. HL-1 cells were maintained in Claycomb medium supplemented with 10% foetal bovine serum, 4 mM L-glutamine, 100 μM noradrenaline (from 10 mM stock solution in L-ascorbic acid 30 mM) and 1 × antibiotic/antimycotic solution (standard medium). The medium was changed every 24–48 h.
To mimic a pathological microenvironment, HL-1 cells were cultured in standard medium up to 90% of confluence and then shifted to a standard medium with low serum (0.1% serum), in the absence of noradrenaline, but with the addition of angiotensin II (100 nM). Cell exposure to angiotensin II was stopped after 18 h of culturing at 37 °C in 5% CO2. Where stated, angiotensin II exposure was carried out in the presence of either an AT1 (irbesartan; 1 μM) or angiotensin II type 2 receptor (AT2) antagonist (PD123319; 1 μM) or an inhibitor of extracellular signal-regulated kinase (ERK)1/2 activation (PD98059; 10 μM).
Glucose and fatty acid uptake in HL-1 cells
The basal and insulin-stimulated glucose and long-chain fatty acid transports were measured radiochemically in HL-1 cells seeded in 96-multiwell plates and cultured up to 90% of confluence (1.8±0.4 × 104 cells). In some plates, angiotensin II was added as described above; other plates were cultured for the same time in Claycomb medium (containing 0.1% serum) and referred to as ‘control'. At the end of the incubation, plates were thoroughly washed with saline solution.
For glucose uptake experiments, cells were diluted with glucose-free Krebs phosphate buffer (pH 7.8; Raimondi et al., 2004), whereas for palmitic acid uptake, cells were incubated in the same buffer containing 5 mM glucose and ranolazine (1 μM), an inhibitor of fatty acid oxidation (Stanley, 2005). The effect of insulin on energy substrate uptake was evaluated by exposing cells to the hormone (humulin) from Eli Lilly (Toronto, Canada) for 30 min before adding [3H]2-deoxy-D-glucose (10 μM; 1 μCi per well) or a [14C]palmitate/BSA complex (1 μCi per well; molar ratio 0.3; final concentration 100 μM) (Chabowski et al., 2005). The uptake of glucose and palmitic acid was measured 10 and 5 min, respectively, after the addition of labelled substrates. Cells were then extensively washed with cold Krebs buffer, and the radioactivity incorporated was measured by scintillation counting (Raimondi et al. 2004).
Non-transporter-dependent glucose and palmitic acid uptake were determined in cells pretreated for 30 min with phloretin (10 μM) before the addition of labelled substrates. The residual (phloretin-insensitive) radioactivity was subtracted from each measurement obtained. The involvement of inositol triphosphate kinase activation in both glucose and fatty acid uptake was evaluated by pretreating the cells for 30 min with wortmannin (100 nM) before the addition of insulin.
Each pharmacological intervention (angiotensin receptor antagonists and ERK1/2 inhibitor) was tested for its ability to modify glucose and fatty acid uptake. As none of the drugs used affected the basal uptake of either glucose or fatty acid, the results obtained from the cells exposed to each pharmacological treatment were pooled with the basal values obtained in control cells. Results are presented as pmol per 104 cells per min for glucose and fatty acid uptake or as arbitrary units (where the effect of each stimulation is compared to the respective basal uptake which is taken as 100). The values shown represent the means±s.e.mean of 5–18 different experiments, each run in triplicate.
HL-1 viability, hypertrophy and cell surface
Viability, protein and DNA synthesis were evaluated in sub-confluent HL-1 cells, 1.7±0.8 × 105 cells cultured in six multiwell plates, that had or had not been exposed to angiotensin II. Cells were then washed with 0.1 M phosphate buffer pH 7.8 containing 0.1 M NaCl (phosphate-buffered saline) before the addition of phosphate-buffered saline containing 0.5 mg ml−1 of 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyltetrazolium bromide (Mosmann, 1983). The formazan production after 2 h was evaluated colorimetrically at 570 nm. Results are expressed as the mean±s.e.mean of the OD recovered from three experiments, each run in quadruplicate.
DNA and new protein synthesis
Cells were plated as above and then exposed to angiotensin II. At the beginning of the exposure, cells were labelled with [3H]thymidine or [3H]leucine (0.5 μCi per well). After 18 h, the radioactive medium was discarded, cells were washed three times with ice-cold phosphate-buffered saline and incubated with 5% trichloroacetic acid (TCA) on ice for 30 min and solubilized in 0.5 N NaOH. After the sample had been neutralized with 0.5 N HCl, the radioactivity incorporated was counted in a scintillation counter (Packard Instruments, Minneapolis, MN, USA). Results are shown as the counts per minute (CPM) per well, and they represent the mean±s.e.mean of three experiments, each run in triplicate. Cells deprived of serum for 24 h before the addition of [3H]thymidine or [3H]leucine did not incorporate significantly different amounts of radioactivity compared to those with normal serum (data not shown).
Morphology
For these measurements, cells cultured in 75 cm2 flasks and exposed to angiotensin II as described above were used. Briefly, control and exposed cells were stained with haematoxylin/eosin, and four randomly chosen microscopic fields per flask were used for analysis. The microscopic fields were registered by a closed-circuit television camera (WPI, Sarasota, FL, USA) applied to a Reichert-Jung Microstar IV light microscope (Cambridge Instruments, Buffalo, NY, USA) and interfaced with a personal computer through a Matrox Marvel G400-TV digitizing card (Matrox Graphics, Dorval, QC, Canada). On the digitized images, cell outlines were traced and the surface area was calculated using the Scion Image Beta 4.0.2 image analysis programme (Scion Corp., Frederick, MD, USA) thresholding on blank, cell-free aerial spaces. The mean values (±s.e.mean) of cell areas were then calculated for each experimental group. From 80 to 120 cells were measured in each randomly selected field (Bani et al., 2006).
Expression of angiotensin II type 1 receptor
Protein lysates from 75 cm2 flasks of HL-1 cells (90–95% confluent) not exposed or exposed to angiotensin II were prepared. Briefly, after the exposure medium had been removed and the cells extensively washed with ice-cold phosphate-buffered saline, the cells were scraped off the flasks with lysis buffer of the composition (in mM) Tris-HCl (50) (pH 7.5), EDTA (1), NaCl (150), complete protease inhibitor cocktail tablet, sodium orthovanadate (1) and NaF (10) and then homogenized. The suspension was centrifuged (1000 g for 10 min at 4 °C) to remove cell debris. The resulting supernatant was frozen at –80 °C for later analysis by immunoblotting. The protein concentration of the crude lysate was determined by the BCA protein assay reagent kit from Pierce (Rockford, IL, USA).
Protein samples were subjected to 4–12% (w/v) acrylamide gel and transferred to polyvinylidene difluoride membranes. The membrane was probed with the primary antibody (1:1000 dilution) against AT1 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1000 dilution) overnight at 4 °C. Antibody binding was detected with a goat-anti-rabbit horseradish peroxidase (1:10 000 dilution) secondary antibody. Detection of the immunocomplex was carried out by chemiluminescence. GAPDH expression was used as a ‘housekeeping protein' and to evaluate transfer efficiency (Rajapurohitam et al., 2006).
Data analysis
Data are expressed as mean±s.e.mean. Results were analysed using Student's unpaired t-test with Welch's correction or with one-way ANOVA (Dunnett's test). A P-value<0.05 was considered to be significant. Calculations were made using GraphPad Prism and Instat version 4.0 for Windows (GraphPad Software, San Diego, CA, USA).
Materials
HL-1 cells were obtained from Dr W Claycomb (Louisiana State University Medical Center, New Orleans, LA, USA). Fibronectin, 0.02% gelatin solution, noradrenaline, ranolazine, phloretin, wortmannin, insulin, 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyltetrazolium bromide, complete protease inhibitor cocktail tablet, sodium orthovanadate and goat-anti-rabbit horseradish peroxidase were obtained from Sigma (St Louis, MO, USA); Claycomb medium and foetal bovine serum from JRH Biosciences (Andover, UK); humulin from Eli Lilly; [3H]2-deoxy-D-glucose, [14C]palmitate/BSA complex, [3H]thymidine and [3H]leucine from Amersham Life Science (Little Chalfont, UK); BCA protein assay reagent kit from Pierce; acrylamide gel (Nu-Page Gel) from Invitrogen (Milan, Italy); GAPDH from St Cruz Biotechnology Inc. (St Cruz, CA, USA).
Results
HL-1 cardiomyocytes present insulin-sensitive glucose and palmitic acid uptakes
As described previously (Chaudary et al., 2002; Palanivel et al., 2006), phloretine-sensitive mechanisms govern the basal supply of glucose and long-chain fatty acids, measured as palmitate uptake, in HL-1 cardiomyocytes in our experimental conditions. In particular, the palmitate uptake accounted for about 95% and the glucose for 5% of the total energy substrate available (Table 1).
Table 1.
Basal glucose and palmitic acid uptake rate in HL-1 cells challenged with different treatments
| Treatments | Glucose uptake (pmol per104 cells per min) | Palmitic acid uptake (pmol per104 cells per min) |
|---|---|---|
| Control (n=18) | 0.37±0.07 | 7.31±0.22 |
| Angiotensin II (n=8) | 0.28±0.09 | 11.41±0.46** |
| Angiotensin II+IRB (n=5) | 0.33±0.130 | 5.88±0.62 |
| Angiotensin II+PD98059 (n=6) | 0.38±0.10 | 6.52±0.32 |
| Angiotensin II+PD123319 (n=5) | 0.22±0.08 | 8.94±0.64* |
Abbreviation: IRB, irbesartan.
**P<0.01, *P<0.05 vs cells not exposed.
The basal glucose and palmitic acid uptake rate was measured in HL-1 cells not exposed and exposed to angiotensin II as described in Methods.
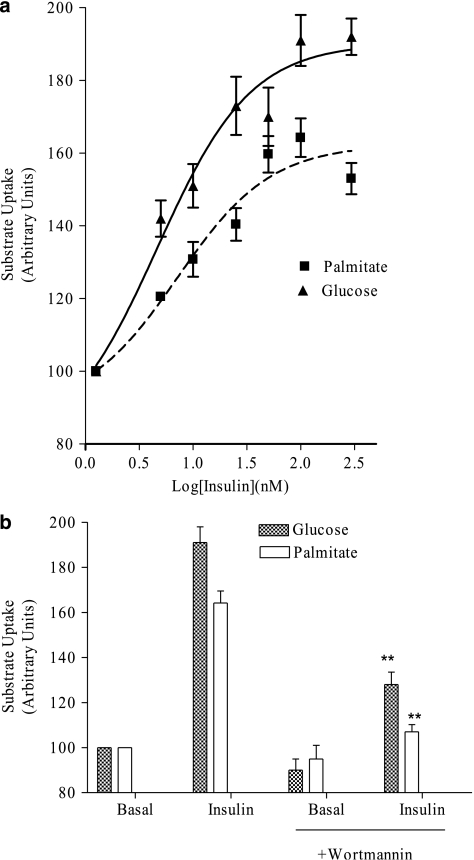
The rate of the two uptakes increased, in a concentration-dependent manner, following acute exposure of cells to insulin. As shown in Figure 1a, insulin increased, in a concentration-dependent manner, the transport of glucose and of palmitate. Similar values of potency were obtained (EC50 values being 7.17±1.71 nM, (confidence limit) CL: 2.57–14.14; and 4.65±1.51 nM, CL: 1.42–15.22 for glucose and palmitate, respectively) and maximum effect (+91% and +64% for glucose and palmitate, respectively) was seen with 100 nM insulin (Figure 1b). Pre-incubating the cells with wortmannin significantly (P<0.05 vs insulin effect; n=6) reduced the increased uptake of glucose and palmitate induced by insulin (Figure 1b). This suggests that inositol triphosphate kinase is involved in the intracellular signalling regulating the docking towards plasmalemma of both transporters.
Figure 1.
The insulin-stimulated glucose and palmitate transport in (immortalized cardiomyocytes) HL-1 cells. (a) Glucose and palmitate uptakes were measured radiochemically as described in Methods. Insulin stimulates in a concentration-dependent manner the rate of both transports. Insulin potency (EC50) was calculated by the fitting of experimental points. (b) Pre-incubation of cells with wortmannin reduced the insulin stimulation of glucose and palmitic acid uptake. Results are presented as stimulated rate compared to the basal transport rate, taken as 100, and they represent the mean±s.e.mean of at least eight (a) or six (b) experiments run in triplicate. For the basal uptake values see Table 1. *P<0.05 vs insulin effect in the absence of wortmannin.
Effects of long-standing exposure to angiotensin II
Cell viability
Exposing cells to angiotensin II (100 nM) for 18 h did not affect cell viability; indeed, similar values of oxidized formazan were recovered in control and in angiotensin II-exposed cells (OD: 0.55±0.07 and 0.58±0.02, respectively, n=3 different cell passages, each determination run in quadruplicate).
The basal transport rate of glucose and palmitate
While angiotensin II exposure for 18 h did not change the basal uptake of glucose, it maximally increased cell palmitate uptake rate. In fact, as shown in Table 1, the palmitate uptake of exposed cells achieved the same rate as that induced by 100 nM insulin in control cells (see above results).
Insulin stimulation of energy substrate available
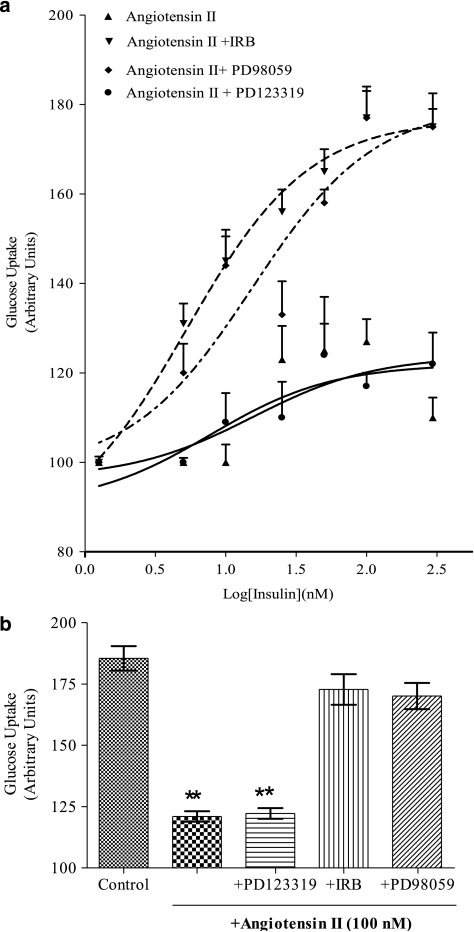
In exposed cells, acute administration of insulin failed to stimulate the uptake of either glucose or palmitate (Figures 2 and 3). In fact, the dose that induced maximum stimulation (100 nM insulin) in control cells produced a glucose uptake of 0.44±0.05 pmol per 104 cells per min (+20% of basal; Figure 2) and a fatty acid uptake of 12.50±0.47 pmol per 104 cells per min (+13% of cell uptake in the absence of insulin).
Figure 2.
Pharmacological modulation of angiotensin II-induced insulin resistance of glucose uptake. (a) Concentration-dependent effect of insulin (from 5 to 300 nM) on glucose transport in cells exposed to angiotensin II, angiotensin II plus irbesartan (IRB, 1 μM) or PD98059 (10 μM) or angiotensin II plus PD123319 (1 μM). Results are the mean±s.e.mean of 8–18 experiments run in triplicate and are presented as stimulation of their basal uptake rate (taken as 100). Lines represent fitting of data points. (b) The maximum value of insulin (100 nM)-stimulated uptake (as calculated by fitting curves from (a) and from Figure 1) in HL-1 cells challenged with different compounds. Each column is the mean±s.e.mean of 5–18 experiments run in triplicate. **P<0.01, compared to insulin maximum effect in control cells.
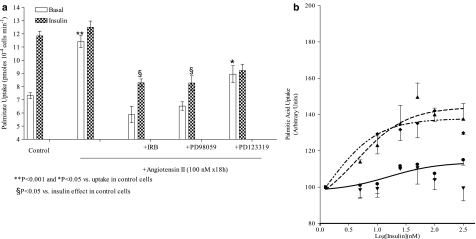
Figure 3.
Pharmacological modulation of angiotensin II-induced insulin resistance of palmitate uptake. (a) The maximum absolute value of insulin (100 nM)-stimulated uptake (pmol per 104 cells per min) in HL-1 cells challenged with different compounds is presented. Each column is the mean±s.e.mean of 5–18 experiments run in triplicate. **P<0.01 and *P<0.05 compared to uptake in control cells, and §P<0.05 compared to insulin effect in control cells. (b) Concentration-dependent stimulation of palmitic acid transport induced by insulin (from 5 to 300 nM) was evaluated radiochemically, as described in Methods, in cells exposed to angiotensin II, angiotensin II plus irbesartan (IRB, 1 μM) or PD98059 (10 μM) or angiotensin II plus PD123319 (1 μM). Results are the mean±s.e.mean of 5–18 experiments run in triplicate and are presented as arbitrary units (see text for details). Lines represent fitting of data points.
Angiotensin II type 1 receptor expression
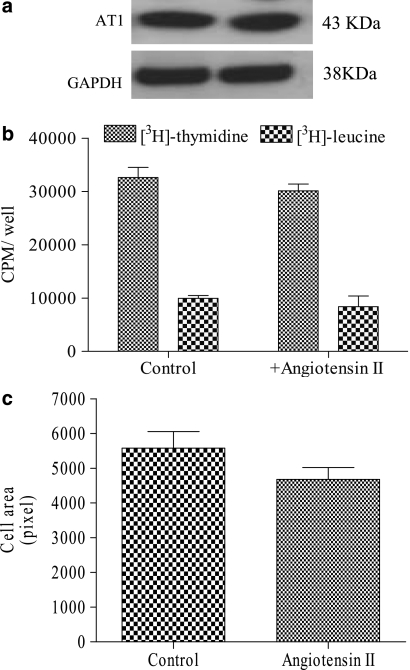
The expression level of the AT1 receptor did not change in cells exposed for 18 h to angiotensin II (Figure 4a).
Figure 4.
Angiotensin II type 1 receptor (AT1 receptor) levels and evaluation of hypertrophy in HL-1 cells exposed to angiotensin II. (a) Western blot analysis of AT1 receptors in lysates (30 μg of cell proteins) from control and angiotensin II-treated HL-1 cells. A representative experiment is shown, repeated twice with similar results. (b) DNA and protein labelled with [3H]thymidine and [3H]leucine were evaluated in cells not exposed (control) and exposed to angiotensin II (100 nM) as described in Methods. Results are expressed as counts per minute (CPM) per well and represent the mean±s.e.mean of three experiments run in triplicate. (c) Cell surface area was measured as described in Methods. The means±s.e.mean of the area of 80–120 cells per field analysed are presented.
Cell hypertrophy
As an index of hypertrophy, the total cell protein content, the cell surface area, the DNA and protein synthesis occurring during angiotensin II exposure were measured.
Our results show that exposure to angiotensin II did not modify either the total cell protein content (0.34±0.15 vs 0.28±0.08 mg ml−1 in control cells), the 18 h related DNA and protein synthesis (Figure 4b) or the cell surface area (Figure 4c). Altogether these findings suggest that angiotensin II does not induce hypertrophy in these cells.
The effect of blocking angiotensin II type 1 receptors on insulin resistance of the energy substrate uptake
As AT1 receptors are clearly present in our cells, we investigated the effect of blocking these receptors on the insulin resistance elicited by angiotensin II exposure. Irbesartan (1 μM), an AT1 antagonist, or PD98059 (10 μM), an inhibitor of ERK1/2 activation, were added during the exposure of the cells to angiotensin II. In addition, since the presence of AT2 receptors could not be excluded, experiments using an AT2 receptor antagonist were also run for comparison.
The effect of irbesartan and ERK1/2 inhibition on the basal and insulin-stimulated glucose and palmitate uptake
The presence of irbesartan and PD 98059 prevented most of the changes in energy substrate uptake induced by angiotensin II. In fact, under these conditions, cell palmitate uptake rate did not increase (Table 1) and, more importantly, cells regained some responsiveness to insulin. In fact, 100 nM insulin stimulated further (+41%) cell palmitate uptake; in terms of pmol, this represents 70% of the maximum insulin effect measured in control cells (Figure 3a).
As regard to glucose uptake, in the presence of irbesartan, the uptake induced by insulin was 0.58±0.08 pmol per 104 cells per min (+77% of basal), a value not significantly different from that induce by insulin in control cells (0.70±0.11 pmol per 104 cells per min; +90% of basal; Figure 2).
Similar results were obtained in cells exposed to PD98059. As with irbesartan, inhibition of ERK1/2 activation prevented the angiotensin II-dependent increase of cell palmitic acid uptake (Table 1). This allowed insulin to fuel the uptake further +37% (Figure 3b). As with irbesartan, in terms of pmol produced, this stimulation represented 69.7% of those produced by insulin in control cells (Figure 3a).
Again, the incubation with angiotensin II in the presence of PD98059 restored the maximum insulin stimulation on the glucose uptake (0.65±0.11 pmol per 104 cells per min; Figure 2b).
Conversely, in cells exposed to angiotensin II and PD123319, palmitic uptake remained high (see Table 1), and the effect of insulin on the uptake of both energy substrates uptakes was attenuated (Figures 2 and 3).
Discussion
Our results demonstrate that an 18 h exposure to angiotensin II has the potential to hamper the supply of energy substrates of HL-1 cardiomyocytes. In particular, angiotensin II exposure increased cell palmitate uptake rate and reduced the insulin-stimulating activity of insulin (insulin resistance) on the uptake of both glucose and palmitate. Furthermore, angiotensin II-related insulin resistance of substrate uptakes appeared to involve different mechanisms and occurred independently of cell hypertrophy. In our experimental conditions, in contrast to that observed in neonatal cardiomyocytes (Rajapurohitam et al., 2006), angiotensin II did not evoke any change either in the cell surface area or in DNA and protein synthesis. Taken together, these results suggest that the onset of this metabolic re-arrangement is an early indication of a predisposition to cardiomyopathies.
Our results confirm that HL-1 cells possess, in addition to glucose transport systems (Chaudary et al., 2002), a phloretin-sensitive mechanism involved in the intracellular transport of long-chain fatty acids. Both transporters respond similarly to insulin stimulation; insulin increased the rate of uptake of glucose and palmitate in a wortmannin-sensitive manner. While these results clearly indicate the involvement of GLUT4 and GLUT1 in the transport of glucose, they do not allow us to draw conclusions regarding the nature of the fatty acid carrier (Fischer et al., 1997). However, since FAT/CD36 is, so far, the only insulin-sensitive fatty acid carrier known (Chabowski et al., 2005), it is likely that it is involved in our experimental model.
Our data allow us to hypothesize that angiotensin II has different effects on FAT/CD36 and GLUT1/GLUT4 recruitment and, because of this, the insulin resistance induced by angiotensin II involves different mechanisms. In fact, cells exposed to angiotensin II showed the same GLUT1 activity as those not exposed, whereas the uptake of fatty acid by the exposed cells was increased maximally. This indicates that angiotensin II has ‘insulin-like' effects at FATCD36, stimulating its trafficking maximally and additionally stabilizing the transporters at the plasma membrane (Coort et al., 2004). As a consequence of this, insulin cannot further stimulate FAT/CD36 in cells that have been in contact with angiotensin II, even when angiotensin II is not present in cell medium. The reduction of insulin function resulted from changes in FAT/CD36 homeostasis occurring during the 18 h of exposure to angiotensin II. In addition, since cell surface area and protein synthesis did not change following angiotensin II, alterations in the sarcolemmic density of the transporters is unlikely to play a major role. However, whatever the mechanism is (saturation, desensitization for overactivation), a previous exposure to angiotensin II reduced the susceptibility of the fatty acid transporters to the effects of insulin.
In contrast, insulin resistance of glucose uptake occurred in the absence of any increase in the basal transport rate of this sugar. In most cells, the ubiquitous GLUT1 mainly accounts for the basal transport rate, but this transporter is much less responsive to insulin stimulation than GLUT4 (Fischer et al., 1997). Thus, the finding that angiotensin II exposure did not affect the basal transport of glucose (GLUT1) suggests that this hormone interferes preferentially with the insulin receptor cascade controlling GLUT4 recruitment in insulin-sensitive cells. A lack of an effect on GLUT1 rate is in line with the absence of hypertrophy in our cell model. Hypertrophic cardiomyocytes have been shown to exhibit a clear-cut increase in glucose consumption and GLUT1 activity (Montessuit and Thorburn, 1999).
If angiotensin II promotes cardiomyocyte insulin resistance, recovery of insulin sensitivity may be induced by drugs interfering with the production or effects of angiotensin II. Our results suggest that this is the case. The insulin resistance of glucose uptake caused by angiotensin II was removed or significantly reduced by inhibiting the AT1 cascade. Alterations due to angiotensin II exposure were similarly reversed by blocking AT1 receptors with irbesartan or by inhibiting ERK1/2 activation, a downstream effector of the receptor cascade (Izawa et al., 2005). Moreover, irbesartan and PD98059 prevented the angiotensin II stimulation of cell fatty acid uptake confirming that this effect is under the control of AT1. However, in contrast to glucose uptake, the reduction of insulin stimulation of fatty acid uptake was not completely attenuated by these drugs but significantly reduced. The reason for this difference is unknown at the present and different hypotheses have been proposed, such as inappropiate irbesartan concentration, angiotensin II receptor-independent effects, type 2 receptor effects unmasked by AT1 blockade or different pools of FATCD36 in cell populations, alteration of the cycling–recycling rate of FAT/CD36 at the plasma membrane. Moreover, while the involvement of ERK1/2 in reducing insulin-dependent glucose uptake has been described previously in vascular cells (Izawa et al., 2005), this is the first time that this kinase has been shown to play a role in the docking of both glucose and palmitic acid transporters in cardiomyocytes. This suggests that similar intracellular mechanisms govern the cycling of GLUT4 and FAT/CD36 and further supports the hypothesis that they coexist in the same vesicles (Luiken et al., 2002a).
On the whole, prolonged exposure to angiotensin II causes metabolic responses in HL-1 cells that are largely—if not solely—dependent on AT1 receptors. On the basis of the present results, it is unlikely that AT2 receptors are involved in the control of insulin-mediated modulation of substrate uptake.
In conclusion, our data demonstrate that, in addition to hyperglycemia (Fang et al., 2004), angiotensin II may be key factor in the onset of cardiac insulin resistance, an early metabolic defect leading to the occurrence of metabolic adaptations in the myocardium that progressively worsen its performance (Chen et al., 1984).
Angiotensin II, by hampering insulin-regulated fatty acid (that is, FAT/CD36) and glucose transport, is a probable candidate for modifying the insulin response of energy substrates in cardiomyocytes. Quite obviously, myocardial insulin-induced glucose disposal can hardly be of relevance for carbohydrate metabolism in the whole body; however, the onset of myocardial insulin resistance may play a crucial role in the development of cardiomyopathies.
Our observations in HL-1 cardiomyocytes have all the limitations of an in vitro study, but our results identify a direct role of AT1 blockers in removing or ameliorating the angiotensin II-dependent dysregulation of energy substrate supply. The finding that irbesartan did not exert any effect per se (that is, in the absence of angiotensin II), strongly indicates that it opposes the adverse metabolic effects elicited by angiotensin II by blocking AT1 receptors. Hence, these findings support a local effect of angiotensin II and indicate an additional mechanism involved in the cardiovascular protection offered by AT1 blockers
Acknowledgments
This work was supported by grants from Telethon (GGP05093). We thank Bristol Meyer Squibb for providing irbesartan.
Abbreviations
- AT1
angiotensin II type 1 receptor
- AT2
angiotensin II type 2 receptor
- CPM
counts per minute
- ERK1/2
extracellular signal-regulated kinase
- FAT/CD36
fatty acid transporter, insulin-sensitive
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GLUT1
type 1 glucose transporter
- GLUT4
type 4 glucose transporter
- HL-1 cells
immortalized cardiomyocytes
Conflict of interest
The authors state no conflict of interest.
References
- Bani D, Giannini L, Ciampa A, Masini E, Suzuki Y, Menegazzi M, et al. Epigallocatechin-3-gallate reduces allergen-induced asthma-like reaction in sensitized guinea pigs. J Pharmacol Exp Ther. 2006;317:1002–1011. doi: 10.1124/jpet.106.102178. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJ, Glatz JF. Regulation of fatty acid transport and membrane transporters in health and disease. Mol Cell Biochem. 2002;239:181–192. [PubMed] [Google Scholar]
- Chabowski A, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, et al. The subcellular compartmentation of fatty acid transporters is regulated differently by insulin and by AICAR. FEBS Lett. 2005;579:2428–2432. doi: 10.1016/j.febslet.2004.11.118. [DOI] [PubMed] [Google Scholar]
- Chaudary N, Shuralyova I, Liron T, Sweeney G, Coe IR. Transport characteristics of HL-1 cells: a new model for the study of adenosine physiology in cardiomyocytes. Biochem Cell Biol. 2002;80:655–665. doi: 10.1139/o02-143. [DOI] [PubMed] [Google Scholar]
- Chen V, Ianuzzo CD, Fong BC, Spitzer JJ. The effects of acute and chronic diabetes on myocardial metabolism in rats. Diabetes. 1984;33:1078–1084. doi: 10.2337/diab.33.11.1078. [DOI] [PubMed] [Google Scholar]
- Clarke TC, Thomas D, Petersen JS, Evans WH, Martin PEM. The antiarrhythmic peptide rotigaptide (ZP123) increases gap junction intercellular communication in cardiac myocytes and HeLa cells expressing connexin. Br J Pharmacol. 2006;147:486–495. doi: 10.1038/sj.bjp.0706631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coort SL, Luiken JJ, Van der Vusse GJ, Bonen A, Glatz JF. Increased FAT (fatty acid translocase)/CD36-mediated long-chain fatty acid uptake in cardiac myocytes from obese Zucker rats. Biochem Soc Trans. 2004;32:83–85. doi: 10.1042/bst0320083. [DOI] [PubMed] [Google Scholar]
- Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–567. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Claycomb WC, Wu G. Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic. J Biol Chem. 2004;279:41077–41084. doi: 10.1074/jbc.M405988200. [DOI] [PubMed] [Google Scholar]
- Fischer Y, Thomas J, Sevilla L, Munoz P, Becker C, Holman G, et al. Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes. Evidence of the existence of different intracellular GLUT4 vesicle populations. J Biol Chem. 1997;272:7085–7092. doi: 10.1074/jbc.272.11.7085. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Davies SS, Nakajima T, Ong BH, Kupershmidt S, Fessel J, et al. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ Res. 2005;97:1262–1269. doi: 10.1161/01.RES.0000195844.31466.e9. [DOI] [PubMed] [Google Scholar]
- Izawa Y, Yoshizumi M, Fujita Y, Ali N, Kanematsu Y, Ishizawa K, et al. ERK1/2 activation by angiotensin II inhibits insulin-induced glucose uptake in vascular smooth muscle cells. Exp Cell Res. 2005;308:291–299. doi: 10.1016/j.yexcr.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Juan CC, Chien Y, Wu LY, Yang WM, Chang CL, Lai YH, et al. Angiotensin II enhances insulin sensitivity in vitro and in vivo. Endocrinology. 2005;146:2246–2254. doi: 10.1210/en.2004-1136. [DOI] [PubMed] [Google Scholar]
- Lerch M, Teuscher AU, Beissner P, Schneider M, Shaw SG, Weidmann P. Effects of angiotensin II-receptor blockade with losartan on insulin sensitivity, lipid profile, and endothelin in normotensive offspring of hypertensive parents. J Cardiovasc Pharmacol. 1998;31:576–580. doi: 10.1097/00005344-199804000-00016. [DOI] [PubMed] [Google Scholar]
- Luiken JJ, Bonen A, Glatz JF. Cellular fatty acid uptake is acutely regulated by membrane-associated fatty acid-binding proteins. Prostaglandins Leukot Essent Fatty Acids. 2002a;67:73–78. doi: 10.1054/plef.2002.0401. [DOI] [PubMed] [Google Scholar]
- Luiken JJ, Coort SL, Koonen DP, van der Horst DJ, Bonen A, Zorzano A, et al. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch. 2004;448:1–15. doi: 10.1007/s00424-003-1199-4. [DOI] [PubMed] [Google Scholar]
- Luiken JJ, Koonen DP, Willems J, Zorzano A, Becker C, Fischer Y, et al. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes. 2002b;51:3113–3119. doi: 10.2337/diabetes.51.10.3113. [DOI] [PubMed] [Google Scholar]
- Montessuit C, Thorburn A. Transcriptional activation of the glucose transporter GLUT1 in ventricular cardiac myocytes by hypertrophic agonists. J Biol Chem. 1999;274:9006–9012. doi: 10.1074/jbc.274.13.9006. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Opie LH. The metabolic vicious cycle in heart failure. Lancet. 2004;364:1733–1734. doi: 10.1016/S0140-6736(04)17412-6. [DOI] [PubMed] [Google Scholar]
- Palanivel R, Eguchi M, Shuralyova I, Coe I, Sweeney G. Distinct effects of short- and long-term leptin treatment on glucose and fatty acid uptake and metabolism in HL-1 cardiomyocytes. Metabolism. 2006;55:1067–1075. doi: 10.1016/j.metabol.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Raimondi L, De Paoli P, Mannucci E, Lonardo G, Sartiani L, Banchelli G, et al. Restoration of cardiomyocyte functional properties by angiotensin II receptor blockade in diabetic rats. Diabetes. 2004;53:1927–1933. doi: 10.2337/diabetes.53.7.1927. [DOI] [PubMed] [Google Scholar]
- Rajapurohitam V, Javadov S, Purdham DM, Kirshenbaum LA, Karmazyn M. An autocrine role for leptin in mediating the cardiomyocyte hypertrophic effects of angiotensin II and endothelin-1. J Mol Cell Cardiol. 2006;41:265–274. doi: 10.1016/j.yjmcc.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Sartiani L, Bochet P, Cerbai E, Mugelli A, Fishmeister R. Functional expression of the hyperpolarization-activated, non selective cation current I(f) in immortalized HL-1 cardiomyocytes. J Physiol. 2002;545:81–92. doi: 10.1113/jphysiol.2002.021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder RE. Optimizing therapeutic strategies to achieve renal and cardiovascular risk reduction in diabetic patients with angiotensin receptor blockers. J Hypertens. 2005;23:905–911. doi: 10.1097/01.hjh.0000166826.17570.86. [DOI] [PubMed] [Google Scholar]
- Stanley WC. Ranolazine: new approach for the treatment of stable angina pectoris. Expert Rev Cardiovasc Ther. 2005;3:821–829. doi: 10.1586/14779072.3.5.821. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- White SM, Costantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle structure and function. Am J Physiol Heart Circ Physiol. 2004;286:H823–H829. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]