Abstract
Background and purpose:
Reactive oxygen species (ROS) have been postulated to play a crucial role in the pathogenesis of ischaemia-reperfusion injury. Among these, hydrogen peroxide (H2O2) is known to be a toxic compound responsible for free-radical-dependent neuronal damage. In recent years, however, the ‘bad reputation' of H2O2 and other ROS molecules has changed. The aim of this study was to assess the protective role of H2O2 and modification in its endogenous production on the electrophysiological and morphological changes induced by oxygen/glucose deprivation (OGD) on CA1 hippocampal neurons.
Experimental approach:
Neuroprotective effects of exogenous and endogenous H2O2 were determined using extracellular electrophysiological recordings of field excitatory post synaptic potentials (fEPSPs) and morphological studies in a hippocampal slice preparation. In vitro OGD was delivered by switching to an artificial cerebrospinal fluid solution with no glucose and with oxygen replaced by nitrogen.
Key results:
Neuroprotection against in vitro OGD was observed in slices treated with H2O2 (3 mM). The rescuing action of H2O2 was mediated by catalase as pre-treatment with the catalase inhibitor 3-amino-1,2,4-triazole blocked this effect. More interestingly, we showed that an increase of the endogenous levels of H2O2, due to a combination of an inhibitor of the glutathione peroxidase enzyme and addition of Cu,Zn-superoxide dismutase in the tissue bath, prevented the OGD-induced irreversible depression of fEPSPs.
Conclusions and implications:
Taken together, our results suggest new possible strategies to lessen the damage produced by a transient brain ischaemia by increasing the endogenous tissue level of H2O2.
Keywords: hydrogen peroxide, ischaemia, electrophysiology, hippocampus, neuroprotection
Introduction
The brain requires a continuous supply of oxygen and glucose to maintain function. Deprivation of energy sources following stroke, global ischaemia or respiratory failure, can lead rapidly to transient or permanent injury of neurons by affecting the cells' energy requirements, pump function or membrane integrity (Lo et al., 2003). The selective vulnerability of pyramidal neurons in the hippocampal subregion CA1 is one of the hallmarks of the rodent models of global cerebral ischaemia (Kirino, 1982; Pulsinelli et al., 1982). In humans, a similar pattern, with selective and delayed degeneration of CA1 neurons, has been documented in autopsy studies on survivors after a cardiac arrest (Petito et al., 1987; Horn and Schlote, 1992). Although reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), superoxide anion (O2−) and/or hydroxyl radical (OH−), have been shown to cause damage to protein, lipids and nucleic acids and may contribute to the harmful side effect of hypoxia/reoxygenation (Traystman et al., 1991), there is also evidence that ROS have normal regulatory actions and play a major role in the modulation of cellular signal transduction cascades in a variety of physiological processes (Sen and Packer, 1996; Finkel, 2003). Hydrogen peroxide is a diffusible molecule that can be synthesized and destroyed rapidly in response to external stimuli, and, only recently, it has become clear that it meets all the important criteria for an intracellular messenger for signal transduction and signal amplification (Rhee et al., 2005; Stone and Yang, 2006) and acts as an ubiquitous second messenger under subtoxic conditions (Finkel, 1998; Rhee et al., 2000). Moreover, H2O2 can modulate synaptic transmission (Pellmar, 1987; Chen et al., 2001) and synaptic plasticity (Auerbach and Segal, 1997; Klann and Thiels, 1999).
Several antioxidant mechanisms serve to counterbalance the potential deleterious effects of ROS. Among these, the enzymatic scavengers superoxide dismutase (SOD), catalase and glutathione peroxidases (GPXs) (Brigelius-Flohe, 1999) are responsible for the balance of formation and conversion of H2O2, maintaining the intracellular concentration of H2O2 at a constant level (Halliwell, 1999). When these downstream enzymatic pathways are impaired and H2O2 is not degraded, cellular metabolism may be affected. However, in conditions of low oxygen supply, such that occurring during hypoxia or ischaemia, metabolic degradation of H2O2 through catalase results in production of H2O and O2, therefore providing an alternative source for O2 (Auerbach and Segal, 1997; Klann and Thiels, 1999). Indeed, previous observations in vitro have shown that H2O2 exerts a protective role by restoring synaptic transmission after an episode of hypoxia in the hippocampus (Fowler, 1997), and that it may act as a supplementary source of O2 in the spinal cord (Walton and Fulton, 1983). More recently, it has been suggested that endogenous H2O2 plays an important cardioprotective (Yaguchi et al., 2003) and neuroprotective role in several in vitro models of ischaemic preconditioning (Furuichi et al., 2005; Xiao-Qing et al., 2005). We also have recently shown a protective role of H2O2 in oxygen-deprived dopaminergic neurons of the rat substantia nigra (Geracitano et al., 2005). The goal of the present study was to evaluate the neuroprotective role of exogenous H2O2 and the potential of modifying the activity of key enzymes (Cu,Zn-SOD, catalase and GPX) involved in the endogenous production and degradation of H2O2. To this aim, we used both electrophysiological and morphological techniques, in an in vitro model of hippocampal ischaemia.
Methods
Brain slice preparation
All animal procedures were in compliance with the European Council Directive (86/609/EEC). Male Wistar rats, (140–180 g body weight) under anaesthesia with halothane, were killed by decapitation. Their hippocampi were rapidly removed and placed in an ice-cold, oxygenated (95% O2 to 5% CO2), artificial cerebral spinal fluid (ACSF) of the following composition (mM): 126 NaCl, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, 24 NaHCO3 and 10 glucose. Parasagittal slices (400 μm thickness) were cut using a vibratome and kept in oxygenated ACSF for at least 1 h at room temperature.
Extracellular recordings
A single slice was placed on a nylon mesh, completely submerged in a small chamber (0.5 ml) and superfused with oxygenated ACSF (∼30 °C) at a constant flow rate of 3 ml min−1. Test pulses (80 ms, 0.06 Hz) were delivered through a bipolar nichrome electrode positioned in the stratum radiatum. Evoked extracellular potentials were recorded with glass microelectrodes (2–10 MΩ), filled with 3 M NaCl, placed in the CA1 region of the stratum radiatum. In vitro oxygen/glucose deprivation (OGD) was obtained by perfusing the slice with ACSF without glucose and gassed with nitrogen (95% N2 to 5% CO2). At the end of the ischaemic period, the slice was again superfused with normal (glucose-containing) oxygenated ACSF. Hypoglycaemic solutions were obtained by omitting glucose from standard ACSF.
Morphological studies
After the recording session, slices were stained with Cresyl Fast Violet solution to evaluate the number of cells irreversibly damaged either by the handling procedures alone (control) or by the putative ischaemic insult (untreated and treated with 3 mM H2O2). Immediately after each treatment, slices were collected from the nylon mesh and placed for 30 min in normal ACSF warmed to 35 °C, and saturated with 95% O2, 5% CO2. This time window was chosen for all groups to allow sufficient time for recovery after each treatment. Then, they were fixed overnight in 4% paraformaldehyde in 0.1 M phosphate buffer, washed and dehydrated in 30% sucrose solution for 3 h at room temperature. Finally, the slices were frozen in liquid nitrogen and cut (20 μm thickness) with a cryostat in serial sections. Sections were subsequently mounted on slides, stained with Cresyl Fast Violet and analysed under light microscopy (Zeiss-Axioplan 2). Neuronal cells count was performed blind and concerned the CA1 pyramidal cell layer of the hippocampal formation. A 25-μm2 grid was positioned on identical areas of the hippocampus in each section (× 40 objective) and the viable cells were counted in two fields of each brain section.
Statistical analysis
Data are expressed as mean±s.e.mean and were tested for statistical significance with a paired, two-tailed Student's t-test or by one-way ANOVA followed by Tukey–Kramer multiple comparisons test, as appropriate. When significant differences were observed, Newman–Keuls multiple comparison test was inferred. A value of P<0.01 was considered significant.
Drugs
All the compounds were obtained from Sigma-Aldrich (Milan, Italy). H2O2 was diluted daily from a 30% stock solution. The concentration of the stock solution was 8.8 M.
Results
The effect of different periods of OGD on hippocampal synaptic transmission
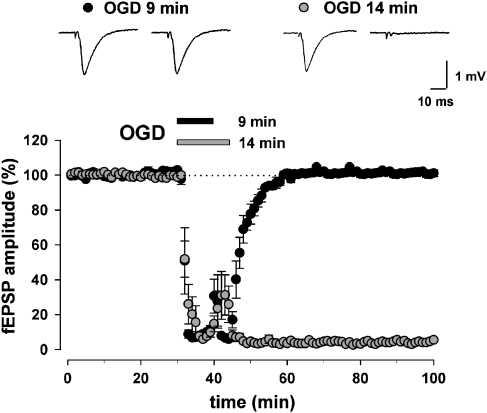
The ability of hippocampal slices to recover synaptic function on return of normal oxygenation depends on the duration of the OGD; the longer the OGD lasted, the less synaptic function could recover. Figure 1 illustrates the time course of the field excitatory postsynaptic potential (fEPSP) depression caused by OGD applied for a period of 9 and 14 min. OGD for 9 min caused a transient fEPSP depression that was always reversible after returning to normal oxygenated ACSF (101±1%, n=9, paired Student's t-test P<0.0001). During OGD, the amplitude of the field potential rapidly decreased and in 5 min was almost abolished. Prolonging the OGD to 11 min caused a loss of the field potentials in 50% of the experiments recorded (6/12, data not shown). Therefore, 14 min of OGD, which always provided an irreversible loss of the field potentials (2±0.09%, n=15, paired Student's t-test P<0.0001), was chosen as the standard ischaemic insult to test, in subsequent experiments, the neuroprotective potential of various compounds. After 14 min of OGD, no recovery of the field potentials was observed during reoxygenation up to 180-min perfusion in normal ACSF (data not shown). Towards the end of the OGD, a transient reappearance of the fEPSP was often observed, being ascribed to the increase in extracellular potassium concentration (∼8 mM) caused by the anoxic episode (Sick et al., 1987).
Figure 1.
Effects of oxygen/glucose deprivation (OGD) on field excitatory postsynaptic potentials (fEPSPs) evoked by electrical stimulation of the stratum radiatum in the CA1 hippocampal region. The recovery of fEPSP amplitude following 9 min (n=9) or 14 min (n=15) of OGD. Exposure to 9 min of OGD (black circles) causes a transient fEPSP depression that was always reversible after returning to normal oxygenated artificial cerebral spinal fluid (ACSF), whereas exposure to 14 min of OGD (grey circles) caused an fEPSP depression that was always irreversible even after a prolonged re-exposure to normal oxygenated ACSF. Each point represents fEPSP amplitude expressed as percentage of the mean baseline responses recorded before OGD application. Bars indicate the time duration of OGD. Insets display representative traces immediately before the OGD and at the end of the experiment.
Neurotoxic effect vs neuroprotective effect of exogenous H2O2 on fEPSPs
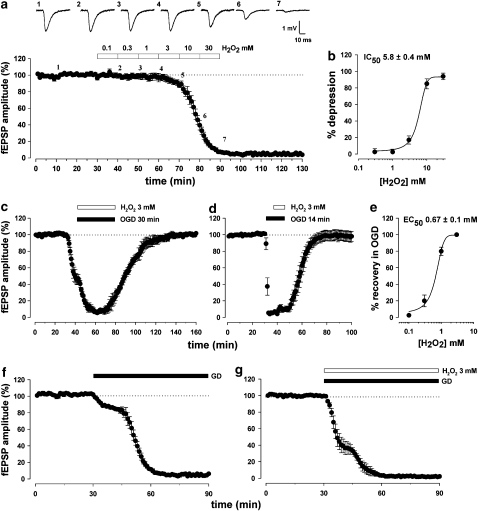
In the present study, we examined the effect of H2O2 added to the bathing solution on the synaptic responses recorded from rat hippocampal slices in control conditions and during OGD. In the CA1 region, the sensitivity of fEPSPs to H2O2 was assessed by applying increasing concentrations of this compound. H2O2 irreversibly depressed the synaptic AMPA receptor-mediated response with an IC50 value of 5.8±0.4 mM (n=4; Figures 2a and b), suggesting that H2O2 is a neurotoxic agent in normoxic conditions. Conversely, as illustrated in Figure 2c, exposure to combined OGD and H2O2 (3 mM) resulted in a transient suppression of the fEPSP to 6±3%, and a subsequent return to 101±1% (n=6, paired Student's t-test P<0.0001) after 60 min reoxygenation. Notably, H2O2 was able to confer neuroprotection even when the duration of OGD was extended up to 30 min. Surprisingly, H2O2 was able to rescue the irreversible loss of fEPSP, even when applied 7 min after OGD had started, highlighting its potent neuroprotective effect once the ischaemic conditions had been imposed. In fact, fEPSP fully recovered to 97.4±6% (n=6, paired Student's t-test P<0.0001, Figure 2d). The EC50 value of H2O2-mediated fEPSPs recovery in OGD was 0.67±0.1 mM (n=4; Figure 2e). For our experiments we used 3 mM H2O2, a concentration that always resulted in neuroprotection.
Figure 2.
The effects of hydrogen peroxide (3 mM) on field excitatory postsynaptic potentials (fEPSPs) in the CA1 region of the hippocampus. (a) Pooled data from four experiments showing the effects of increasing concentrations of H2O2 on the synaptic depression of fEPSPs. (b) Concentration–response curve (n=4) showing the peak synaptic fEPSP depression vs [H2O2]. (c–d) Pooled data from six experiments showing the protective effect of H2O2 (3 mM) over a 30-min oxygen/glucose deprivation (OGD) exposure and its ability to rescue synaptic transmission even, when applied after OGD administration. (e) Concentration–response curve (n=4) showing the peak synaptic fEPSP recovery vs [H2O2] during OGD. (f) Pooled data from six experiments showing the complete loss of fEPSPs as a consequence of perfusing the slices in a glucose-deprived (GD) medium. (g) The same effect was insensitive to treatment with H2O2 (3 mM).
H2O2 specifically counteracted hypoxia and did not oppose the effects of hypoglycaemia
The CA1 pyramidal neurons are among the most vulnerable in the brain to hypoglycaemic stress. Although studies on brain slices have demonstrated that exposure to glucose-free medium enhances glutamate and aspartate release (Burke and Nadler, 1989), synaptic transmission inevitably fails during hypoglycaemia (Fowler, 1993). Our next step was to test whether H2O2 may also prevent the loss of synaptic function induced by a period of glucose deprivation (GD). As shown in Figure 4, perfusion of slices in a glucose-free ACSF caused a continually decaying loss of the field potentials that was complete after 30 min (6±1%, n=6, paired Student's t-test P<0.0001, Figure 2f). However, in contrast to what we found during OGD, no recovery was observed following perfusion with H2O2 (3 mM) in aglycaemic ACSF (2±0.5%, n=5, paired Student's t-test P<0.0001, Figure 2g), suggesting that hydrogen peroxide exerts its protective role against OGD by compensating for the lack of oxygen. The loss of fEPSP observed in the presence of H2O2 occurred even more rapidly, confirming that hydrogen peroxide may paradoxically result a toxic compound in a normoxic environment. In both conditions, the GD-mediated loss of fEPSPs was irreversible following reperfusion with normoglycaemic ACSF (data not shown).
Morphological studies of the protective effect of H2O2
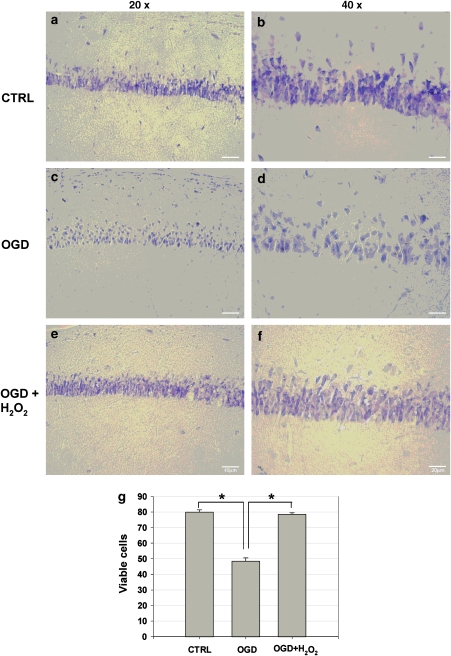
The protective effect of H2O2 observed with the electrophysiological in vitro model of cerebral ischaemia was also confirmed by histological experiments. We analysed viable CA1 cells in hippocampal slices subdivided into three experimental groups (n=6): control slices, slices exposed to ischaemic ACSF and slices exposed to ischaemic ACSF treated with H2O2 (3 mM). In the control group, the viable neurons in the pyramidal cell layer of the CA1 hippocampal area show a good preservation of cell morphology (Figures 3a and b). In contrast, in the ischaemic group, the damaged neurons could be easily detected as round cell bodies with no distinction between cytoplasm and nucleus (Figures 3c and d). Interestingly, treatment with H2O2 in ischaemic conditions minimized neuronal damage caused by OGD alone (Figures 3e and f). Quantitative statistical analysis carried out by counting viable cells showed that the CA1 region of slices exposed to 14 min of OGD exhibited a marked decline in viable neurons when compared to control animals (Figure 3g). However, histological examinations revealed that H2O2 was able to rescue neurons that were otherwise destined to degenerate during OGD (Figure 3g).
Figure 3.
Representative photomicrographs showing the protective effect of H2O2 (3 mM) against 14 min of oxygen/glucose deprivation (OGD). (a–f) Photomicrographs of rat hippocampal sections stained with Cresyl Violet; (a, c and e) low magnification (× 20); (b, d and f) high magnification (× 40). (a and b) Refer to slices taken from a control animal; (c and d) refer to slices treated with 14 min of OGD; finally, (e and f) show the effect of pretreatment with H2O2 (3 mM). Slices were post-fixed 30 min after each treatment. (g) Density of Cresyl Violet viable cells per unit test area (25 μm2) in the CA1 hippocampal area. Slices exposed for 14 min to OGD contained more damaged cells than control slices (OGD-treated vs untreated, *P<0.001, n=6). A pretreatment with H2O2 (3 mM) significantly reduced the severity of cell damage after OGD compared with the untreated ischaemic slices (H2O2-treated vs OGD-treated, *P<0.001, n=6).
Catalase mediates the protective effect of H2O2
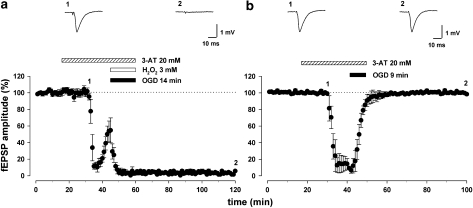
We then investigated whether the protective effect of H2O2 was secondary to intracellular generation of O2 through the catalase pathway (Llinas and Sugimori, 1980; Walton and Fulton, 1983). To this aim, we evaluated the consequence of H2O2 administration on 14 min of OGD in the continuous presence of the irreversible catalase inhibitor 3-amino-1,2,4-triazole (3-AT; 20 mM). Slices were preincubated with 3-AT (20 mM) for ∼60 min, and were continuously perfused with this inhibitor during recordings. Under these conditions, the recovery of synaptic transmission usually provided by the presence of H2O2 (3 mM) during exposure to 14 min of OGD was completely abolished (with 3-AT, 1±1%; without 3-AT, 101±1%, n=6, paired Student's t-test P<0.0001; Figure 4a), suggesting that catalase mediated the protective effect of H2O2. Next, we wanted to ascertain that 3-AT did not accentuate per se the ischaemic damage, and hence, block the protective effect of H2O2. For this, we added 3-AT (20 mM) alone to slices exposed to 9 min of OGD, an insult from which the fEPSPs always recover (see Figure 1). As shown in Figure 4b, synaptic transmission still fully recovered to baseline following this procedure with 3-AT (101±1%, n=6, paired Student's t-test P<0.0001).
Figure 4.
Catalase is involved in the protective effect of H2O2. (a) Pooled data from six experiments showing that the protective effect of H2O2 (3 mM) was prevented by superfusion, both in pretreatment and during oxygen/glucose deprivation (OGD), with the catalase inhibitor 3-amino-1,2,4-triazole (3-AT; 20 mM), suggesting that the protective role of H2O2 was mediated by catalase. The field excitatory postsynaptic potential (fEPSP) traces were taken at the time points indicated by numbers in the corresponding graph. (b) Treatment of slices with 3-AT alone was not able to alter fEPSPs recorded from slices exposed to 9 min of OGD compared to control conditions (n=6).
Pharmacological strategies aimed to increase the endogenous levels of H2O2 prevent OGD-induced irreversible depression of synaptic transmission
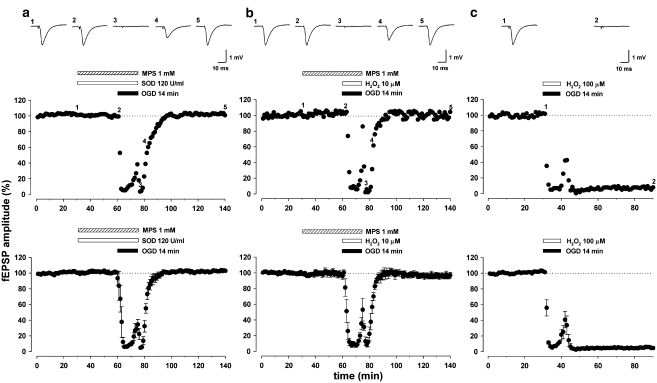
Given that H2O2 exhibited a strong neuroprotective effect against OGD, in a subsequent set of experiments, we aimed to pharmacologically enhance the endogenous levels of H2O2, thus favouring the catalase pathway. We achieved this enhancement by blocking the GPX pathway with mercaptosuccinate (MPS, 1 mM) or by applying exogenous Cu,Zn-SOD (120 U ml−1), the generator of H2O2. Various degrees of success and failure were obtained in neuroprotection when either of these treatments was used alone. In our experiments, exogenous Cu,Zn-SOD (120 U ml−1), when added to the bathing solution of hippocampal slices, was able to prevent only four times out of seven (data not shown) the irreversible loss of synaptic transmission typically obtained following 14 min of OGD exposure. A comparable variability of response was seen when applying MPS (1 mM) alone (protection occurring in five out of seven slices). The reasons for such failures are not clear, but could be related to various factors including the difficulty of these molecules to cross the plasmalemmal membrane. Interestingly, when Cu,Zn-SOD (120 U ml−1) was applied together with MPS (1 mM), both in pretreatment and during the 14-min OGD, the irreversible loss of synaptic transmission usually obtained with 14-min OGD was always prevented (103±0,5 %, n=6, paired Student's t-test P<0.0001; Figure 5a). Furthermore, full recovery of synaptic transmission after 14-min OGD was also observed when applying MPS (1 mM), with micromolar doses of hydrogen peroxide (H2O2, 10 μM), both in pretreatment and during OGD (96±3%, n=6, paired Student's t-test P<0.0001; Figure 5b). This may suggest that the required amount of endogenous H2O2, supposedly protective during OGD, could not be reached by applying either MPS or Cu,Zn-SOD alone, but only by their combined interaction. However, H2O2 alone, even when applied at a 10-fold higher concentration (100 μM), failed to restore synaptic transmission after 14 min of OGD (5±2%, n=6, paired Student's t-test P<0.0001; Figure 5c).
Figure 5.
Pharmacological strategies aimed to increase the endogenous production of H2O2. (a) A single example (upper graph) and pooled data from six experiments (lower graph) showing the protective effect of mercaptosuccinate (MPS; 1 mM) and superoxide dismutase (SOD; 120 U ml) on the irreversible field excitatory postsynaptic potential (fEPSP) depression induced by 14 min of oxygen/glucose deprivation (OGD). Bars indicate the duration of exposure to the compounds and the OGD. The fEPSP traces were taken at the time points indicated by numbers in the corresponding graph. Each point represents fEPSP amplitude expressed as percentage of the mean baseline responses recorded before drug application. Data in the lower graph, expressed as percentage of baseline values, are means±s.e.mean. (b) A single example (upper graph) and pooled data from six experiments (lower graph) showing the recovery of synaptic transmission after 14 min of OGD when applying a combination of MPS (1 mM) and a low concentration of H2O2 (10 μM), both in pretreatment and during OGD. (c) A single example (upper graph) and pooled data from six experiments (lower graph) indicating how a higher concentration of H2O2 (100 μM) was unable to prevent the irreversible loss of synaptic transmission following 14 min of OGD.
Discussion and conclusions
Since its discovery, H2O2 has been known as a toxic agent for human tissues. However, in the light of recent findings, its role should now be re-evaluated. In fact, this molecule, despite its bad reputation, is now being recognized as an endogenous molecule of life, which is essential for the proper development and proliferation of cells. Therefore, a low concentration of H2O2 is vital for some physiological processes, while a high concentration is toxic for human cells. The enzyme catalase is the main regulator of hydrogen peroxide metabolism and, due to its structure and function, is very effective in degrading toxic concentrations of hydrogen peroxide, producing H2O and O2, without changing its low, physiological concentration. Therefore, activation of catalase occurring during conditions where H2O2 accumulates in the brain (Cino and Del Maestro, 1989; Simonson et al., 1993), in the heart (Shlafer et al., 1987; Vandeplassche et al., 1989; Chen and Lesnefsky, 2006), in the kidney (Kunduzova et al., 2002) and during perinatal hypoxia ischaemia (Fullerton et al., 1998) may represent an early endogenous defence mechanism aimed to compensate for the lack of oxygen supply. Indeed, it was shown that a higher activity of catalase occurs in the substantia nigra pars compacta (Hung and Lee, 1998; Avshalumov et al., 2005), and that H2O2 degradation through catalase is able to overcome the metabolic stress typically associated with episodes of energy deprivation (Geracitano et al., 2005).
The aim of this study was to assess the protective role of H2O2 and the potential of manipulating its endogenous production on the electrophysiological and morphological changes induced by OGD on CA1 hippocampal neurons.
We used field potential recordings in the hippocampus as it represents an ideal way to monitor the activity of a large population of neurons, hence to detect the functional changes that occur during a period of energy failure (Whittingham et al., 1984; Lobner and Lipton, 1993; Fowler, 1997). Accordingly, we have found that a sustained short period of OGD induced an irreversible functional impairment of synaptic neurotransmission that represents an index of neuronal injury. This is also confirmed by the morphological observation of the tissue using Cresyl Violet staining. Thus, the irreversible electrophysiological changes that closely correlate with the histologically defined neuronal damage, confirmed that H2O2 exerts a strong neuroprotective effect by compensating for the lowered levels of O2 during OGD. This is demonstrated by the lack of neuroprotection when the production of O2 from H2O2 was blocked by the catalase inhibitor 3-AT and by the failure of H2O2 to oppose hypoglycaemia-induced loss of synaptic function. Nevertheless, we adopted a model of combined oxygen and GD to mimic ischaemic conditions in vivo. We were also able to show clear protection against the ischaemic insult by increasing the endogenous content of H2O2 in the tissue. Therefore, we have concluded that manipulation of the enzymic production of H2O2 was neuroprotective in the slice preparations, probably due to a supplementary source of O2 generated by breakdown of H2O2 during the ischaemic episode. In fact, increasing the endogenous level of H2O2 by the simultaneous reduction of the activity of GPX and by exogenously applying Cu,Zn-SOD also allowed the hippocampal tissue to be rescued from the damage caused by OGD. Inhibition of GPX alone appeared not to raise endogenous H2O2 to a level that was high enough to rescue the neurons. We therefore had to add Cu,Zn-SOD, further boosting the production of H2O2 in the hippocampal tissue and, under these conditions, a considerable protection was achieved.
Our observation that we could rescue neurons from injury caused by OGD through manipulation of the cellular content of H2O2 suggests that a valuable protective strategy could be to raise the endogenous level of this compound in the ischaemic tissue. In fact, H2O2 is highly diffusible and rapidly metabolized through the action of catalase into free O2, thus acting as a donor of molecular O2 that we think could be vital in a pathological condition such as ischaemia.
Therefore, we would propose that H2O2 can act in a protective role, as the application of exogenous H2O2, as well as the pharmacological manipulation of the enzymes involved in its production and degradation, conferred neuroprotection in an in vitro model of OGD. Of course, at this stage, we would not suggest therapeutic use of exogenous H2O2, which would be potentially toxic if directly administered in humans. However, the combination of the blockade of the GPX pathway together with Cu,Zn-SOD, by transiently increasing the endogenous concentrations of H2O2 to below toxic levels, would seem to be a protective strategy in conditions of energy deprivation.
Acknowledgments
This work was supported by the Italian Ministry of Health. We thank Mr Mauro Federici for excellent technical assistance.
Abbreviations
- ACSF
artificial cerebral spinal fluid
- 3-AT
3-amino-1,2,4-triazole
- fEPSP
field excitatory postsynaptic potentials
- GD
glucose deprivation
- GPXs
glutathione peroxidases
- MPS
mercaptosuccinate
- O2−
superoxide anion
- OGD
oxygen/glucose deprivation
- OH−
hydroxyl radical
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Conflict of interest
The authors state no conflict of interest.
References
- Auerbach JM, Segal M. Peroxide modulation of slow onset potentiation in rat hippocampus. J Neurosci. 1997;17:8695–8701. doi: 10.1523/JNEUROSCI.17-22-08695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Koos T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci. 2005;25:4222–4231. doi: 10.1523/JNEUROSCI.4701-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999;27:951–965. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- Burke SP, Nadler JV. Effects of glucose deficiency on glutamate/aspartate release and excitatory synaptic responses in the hippocampal CA1 area in vitro. Brain Res. 1989;500:333–342. doi: 10.1016/0006-8993(89)90329-6. [DOI] [PubMed] [Google Scholar]
- Chen BT, Avshalumov MV, Rice ME. H2O2 is a novel, endogenous modulator of synaptic dopamine release. J Neurophysiol. 2001;85:2468–2476. doi: 10.1152/jn.2001.85.6.2468. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lesnefsky EJ. ‘Hiding out' from chronic ischemia with help from the mitochondria. J Mol Cell Cardiol. 2006;41:956–958. doi: 10.1016/j.yjmcc.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Cino M, Del Maestro RF. Generation of hydrogen peroxide by brain mitochondria: the effect of reoxygenation following postdecapitative ischemia. Arch Biochem Biophys. 1989;269:623–638. doi: 10.1016/0003-9861(89)90148-3. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Fowler JC. Purine release and inhibition of synaptic transmission during hypoxia and hypoglycemia in rat hippocampal slices. Neurosci Lett. 1993;157:83–86. doi: 10.1016/0304-3940(93)90648-5. [DOI] [PubMed] [Google Scholar]
- Fowler JC. Hydrogen peroxide opposes the hypoxic depression of evoked synaptic transmission in rat hippocampal slices. Brain Res. 1997;766:255–258. doi: 10.1016/s0006-8993(97)00699-9. [DOI] [PubMed] [Google Scholar]
- Fullerton HJ, Ditelberg JS, Chen SF, Sarco DP, Chan PH, Epstein CJ, et al. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann Neurol. 1998;44:357–364. doi: 10.1002/ana.410440311. [DOI] [PubMed] [Google Scholar]
- Furuichi T, Liu W, Shi H, Miyake M, Liu KJ. Generation of hydrogen peroxide during brief oxygen-glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. J Neurosci Res. 2005;79:816–824. doi: 10.1002/jnr.20402. [DOI] [PubMed] [Google Scholar]
- Geracitano R, Tozzi A, Berretta N, Florenzano F, Guatteo E, Viscomi MT, et al. Protective role of hydrogen peroxide in oxygen-deprived dopaminergic neurones of the rat substantia nigra. J Physiol. 2005;568:97–110. doi: 10.1113/jphysiol.2005.092510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Antioxidant defence mechanisms: from the beginning to the end (of the beginning) Free Radic Res. 1999;31:261–272. doi: 10.1080/10715769900300841. [DOI] [PubMed] [Google Scholar]
- Horn M, Schlote W. Cerebral resuscitation. Z Gesamte Inn Med. 1992;47:115–126. [PubMed] [Google Scholar]
- Hung HC, Lee EH. MPTP produces differential oxidative stress and antioxidative responses in the nigrostriatal and mesolimbic dopaminergic pathways. Free Radic Biol Med. 1998;24:76–84. doi: 10.1016/s0891-5849(97)00206-2. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:359–376. doi: 10.1016/s0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Kunduzova OR, Bianchi P, Parini A, Cambon C. Hydrogen peroxide production by monoamine oxidase during ischemia/reperfusion. Eur J Pharmacol. 2002;448:225–230. doi: 10.1016/s0014-2999(02)01913-1. [DOI] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lobner D, Lipton P. Intracellular calcium levels and calcium fluxes in the CA1 region of the rat hippocampal slice during in vitro ischemia: relationship to electrophysiological cell damage. J Neurosci. 1993;13:4861–4871. doi: 10.1523/JNEUROSCI.13-11-04861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmar TC. Peroxide alters neuronal excitability in the CA1 region of guinea-pig hippocampus in vitro. Neuroscience. 1987;23:447–456. doi: 10.1016/0306-4522(87)90068-6. [DOI] [PubMed] [Google Scholar]
- Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Waldman S, Rawlinson D, Plum F. Moderate hyperglycemia augments ischemic brain damage: a neuropathologic study in the rat. Neurology. 1982;32:1239–1246. doi: 10.1212/wnl.32.11.1239. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid Redox Signal. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996. pp. 709–720. [DOI] [PubMed]
- Shlafer M, Myers CL, Adkins S. Mitochondrial hydrogen peroxide generation and activities of glutathione peroxidase and superoxide dismutase following global ischemia. J Mol Cell Cardiol. 1987;19:1195–1206. doi: 10.1016/s0022-2828(87)80530-8. [DOI] [PubMed] [Google Scholar]
- Sick TJ, Solow EL, Roberts EL., Jr Extracellular potassium ion activity and electrophysiology in the hippocampal slice: paradoxical recovery of synaptic transmission during anoxia. Brain Res. 1987;418:227–234. doi: 10.1016/0006-8993(87)90090-4. [DOI] [PubMed] [Google Scholar]
- Simonson SG, Zhang J, Canada AT, Jr, Su YF, Benveniste H, Piantadosi CA. Hydrogen peroxide production by monoamine oxidase during ischemia-reperfusion in the rat brain. J Cereb Blood Flow Metab. 1993;13:125–134. doi: 10.1038/jcbfm.1993.15. [DOI] [PubMed] [Google Scholar]
- Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Traystman RJ, Kirsch JR, Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol. 1991;71:1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- Vandeplassche G, Hermans C, Thone F, Borgers M. Mitochondrial hydrogen peroxide generation by NADH-oxidase activity following regional myocardial ischemia in the dog. J Mol Cell Cardiol. 1989;21:383–392. doi: 10.1016/0022-2828(89)90649-4. [DOI] [PubMed] [Google Scholar]
- Walton K, Fulton B. Hydrogen peroxide as a source of molecular oxygen for in vitro mammalian CNS preparations. Brain Res. 1983;278:387–393. doi: 10.1016/0006-8993(83)90280-9. [DOI] [PubMed] [Google Scholar]
- Whittingham TS, Lust WD, Passonneau JV. An in vitro model of ischemia: metabolic and electrical alterations in the hippocampal slice. J Neurosci. 1984;4:793–802. doi: 10.1523/JNEUROSCI.04-03-00793.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao-Qing T, Jun-Li Z, Yu C, Jian-Qiang F, Pei-Xi C. Hydrogen peroxide preconditioning protects PC12 cells against apoptosis induced by dopamine. Life Sci. 2005;78:61–66. doi: 10.1016/j.lfs.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Yaguchi Y, Satoh H, Wakahara N, Katoh H, Uehara A, Terada H, et al. Protective effects of hydrogen peroxide against ischemia/reperfusion injury in perfused rat hearts. Circ J. 2003;67:253–258. doi: 10.1253/circj.67.253. [DOI] [PubMed] [Google Scholar]