Abstract
The lower oesophageal sphincter (LOS) is a specialized region of the oesophageal circular smooth muscle that allows the passage of a swallowed bolus to the stomach and prevents the reflux of gastric contents into the oesophagus. The anatomical arrangement of the LOS includes semicircular clasp fibres adjacent to the lesser gastric curvature and sling fibres following the greater gastric curvature. Such anatomical arrangement together with an asymmetric intrinsic innervation and distinct proportion of neurotransmitters in both regions produces an asymmetric pressure profile. The LOS tone is myogenic in origin and depends on smooth muscle properties that lead to opening of L-type Ca2+ channels; however it can be modulated by enteric motor neurons, the parasympathetic and sympathetic extrinsic nervous system and several neurohumoral substances. Nitric oxide synthesized by neuronal NOS is the main inhibitory neurotransmitter involved in LOS relaxation. Different putative neurotransmitters have been proposed to play a role together with NO. So far, only ATP or related purines have shown to be co-transmitters with NO. Acetylcholine and tachykinins are involved in the LOS contraction acting through acetylcholine M3 and tachykinin NK2 receptors. Nitric oxide can also be involved in the regulation of LOS contraction. The understanding of the mechanisms that originate and modulate LOS tone, relaxation and contraction and the characterization of neurotransmitters and receptors involved in LOS function are important to develop new pharmacological tools to treat primary oesophageal motor disorders and gastro-oesophageal reflux disease.
Keywords: lower oesophageal sphincter, NANC, muscle tone, inhibitory neurotransmitters, excitatory neurotransmitters, interstitial cells of Cajal
Introduction
The high-pressure zone at the junction between the oesophagus and the stomach is a specialized region composed of the lower oesophageal sphincter (LOS) and the crural diaphragm. The action of these structures on the one hand allows passage of a swallowed bolus to the stomach and on the other hand prevents reflux of gastric contents into the oesophagus. A fine regulation of LOS basal tone, relaxation and contraction is very important to accomplish these functions. In spite of its physiological relevance and implications in the pathophysiology of gastro–oesophageal diseases, the basic mechanisms involved in the control of LOS activity are still incompletely understood. Significant progress has been made in understanding the nature of inhibitory and excitatory neurotransmission in the LOS. Nitric oxide (NO) plays a major role but other inhibitory neurotransmitters may be involved such as ATP, vasoactive intestinal polypeptide (VIP), pituitary adenylate cyclase-activating peptide (PACAP), calcitonin gene-related peptide (CGRP) and carbon monoxide (CO). ACh and tachykinins are the main excitatory neurotransmitters; however, their contribution and the type of receptors involved in LOS excitation are not completely known.
This article reviews current knowledge on the regulation of the LOS activity, with particular emphasis on the evidence for neurotransmission and the receptors and channels involved in mediating relaxation or contraction.
Motor neuronal anatomy of the LOS
The LOS is innervated by both the parasympathetic and sympathetic systems through afferent (sensitive pathway) and efferent fibres (motor pathway). Sensory information from the LOS to the brain is conveyed by both spinal and vagal sensory afferents (Goyal and Rattan, 1975; Sohn et al., 1993; Hornby and Abrahams, 2000). The spinal afferents have their cell bodies in the dorsal root ganglia at T1–L3 (Collman et al., 1992) and are specialized to detect mainly nociceptive stimuli. The vagal afferents with their cell bodies in the nodose ganglia transmit non-painful information to the brain, synapsing mostly in the nucleus of tractus solitarius. The neurons of this nucleus are connected with the dorsal motor nucleus of the vagus that is the origin of vagal motor neurons that innervate LOS and the smooth muscle part of the oesophageal body. The motor neurons that innervate the striated muscle portion of the oesophagus are located mainly in the rostral part of the nucleus ambiguous (Collman et al., 1993). In contrast with the striated muscle, vagal efferent fibres do not innervate the smooth muscle directly; they synapse with inhibitory and excitatory motorneurons in the myenteric plexus. The distribution of the neuronal bodies in the dorsal motor nucleus depends on whether or not they synapse with inhibitory or excitatory motorneurons. The neuronal bodies of the excitatory pathway are located in the rostral part of the dorsal motor nucleus whereas neuronal bodies of the inhibitory pathway are in the caudal part (Rossiter et al., 1990). Although the vagus nerve innervates both inhibitory and excitatory myenteric motor neurons, vagal stimulation in animal studies provokes always an initial LOS relaxation that is followed by a contraction (Rattan, 1986; Blackshaw et al., 1997; Kawahara et al., 1997). The synaptic transmission between the efferent neurons and the inhibitory enteric neurons is mainly mediated by nicotinic and M1 muscarinic receptors (Goyal and Rattan, 1975; Smid and Blackshaw, 2000b); however, 5-HT can also play a role in the synaptic transmission at this level (Rattan and Goyal, 1978; Paterson et al., 1992).
Neuronal bodies of the efferent sympathetic pathway are located in the spinal segments T6–T10 and they are sending the axons to the celiac ganglia through the major splachnic nerve. Preganglionic neurons are cholinergic and activate ACh nicotinic receptors located in the noradrenergic postganglionic neurons. These adrenergic fibres can innervate the LOS smooth muscle directly or via the enteric motor neurons (Papasova, 1989). The sympathetic response of the LOS may vary according to species. In ferret, stimulation of the splanchnic nerve activates adrenergic neurons and relaxes LOS through β-adrenoceptors (Blackshaw et al., 1997). In cats, the stimulation of the peripheral end of the greater splachnic nerve induces an increase in LOS pressure and is the final result of the stimulation of adrenoceptors located in the smooth muscle and in cholinergic myenteric neurons (Fournet et al., 1979; Gonella et al., 1979). In contrast, the stimulation of the central end of splanchnic nerve produces a decrease in LOS pressure not affected by the β-adrenoceptor antagonist propanolol.
Asymmetry of the LOS: circular and oblique fibres
The human LOS is a specialized 4-cm long, thickened region of the circular muscle layer at the end of the distal oesophagus. In humans, the LOS does not form a complete ring and it is composed of semicircular clasp fibres adjacent to the lesser gastric curvature (incomplete ring in U shape) and by oblique muscular bundles coming from the greater gastric curvature (sling fibres) (Liebermann-Meffert et al., 1979). This morphological asymmetry is associated with an asymmetric pressure profile as measured with manometry (Stein et al., 1995). In other species such as dog (Friedland et al., 1971), opossum (Christensen and Torres, 1975) and cat (Friedland et al., 1971; Preiksaitis et al., 1994), the circular muscle forms a complete ring. Only in pigs the distribution of clasp and sling fibres is the same than that observed in humans (Vicente et al., 2001). Manometric studies in humans and cats show that the LOS pressure distribution is asymmetric; the pressure at the left side is higher (Preiksaitis et al., 1994). In addition, atropine decreases LOS tone in humans (Richardson and Welch, 1981) and cats (Preiksaitis et al., 1994) but the drop is more pronounced in the left part of the LOS. This finding might reflect asymmetric sensitivity to cholinergic stimulation. Ach muscarinic receptor agonists induce a higher concentration-dependent contraction in isolated sling fibres compared with clasp fibres in different species including humans (Preiksaitis et al., 1994; Preiksaitis and Diamant, 1997; Farre et al., 2007a). The higher sensitivity of sling muscle fibres can be due to the different expression of M3 muscarinic receptors (Muinuddin et al., 2003). Regional myogenic differences in the expression of L-type Ca2+ (Ca(L)) channels and in the Ca(L) channel current (ICa(L)) density can explain also the different contribution of both types of fibres to LOS contractibility (Muinuddin et al., 2004b). Compared with the LOS, clasp and sling fibres have also different sensitivity to dopamine, phenylephrine and isoprenaline (Tian et al., 2004).
The presence of important functional asymmetry between both halves of the LOS has to be considered to evaluate the involvement of neurotransmitters and the effect of drugs on LOS smooth muscle function.
LOS basal tone
The difference between the LOS and the oesophageal body smooth muscle is the development of an increased basal tone at the LOS, which in vivo can be identified as a zone of higher intraluminal pressure between the stomach and the oesophagus. In healthy volunteers, the LOS generates a tonic intraluminal pressure of 15–30 mm Hg above the intragastric pressure (Richter et al., 1987). The LOS basal tone contributes significantly to the increased pressure at the oesophago–gastric junction; however, the contraction of crural diaphragm around the LOS further increases that pressure, particularly during inspiration and increased intra-abdominal pressure (Pandolfino et al., 2006). Unlike humans, cats (Boyle et al., 1985), dogs (Martin et al., 1992) and pigs (Vicente et al., 2001), rats (the most widely used laboratory animal) have an oesophago–gastric junction with a significant separation between LOS and crural diaphragm (Soto et al., 1997; Montedonico et al., 1999) making this species less useful for in vivo studies of antireflux barrier function.
Basal LOS tone is primarily myogenic in origin because of specialized properties of the smooth muscle cells at this level. The LOS smooth muscle cells are thought to be more depolarized than the oesophageal body muscle cells with a resting membrane potential less negative (respectively, −41 mV instead of −50 mV). This difference may lead to spontaneous spike-like action potentials and generation of basal tone (Zhang et al., 2000), perhaps via the activation of voltage-gated Ca2+ channels, which in turn leads to entry of the extracellular calcium. Another explanation may be related to differences in structural proteins such as α-actin, basic essential light chains LC17b and caldesmon (Szymanski et al., 1998).
It is well established in different species, including humans, that LOS tone is maintained by the influx of Ca2+ trough Ca(L) channels (Fox and Daniel, 1979; Biancani et al., 1987; Salapatek et al., 1998; Kovac et al., 2005). Experiments with Ca2+-free buffer, nifedipine (a Ca(L) channel blocker), cyclopiazonic acid (sarcoplasmic reticulum Ca2+-ATPase inhibitor) and 2-APB (inhibition of Ca2+ release from SR Ca2+ stores) showed that clasp and sling LOS fibres use intracellular and extracellular Ca2+ sources to different degrees in the generation of spontaneous tone (Muinuddin et al., 2004c). The greater expression of Ca(L) channels and the ICa(L) density in cells from clasp muscle than sling muscle might contribute to the different involvement of these areas in the maintenance of smooth muscle LOS tone (Muinuddin et al., 2004b).
Lower oesophageal sphincter tone is modulated by excitatory and inhibitory vagal pathways. Interventions that affect both pathways simultaneously that is administration of tetrodotoxin (Na+ channel blocker) (Goyal and Rattan, 1976) or bilateral vagotomy (Rattan and Goyal, 1974) do not modify LOS pressure in vivo. However, when only one pathway is blocked, it leads to unopposed effects of the other pathway. For example, suppression of a nitrergic inhibitory pathway with NOS inhibitors results in rise of LOS pressure due to the unopposed action of excitatory nerves (Yamato et al., 1992; Xue et al., 1996; Konturek et al., 1997). Similarly, suppression of a cholinergic excitatory pathway by an anticholinergic agent decreases LOS pressure due to unopposed action of the inhibitory nerves. This effect seems to be species specific. Unlike human and dogs, in opossums and monkeys, atropine failed to reduce LOS pressure (Dodds et al., 1981). Similar results were observed in vitro with LOS muscle strips. NOS inhibitors increase tone of muscle strips from LOS of different species including humans (Gonzalez et al., 2004; Farre et al., 2006) and atropine decreases tone of muscle strips from LOS in pigs (Farre et al., 2006), but not in humans (Preiksaitis and Diamant, 1997; Gonzalez et al., 2004) suggesting that effect of atropine on human LOS pressure in vivo may be at the CNS level. We cannot exclude that cholinergic tone has been removed by isolation of the tissue in man. The contribution of nitrergic myenteric neurons to LOS basal tone was confirmed by manometric studies in neuronal NOS-deficient mice showing a high LOS basal pressure and impaired LOS relaxation similar to that observed in human achalasia (Sivarao et al., 2001). Experiments in pigs suggest that basal LOS tone is modulated asymmetrically that is the sling fibres are more controlled by both cholinergic and nitrergic inputs whereas basal tone from LOS clasp fibres seems to be more myogenically regulated (Farre et al., 2007a).
The role of the sympathetic nervous system on basal LOS pressure is not clear. Bilateral cervical sympathectomy or greater splanchnic nerve sectioning has no effect on basal LOS pressure (Fournet et al., 1979). In contrast, experiments using α- and β-adrenoceptor antagonists suggest that a significant portion of basal LOS pressure (30%) is dependent upon the stimulation of α- but not β-adrenoceptors (DiMarino and Cohen, 1973).
Together with the myogenic nature of LOS tone and its modulation by parasympathetic and sympathetic nervous system, a number of neurohumoral substances are known to affect basal LOS tone (Table 1). Other substances as somatostatin and thyrotropin-releasing hormone do not affect basal LOS pressure but inhibit the LOS contraction induced by other agents (Parkman and Reynolds, 1990; Parkman et al., 1993).
Table 1.
Neurohumoral substances affecting basal LOS tone
| Substances | Effects and references | Species |
|---|---|---|
| α-adrenoceptor agonist (phenylephrine) | ↑ (Tian et al., 2004) | Humana |
| β-adrenoceptor agonist for β1, β2 and β3: isoprenaline, isoproterenol, dobutamine, terbutaline, albuterol, CL 316243 | ↓ (Allescher et al., 1988) | Doga |
| (Tottrup et al., 1990b) | Humana | |
| (Crowell et al., 2001) | Humanb | |
| (Sarma et al., 2003) | Opossuma | |
| (Tian et al., 2004) | Humana | |
| Dopamine | ↓ or ↑ (Tian et al., 2004) | Humana |
| Angiotensin II | ↑ (Rattan et al., 2003) | Rata |
| Prostanoids (PGFalpha, PGH2, TXB2, PGE1 and PGF1, arachidonic acid) | ↑ (Daniel et al., 1979a) | Opossumb |
| (Daniel et al., 1979b) | Opossuma | |
| (Cao et al., 2002) | Cata | |
| (Velkova et al., 1981) | Cata | |
| Xanthines (theophylline) | ↓ (Tottrup et al., 1990b) | Humana |
| Motilin | ↑ (Tomita et al., 1997) | Humana |
| Galanin | ↑ (Rattan and Goyal, 1987) | Opossumb |
| Bombesin | ↑ (Mukhopadhyay and Kunnemann, 1979) | Opossumb |
| (Kortesova et al., 1990) | Cata | |
| CCK-8 | ↓ In vivo (Behar and Biancani, 1977) | Humanb |
| ↑ In vitro (Gonzalez et al., 2000) | Humana | |
| Pentagstrin | ↑ (Zwick et al., 1976) | Dogsb |
| IL-1β | ↓ (Cheng et al., 2005) | Cata |
| (Cao et al., 2006) | Cata | |
| PAR receptor agonists for PAR1 and PAR2: TFLLR-NH2, SFLLRN-NH2 SLIGKV-NH2 and SLIGRL-NH2 | ↓ (Huang, 2007) | Guinea-piga |
Abbreviations: ↓, relaxation; ↑, contraction; IL, interleukin; LOS, lower oesophageal sphincter; PAR, protease-activated receptor; PG, prostaglandin; TX, thromboxane.
In vitro.
In vivo.
LOS relaxation
Lower oesophageal sphincter relaxation is an integral part of swallow-induced primary peristalsis in the oesophagus. However, swallow-induced LOS relaxation can happen without pharyngeal or oesophageal peristalsis as well. The distension of the smooth muscle part of the oesophagus can induce LOS relaxation with or without associated secondary peristalsis. Such relaxation is due to local reflexes and is not vagally mediated. Transient lower oesophageal sphincter relaxations last 10–30 s and are not associated with swallowing (Mittal and Balaban, 1997) but triggered by gastric distention (Holloway et al., 1985, 1989, 1991). Transient lower oesophageal sphincter relaxations are responsible for the postprandial increase in gastro–oesophageal reflux and may play an important role in the gastro–oesophageal reflux disease (Kahrilas et al., 2000).
NANC inhibitory neurotransmitters in the LOS
The NANC neurotransmitters NO, VIP, PACAP, ATP, CGRP and CO have inhibitory effects in the gastrointestinal tract. Their contribution shows regional variations and sometimes in the same region their individual contribution is species specific.
Immunohistochemistry of the LOS myenteric neurons shows the presence of the NOS, the peptides VIP, PACAP and CGRP and the constitutive enzyme haem oxygenase type 2 that is involved in the synthesis of CO. NO is considered the main inhibitory mediator. The role of other neurotransmitters is still controversial.
Vasoactive intestinal polypeptide (Fahrenkrug et al., 1978) and ATP (Burnstock et al., 1970) were the first neurotransmitters proposed to mediate NANC relaxation in the oesophagus. Few years later, Rattan (1986) and Goyal et al. (1980) suggested that neither ATP nor adenosine were the inhibitory neurotransmitters involved in LOS relaxation and VIP was proposed to be the main oesophageal NANC neurotransmitter (Behar et al., 1989). VIP was also proposed as the substance that mediated the relaxation induced by electrical field stimulation (EFS) of muscle strips from the LOS (Biancani et al., 1984). Other studies, however, suggested that neither a purine nor VIP was the mediator of inhibitory nerves to the oesophageal smooth muscle (Daniel et al., 1983). The scenario changed when in the late 1980s it was demonstrated that NO is released from endothelial cells (Palmer et al., 1987), selective NOS inhibitors were developed (Mulsch and Busse, 1990) and NO was identified as the inhibitory mediator of NANC neurotransmission in the gastrointestinal tract (Bult et al., 1990).
Nitric oxide
The important role of NO in LOS relaxation was suggested for the first time in 1991 from experiments studying the effect of NOS inhibitors on opossum LOS muscle strips (Murray et al., 1991; Tottrup et al., 1991) and later confirmed on human tissue (Oliveira et al., 1992; Tottrup et al., 1993). Further experiments showed that NO is the main neurotransmitter that mediates smooth muscle LOS relaxation in different species such as dogs, guinea-pigs, rats, cats and pigs (de Man et al., 1991; Murray et al., 1991; Kortezova et al., 1996; Yuan et al., 1998; Farre et al., 2006, 2007b).
In vivo experiments showed that NOS inhibitors can abolish or reduce swallow-induced LOS relaxation (Yamato et al., 1992; Xue et al., 1996; Konturek et al., 1997; Sivarao et al., 2001) and patients with achalasia have a reduction in the number of myenteric nitrergic neurons (Mearin et al., 1993), consistent with a physiological role for NO.
The role of NO in relaxation of LOS smooth muscle has been extensively studied in vitro. Stimulation of the intrinsic nerves by EFS induces an ‘on' contraction instead an ‘on' relaxation in LOS strips from achalasic patients suggesting that nitrergic neurotransmission is severely impaired (Tottrup et al., 1990a). Studies using mice with genetically engineered endothelial and neuronal NOS showed that neuronal NOS rather than eNOS is the enzymatic source of the NO that mediates NANC relaxation (Kim et al., 1999; Sivarao et al., 2001).
In different species, pure NO, NO donors and EFS can induce relaxation/hyperpolarization of smooth muscle strips from the LOS by a cGMP pathway. Experiments using GC inhibitors and direct measurements of cGMP (Torphy et al., 1986; Barnette et al., 1989; Rattan and Moummi, 1989; Conklin and Du, 1992; Jun et al., 2003; Farre et al., 2006) confirmed that activation of nitrergic myenteric neurons induces smooth muscle LOS relaxation via the GC–cGMP pathway.
It remains unclear what type of membrane channels are involved in nitrergic inhibition of the LOS. It was previously proposed that NO relaxes gastrointestinal smooth muscle by either opening of K+ channels or closing of Ca2+-activated Cl− channels (Cl(Ca)) (Cayabyab and Daniel, 1995; Koh et al., 1995; Zhang et al., 1998). Neural NO causes relaxation of LOS muscle strips that does not require K+ channels because the non-specific K+-channel blocker tetraethylammonium does not modify EFS-induced relaxation (Daniel et al., 2002). The putative Cl(Ca) blockers 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid, 9-anthroic acid (A-9-C) and niflumic acid significantly reduced the amplitude of the inhibitory junction potentials. These findings suggest that the inhibitory junction potentials observed in LOS circular smooth muscle is due to a decrease in membrane chloride conductance (Crist et al., 1991; Zhang and Paterson, 2003). A current hypothesis proposes that neural NO inactivates Cl(Ca) channels via intracellular increase of cGMP, leading to an inhibitory junction potential and relaxation (Zhang and Paterson, 2003). However, such hypothesis should be carefully considered because of the non-specific nature of chloride channel blockers, and because the role of K channels cannot be so far completely excluded (Jury et al., 2001).
Nitric oxide is the main inhibitory neurotransmitter at the LOS. However, NOS inhibitors cannot block completely the LOS relaxation, suggesting that other non-nitrergic neurotransmitter/s are involved.
Purines
Purine receptors are classified as P1, activated by adenosine and P2, activated by ATP, ADP, UTP and UDP. P2 receptors are divided in two families: P2X and P2Y that are ligand-gated ion channels and G protein-coupled receptors respectively.
ATP, ADP and adenosine decrease LOS pressure in opossum (Rattan and Goyal, 1980), induce hyperpolarization (Daniel et al., 1983) and relax smooth muscle strips from the LOS (Farre et al., 2006). ATP-induced relaxation is characterized by a fast component followed by a slow relaxation period (Farre et al., 2006). Suramin, a P2-unspecific receptor antagonist, and apamin, a blocker of small conductance Ca2+-activated K+ (K(Ca,slow)) channels, reduce the hyperpolarization/relaxation induced by ATP in LOS smooth muscle (Imaeda et al., 1998; Farre et al., 2006). In addition, apamin and the P2Y1 receptor antagonist MRS2179 (Alexander et al., 2005) block the fast component of the ATP-induced relaxation (Farre et al., 2006). Smooth muscle relaxation along the gastrointestinal tract includes an apamin-sensitive component that might involve ATP or related purines as a mediator (Costa et al., 1986; Lefebvre et al., 1991; Ohno et al., 1996; Rae and Muir, 1996). The apamin-sensitive component was described in EFS-induced relaxation of LOS muscle strips after NOS blockade. This observation was made in different species including humans (Imaeda et al., 1998; Gonzalez et al., 2004; Farre et al., 2006, 2007b). It was also observed during vagal stimulation (Yuan et al., 1998; Yuan and Brookes, 1999). The non-nitrergic component could be reduced by MRS2179 in pigs (Farre et al., 2006) and humans (Estrada et al., 2006). In addition, the selective P2Y1 receptor agonist ADPβS and 2-MeSATP induces a relaxation in human muscle strips from the LOS. All these results suggest that ATP or related purines play a role in the non-nitrergic component of the relaxation of the LOS acting through P2Y1 receptors and activating K(Ca,slow) channels. The role of purines during swallow-induced and or transient LOS relaxations is unknown.
VIP/PACAP
Vasoactive intestinal polypeptide and/or PACAP receptors belong to the family of the seven transmembrane G protein-coupled receptors and are classified as VPAC1, VPAC2 and PAC1 according to their affinity for VIP and PACAP. VPAC1 and the VPAC2 receptors have similar affinities for both VIP and PACAP, in contrast, PAC1 exhibits high affinity for PACAP and a low affinity for VIP (Harmar et al., 1998).
Vasoactive intestinal polypeptide is present and localizes with NOS in LOS myenteric neurons in different species including humans (Aggestrup et al., 1985; Uddman et al., 1991; Ny et al., 1995a). The role of VIP in LOS relaxation remains controversial. VIP decreases LOS pressure (Rattan et al., 1982; Guelrud et al., 1992) in vivo and induces smooth muscle relaxation in different species (Jury et al., 1992; Kohjitani et al., 1996b; Kortezova et al., 1996; Uc et al., 1999; Farre et al., 2006). Different antagonists were tested on VIP-induced smooth muscle relaxation including [Lys1,Pro2,5,Arg3,4,Tyr6]VIP, VIP(10–28) (Jun et al., 2003), VIP(6–28), [D-p-Cl-Phe6,Leu17]VIP and [Ac-Tyr1,D-Phe2] growth hormone-releasing factor-(1–29) amide. Only the first two inconsistently antagonized the effects of VIP but they had no effect on EFS-induced LOS relaxation (Uc et al., 1999; Farre et al., 2006). These results suggest that more potent and specific VIP receptor antagonists are needed to evaluate the role of endogenous VIP in LOS relaxation. VIP antiserum was able to block the non-nitrergic LOS relaxation but it was later demonstrated that the effect was due to the solvent rather than due to VIP antibodies (Uc et al., 1999).
Further evidence against VIP as an NANC inhibitory neurotransmitter in LOS is suggested by the nature of the second messenger involved in LOS relaxation. VIP-induced LOS relaxation is associated with increase of cAMP levels without increase of cGMP (Torphy et al., 1986; Szewczak et al., 1990). Furthermore, the response to VIP is either poor or non-modified by the GC inhibitor 1H-(1,2,4)-oxadiaziol-(4,3-a)quinoxalin-1-one (Uc et al., 1999; Shahin et al., 2000; Jun et al., 2003). In contrast, EFS-induced relaxation of LOS smooth muscle has no effect on cAMP but increases cGMP levels in a frequency-dependent manner (Torphy et al., 1986). These data are against the hypothesis that endogenous VIP may play a significant role in NANC neurotransmission to the LOS.
It has been proposed that a significant portion of NO production is induced by VIP presynaptically or in smooth muscle cells (Grider et al., 1992). In the LOS, VIP-induced relaxation is poor or non-modified by NOS inhibitors (Jury et al., 1992; Jun et al., 2003; Farre et al., 2006) indicating that VIP does not produce LOS relaxation via generation of NO.
PACAP-immunoreactive nerve fibres are running through the circular muscle layer of the LOS in different species (Uddman et al., 1991; Ny et al., 1995b). These fibres are also immunoreactive to NOS (Ny et al., 1994). The role of PACAP in LOS NANC neurotransmission is controversial due to species differences.
PACAP 27 and 38 relax muscle strips from the LOS (Ny et al., 1995b; Farre et al., 2006). In ferrets, the PACAP receptor antagonist, PACAP 6–38, does not affect vagal or EFS-induced relaxations (Smid and Blackshaw, 2000b) in spite of blocking the effect of exogenous PACAP in other parts of the gastrointestinal tract (Baccari and Calamai, 2001; Rozsai et al., 2001; Zizzo et al., 2004). These data suggested that PACAP does not mediate in vivo or EFS-induced LOS relaxation in ferrets.
In pigs, PACAP 27 induces LOS relaxation mediated via K(Ca,slow) channels (Farre et al., 2006). Identical pathway is involved in the apamin-sensitive component of the EFS-induced relaxation, suggesting that in pigs, PACAP may play a role as an NANC inhibitory neurotransmitter in LOS relaxation. Further studies with selective PACAP receptor antagonists are needed to confirm this hypothesis.
The intracellular cascade involved in PACAP-induced smooth muscle LOS relaxation is still uncertain. PACAP 27 increases the production of cAMP via activation of adenyl cyclase (Ny et al., 1995b). However, activation of VIP/PACAP receptors is not exclusively coupled via the adenylate cyclase system, but it can also stimulate the PLC pathway (Christophe, 1993). For example, in the small intestine, the hyperpolarization and relaxation of smooth muscle induced by PACAP is coupled to apamin-sensitive K+ channel (Kishi et al., 1996; Ekblad, 1999; McCloskey and Gurney, 2002). This pathway involves stimulation of PLC, increased production of 1,4,5-trisphosphate, localized Ca2+ release from intracellular stores and opening of K(Ca,slow) channel (Zizzo et al., 2005). The subtype of receptor involved in the PACAP-induced LOS relaxation and the intracellular mediators require further investigation.
Calcitonin gene-related peptide
CGRP-immunoreactive neurons are present within the myenteric LOS neurons and CGRP decreases LOS pressure in vivo (Rattan et al., 1988; Parkman et al., 1989).
The in vitro effect of CGRP varies between species. CGRP does not relax muscle strips from rabbit or pig LOS (Kohjitani et al., 1996a; Farre et al., 2006) but induces a concentration-dependent relaxation in muscle strips from opossum LOS that is antagonized by CGRP 8–37. In the same specie, this antagonist, however, does not prevent the relaxation induced by EFS (Uc et al., 1997, 1999). The role of CGRP is still unknown, but the current data do not support the hypothesis that CGRP is a primary mediator of nerve-induced LOS relaxation.
Carbon monoxide
Like NO, CO is a gas that has been suggested to play a role in smooth muscle relaxation in other areas of the gastrointestinal tract (Watkins et al., 2004; Lim et al., 2005). The role of CO in LOS relaxation has not been extensively studied and results are not conclusive. The constitutive enzyme responsible for the synthesis of CO, haem oxygenase type 2, is present in the enteric nervous system (ENS) of the LOS colocalizing in neurons containing NOS and VIP (Ny et al., 1996). Both the CO-releasing molecule CORM-1 and pure CO can relax muscle strips opossum, feline and porcine LOS, but the selective haem oxygenase inhibitor, zinc protoporphyrin IX, does not modify EFS-induced relaxation (Ny et al., 1996; Fan et al., 1998; Farre et al., 2006). CO uses the same intracellular pathway as NO that is activation of GC and cGMP production (Ny et al., 1996). The role of CO as an NANC inhibitory neurotransmitter at the LOS requires further investigation.
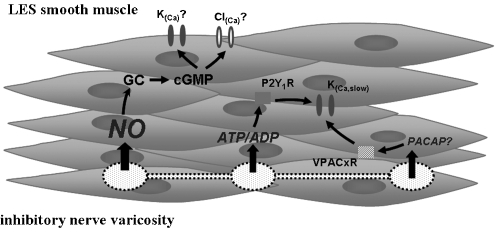
Figure 1 summarizes the main neurotransmitters, receptors and channels involved in mediating LOS relaxation.
Figure 1.
Schematic representation of the neurotransmitters and K+ channels involved in LOS smooth muscle relaxation. Nitric oxide (NO) released by nerve varicosities activates guanylate cyclase, which increases cGMP levels followed by activation of membrane Cl(Ca) and/or K(Ca) channels, hyperpolarizing the cell and inducing LOS relaxation. Purines (ATP/ADP) can relax LOS by activation of P2Y1 receptor, which is associated with activation of a K(Ca,slow) channel. PACAP may also provoke relaxation by activation of K(Ca,slow) channel acting through an unknown VIP/PACAP receptor. LOS, lower oesophageal sphincter; PACAP, pituitary adenylate cyclase-activating peptide; VIP, vasoactive intestinal polypeptide.
LOS contraction
The LOS relaxations are followed by an after contraction that restores basal LOS tone. After a swallow-induced or a transient LOS relaxation, the oral part of the sphincter contracts in continuity with peristalsis in the oesophageal body. The lower part of the LOS does not show after contraction and the sphincter pressure simply returns to the resting level. An identical pattern is obtained by EFS of muscle strips from the LOS in different species. EFS induces relaxation during the stimulus (‘on' relaxation) followed by a contraction (‘off' contraction) when strips have been obtained from the proximal part of the LOS and an isolated ‘on' relaxation when the strips were obtained from the distal part (Christensen et al., 1973; Gaumnitz et al., 1995; Gonzalez et al., 2004).
In vivo, the LOS contraction observed after relaxation is partially peripheral (rebound) and partially centrally mediated (cholinergic excitatory innervation of the LOS) (Beyak et al., 1997).
The excitatory neurotransmitters and subtypes of receptors involved in LOS after contraction are not completely known.
Excitatory neurotransmitters in the LOS
Motor neurons of the entire gastrointestinal tract contain acetylcholinetransferase (Ratcliffe et al., 1998; Konomi et al., 2002; Porter et al., 2002) and the tachykinins such as substance P (SP), neurokinin A (NKA) and B (NKB) (Sandler et al., 1991; Shuttleworth et al., 1991; Yunker et al., 1999; Yip et al., 2003). ACh is the main excitatory neurotransmitter in the gastrointestinal tract but tachykinins can also play an important role in specific gastrointestinal regions (Cao et al., 2000; Krysiak and Preiksaitis, 2001; El-Mahmoudy et al., 2003).
Acetylcholine
Excitatory motor neurons, immunoreactive for acetylcholinetransferase, are present in the LOS myenteric plexus (Seelig et al., 1984; Brookes et al., 1996). Carbachol and bethanechol induce a dose-dependent contraction of LOS smooth muscle (Huber et al., 1993; Smid and Blackshaw, 2000a). Atropine inhibits the ‘off' contraction of LOS muscle strips observed after EFS (Gonzalez et al., 2004; Farre et al., 2006). Gilbert et al. (1984) proposed that M2 muscarinic receptors are located directly on the smooth muscle of the opossum LOS. In cat LOS, however, carbachol-induced contractions seem to be mediated by M3 receptors, as suggested by the sensitivity to the antagonists methoctramine, 4-DAMP and pirenzipine (Preiksaitis et al., 1994). These results were sustained by experiments with cat LOS-isolated muscle cells. Contraction of these cells was inhibited by the ACh muscarinic M3 receptor antagonist p-fluoro-hexa-hydro-sila-difenidol (Sohn et al., 1993). The activation of M3 receptors is linked to Gq and to phosphatidylinositol-specific phospholipase C and to the production of inositol 1,4,5-trisphosphate and DAG. 1,4,5-Trisphosphate causes release of Ca2+ from endoplasmic reticulum and activation of calmodulin. Ca2+-calmodulin causes activation of myosin light-chain kinase inducing the contraction (Harnett et al., 2005).
Tachykinins
Substance P and NKA immunoreactive nerve fibres are present in the LOS of human, pigs and dogs (Aggestrup et al., 1986; Sandler et al., 1991). Intravenous administration of SP increases LOS pressure, dose-dependent, using a pathway that partially involves ACh muscarinic receptors. In contrast, NKA increases LOS tone independently of neural cholinergic mechanisms (Mukhopadhyay, 1978; Sandler et al., 1991).
Substance P, NKA and NKB induce a concentration-dependent contractile response of muscle strips from human LOS with a rank order of potency NKA>NKB>SP. The selective NK2 receptor antagonist, SR 48968, but not the selective NK1 receptor antagonist, CP-96345, inhibits the response to NKA (Huber et al., 1993). These results suggest that the tachykinin-induced contraction of the LOS is mediated by tachykinin NK2 receptor stimulation.
Nitric oxide
Although it is well established that NO is the main NANC inhibitory neurotransmitter to the LOS, it can also play a role in the LOS contraction. In LOS muscle strips, EFS induces relaxation followed by an ‘off' contraction. In opossum (Gaumnitz et al., 1995), pig (Farre et al., 2006) and mouse (Kim et al., 1999), both responses are impaired by the NOS inhibitor N-nitro-L-arginine methyl ester and by the GC inhibitor 1H-(1,2,4)-oxadiaziol-(4,3-a)quinoxalin-1-one, suggesting that NO is involved not only in the relaxation but also in the contraction (rebound contractions). Studies using genetically engineered mice without neuronal NOS confirmed these results (Kim et al., 1999). Furthermore, NO may contract oesophageal longitudinal muscle (Zhang and Paterson, 2001).
This effect seems to be species dependent. In cat, rat and human (Gonzalez et al., 2004; L'Heureux et al., 2006; Farre et al., 2007b), NOS inhibitors do not affect the ‘off' contraction. In vivo experiments in opossums and cats showed that NG-nitro-L-arginine (i.v.) inhibit LOS relaxation and decrease the amplitude of LOS after contraction (Paterson et al., 1992; Yamato et al., 1992; Xue et al., 1996; Sifrim and Lefebvre, 2001). The effect of NOS inhibitors on LOS relaxation seems to be peripheral because it is similar in vivo and in isolated LOS muscle strips. In contrast, the effect of NOS inhibitors on LOS after contraction might be central. In cats, the LOS after contraction mainly results from central release of NO, neural excitation at the dorsal motor nucleus of the vagus and activation of cholinergic excitatory innervation to the LOS (Beyak et al., 1997).
Figure 2 summarizes the main neurotransmitters and receptors involved in mediating the LOS contraction.
Figure 2.
Schematic representation of the neurotransmitters involved in LOS smooth muscle contraction. ACh and tachykinins (substance P, neurokinin A and B) released by nerve varicosities induce LOS contraction by activation of M3 and NK2 receptors respectively. Nitric oxide (NO) can also participate in the LOS contraction by a cGMP pathway. LOS, lower oesophageal sphincter.
Asymmetry of the inhibitory and excitatory neurotransmission to the LOS
There is a marked asymmetry in the contribution of the inhibitory and excitatory neurotransmitters to the LOS. This phenomena was described in pigs (Farre et al., 2004) and cats. EFS of different muscle strips from the LOS showed that in clasp fibres, there is a strong nitrergic (L'Heureux et al., 2006; Farre et al., 2007a) and an apamin-sensitive component mediated by ATP. In contrast, this type of neurotransmission is reduced in sling fibres. Although NO and ATP mediate LOS smooth muscle relaxation in both types of fibres, there is a major contribution of the inhibitory components in clasp fibres (Farre et al., 2006). This variation can be explained by different inhibitory innervation between both sides of the LOS (Brookes et al., 1996; Yuan et al., 1998; Yuan and Brookes, 1999) and/or a higher sensitivity of the smooth muscle to exogenous application of sodium nitroprusside and ATP in the clasp fibres (Farre et al., 2006). Morphological studies mapping the regional distribution of myenteric neurons in the LOS showed a majority of inhibitory neurons in the clasp region. Unlike the inhibitory components, the cholinergic one is stronger in sling fibres (L'Heureux et al., 2006; Farre et al., 2007a). Similarly, the variation can be explained by different cholinergic innervation (Yuan and Brookes, 1999) and/or different sensitivity of both sides of the LOS to muscarinic receptors agonists as it was commented above. The asymmetrical contribution of the inhibitory and excitatory neurotransmission might have an impact during LOS relaxations in vivo and should be considered for the development of pharmacological strategies aiming to influence both LOS basal pressure and transient LOS relaxations.
Nerve ending–smooth muscle interaction in the LOS. Role of interstitial cells of Cajal
Interstitial cells of Cajal (ICC) were described for the first time by Ramón y Cajal (1904) at the end of the eighteen century. Immunohistochemical studies showed that they do not have classical markers of myenteric neurons, glial cells, fibroblasts or smooth muscle, but they do have neuronal enolase, suggesting a connection with some neuronal type (Prosser et al., 1989). At the LOS, only intramuscular ICC (ICC-IM) can be identified and are located in both longitudinal and circular smooth muscle layers. Daniel and Posey-Daniel (1984) described for the first time the ultrastructural arrangement of the LOS neuromuscular junction. Nerve endings with varicosities can innervate the smooth muscle directly or indirectly through ICC-IM. The location of ICC-IM suggested that they may play a role in transducing the effects of neurotransmitters released from nerve ending to smooth muscle cells. The role of ICC at the LOS has been recently evaluated using a W/WV mutant mice (mutation associated with a lack of ICC-IM) (Ward et al., 1998; Sivarao et al., 2001). Smooth muscle strips from LOS of mutant mice show a reduced NANC nitrergic neurotransmission suggesting that ICC-IM may play a significant role in the inhibitory neural pathway. The gastric fundus of W/WV mutant mice have impaired cholinergic neurotransmission (Ward et al., 2000) suggesting that ICC-IM play a major role in cholinergic excitatory inputs. However, swallow- and vagal-induced LOS relaxation is not modified in the W/WV mutant mice (Sivarao et al., 2001) suggesting that in vivo, the role of ICC-IM is not critical for LOS nitrergic relaxation. In contrast, W/WV mutant mice have a hypotensive LOS (Sivarao et al., 2001), suggesting that deficiency in the ICC-IM may impair the LOS smooth muscle function.
Further experiments were performed using another mutant animal without ICC-IM (Ws/Ws rats) (Farre et al., 2007b). In these animals, ultrastructural analysis shows the presence of fibroblast-like ICC instead of ICC-IM. Fibroblast-like ICC forms a direct apposition with nerve endings varicosities and a GAP junction with smooth muscle cells. The presence of these cells is rare in control rats. In this model, the nitrergic and the apamin-sensitive relaxation of muscle strips from the LOS was not affected. In Ws/Ws rats, the cholinergic component of the response of LOS muscle strips to EFS is preserved. These findings suggest that the presence of fibroblast-like ICC in Ws/Ws rats may accomplish the same role of ICC-IM serving as the mediator of inhibitory and excitatory neurotransmission in the LOS.
Acknowledgments
Ricard Farré and Daniel Sifrim were supported by a ‘Geconcerteerde Onderzoeksactie' grant from the Catholic University of Leuven, Belgium. Ricard Farré is a postdoctoral fellow of the Flanders Research Foundation (FWO).
Abbreviations
- Ca(L)
L-type Ca2+ channel
- Cl(Ca)
Ca2+-activated Cl− channel
- EFS
electrical field stimulation
- ICa(L)
L-type Ca2+ channel current
- ICC-IM
intramuscular interstitial cells of Cajal
- K(Ca,slow)
small conductance Ca2+-activated K+ channel
- LOS
lower oesophageal sphincter
Conflict of interest
The authors state no conflict of interest.
References
- Aggestrup S, Uddman R, Jensen SL, Hakanson R, Sundler F, Schaffalitzky de MO, et al. Regulatory peptides in lower esophageal sphincter of pig and man. Dig Dis Sci. 1986;31:1370–1375. doi: 10.1007/BF01299816. [DOI] [PubMed] [Google Scholar]
- Aggestrup S, Uddman R, Jensen SL, Sundler F, Schaffalitzky de MO, Holst JJ, et al. Regulatory peptides in the lower esophageal sphincter of man. Regul Pept. 1985;10:167–178. doi: 10.1016/0167-0115(85)90011-4. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels, 1st edition (2005 revision) Br J Pharmacol. 2005;144 Suppl 1:S1–S128. doi: 10.1038/sj.bjp.0706158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allescher HD, Berezin I, Jury J, Daniel EE. Characteristics of canine lower esophageal sphincter: a new electrophysiological tool. Am J Physiol. 1988;255:G441–G453. doi: 10.1152/ajpgi.1988.255.4.G441. [DOI] [PubMed] [Google Scholar]
- Baccari MC, Calamai F. Modulation of nitrergic relaxant responses by peptides in the mouse gastric fundus. Regul Pept. 2001;98:27–32. doi: 10.1016/s0167-0115(00)00225-1. [DOI] [PubMed] [Google Scholar]
- Barnette M, Torphy TJ, Grous M, Fine C, Ormsbee HS., III Cyclic GMP: a potential mediator of neurally- and drug-induced relaxation of opossum lower esophageal sphincter. J Pharmacol Exp Ther. 1989;249:524–528. [PubMed] [Google Scholar]
- Behar J, Biancani P. Effect of cholecystokinin-octapeptide on lower esophageal sphincter. Gastroenterology. 1977;73:57–61. [PubMed] [Google Scholar]
- Behar J, Guenard V, Walsh JH, Biancani P. VIP and acetylcholine: neurotransmitters in esophageal circular smooth muscle. Am J Physiol. 1989;257:G380–G385. doi: 10.1152/ajpgi.1989.257.3.G380. [DOI] [PubMed] [Google Scholar]
- Beyak MJ, Collman PI, Valdez DT, Xue S, Diamant NE. Superior laryngeal nerve stimulation in the cat: effect on oropharyngeal swallowing, oesophageal motility and lower oesophageal sphincter activity. Neurogastroenterol Motil. 1997;9:117–127. doi: 10.1046/j.1365-2982.1997.d01-22.x. [DOI] [PubMed] [Google Scholar]
- Biancani P, Hillemeier C, Bitar KN, Makhlouf GM. Contraction mediated by Ca2+ influx in esophageal muscle and by Ca2+ release in the LES. Am J Physiol. 1987;253:G760–G766. doi: 10.1152/ajpgi.1987.253.6.G760. [DOI] [PubMed] [Google Scholar]
- Biancani P, Walsh JH, Behar J. Vasoactive intestinal polypeptide. A neurotransmitter for lower esophageal sphincter relaxation. J Clin Invest. 1984;73:963–967. doi: 10.1172/JCI111320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Haupt JA, Omari T, Dent J. Vagal and sympathetic influences on the ferret lower oesophageal sphincter. J Auton Nerv Syst. 1997;66:179–188. doi: 10.1016/s0165-1838(97)00082-9. [DOI] [PubMed] [Google Scholar]
- Boyle JT, Altschuler SM, Nixon TE, Tuchman DN, Pack AI, Cohen S. Role of the diaphragm in the genesis of lower esophageal sphincter pressure in the cat. Gastroenterology. 1985;88:723–730. doi: 10.1016/0016-5085(85)90143-x. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Chen BN, Hodgson WM, Costa M. Characterization of excitatory and inhibitory motor neurons to the guinea pig lower esophageal sphincter. Gastroenterology. 1996;111:108–117. doi: 10.1053/gast.1996.v111.pm8698189. [DOI] [PubMed] [Google Scholar]
- Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Cheng L, Behar J, Biancani P, Harnett KM. IL-1beta signaling in cat lower esophageal sphincter circular muscle. Am J Physiol Gastrointest Liver Physiol. 2006;291:G672–G680. doi: 10.1152/ajpgi.00110.2006. [DOI] [PubMed] [Google Scholar]
- Cao W, Harnett KM, Behar J, Biancani P. PGF(2alpha)-induced contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2002;283:G282–G291. doi: 10.1152/ajpgi.00357.2001. [DOI] [PubMed] [Google Scholar]
- Cao W, Pricolo VE, Zhang L, Behar J, Biancani P, Kirber MT. Gq-linked NK(2) receptors mediate neurally induced contraction of human sigmoid circular smooth muscle. Gastroenterology. 2000;119:51–61. doi: 10.1053/gast.2000.8552. [DOI] [PubMed] [Google Scholar]
- Cayabyab FS, Daniel EE. K+ channel opening mediates hyperpolarizations by nitric oxide donors and IJPs in opossum esophagus. Am J Physiol. 1995;268:G831–G842. doi: 10.1152/ajpgi.1995.268.5.G831. [DOI] [PubMed] [Google Scholar]
- Cheng L, Cao W, Behar J, Biancani P, Harnett KM. Inflammation induced changes in arachidonic acid metabolism in cat LES circular muscle. Am J Physiol Gastrointest Liver Physiol. 2005;288:G787–G797. doi: 10.1152/ajpgi.00327.2004. [DOI] [PubMed] [Google Scholar]
- Christensen J, Freeman BW, Miller JK. Some physiological characteristics of the esophagogastric junction in the opossum. Gastroenterology. 1973;64:1119–1125. [PubMed] [Google Scholar]
- Christensen J, Torres EI. Three layers of the opossum stomach: responses to nerve stimulation. Gastroenterology. 1975;69:641–648. [PubMed] [Google Scholar]
- Christophe J. Type I receptors for PACAP (a neuropeptide even more important than VIP. Biochim Biophys Acta. 1993;1154:183–199. doi: 10.1016/0304-4157(93)90011-c. [DOI] [PubMed] [Google Scholar]
- Collman PI, Tremblay L, Diamant NE. The distribution of spinal and vagal sensory neurons that innervate the esophagus of the cat. Gastroenterology. 1992;103:817–822. doi: 10.1016/0016-5085(92)90012-n. [DOI] [PubMed] [Google Scholar]
- Collman PI, Tremblay L, Diamant NE. The central vagal efferent supply to the esophagus and lower esophageal sphincter of the cat. Gastroenterology. 1993;104:1430–1438. doi: 10.1016/0016-5085(93)90352-d. [DOI] [PubMed] [Google Scholar]
- Conklin JL, Du C. Guanylate cyclase inhibitors: effect on inhibitory junction potentials in esophageal smooth muscle. Am J Physiol. 1992;263:G87–G90. doi: 10.1152/ajpgi.1992.263.1.G87. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB, Humphreys CM. Apamin distinguishes two types of relaxation mediated by enteric nerves in the guinea-pig gastrointestinal tract. Naunyn Schmiedebergs Arch Pharmacol. 1986;332:79–88. doi: 10.1007/BF00633202. [DOI] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Chloride-mediated inhibitory junction potentials in opossum esophageal circular smooth muscle. Am J Physiol. 1991;261:G752–G762. doi: 10.1152/ajpgi.1991.261.5.G752. [DOI] [PubMed] [Google Scholar]
- Crowell MD, Zayat EN, Lacy BE, Schettler-Duncan A, Liu MC. The effects of an inhaled beta(2)-adrenergic agonist on lower esophageal function: a dose–response study. Chest. 2001;120:1184–1189. doi: 10.1378/chest.120.4.1184. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Bowes TJ, Jury J. Roles of guanylate cyclase in responses to myogenic and neural nitric oxide in canine lower esophageal sphincter. J Pharmacol Exp Ther. 2002;301:1111–1118. doi: 10.1124/jpet.301.3.1111. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Crankshaw J, Sarna S. Prostaglandins and myogenic control of tension in lower esophageal sphincter in vitro. Prostaglandins. 1979a;17:629–639. doi: 10.1016/0090-6980(79)90014-5. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Helmy-Elkholy A, Jager LP, Kannan MS. Neither a purine nor VIP is the mediator of inhibitory nerves of opossum oesophageal smooth muscle. J Physiol. 1983;336:243–260. doi: 10.1113/jphysiol.1983.sp014579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel EE, Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol. 1984;246:G305–G315. doi: 10.1152/ajpgi.1984.246.3.G305. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Sarna S, Waterfall W, Crankshaw J. Role of endogenous prostaglandins in regulating the tone of opossum lower esophageal sphincter in vivo. Prostaglandins. 1979b;17:641–648. doi: 10.1016/0090-6980(79)90015-7. [DOI] [PubMed] [Google Scholar]
- de Man JG, Pelckmans PA, Boeckxstaens GE, Bult H, Oosterbosch L, Herman AG, et al. The role of nitric oxide in inhibitory non-adrenergic non-cholinergic neurotransmission in the canine lower oesophageal sphincter. Br J Pharmacol. 1991;103:1092–1096. doi: 10.1111/j.1476-5381.1991.tb12305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarino AJ, Cohen S. The adrenergic control of lower esophageal sphincter function. An experimental model of denervation supersensitivity. J Clin Invest. 1973;52:2264–2271. doi: 10.1172/JCI107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds WJ, Dent J, Hogan WJ, Arndorfer RC. Effect of atropine on esophageal motor function in humans. Am J Physiol. 1981;240:G290–G296. doi: 10.1152/ajpgi.1981.240.4.G290. [DOI] [PubMed] [Google Scholar]
- Ekblad E. Pharmacological evidence for both neuronal and smooth muscular PAC1 receptors and a VIP-specific receptor in rat colon. Regul Pept. 1999;85:87–92. doi: 10.1016/s0167-0115(99)00080-4. [DOI] [PubMed] [Google Scholar]
- El-Mahmoudy A, Matsuyama H, Khalifa M, Shimizu Y, Takewaki T. Tachykinins mediate non-adrenergic, non-cholinergic excitatory neurotransmission to the hamster ileum via NK1 and NK2 receptors. Life Sci. 2003;73:1939–1951. doi: 10.1016/s0024-3205(03)00545-9. [DOI] [PubMed] [Google Scholar]
- Estrada O, Lecea B, Aulí M, Farré R, Suñol X, Clave P. Inhibitory purinergic neurotransmission in human lower esophageal sphincter. Neurogastroenterol Motil. 2006;18:780. [Google Scholar]
- Fahrenkrug J, Haglund U, Jodal M, Lundgren O, Olbe L, de Muckadell OB. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. J Physiol. 1978;284:291–305. doi: 10.1113/jphysiol.1978.sp012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YP, Chakder S, Rattan S. Inhibitory effect of zinc protoporphyrin IX on lower esophageal sphincter smooth muscle relaxation by vasoactive intestinal polypeptide and other receptor agonists. J Pharmacol Exp Ther. 1998;285:468–474. [PubMed] [Google Scholar]
- Farre R, Auli M, Lecea B, Estrada O, Sunol X, Clave P. Mechanisms controlling function in the clasp and sling regions of porcine lower oesophageal sphincter. Br J Surg. 2007a;94:1427–1436. doi: 10.1002/bjs.5831. [DOI] [PubMed] [Google Scholar]
- Farre R, Auli M, Lecea B, Martinez E, Clave P. Pharmacologic characterization of intrinsic mechanisms controlling tone and relaxation of porcine lower esophageal sphincter. J Pharmacol Exp Ther. 2006;316:1238–1248. doi: 10.1124/jpet.105.094482. [DOI] [PubMed] [Google Scholar]
- Farre R, Martinez E, Sunyol X, Clave P. Asymmetrical mechanisms controlling resting tone, relaxation and contraction in clasp and sling regions of porcine LES. Gastroenterology. 2004;126:A636–A637. [Google Scholar]
- Farre R, Wang X-Y, Vidal E, Domenech A, Pumarola M, Clave P, et al. Interstitial cells of Cajal and neuromuscular transmission in the rat lower oesophageal sphincter. Neurogastroenterol Motil. 2007b;19:484–496. doi: 10.1111/j.1365-2982.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- Fournet J, Snape WJ, Jr, Cohen S. Sympathetic control of lower esophageal sphincter function in the cat. Action of direct cervical and splanchnic nerve stimulation. J Clin Invest. 1979;63:562–570. doi: 10.1172/JCI109337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JA, Daniel EE. Role of Ca2+ in genesis of lower esophageal sphincter tone and other active contractions. Am J Physiol. 1979;237:E163–E171. doi: 10.1152/ajpendo.1979.237.2.E163. [DOI] [PubMed] [Google Scholar]
- Friedland GW, Kohatsu S, Lewin K. Comparative anatomy of feline and canine gastric sling fibers. Analogy to human anatomy. Am J Dig Dis. 1971;16:493–507. doi: 10.1007/BF02235539. [DOI] [PubMed] [Google Scholar]
- Gaumnitz EA, Bass P, Osinski MA, Sweet MA, Singaram C. Electrophysiological and pharmacological responses of chronically denervated lower esophageal sphincter of the opossum. Gastroenterology. 1995;109:789–799. doi: 10.1016/0016-5085(95)90386-0. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Rattan S, Goyal RK. Pharmacologic identification, activation and antagonism of two muscarine receptor subtypes in the lower esophageal sphincter. J Pharmacol Exp Ther. 1984;230:284–291. [PubMed] [Google Scholar]
- Gonella J, Niel JP, Roman C. Sympathetic control of lower oesophageal sphincter motility in the cat. J Physiol. 1979;287:177–190. doi: 10.1113/jphysiol.1979.sp012653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AA, Farre R, Clave P. Different responsiveness of excitatory and inhibitory enteric motor neurons in the human esophagus to electrical field stimulation and to nicotine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G299–G306. doi: 10.1152/ajpgi.00534.2003. [DOI] [PubMed] [Google Scholar]
- Gonzalez AA, Farre R, Mones J, Capella G, Clave P. Pharmacological and molecular characterization of muscular cholecystokinin receptors in the human lower oesophageal sphincter. Neurogastroenterol Motil. 2000;12:539–546. doi: 10.1046/j.1365-2982.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Goyal RK, Rattan S. Nature of the vagal inhibitory innervation to the lower esophageal sphincter. J Clin Invest. 1975;55:1119–1126. doi: 10.1172/JCI108013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal RK, Rattan S. Genesis of basal sphincter pressure: effect of tetrodotoxin on lower esophageal sphincter pressure in opossum in vivo. Gastroenterology. 1976;71:62–67. [PubMed] [Google Scholar]
- Goyal RK, Rattan S, Said SI. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature. 1980;288:378–380. doi: 10.1038/288378a0. [DOI] [PubMed] [Google Scholar]
- Grider JR, Murthy KS, Jin JG, Makhlouf GM. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am J Physiol. 1992;262:G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- Guelrud M, Rossiter A, Souney PF, Rossiter G, Fanikos J, Mujica V. The effect of vasoactive intestinal polypeptide on the lower esophageal sphincter in achalasia. Gastroenterology. 1992;103:377–382. doi: 10.1016/0016-5085(92)90824-i. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function ISignal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G407–G416. doi: 10.1152/ajpgi.00398.2004. [DOI] [PubMed] [Google Scholar]
- Holloway RH, Hongo M, Berger K, McCallum RW. Gastric distention: a mechanism for postprandial gastroesophageal reflux. Gastroenterology. 1985;89:779–784. doi: 10.1016/0016-5085(85)90572-4. [DOI] [PubMed] [Google Scholar]
- Holloway RH, Kocyan P, Dent J. Provocation of transient lower esophageal sphincter relaxations by meals in patients with symptomatic gastroesophageal reflux. Dig Dis Sci. 1991;36:1034–1039. doi: 10.1007/BF01297443. [DOI] [PubMed] [Google Scholar]
- Holloway RH, Wyman JB, Dent J. Failure of transient lower oesophageal sphincter relaxation in response to gastric distension in patients with achalasia: evidence for neural mediation of transient lower oesophageal sphincter relaxations. Gut. 1989;30:762–767. doi: 10.1136/gut.30.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby PJ, Abrahams TP. Central control of lower esophageal sphincter relaxation. Am J Med. 2000;108 Suppl 4a:90S–98S. doi: 10.1016/s0002-9343(99)00345-9. [DOI] [PubMed] [Google Scholar]
- Huang SC. Protease-activated receptor-1 (PAR1) and PAR2 but not PAR4 mediate relaxations in lower esophageal sphincter. Regul Pept. 2007;142:37–43. doi: 10.1016/j.regpep.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Huber O, Bertrand C, Bunnett NW, Pellegrini CA, Nadel JA, Nakazato P, et al. Tachykinins mediate contraction of the human lower esophageal sphincter in vitro via activation of NK2 receptors. Eur J Pharmacol. 1993;239:103–109. doi: 10.1016/0014-2999(93)90982-n. [DOI] [PubMed] [Google Scholar]
- Imaeda K, Joh T, Yamamoto Y, Itoh M, Suzuki H. Properties of inhibitory junctional transmission in smooth muscle of the guinea pig lower esophageal sphincter. Jpn J Physiol. 1998;48:457–465. doi: 10.2170/jjphysiol.48.457. [DOI] [PubMed] [Google Scholar]
- Jun CH, Lee TS, Sohn UD. NO/cyclic GMP pathway mediates the relaxation of feline lower oesophageal sphincter. Auton Autacoid Pharmacol. 2003;23:159–166. doi: 10.1046/j.1474-8673.2003.00291.x. [DOI] [PubMed] [Google Scholar]
- Jury J, Ahmedzadeh N, Daniel EE. A mediator derived from arginine mediates inhibitory junction potentials and relaxations in lower esophageal sphincter: an independent role for vasoactive intestinal peptide. Can J Physiol Pharmacol. 1992;70:1182–1189. doi: 10.1139/y92-164. [DOI] [PubMed] [Google Scholar]
- Jury J, Patel M, Bowes T, Daniel EE. Actions of putative chloride channel blocking agents on canine lower esophageal sphincter (LES) Can J Physiol Pharmacol. 2001;79:1007–1014. [PubMed] [Google Scholar]
- Kahrilas PJ, Shi G, Manka M, Joehl RJ. Increased frequency of transient lower esophageal sphincter relaxation induced by gastric distention in reflux patients with hiatal hernia. Gastroenterology. 2000;118:688–695. doi: 10.1016/s0016-5085(00)70138-7. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Blackshaw LA, Lehmann A, Dent J. Responses of the rat lower oesophageal sphincter (LOS) to vagal efferent activation. Neurogastroenterol Motil. 1997;9:85–97. doi: 10.1046/j.1365-2982.1997.d01-24.x. [DOI] [PubMed] [Google Scholar]
- Kim CD, Goyal RK, Mashimo H. Neuronal NOS provides nitrergic inhibitory neurotransmitter in mouse lower esophageal sphincter. Am J Physiol. 1999;277:G280–G284. doi: 10.1152/ajpgi.1999.277.2.G280. [DOI] [PubMed] [Google Scholar]
- Kishi M, Takeuchi T, Suthamnatpong N, Ishii T, Nishio H, Hata F, et al. VIP- and PACAP-mediated nonadrenergic, noncholinergic inhibition in longitudinal muscle of rat distal colon: involvement of activation of charybdotoxin- and apamin-sensitive K+ channels. Br J Pharmacol. 1996;119:623–630. doi: 10.1111/j.1476-5381.1996.tb15719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Campbell JD, Carl A, Sanders KM. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. J Physiol. 1995;489 Part 3:735–743. doi: 10.1113/jphysiol.1995.sp021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohjitani A, Shirakawa J, Okada S, Obara H. Effects of various peptides on isolated rabbit lower esophageal sphincter. Peptides. 1996b;17:927–931. doi: 10.1016/0196-9781(96)00137-4. [DOI] [PubMed] [Google Scholar]
- Kohjitani A, Shirakawa J, Okada S, Obara H. Effects of various peptides on isolated rabbit lower esophageal sphincter. Peptides. 1996a;17:927–931. doi: 10.1016/0196-9781(96)00137-4. [DOI] [PubMed] [Google Scholar]
- Konomi H, Meedeniya AC, Simula ME, Toouli J, Saccone GT. Characterization of circular muscle motor neurons of the duodenum and distal colon in the Australian brush-tailed possum. J Comp Neurol. 2002;443:15–26. doi: 10.1002/cne.10094. [DOI] [PubMed] [Google Scholar]
- Konturek JW, Thor P, Lukaszyk A, Gabryelewicz A, Konturek SJ, Domschke W. Endogenous nitric oxide in the control of esophageal motility in humans. J Physiol Pharmacol. 1997;48:201–209. [PubMed] [Google Scholar]
- Kortesova NI, Kimova VS, Bagaev VA, Papasova MP. The mechanism of action of bombesin on cat lower esophageal sphincter. Regul Pept. 1990;29:93–101. doi: 10.1016/0167-0115(90)90072-5. [DOI] [PubMed] [Google Scholar]
- Kortezova N, Mizhorkova Z, Milusheva E, Varga G, Vizi ES, Papasova M. Non-adrenergic non-cholinergic neuron stimulation in the cat lower esophageal sphincter. Eur J Pharmacol. 1996;304:109–115. doi: 10.1016/0014-2999(96)00093-3. [DOI] [PubMed] [Google Scholar]
- Kovac JR, Preiksaitis HG, Sims SM. Functional and molecular analysis of L-type calcium channels in human esophagus and lower esophageal sphincter smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2005;289:G998–G1006. doi: 10.1152/ajpgi.00529.2004. [DOI] [PubMed] [Google Scholar]
- Krysiak PS, Preiksaitis HG. Tachykinins contribute to nerve-mediated contractions in the human esophagus. Gastroenterology. 2001;120:39–48. doi: 10.1053/gast.2001.20910. [DOI] [PubMed] [Google Scholar]
- L'Heureux MC, Muinuddin A, Gaisano HY, Diamant NE. Feline lower esophageal sphincter sling and circular muscles have different functional inhibitory neuronal responses. Am J Physiol Gastrointest Liver Physiol. 2006;290:G23–G29. doi: 10.1152/ajpgi.00303.2005. [DOI] [PubMed] [Google Scholar]
- Lefebvre RA, de Beurme FA, Sas S. Effect of apamin on the responses to VIP, ATP and NANC neurone stimulation in the rat and cat gastric fundus. J Auton Pharmacol. 1991;11:73–83. doi: 10.1111/j.1474-8673.1991.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Liebermann-Meffert D, Allgower M, Schmid P, Blum AL. Muscular equivalent of the lower esophageal sphincter. Gastroenterology. 1979;76:31–38. [PubMed] [Google Scholar]
- Lim I, Gibbons SJ, Lyford GL, Miller SM, Strege PR, Sarr MG, et al. Carbon monoxide activates human intestinal smooth muscle L-type Ca2+ channels through a nitric oxide-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2005;288:G7–G14. doi: 10.1152/ajpgi.00205.2004. [DOI] [PubMed] [Google Scholar]
- Martin CJ, Dodds WJ, Liem HH, Dantas RO, layman RD, Dent J. Diaphragmatic contribution to gastroesophageal competence and reflux in dogs. Am J Physiol. 1992;263:G551–G557. doi: 10.1152/ajpgi.1992.263.4.G551. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- Mearin F, Mourelle M, Guarner F, Salas A, Riveros-Moreno V, Moncada S, et al. Patients with achalasia lack nitric oxide synthase in the gastro–oesophageal junction. Eur J Clin Invest. 1993;23:724–728. doi: 10.1111/j.1365-2362.1993.tb01292.x. [DOI] [PubMed] [Google Scholar]
- Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997;336:924–932. doi: 10.1056/NEJM199703273361306. [DOI] [PubMed] [Google Scholar]
- Montedonico S, Godoy J, Mate A, Possogel AK, ez-Pardo JA, Tovar JA. Muscular architecture and manometric image of gastroesophageal barrier in the rat. Dig Dis Sci. 1999;44:2449–2455. doi: 10.1023/a:1026678820384. [DOI] [PubMed] [Google Scholar]
- Muinuddin A, Chrones T, Preiksaitis H, Diamant NE. Distinct expression of M3 muscarinic receptors in feline lower esophageal sphincter smooth muscle. Neurogastroenterol Motil. 2003;15:21–22. [Google Scholar]
- Muinuddin A, Kang Y, Gaisano HY, Diamant NE. Regional differences in L-type Ca2+ channel expression in feline lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol. 2004b;287:G772–G781. doi: 10.1152/ajpgi.00102.2004. [DOI] [PubMed] [Google Scholar]
- Muinuddin A, Neshatian L, Gaisano HY, Diamant NE. Calcium source diversity in feline lower esophageal sphincter circular and sling muscle. Am J Physiol Gastrointest Liver Physiol. 2004c;286:G271–G277. doi: 10.1152/ajpgi.00291.2003. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay AK. Effect of substance P on the lower esophageal sphincter of the opossum. Gastroenterology. 1978;75:278–282. [PubMed] [Google Scholar]
- Mukhopadhyay AK, Kunnemann M. Mechanism of lower esophageal sphincter stimulation by bombesin in the opossum. Gastroenterology. 1979;76:1409–1414. [PubMed] [Google Scholar]
- Mulsch A, Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990;341:143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Murray J, Du C, Ledlow A, Bates JN, Conklin JL. Nitric oxide: mediator of nonadrenergic noncholinergic responses of opossum esophageal muscle. Am J Physiol. 1991;261:G401–G406. doi: 10.1152/ajpgi.1991.261.3.G401. [DOI] [PubMed] [Google Scholar]
- Ny L, Alm P, Ekstrom P, Hannibal J, Larsson B, Andersson KE. Nitric oxide synthase-containing, peptide-containing, and acetylcholinesterase-positive nerves in the cat lower oesophagus. Histochem J. 1994;26:721–733. doi: 10.1007/BF00158204. [DOI] [PubMed] [Google Scholar]
- Ny L, Alm P, Ekstrom P, Larsson B, Grundemar L, Andersson KE. Localization and activity of haem oxygenase and functional effects of carbon monoxide in the feline lower oesophageal sphincter. Br J Pharmacol. 1996;118:392–399. doi: 10.1111/j.1476-5381.1996.tb15415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny L, Alm P, Larsson B, Ekstrom P, Andersson KE. Nitric oxide pathway in cat esophagus: localization of nitric oxide synthase and functional effects. Am J Physiol. 1995a;268:G59–G70. doi: 10.1152/ajpgi.1995.268.1.G59. [DOI] [PubMed] [Google Scholar]
- Ny L, Larsson B, Alm P, Ekstrom P, Fahrenkrug J, Hannibal J, et al. Distribution and effects of pituitary adenylate cyclase activating peptide in cat and human lower oesophageal sphincter. Br J Pharmacol. 1995b;116:2873–2880. doi: 10.1111/j.1476-5381.1995.tb15939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno N, Xue L, Yamamoto Y, Suzuki H. Properties of the inhibitory junction potential in smooth muscle of the guinea-pig gastric fundus. Br J Pharmacol. 1996;117:974–978. doi: 10.1111/j.1476-5381.1996.tb15290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RB, Matsuda NM, Antoniolli AR, Ballejo G. Evidence for the involvement of nitric oxide in the electrically induced relaxations of human lower esophageal sphincter and distal pylorus. Braz J Med Biol Res. 1992;25:853–855. [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pandolfino JE, Ghosh SK, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying EGJ morphology and relaxation with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1033–G1040. doi: 10.1152/ajpgi.00444.2005. [DOI] [PubMed] [Google Scholar]
- Papasova M.Sphincteric function Handbook of Physiology, The Gastrointestinal System 1989American Physiological Society: Washington, DC; 987–2023.In: Wood JD (ed) [Google Scholar]
- Parkman HP, Reynolds JC. Somatostatin selectively inhibits excitatory contractile pathways of the feline lower esophageal sphincter. Regul Pept. 1990;27:325–334. doi: 10.1016/0167-0115(90)90121-c. [DOI] [PubMed] [Google Scholar]
- Parkman HP, Reynolds JC, Elfman KS, Ogorek CP. Calcitonin gene-related peptide: a sensory and motor neurotransmitter in the feline lower esophageal sphincter. Regul Pept. 1989;25:131–146. doi: 10.1016/0167-0115(89)90255-3. [DOI] [PubMed] [Google Scholar]
- Parkman HP, Reynolds JC, Ogorek CP, Kreider MS. Thyrotropin-releasing hormone: an inhibitory regulatory peptide of feline lower esophageal sphincter. Am J Physiol. 1993;264:G522–G527. doi: 10.1152/ajpgi.1993.264.3.G522. [DOI] [PubMed] [Google Scholar]
- Paterson WG, Anderson MA, Anand N. Pharmacological characterization of lower esophageal sphincter relaxation induced by swallowing, vagal efferent nerve stimulation, and esophageal distention. Can J Physiol Pharmacol. 1992;70:1011–1015. doi: 10.1139/y92-139. [DOI] [PubMed] [Google Scholar]
- Porter AJ, Wattchow DA, Brookes SJ, Costa M. Cholinergic and nitrergic interneurones in the myenteric plexus of the human colon. Gut. 2002;51:70–75. doi: 10.1136/gut.51.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiksaitis HG, Diamant NE. Regional differences in cholinergic activity of muscle fibers from the human gastroesophageal junction. Am J Physiol. 1997;272:G1321–G1327. doi: 10.1152/ajpgi.1997.272.6.G1321. [DOI] [PubMed] [Google Scholar]
- Preiksaitis HG, Tremblay L, Diamant NE. Cholinergic responses in the cat lower esophageal sphincter show regional variation. Gastroenterology. 1994;106:381–388. doi: 10.1016/0016-5085(94)90596-7. [DOI] [PubMed] [Google Scholar]
- Prosser CL, Holzwarth MA, Barr L. Immunocytochemistry of the interstitial cells of Cajal in the rat intestine. J Auton Nerv Syst. 1989;27:17–25. doi: 10.1016/0165-1838(89)90124-0. [DOI] [PubMed] [Google Scholar]
- Rae MG, Muir TC. Neuronal mediators of inhibitory junction potentials and relaxation in the guinea-pig internal anal sphincter. J Physiol. 1996;493 Part 2:517–527. doi: 10.1113/jphysiol.1996.sp021400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal R. Textura del sistema nervioso del hombre y de los vertebrados. Madrid: Imprenta y libreria de Nicolas Moya; 1904. [Google Scholar]
- Ratcliffe EM, deSa DJ, Dixon MF, Stead RH. Choline acetyltransferase (ChAT) immunoreactivity in paraffin sections of normal and diseased intestines. J Histochem Cytochem. 1998;46:1223–1231. doi: 10.1177/002215549804601102. [DOI] [PubMed] [Google Scholar]
- Rattan S. The non-adrenergic non-cholinergic innervation of the esophagus and the lower esophageal sphincter. Arch Int Pharmacodyn Ther. 1986;280:62–83. [PubMed] [Google Scholar]
- Rattan S, Gonnella P, Goyal RK. Inhibitory effect of calcitonin gene-related peptide and calcitonin on opossum esophageal smooth muscle. Gastroenterology. 1988;94:284–293. doi: 10.1016/0016-5085(88)90414-3. [DOI] [PubMed] [Google Scholar]
- Rattan S, Goyal RK. Neural control of the lower esophageal sphincter: influence of the vagus nerves. J Clin Invest. 1974;54:899–906. doi: 10.1172/JCI107829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Goyal RK. Evidence of 5-HT participation in vagal inhibitory pathway to opossum LES. Am J Physiol. 1978;234:E273–E276. doi: 10.1152/ajpendo.1978.234.3.E273. [DOI] [PubMed] [Google Scholar]
- Rattan S, Goyal RK. Evidence against purinergic inhibitory nerves in the vagal pathway to the opossum lower esophageal sphincter. Gastroenterology. 1980;78:898–904. [PubMed] [Google Scholar]
- Rattan S, Goyal RK. Effect of galanin on the opossum lower esophageal sphincter. Life Sci. 1987;41:2783–2790. doi: 10.1016/0024-3205(87)90423-1. [DOI] [PubMed] [Google Scholar]
- Rattan S, Grady M, Goyal RK. Vasoactive intestinal peptide causes peristaltic contractions in the esophageal body. Life Sci. 1982;30:1557–1563. doi: 10.1016/0024-3205(82)90244-2. [DOI] [PubMed] [Google Scholar]
- Rattan S, Moummi C. Influence of stimulators and inhibitors of cyclic nucleotides on lower esophageal sphincter. J Pharmacol Exp Ther. 1989;248:703–709. [PubMed] [Google Scholar]
- Rattan S, Puri RN, Fan YP. Involvement of rho and rho-associated kinase in sphincteric smooth muscle contraction by angiotensin II. Exp Biol Med (Maywood) 2003;228:972–981. doi: 10.1177/153537020322800814. [DOI] [PubMed] [Google Scholar]
- Richardson BJ, Welch RW. Differential effect of atropine on rightward and leftward lower esophageal sphincter pressure. Gastroenterology. 1981;81:85–89. [PubMed] [Google Scholar]
- Richter JE, Wu WC, Johns DN, Blackwell JN, Nelson JL, III, Castell JA, et al. Esophageal manometry in 95 healthy adult volunteers. Variability of pressures with age and frequency of ‘abnormal' contractions. Dig Dis Sci. 1987;32:583–592. doi: 10.1007/BF01296157. [DOI] [PubMed] [Google Scholar]
- Rossiter CD, Norman WP, Jain M, Hornby PJ, Benjamin S, Gillis RA. Control of lower esophageal sphincter pressure by two sites in dorsal motor nucleus of the vagus. Am J Physiol. 1990;259:G899–G906. doi: 10.1152/ajpgi.1990.259.6.G899. [DOI] [PubMed] [Google Scholar]
- Rozsai B, Lazar Z, Benko R, Bartho L. Inhibition of the NANC relaxation of the guinea-pig proximal colon longitudinal muscle by the purinoceptor antagonist PPADS, inhibition of nitric oxide synthase, but not by a PACAP/VIP antagonist. Pharmacol Res. 2001;43:83–87. doi: 10.1006/phrs.2000.0742. [DOI] [PubMed] [Google Scholar]
- Salapatek AM, Lam A, Daniel EE. Calcium source diversity in canine lower esophageal sphincter muscle. J Pharmacol Exp Ther. 1998;287:98–106. [PubMed] [Google Scholar]
- Sandler AD, Maher JW, Weinstock JV, Schmidt CD, Schlegel JF, Jew JY, et al. Tachykinins in the canine gastroesophageal junction. Am J Surg. 1991;161:165–170. doi: 10.1016/0002-9610(91)90379-r. [DOI] [PubMed] [Google Scholar]
- Sarma DN, Banwait K, Basak A, DiMarino AJ, Rattan S. Inhibitory effect of beta3-adrenoceptor agonist in lower esophageal sphincter smooth muscle: in vitro studies. J Pharmacol ExpTher. 2003;304:48–55. doi: 10.1124/jpet.102.040501. [DOI] [PubMed] [Google Scholar]
- Seelig LL, Jr, Doody P, Brainard L, Gidda JS, Goyal RK. Acetylcholinesterase and choline acetyltransferase staining of neurons in the opossum esophagus. Anat Rec. 1984;209:125–130. doi: 10.1002/ar.1092090115. [DOI] [PubMed] [Google Scholar]
- Shahin W, Murray JA, Clark E, Conklin JL. Role of cGMP as a mediator of nerve-induced motor functions of the opossum esophagus. Am J Physiol Gastrointest Liver Physiol. 2000;279:G567–G574. doi: 10.1152/ajpgi.2000.279.3.G567. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CW, Murphy R, Furness JB, Pompolo S. Comparison of the presence and actions of substance P and neurokinin A in guinea-pig taenia coli. Neuropeptides. 1991;19:23–34. doi: 10.1016/0143-4179(91)90070-y. [DOI] [PubMed] [Google Scholar]
- Sifrim D, Lefebvre R. Role of nitric oxide during swallow-induced esophageal shortening in cats. Dig Dis Sci. 2001;46:822–830. doi: 10.1023/a:1010760619615. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(−/−) and hypotensive in W/W(v) mutant mice. Gastroenterology. 2001;121:34–42. doi: 10.1053/gast.2001.25541. [DOI] [PubMed] [Google Scholar]
- Smid SD, Blackshaw LA. Neuromuscular function of the human lower oesophageal sphincter in reflux disease and Barrett's oesophagus. Gut. 2000a;46:756–761. doi: 10.1136/gut.46.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid SD, Blackshaw LA. Vagal ganglionic and nonadrenergic noncholinergic neurotransmission to the ferret lower oesophageal sphincter. Auton Neurosci. 2000b;86:30–36. doi: 10.1016/S1566-0702(00)00210-1. [DOI] [PubMed] [Google Scholar]
- Sohn UD, Harnett KM, De PG, Behar J, Biancani P. Distinct muscarinic receptors, G proteins and phospholipases in esophageal and lower esophageal sphincter circular muscle. J Pharmacol Exp Ther. 1993;267:1205–1214. [PubMed] [Google Scholar]
- Soto C, Qi B, Diez-Pardo JA, Tovar JA. Identification of diaphragmatic crural component of gastroesophageal barrier in the rat. Dig Dis Sci. 1997;42:2420–2425. doi: 10.1023/a:1018831705342. [DOI] [PubMed] [Google Scholar]
- Stein HJ, Liebermann-Meffert D, DeMeester TR, Siewert JR. Three-dimensional pressure image and muscular structure of the human lower esophageal sphincter. Surgery. 1995;117:692–698. doi: 10.1016/s0039-6060(95)80014-x. [DOI] [PubMed] [Google Scholar]
- Szewczak SM, Behar J, Billett G, Hillemeier C, Rhim BY, Biancani P. VIP-induced alterations in cAMP and inositol phosphates in the lower esophageal sphincter. Am J Physiol. 1990;259:G239–G244. doi: 10.1152/ajpgi.1990.259.2.G239. [DOI] [PubMed] [Google Scholar]
- Szymanski PT, Chacko TK, Rovner AS, Goyal RK. Differences in contractile protein content and isoforms in phasic and tonic smooth muscles. Am J Physiol. 1998;275:C684–C692. doi: 10.1152/ajpcell.1998.275.3.C684. [DOI] [PubMed] [Google Scholar]
- Tian ZQ, Liu JF, Wang GY, Li BQ, Wang FS, Wang QZ, et al. Responses of human clasp and sling fibers to neuromimetics. J Gastroenterol Hepatol. 2004;19:440–447. doi: 10.1111/j.1440-1746.2003.03307.x. [DOI] [PubMed] [Google Scholar]
- Tomita R, Tanjoh K, Munakata K. The role of motilin and cisapride in the enteric nervous system of the lower esophageal sphincter in humans. Surg Today. 1997;27:985–992. doi: 10.1007/BF02385776. [DOI] [PubMed] [Google Scholar]
- Torphy TJ, Fine CF, Burman M, Barnette MS, Ormsbee HS., III Lower esophageal sphincter relaxation is associated with increased cyclic nucleotide content. Am J Physiol. 1986;251:G786–G793. doi: 10.1152/ajpgi.1986.251.6.G786. [DOI] [PubMed] [Google Scholar]
- Tottrup A, Forman A, Funch-Jensen P, Raundahl U, Andersson KE. Effects of postganglionic nerve stimulation in oesophageal achalasia: an in vitro study. Gut. 1990a;31:17–20. doi: 10.1136/gut.31.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottrup A, Forman A, Madsen G, Andersson KE. The actions of some beta-receptor agonists and xanthines on isolated muscle strips from the human oesophago–gastric junction. Pharmacol Toxicol. 1990b;67:340–343. doi: 10.1111/j.1600-0773.1990.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Tottrup A, Ny L, Alm P, Larsson B, Forman A, Andersson KE. The role of the L-arginine/nitric oxide pathway for relaxation of the human lower oesophageal sphincter. Acta Physiol Scand. 1993;149:451–459. doi: 10.1111/j.1748-1716.1993.tb09642.x. [DOI] [PubMed] [Google Scholar]
- Tottrup A, Svane D, Forman A. Nitric oxide mediating NANC inhibition in opossum lower esophageal sphincter. Am J Physiol. 1991;260:G385–G389. doi: 10.1152/ajpgi.1991.260.3.G385. [DOI] [PubMed] [Google Scholar]
- Uc A, Murray JA, Conklin JL. Effects of calcitonin gene-related peptide on opossum esophageal smooth muscle. Gastroenterology. 1997;113:514–520. doi: 10.1053/gast.1997.v113.pm9247471. [DOI] [PubMed] [Google Scholar]
- Uc A, Oh ST, Murray JA, Clark E, Conklin JL. Biphasic relaxation of the opossum lower esophageal sphincter: roles of NO., VIP, and CGRP. Am J Physiol. 1999;277:G548–G554. doi: 10.1152/ajpgi.1999.277.3.G548. [DOI] [PubMed] [Google Scholar]
- Uddman R, Luts A, Absood A, Arimura A, Ekelund M, Desai H, et al. PACAP, a VIP-like peptide, in neurons of the esophagus. Regul Pept. 1991;36:415–422. doi: 10.1016/0167-0115(91)90074-q. [DOI] [PubMed] [Google Scholar]
- Velkova V, Papasova M, Radomirov R. Effects of PGE1 and PGF2 alpha on the responses of gastrointestinal sphincters to field stimulation. Eur J Pharmacol. 1981;75:297–303. doi: 10.1016/0014-2999(81)90557-4. [DOI] [PubMed] [Google Scholar]
- Vicente Y, Da RC, Yu J, Hernandez-Peredo G, Martinez L, Perez-Mies B, et al. Architecture and function of the gastroesophageal barrier in the piglet. Dig Dis Sci. 2001;46:1899–1908. doi: 10.1023/a:1010631030320. [DOI] [PubMed] [Google Scholar]
- Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- Watkins CC, Boehning D, Kaplin AI, Rao M, Ferris CD, Snyder SH. Carbon monoxide mediates vasoactive intestinal polypeptide-associated nonadrenergic/noncholinergic neurotransmission. Proc Natl Acad Sci USA. 2004;101:2631–2635. doi: 10.1073/pnas.0308695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Valdez D, Collman PI, Diamant NE. Effects of nitric oxide synthase blockade on esophageal peristalsis and the lower esophageal sphincter in the cat. Can J Physiol Pharmacol. 1996;74:1249–1257. doi: 10.1139/cjpp-74-11-1249. [DOI] [PubMed] [Google Scholar]
- Yamato S, Saha JK, Goyal RK. Role of nitric oxide in lower esophageal sphincter relaxation to swallowing. Life Sci. 1992;50:1263–1272. doi: 10.1016/0024-3205(92)90326-k. [DOI] [PubMed] [Google Scholar]
- Yip L, Kwok YN, Buchan AM. Cellular localization and distribution of neurokinin-1 receptors in the rat stomach. Auton Neurosci. 2003;104:95–108. doi: 10.1016/S1566-0702(02)00293-X. [DOI] [PubMed] [Google Scholar]
- Yuan S, Brookes SJ. Neuronal control of the gastric sling muscle of the guinea pig. J Comp Neurol. 1999;412:669–680. doi: 10.1002/(sici)1096-9861(19991004)412:4<669::aid-cne8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Yuan S, Costa M, Brookes SJ. Neuronal pathways and transmission to the lower esophageal sphincter of the guinea Pig. Gastroenterology. 1998;115:661–671. doi: 10.1016/s0016-5085(98)70145-3. [DOI] [PubMed] [Google Scholar]
- Yunker AM, Krause JE, Roth KA. Neurokinin B- and substance P-like immunoreactivity are co-localized in enteric nerves of rat ileum. Regul Pept. 1999;80:67–74. doi: 10.1016/s0167-0115(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Miller DV, Paterson WG. Opposing roles of K(+) and Cl(−) channels in maintenance of opossum lower esophageal sphincter tone. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1226–G1234. doi: 10.1152/ajpgi.2000.279.6.G1226. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG. Nitric oxide contracts longitudinal smooth muscle of opossum oesophagus via excitation–contraction coupling. J Physiol. 2001;536:133–140. doi: 10.1111/j.1469-7793.2001.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Paterson WG. Role of sarcoplasmic reticulum in control of membrane potential and nitrergic response in opossum lower esophageal sphincter. Br J Pharmacol. 2003;140:1097–1107. doi: 10.1038/sj.bjp.0705537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Vogalis F, Goyal RK. Nitric oxide suppresses a Ca(2+)-stimulated Cl− current in smooth muscle cells of opossum esophagus. Am J Physiol. 1998;274:G886–G890. doi: 10.1152/ajpgi.1998.274.5.G886. [DOI] [PubMed] [Google Scholar]
- Zizzo MG, Mule F, Serio R. Interplay between PACAP and NO in mouse ileum. Neuropharmacology. 2004;46:449–455. doi: 10.1016/j.neuropharm.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Zizzo MG, Mule F, Serio R. Mechanisms underlying the inhibitory effects induced by pituitary adenylate cyclase-activating peptide in mouse ileum. Eur J Pharmacol. 2005;521:133–138. doi: 10.1016/j.ejphar.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Zwick R, Bowes KL, Daniel EE, Sarna SK. Mechanism of action of pentagastrin on the lower esophageal sphincter. J Clin Invest. 1976;57:1644–1651. doi: 10.1172/JCI108435. [DOI] [PMC free article] [PubMed] [Google Scholar]