Abstract
Background and purpose:
Diabetes-associated vascular dysfunction contributes to increased cardiovascular risk. We investigated whether the phosphodiesterase-5 inhibitor sildenafil would improve vascular function in diabetic rats.
Experimental approach:
Male Wistar rats were injected with streptozotocin (50 mg kg-1, i.v.) to induce insulin-deficient diabetes. Direct effects of sildenafil as well as modification of endothelium-dependent and -independent vasorelaxation were investigated in vitro. The effects of acute and chronic (2 week) treatment in vivo of sildenafil on vascular function were also characterized in isolated aortic segments in organ bath chambers 4 weeks after diabetes induction.
Key results:
Sildenafil induced a concentration-dependent vasorelaxation, which was attenuated by the nitric oxide (NO) synthase inhibitor, NG-nitro-L-arginine. Acetylcholine-induced endothelium-dependent as well as endothelium-independent relaxation induced by the NO donor, DEA-NONOate, was significantly reduced in aortae from diabetic rats. Incubation with sildenafil in vitro normalized both endothelium-dependent and -independent relaxation in aortae from diabetic rats. Acute as well as chronic in vivo treatment with sildenafil resulted in enhanced endothelium-dependent and -independent vasorelaxation. Superoxide formation was increased in diabetes, associated with enhanced membrane expression of the NAD(P)H oxidase subunit gp91phox and Rac, which were both reduced by chronic treatment with sildenafil.
Conclusions and implications:
We demonstrate that sildenafil treatment rapidly and chronically improves vascular relaxation in diabetic rats. Treatment with sildenafil might provide a similarly beneficial effect in diabetic patients.
Keywords: endothelial function, nitric oxide, diabetes, oxygen radicals, phosphodiesterase-5
Introduction
Diabetes is strongly associated with various cardiovascular diseases, which are the primary cause of morbidity and mortality among patients with diabetes, accounting for more than 80% of deaths (Tschoepe and Menart-Houtermans, 2002). Diabetes alone confers a long-term cardiovascular risk similar to that observed among non-diabetic patients with prior myocardial infarction (Haffner et al., 1998). Patients with diabetes have early development of abnormal endothelial function, aggressive atherosclerosis and adverse arterial remodelling (Tschoepe and Menart-Houtermans, 2002). The risk for cardiovascular disease is already substantially elevated before diagnosis of diabetes (Hu et al., 2002) and the progression of atherosclerosis is accelerated in diabetics (Wagenknecht et al., 2003). Reduced nitric oxide (NO) bioavailability and abundant formation of reactive oxygen species (ROS) within the vascular wall are the key determinants in endothelial dysfunction resulting in an imbalance between NO and ROS. Impaired endothelial function has been described in very early stages of diabetes mellitus and hyperglycaemia, and decreased insulin-sensitivity as well as increased oxidative stress have been proposed as possible contributors (reviewed by Guerci et al., 2001). The increased amount of ROS and the lack of NO are deleterious to smooth muscle cell function.
Endothelium-derived NO activates soluble guanylyl cyclase in smooth muscle cells resulting in enhanced cGMP concentrations and vasorelaxation. Sildenafil is a potent and selective inhibitor of phosphodiesterase (PDE)-5, and thus decreases hydrolysis of cGMP. Sildenafil enhances NO/cGMP-mediated signalling in the corpus cavernosum and is approved for treatment of erectile dysfunction. As already noted for endothelial dysfunction, erectile dysfunction is common in diabetic patients and both disorders are clearly linked. Inhibiting cGMP degradation by sildenafil might be a rational approach to treat patients with diabetes, coronary artery disease or heart failure (Gross, 2005). Sildenafil dilates epicardial coronary arteries, improves endothelial dysfunction and inhibits platelet activation in patients with coronary artery disease (Halcox et al., 2002), and acutely enhances flow-mediated vasodilation in patients with heart failure (Hryniewicz et al., 2005). Similarly, PDE-5 inhibition with sildenafil prevents the smoking-induced decrease in flow-mediated vasodilation and increases impaired NO bioactivity (Kimura et al., 2003; Vlachopoulos et al., 2004).
In the present study, we demonstrate that sildenafil improves NO-mediated vasorelaxation in vitro as well as following acute and chronic treatment in vivo, in an experimental model of diabetes, whereby chronic treatment significantly reduces oxidative stress.
Methods
Animals
These investigations conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Male Wistar rats (250–300 g, obtained from Harlan-Winkelmann, Borchen, Germany) were housed in temperature-controlled cages (20–22 °C) with a 12-h light–dark cycle, and given free access to water and formulated diets.
Induction of diabetes by streptozotocin injection
A single dose streptozotocin (STZ) regimen was used to induce pancreatic islet cell destruction and persistent hyperglycaemia. STZ (10 mg ml−1, Sigma, Deisenhofen, Germany) was freshly dissolved in sterile sodium citrate buffer (25 mM, pH 4.5) and used within 10 min. Rats received a single 50 mg kg−1 intravenous injection of STZ or citrate buffer (control). Blood glucose was monitored using a one-touch blood glucose meter (Ascensia Elite, Bayer-Vital GmbH, Leverkusen, Germany). Hyperglycaemia was defined as a random blood glucose level >20 mM at 2 and 4 weeks after injection. Diabetic rats were randomized to placebo or sildenafil (5 mg kg−1 day−1 by gavage; Pfizer) at day 14. Two weeks later, vasomotor function was measured to assess chronic effects of sildenafil. The last dose of sildenafil was given on the day before the experiments were performed. Acute in vivo effects of sildenafil were investigated in diabetic animals 4 weeks after STZ injection 2 h following one single application of the study drug. In vitro effects of sildenafil were evaluated in aortae from 4-week diabetic as well as healthy control rats in separate experiments.
Vascular reactivity studies
The descending thoracic aorta was dissected under deep anaesthesia induced by isoflurane following removal of the heart and cleaned of connective tissue. One section was used for measurement of superoxide production, while the other was cut into 3 mm rings, which were mounted in an organ bath (FMI, Seeheim, Germany) for isometric force measurements. The rings were equilibrated for 30 min under a resting tension of 2 g in oxygenated (95% O2; 5% CO2) Krebs–Henseleit solution (NaCl 118 mM, KCl 4.7 mM, MgSO4 1.2 mM, CaCl2 1.6 mM, KH2PO4 1.2 mM, NaHCO3 25 mM, glucose 12 mM; pH 7.4, 37 °C) containing diclofenac (1 μM). All vascular reactivity studies were performed in vessels with endothelium. Rings were repeatedly contracted by KCl (with a maximum of 100 mM) until reproducible responses were obtained. The relaxant response to cumulative concentrations of acetylcholine was assessed after preconstriction with phenylephrine (0.3–3.0 μM) to comparable levels (in g: control: 1.93±0.04, STZ-placebo: 1.89±0.06, STZ-sildenafil: 1.85±0.06). Furthermore, relaxant responses to the endothelium-independent vasodilator 2-(N,N-diethylamino)-diazenolate-2-oxide (DEA-NONOate; Alexis Biochemicals, San Diego, CA, USA) were determined after preconstriction with phenylephrine (in g: control: 2.05±0.06, STZ-placebo: 2.13±0.05, STZ-sildenafil: 2.11±0.06) in the presence of NG-nitro-L-arginine (L-NNA, 100 nM).
In separate in vitro experiments, the effect of cumulative concentrations of sildenafil as well as preincubation with sildenafil (100 nM, 5 min) prior to endothelium-dependent and -independent vasorelaxation in preconstricted aortic rings was tested.
Measurement of superoxide anion formation
Vascular superoxide formation was measured using lucigenin-enhanced chemiluminescence (Schäfer et al., 2003). The light reaction between superoxide and lucigenin (5 μM) was detected in a luminometer (Wallac, Freiburg, Germany) during incubation of rings in a HEPES-modified Krebs buffer (pH 7.40).
The oxidative fluorescent dye, hydroethidine, was used to evaluate in situ production of superoxide as described previously (Schäfer et al., 2003). Unfixed frozen ring segments were cut into 10-μm-thick sections and placed on a glass slide. Hydroethidine (2 μM) was topically applied to each tissue section and coverslipped. Slides were incubated in a light-protected humidified chamber at 37 °C for 30 min.
Stained sections were investigated using a Nikon Eclipse E600 microscope equipped with a C1 confocal scanning head and a 20-fold oil immersion objective. Pictures were acquired and prepared for presentation using the EZ-C1 3.00 software from Nikon. Image analysis was performed using Image J (Rasband WS, ImageJ, US National Institutes of Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij/, 1997–2006.). Aortic rings from diabetic animals and control tissues were processed and imaged in parallel. Microscope settings and laser intensities were identical for acquisition of images from diabetic and control specimens.
Immunoblotting
Aorta samples with endothelium were homogenized in ice-cold Tris-buffer (30 mM Tris-HCl, pH 7.5, 10 mM EGTA, 5 mM EDTA, 1 mM dithiothreitol and 250 mM sucrose). The homogenates were centrifuged at 8000 g for 10 min at 4 °C. To obtain membranous fractions, the supernatants were then centrifuged at 120 000 g for 45 min at 4 °C. The resulting pellets were resuspended in Tris-buffer and solubilized with 1% Triton X-100 and 1% sodium cholate. Membranous extracts were mixed with sample loading buffer (B7703, BioLabs, Frankfurt, Germany) and separated on 10% sodium dodecyl sulphate (SDS)-polyacrylamide gel under reducing conditions. Proteins were electrotransferred onto polyvinylidine difluoride membrane (Immun-Blot 0.2 μm, Bio-Rad, Munich, Germany). The bands were detected using chemiluminescence assay (ECL+Plus, Amersham, Munich, Germany). Primary antibodies used recognize: gp91phox (611414, BD Biosciences Pharmingen, Heidelberg, Germany), p47phox (610355, BD Bioscience Pharmingen), Rac (05–389, Upstate antibodies) and β-actin (4967, Cell Signaling Technology, Frankfurt, Germany).
Low temperature SDS-polyacrylamide gel electrophoresis
Aorta extracts were mixed with 3 × SDS sample buffer (187.5 mM Tris-HCl (pH 6.8), 6% (w/v) SDS, 30% glycerol and 0.03% (w/v) bromophenol blue and 15% v/v 2-mercaptoethanol) at 0 °C. Samples were loaded on 7.5% polyacrylamide gels and subjected to electrophoresis. Gels and buffers were cooled to 4 °C prior to electrophoresis and the buffer tank placed in an ice-bath during electrophoresis. Endothelial NO synthase (eNOS) dimer/monomer protein was detected by western blot analysis using an anti-eNOS antibody (N-30020, Transduction Laboratories, Lexington, KY, USA).
Immunohistochemistry
For immunohistochemical analysis, frozen 5 μm sections of aorta were stained using primary antibody against CD68 (MCA341R, Serotec, Disseldorf, Germany). Briefly, sections were fixed in cold acetone for 5 min followed by pretreatment with 0.3% hydrogen peroxide for 20 min to inhibit endogenous peroxidase activity. Subsequently, sections were blocked with 2% goat serum for 30 min and incubated with the primary antibody for 1 h at room temperature. After rinsing with PBS, the sections were incubated for 30 min with a biotinylated secondary antibody (550337, BD Biosciences Pharmingen), followed by incubation with Streptavidin-horseradish peroxidase (550946, BD Biosciences Pharmingen) and diaminobenzidine (SK4100, Vector Linaris, Wertheim-Bettingen, Germany). Sections were counterstained with eosin.
Statistics
Values shown are means±s.e.mean for curves and bar graphs. Relaxant responses are given as percentage relaxation relative to the preconstriction level. Statistical analysis was performed using Prism 4 (GraphPad Software, San Diego, CA, USA) by repeated measures analysis of variance followed by Tukey–Kramer multiple comparisons test. Superoxide formation was analysed by analysis of variance followed by a Tukey post hoc test where appropriate; P<0.05 was considered statistically significant.
Materials
Unless otherwise stated, all chemicals were obtained from Sigma (Deisenhofen, Germany) in the highest purity available.
Results
Diabetes-induced increases in blood glucose (control: 10.5±1.9 mM, STZ-placebo: 32.0±0.6 mM, n=15 per group, P<0.01) were unaffected by sildenafil treatment of diabetic animals (32.2±0.7 mM), while the reduction in body weight (control: 336±16 g, STZ-placebo: 252±9 g, n=15 per group, P<0.01) was slightly further lowered following treatment with sildenafil (204±6 g, n=15, P<0.05).
Vasomotor function—in vitro effects of sildenafil
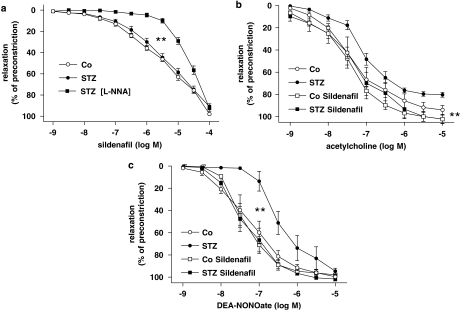
A concentration response to sildenafil in vitro was obtained in isolated preconstricted aortic rings from diabetic and non-diabetic rats. Sildenafil concentration-dependently induced vasorelaxation of preconstricted aortic rings. The concentration response to sildenafil was significantly attenuated in aortic rings following preincubation with the NO synthase inhibitor L-NNA, indicating that enhanced endogenous NO signalling accounts for the majority of sildenafil-induced vasorelaxation at lower and medium concentrations in both groups (Figure 1a).
Figure 1.
(a) Sildenafil-induced concentration-dependent relaxation in the absence and presence of the NO synthase inhibitor L-NNA(100 nM). (b) In vitro effects of sildenafil (100 mM, 5 min) on acetylcholine-induced, endothelium-dependent and (c) endothelium-independent vasorelaxation by the NO donor DEA-NONOate in aorta from control or diabetic (STZ) rats. Data are means±s.e.mean from 10 to 15 experiments, **P<0.01 vs STZ. DEA-NONOate, 2-(N,N-diethylamino)-diazenolate-2-oxide; L-NNA, NG-nitro-L-arginine; STZ, streptozotocin.
Acute in vitro incubation with sildenafil at a threshold concentration (100 mM) enhanced acetylcholine-induced, NO-mediated, endothelium-dependent vasorelaxation of preconstricted aortic rings from diabetic rats to levels comparable to vessels from healthy rats (Figure 1b). This effect seemed to be predominantly attributable to enhanced smooth muscle sensitivity to NO, because sildenafil also markedly enhanced endothelium-independent vasorelaxation to the NO donor DEA-NONOate, which was significantly impaired in aortic rings from diabetic rats (Figure 1c).
Vasomotor function—acute in vivo effects of sildenafil
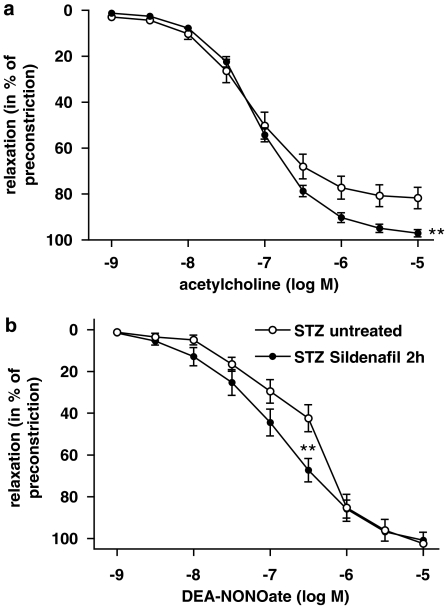
For assessment of acute effects of sildenafil in vivo, aortic rings were harvested from diabetic rats 2 h after a single application of sildenafil (5 mg kg−1 body weight). Endothelium-dependent as well as -independent vasorelaxation was significantly improved (Figure 2).
Figure 2.
(a) Acute in vivo effects of one single dose of sildenafil (5 mg kg−1 by gavage, 2 h before harvesting the aorta) on acetylcholine-induced, endothelium-dependent and (b) endothelium-independent vasorelaxation stimulated by the NO donor DEA-NONOate in aortic rings from control and diabetic (STZ) rats. Data are means±s.e.mean from 10 to 15 experiments, **P<0.01 vs STZ untreated. DEA-NONOate, 2-(N,N-diethylamino)-diazenolate-2-oxide; STZ, streptozotocin.
Vasomotor function—chronic in vivo effects of sildenafil
In addition to the above-mentioned direct (Figure 1) and acute (Figure 2) effects of sildenafil on vascular function, we assessed the impact of chronic sildenafil treatment (2 weeks) with the last dose given >24 h before the experiments. Acetylcholine-induced endothelium-dependent vasorelaxation, which was impaired in diabetic rats, was significantly improved after chronic treatment with sildenafil (Figures 3a and c).
Figure 3.
(a) Concentration–response curves for endothelium-dependent vasorelaxation elicited by cumulative application of acetylcholine and (b) endothelium-independent relaxation by incremental concentrations of DEA-NONOate in isolated aortic rings from control rats and diabetic rats (STZ) treated either with placebo or sildenafil (5 mg kg−1 day−1 for 2 weeks). Respective EC50 values were determined for every single concentration–response curve of acetylcholine (c) and DEA-NONOate (d). Data are means±s.e.mean from 10 to 15 different animals, **P<0.01 vs Control; ##P<0.01 vs STZ-placebo. DEA-NONOate, 2-(N,N-diethylamino)-diazenolate-2-oxide; STZ, streptozotocin.
The concentration–response curve for the NO-donor DEA-NONOate, which was used to assess endothelium-independent vasorelaxation, was shifted to the right in aortae from diabetic rats and was normalized after chronic treatment with sildenafil as indicated by the respective EC50 values (Figures 3b and d).
Vascular ROS
One very important contributor to reduced sensitivity to NO in diabetes is oxidative stress. Aortic superoxide production was therefore assessed by lucigenin-enhanced chemiluminescence. In aortic tissues, luminescence signals were significantly increased in rats with diabetes and significantly reduced by chronic treatment with sildenafil (Figure 4a). In contrast, direct in vitro incubation with sildenafil (100 nM) did not inhibit superoxide generation (superoxide generation: STZ+buffer 0.91±0.11 c.p.m. mg−1 dry weight; STZ+sildenafil 0.90±0.18 c.p.m. mg−1 dry weight). Expression of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunits gp91phox and p47phox was significantly increased in aortae from diabetic rats. gp91phox expression was significantly reduced by chronic treatment with sildenafil (Figure 4b), whereas the increased p47phox expression was not changed (data not shown). Translocation of the small G protein Rac-1 to the plasma membrane activates NADPH oxidase. There was a trend towards increased Rac-1 expression in the aortic membrane in diabetes vs control, and sildenafil significantly reduced Rac-1 expression changes (Figure 4c). Confocal scanning microscopy images, which utilized hydroethidium to visualize superoxide formation, indicated increased ROS production throughout the vascular wall in aortic rings from diabetic vs control animals, and markedly reduced signals in rats treated with sildenafil as compared to untreated diabetic animals (Figure 4d).
Figure 4.
Superoxide production in aortic rings from control rats and diabetic rats (STZ) treated either with placebo or sildenafil (5 mg kg−1 day−1 for 2 weeks) was detected and quantified by lucigenin-enhanced chemiluminescence (a). Expression of the NADPH oxidase subunit gp91phox (b) and membrane expression of the small G protein Rac-1, a NADPH oxidase regulator (c), were assessed by western blot. Mean±s.e.mean from 6 to 10 separate experiments. *P<0.05 vs Control; #P<0.05, ##P<0.01 vs STZ-placebo. Confocal microscopy of 10-μm-thick aortic sections incubated with the fluorescent dye hydroethidium to visualize superoxide formation throughout the vascular wall (d). NADPH, nicotinamide adenine dinucleotide phosphate; STZ, streptozotocin.
In addition to these mechanisms demonstrating ROS production throughout the vascular wall, endothelium-specific increased superoxide formation by uncoupled eNOS with reduced dimer/monomer ratio has been observed in diabetes (Alp et al., 2003; Cai et al., 2005). We found that eNOS dimer/monomer ratio was significantly reduced in the aortae of diabetic animals, which was reversed by chronic treatment with sildenafil (Figure 5a).
Figure 5.
Uncoupling of eNOS in aortic rings from control rats and diabetic rats (STZ) treated either with placebo or sildenafil (5 mg kg−1 day−1 for 2 weeks) was assessed by eNOS dimer/monomer ratio following low temperature SDS-PAGE (a) *P<0.05 vs Control; #P<0.05 vs STZ-placebo. Macrophage infiltration into aortae was examined by immunohistochemistry for CD68+ cells (b). eNOS, endothelial NO synthase; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; STZ, streptozotocin.
To exclude superoxide formation by infiltrating macrophages, we performed immunohistochemistry to detect CD68+ cells. We did not find significant infiltration of aortae from diabetic animals by CD68+ cells (Figure 5b).
Discussion
In this study, we demonstrate that acute and chronic treatment with the PDE-5 inhibitor sildenafil in experimental diabetes improves vascular function and reduces superoxide formation, suggesting a potential general mechanism of improved signalling through the NO/cGMP-signalling cascade.
Patients with erectile dysfunction in the absence of clinical cardiovascular disease have a deficient endothelium-dependent and -independent vasodilation occurring before the development of other overt functional or structural systemic vascular disease (Kaiser et al., 2004). Erectile dysfunction in diabetic men correlates with endothelial dysfunction and reduced NO bioavailability/activity might provide a unifying explanation (De Angelis et al., 2001). Based on these studies, we determined whether sildenafil, which is an approved therapy for erectile dysfunction, beneficially modulates endothelial function in experimental diabetes. We firstly demonstrated that sildenafil exerts a similar concentration-dependent vasorelaxation in isolated aortic rings from diabetic and healthy rats. Prior inhibition of NO synthase by L-NNA showed that this direct effect of sildenafil was truly NO dependent, at least at low and medium concentrations of sildenafil. Based on that concentration response, we performed further experiments, in which a threshold concentration of sildenafil was added before stimulating endogenous NO release or supplementing exogenous NO. Sildenafil enhanced acetylcholine-induced NO-mediated vasorelaxation in aortic rings from diabetic rats. Similarly, the impaired responsiveness to exogenous NO in aortic rings from diabetic rats was significantly improved by pre-incubation with sildenafil. These effects were also observed when sildenafil was given in vivo 2 h before the in vitro experiments. Thus, acute application of sildenafil is sufficient to enhance the impaired vascular NO/cGMP signalling in diabetes.
Chronic treatment of diabetic rats with sildenafil resulted in a significant improvement of endothelium-dependent as well as -independent vasorelaxation indicating improved signalling through the NO/cGMP-signalling cascade, even more than 24 h after the last dosing. A recent study in hypertensive patients demonstrated that sildenafil had the potential for chronic treatment in addition to its specific local indication as acute supportive treatment in erectile dysfunction (Oliver et al., 2006). Endothelial dysfunction is a common feature in cardiovascular diseases characterized by an imbalance between NO and ROS. Oxidant stress is a major cause of reduced endothelial NO bioavailability in diabetes and is involved in the pathogenesis and progression of diabetic tissue damage (Guzik et al., 2002; Landmesser et al., 2006). Increased expression of NAD(P)H oxidase subunits, enhanced NAD(P)H oxidase and protein kinase C activity as well as increased levels of the endogenous eNOS inhibitor asymmetric dimethylarginine result in enhanced oxidative stress and reduced NO bioavailability in diabetes (Hink et al., 2001). Treatment with sildenafil reduced lipid peroxidation and increased total antioxidant capacity in plasma of diabetic rats (Milani et al., 2005). Sildenafil inhibited NAD(P)H oxidase-dependent superoxide formation in corpus cavernosum from hypercholesterinaemic rabbits thereby improving smooth muscle relaxation even in the absence of endogenous NO release (Shukla et al., 2005). Sildenafil also reduced superoxide formation and NAD(P)H oxidase expression in cultured porcine pulmonary artery endothelial cells as well as corpus cavernosum smooth muscle cells (Koupparis et al., 2005; Muzaffar et al., 2005a, 2005b). We demonstrate that chronic treatment with sildenafil reduces the enhanced superoxide formation and expression of the NAD(P)H oxidase subunit gp91phox in aortae from diabetic rats. eNOS becomes uncoupled under certain circumstances such as high glucose challenge to produce superoxide instead of NO (Bauersachs and Schäfer, 2005; Forstermann and Munzel, 2006). However, a major contribution of uncoupled eNOS to superoxide formation in the present experimental condition is unlikely. Enhanced formation of superoxide in all layers of the aortic wall together with the increased expression of certain NAD(P)H oxidase subunits and expression changes of Rac-1 favours NAD(P)H oxidase as the major source of ROS. This is further supported by the fact that L-NNA did not significantly reduce superoxide formation in aortae from diabetic rats (data not shown). Nevertheless, there was a significant shift in eNOS dimer/monomer ratio towards monomer in aortae from diabetic rats as previously reported in mouse aortae (Alp et al., 2003; Cai et al., 2005), which was reversed by chronic treatment with sildenafil.
The reaction product of superoxide and NO, peroxynitrite, may compensate in part for the loss of vascular NO bioavailability in diabetes (Zobali et al., 2001). It has recently been demonstrated that peroxynitrite is capable of inducing cGMP-dependent vasorelaxation, which can be enhanced by the PDE-5 inhibitor sildenafil (Li et al., 2005). Thereby, PDE-5 inhibition would not only result in the enhancement of the residual truly NO-mediated signal but also amplify the peroxynitrite-induced vasorelaxation.
In summary, chronic treatment with sildenafil reduced superoxide formation and enhanced smooth muscle reactivity to exogenous NO in our study, thereby reversing the deleterious events leading to vascular dysfunction, and improving vascular homoeostasis. Chronic regular treatment with sildenafil has recently been shown to constitute an effective antihypertensive therapy and may also be a valuable tool to improve diabetic vascular dysfunction. However, its use for chronic treatment in clinical practice is limited by its short duration of action requiring multiple doses per day (Oliver et al., 2006) and this pharmacological approach is currently only applied for diabetic patients with a very well-defined subset of endothelial dysfunction, namely erectile dysfunction (Basu and Ryder, 2004; Pegge et al., 2006).
Acknowledgments
We thank Meike Leutke for expert technical assistance. The study was partially supported by a research grant from Pfizer and by the Deutsche Forschungsgemeinschaft (SFB 355 and 688 to T.R.).
Abbreviations
- DEA-NONOate
2-(N,N-diethylamino)-diazenolate-2-oxide
- L-NNA
NG-nitro-L-arginine
- PDE
phosphodiesterase
- ROS
reactive oxygen species
- STZ
streptozotocin
Conflict of interest
The study was partially supported by a research grant from Pfizer.
References
- Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I over-expression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Ryder REJ. New treatment options for erectile dysfunction in patients with diabetes mellitus. Drugs. 2004;64:2667–2688. doi: 10.2165/00003495-200464230-00004. [DOI] [PubMed] [Google Scholar]
- Bauersachs J, Schäfer A. Tetrahydrobiopterin and eNOS dimer/monomer ratio—a clue to eNOS uncoupling in diabetes. Cardiovasc Res. 2005;65:768–769. doi: 10.1016/j.cardiores.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48:1933–1940. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- De Angelis L, Marfella MA, Siniscalchi M, Marino L, Nappo F, Giugliano F, et al. Erectile and endothelial dysfunction in Type II diabetes: a possible link. Diabetologia. 2001;44:1155–1160. doi: 10.1007/s001250100616. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Gross GJ. Sildenafil and endothelial dysfunction in humans. Circulation. 2005;111:721–723. doi: 10.1161/01.CIR.0000156407.59837.C1. [DOI] [PubMed] [Google Scholar]
- Guerci B, Bohme P, Kearney-Schwartz A, Zannad F, Drouin P. Endothelial dysfunction and type 2 diabetes. Part 2: altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab. 2001;27:436–447. [PubMed] [Google Scholar]
- Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- Halcox JP, Nour KR, Zalos G, Mincemoyer RA, Waclawiw M, Rivera CE, et al. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol. 2002;40:1232–1240. doi: 10.1016/s0735-1097(02)02139-3. [DOI] [PubMed] [Google Scholar]
- Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:e14–e22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- Hryniewicz K, Dimayuga C, Hudaihed A, Androne AS, Zheng H, Jankowski K, et al. Inhibition of angiotensin-converting enzyme and phosphodiesterase type 5 improves endothelial function in heart failure. Clin Sci (London) 2005;108:331–338. doi: 10.1042/CS20040266. [DOI] [PubMed] [Google Scholar]
- Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002;25:1129–1134. doi: 10.2337/diacare.25.7.1129. [DOI] [PubMed] [Google Scholar]
- Kaiser DR, Billups K, Mason C, Wetterling R, Lundberg JL, Bank AJ. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J Am Coll Cardiol. 2004;43:179–184. doi: 10.1016/j.jacc.2003.07.042. [DOI] [PubMed] [Google Scholar]
- Kimura M, Higashi Y, Hara K, Noma K, Sasaki S, Nakagawa K, et al. PDE5 inhibitor sildenafil citrate augments endothelium-dependent vasodilation in smokers. Hypertension. 2003;41:1106–1110. doi: 10.1161/01.HYP.0000068202.42431.CC. [DOI] [PubMed] [Google Scholar]
- Koupparis AJ, Jeremy JY, Muzaffar S, Persad R, Shukla N. Sildenafil inhibits the formation of superoxide and the expression of gp47 NAD(P)H oxidase induced by the thromboxane A2 mimetic, U46619, in corpus cavernosal smooth muscle cells. BJU Int. 2005;96:423–427. doi: 10.1111/j.1464-410X.2005.05643.x. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Harrison D, Drexler H. Oxidant stress−a major cause of reduced endothelial nitric oxide availability in cardiovascular disease. Eur J Clin Pharmacol. 2006;62:13–19. [Google Scholar]
- Li J, Li W, Altura BT, Altura BM. Peroxynitrite-induced relaxation in isolated rat aortic rings and mechanisms of action. Toxicol Appl Pharmacol. 2005;209:269–276. doi: 10.1016/j.taap.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Milani E, Nikfar S, Khorasani R, Zamani MJ, Abdollahi M. Reduction of diabetes-induced oxidative stress by phosphodiesterase inhibitors in rats. Comp Biochem Physiol C Toxicol Pharmacol. 2005;140:251–255. doi: 10.1016/j.cca.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Jeremy JY. Nicotinamide adenine dinucleotide phosphate oxidase: a promiscuous therapeutic target for cardiovascular drugs. Trends Cardiovasc Med. 2005a;15:278–282. doi: 10.1016/j.tcm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Muzaffar S, Shukla N, Srivastava A, Angelini GD, Jeremy JY. Sildenafil citrate and sildenafil nitrate (NCX 911) are potent inhibitors of superoxide formation and gp91phox expression in porcine pulmonary artery endothelial cells. Br J Pharmacol. 2005b;146:109–117. doi: 10.1038/sj.bjp.0706305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JJ, Melville VP, Webb DJ. Effect of regular phosphodiesterase type 5 inhibition in hypertension. Hypertension. 2006;48:622–627. doi: 10.1161/01.HYP.0000239816.13007.c9. [DOI] [PubMed] [Google Scholar]
- Pegge NC, Twomey AM, Vaughton K, Gravenort MB, Ramsey MW, Price DE. The role of endothelial dysfunction in the pathophysiology of erectile dysfunction in diabetes and in determining response to treatment. Diabet Med. 2006;23:873–878. doi: 10.1111/j.1464-5491.2006.01911.x. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Fraccarollo D, Hildemann S, Tas P, Ertl G, Bauersachs J. Addition of the selective aldosterone receptor antagonist eplerenone to ACE inhibition in heart failure: effect on endothelial function. Cardiovasc Res. 2003;58:655–662. doi: 10.1016/s0008-6363(03)00333-x. [DOI] [PubMed] [Google Scholar]
- Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur J Pharmacol. 2005;517:224–231. doi: 10.1016/j.ejphar.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Tschoepe D, Menart-Houtermans B.Diabetes mellitus Platelets 2002Academic Press: San Diego; 435–445.In: Michelson AD (ed). [Google Scholar]
- Vlachopoulos C, Tsekoura D, Alexopoulos N, Panagiotakos D, Aznaouridis K, Stefanadis C. Type 5 phosphodiesterase inhibition by sildenafil abrogates acute smoking-induced endothelial dysfunction. Am J Hypertens. 2004;17:1040–1044. doi: 10.1016/j.amjhyper.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Wagenknecht LE, Zaccaro D, Espeland MA, Karter AJ, O'Leary DH, Haffner SM. Diabetes and progression of carotid atherosclerosis: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol. 2003;23:1035–1041. doi: 10.1161/01.ATV.0000072273.67342.6D. [DOI] [PubMed] [Google Scholar]
- Zobali F, Cakici I, Karasu C. Effects of peroxynitrite on the reactivity of diabetic rat aorta. Pharmacology. 2001;63:58–64. doi: 10.1159/000056113. [DOI] [PubMed] [Google Scholar]