Abstract
In non-excitable cells, activation of G-protein-coupled phospholipase C (PLC)-linked receptors causes the release of Ca2+ from intracellular stores, which is followed by transmembrane Ca2+ entry. This Ca2+ entry underlies a small and sustained phase of the cellular [Ca2+]i increases and is important for several cellular functions including gene expression, secretion and cell proliferation. This form of transmembrane Ca2+ entry is supported by agonist-activated Ca2+-permeable ion channels that are activated by store depletion and is referred to as store-operated Ca2+ entry (SOCE) and represents a major pathway for agonist-induced Ca2+ entry. In excitable cells such as smooth muscle cells, Ca2+ entry mechanisms responsible for sustained cellular activation are normally considered to be mediated via either voltage-operated or receptor-operated Ca2+ channels. Although SOCE occurs following agonist activation of smooth muscle, this was thought to be more important in replenishing Ca2+ stores rather than acting as a source of activator Ca2+ for the contractile process. This review summarizes our current knowledge of SOCE as a regulator of vascular smooth muscle tone and discusses its possible role in the cardiovascular function and disease. We propose a possible hypothesis for its activation and suggest that SOCE may represent a novel target for pharmacological therapeutic intervention.
Keywords: capacitative calcium entry, cardiovascular disease, store-operated calcium entry, TRP channel, vascular smooth muscle
Introduction
Virtually, every cellular response is regulated by changes in intracellular free calcium levels ([Ca2+]i), making this ion a universal intracellular mediator. Thus, understanding the mechanisms that control Ca2+ entry into cells becomes critically important. Increases in cytoplasmic Ca2+ signals can be generated either by release of Ca2+ from intracellular stores and/or by influx of Ca2+ from the extracellular fluid. The release of intracellular Ca2+ occurs from the endoplasmic reticulum or its specialized counterpart in muscle cells, the sarcoplasmic reticulum (SR), and is generally signaled by the formation of second-messengers, such as inositol 1,4,5-trisphosphate (IP3) (Streb et al., 1983). However, it soon became apparent that the release of Ca2+ from intracellular stores is often followed by a sustained phase of Ca2+ entry from the extracellular space (Putney and McKay, 1999a). This led to the proposal by Putney that depleted Ca2+ stores (primarily in the endoplasmic reticulum) are able to gate the entry of extracellular Ca2+ where intracellular Ca2+ stores act as a capacitor, thus, leading to the term ‘capacitative calcium entry' that has been superceded more recently by the ‘store-operated calcium entry' (SOCE) (Putney, 1999b, Parekh and Putney, 2005). This concept was supported by the identification of a well-characterized store-operated current, the so-called Ca2+ release-activated Ca2+ current (Hoth and Penner, 1992), although the consensus is that the current mechanisms underlying capacitative calcium entry may in fact be a special case of SOCE, and not represent a generalized phenomenon.
In spite of many investigations of SOCE, the molecular details of the activation mechanisms of store-operated Ca2+ channels (SOCCs) remain fragmentary. In fact, the existence of different genes encoding SOCCs may account for the diverse activation mechanisms of this channel. There appears to be no general agreement regarding the nature of the Ca2+ store from which the signal emits, the identity of the Ca2+ sensor that monitors the filling state of the stores, the retrograde signal transduction mechanism that activates SOCCs or the molecular identity of SOCCs. This is probably due to the complexities of mechanisms involved in SOCE, as well as the peculiarity of various experimental methods employed. The effect of SOCE on vascular function may include changes in both the endothelium (Oike et al., 1994; Fasolato and Nilius, 1998; Freichel et al., 2001; Cioffi et al., 2005) and vascular smooth muscle cells (VSMCs). In this review, we will focus our discussion on SOCE in VSMCs. Moreover, we will highlight some of the challenges encountered in creating a unified hypothesis.
SR Ca2+-ATPase inhibitors and SOCE
Exploration of the functional significance of SOCE has been greatly aided by the use of agents, such as cyclopiazonic acid (CPA) and thapsigargin, which act as selective inhibitors of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) (Laporte et al., 2004). These drugs cause depletion of SR Ca2+ stores by inhibiting sequestration of Ca2+ ions without activation of G proteins, and are used to provide an important distinction between Ca2+ entering through SOCCs as opposed to receptor-operated Ca2+ channels (ROCCs). Undoubtedly, sustained Ca2+ influx, or cellular responses, activated by SR Ca2+-ATPase inhibitors can be considered markers for the involvement of SOCE in cell signalling.
In cultured VSMCs, depletion of SR Ca2+ stores with thapsigargin activates Ca2+ influx that is independent of the generation of inositol phosphate and resistant to the L-type voltage-operated Ca2+ channel (VOCC) blocker, nicardipine (Xuan et al., 1992). Numerous studies show that the SERCA inhibitors increase not only Ca2+ influx but also vascular tone of different blood vessels (Table 1). In all such cases, the contractions are sustained and dependent on the presence of extracellular Ca2+. However, the contractile response to SERCA inhibitors shows variable sensitivity to Ca2+ channel-blocking drugs. For instance, in the rat aorta, the majority of the contractions is nifedipine-sensitive (Kwan et al., 1994; Low et al., 1994; Tepel et al., 1994; Xuan and Glass, 1996; Noguera et al., 1997; Tosun et al., 1998), whereas in the rat pulmonary, renal and retinal arteries, the contraction is nifedipine-resistant (Gonzalez De La Fuente et al., 1995; Curtis and Scholfield, 2001; Snetkov et al., 2003). In rat femoral and carotid arteries, mouse anococcygeus, guinea-pig and cat fundus, Ca2+ entry stimulated by store depletion is partially nifedipine-sensitive (Gibson et al., 1994; Sekiguchi et al., 1996; Petkov and Boev, 1996a, 1996b; Nomura et al., 1997). These results suggest that smooth muscle contraction in response to SERCA inhibitors may be caused by Ca2+ entry through both VOCCs and SOCCs, with the relative importance of these entry pathways depending on the smooth muscle type, with SOCCs appearing to be of greater importance in tonic smooth muscles, for example guinea pig and cat gastric fundus, mouse anococcygeus and rat pulmonary artery. The SERCA inhibitors do not contract all smooth muscles, and in some cases, a poor correlation exists between increased intracellular Ca2+ and contraction (Snetkov et al., 2003). Huang et al. (2006) reported that Ca2+ entry through SOCC is not directly coupled to VSMC contraction in renal arteries (Huang et al., 2006). A role for SOCCs other than smooth muscle contraction was first suggested by Flemming et al. (2003) in rabbit cerebral arteries where the application of CPA induces a sustained increase in [Ca2+]i in the presence of a VOCC blocker (D600), which is not associated with contractions. However, membrane depolarization with a K+-rich solution (in the absence of D600) also produces a sustained rise in [Ca2+]i that is associated with smooth muscle contraction (Flemming et al., 2003). Since both CPA and a 35 mM KCl-containing solution raise [Ca2+]i to similar levels, it is possible that CPA activation of SOCCs causes an increase in [Ca2+]i in a cellular compartment that is spatially separated from contractile proteins, indicating that this spatially separate cellular compartment may include internal Ca2+ stores that are able to regulate local Ca2+ levels. Moreover, CPA activates a sustained, non-selective cation conductance in single myocytes isolated from the mouse anococcygeus. The current–voltage relationship for the CPA-induced current is linear with a reversal potential close to +30 mV in near physiological cation gradients. The reversal potential shifts to a more negative value upon removal of extracellular Ca2+, indicating that a large proportion of the current is carried by Ca2+ (Wayman et al., 1996a). This notion was supported by simultaneous recordings of current and intracellular Ca2+ levels, which showed that activation or inhibition of the current is accompanied by rises and falls in [Ca2+]i correspondingly (Wayman et al., 1996b). However, the cellular mechanisms linking store depletion to the opening of SOCCs are not well understood (Castells and Droogmans, 1981; Putney, 2001; Flemming et al., 2002; Wilson et al., 2002).
Table 1.
(Vascular) smooth muscles in which SERCA pump inhibitors raise [Ca2+]i and/or elicit contraction
| Species | Smooth muscle | Remarks | Responses | References |
|---|---|---|---|---|
| Rat | Aorta | Abolished by nifedipine/nicardipine | Contraction and increased [Ca2+]i | Tepel et al., 1994; Kwan et al., 1994; Low et al., 1994; Noguera et al., 1997; Tosun et al., 1998; Xuan and Glass, 1996 |
| Carotid artery | Partially reduced by verapamil | Contraction and increased [Ca2+]i | Sekiguchi et al., 1996 | |
| Coronary artery | No contraction and increased [Ca2+]i | Snetkov et al., 2003 | ||
| Femoral artery | Partially reduced by verpamil | Contraction and increased [Ca2+]i | Nomura et al., 1997 | |
| Femoral artery | A small transient contraction and increased [Ca2+]i | Snetkov et al., 2003 | ||
| Mesenteric artery | No contraction and increased [Ca2+]i | Snetkov et al., 2003 | ||
| Pulmonary artery | Unaffected by nifedipine, verapamil reduced by tyrosine kinase inhibitors. pCa2+/tension curve unaffected | Gonzalez De La Fuente et al., 1995 | ||
| Pulmonary distal arterial smooth muscle | Abolished by nifedipine | Increased [Ca2+]i | Wang et al., 2004 | |
| Intrapulmonary artery | Unaffected by diltiazem or the reverse mode Na+/Ca2+ antiport inhibitor KB-R7943 | Contraction and increased [Ca2+]i | Snetkov et al., 2003 | |
| Renal cortical interlobar arteries | Increased [Ca2+]i | Facemire et al., 2004 | ||
| Renal artery | No contraction | Snetkov et al., 2003 | ||
| Preglomerular vascular smooth muscle | Increased [Ca2+]i | Fellner and Arendshorst, 1999 | ||
| Basilar arteries | Unaffected by verapamil | Contraction | Bergdahl et al., 2005 | |
| Ileum | Unaffected by nifedipine, methoxyverapmail | Increased [Ca2+]i | Ohta et al., 1995 | |
| Spleen | Unaffected by nifedipine reduced by tyrosine kinase inhibitors | Burt et al., 1995 | ||
| Urinary bladder | Very weak contractile | Munro and Wendt, 1994 | ||
| A7r5 rat smooth muscle cell line | Not applicable | Increased [Ca2+]i | Byron and Taylor, 1995; Iwamuro et al., 1999; Iwasawa et al., 1997 | |
| Mouse | Anococcygeus | Partially reduced by nifedipine | Contraction and increased [Ca2+]i | Gibson et al., 1994; Wallace et al., 1999 |
| Guinea-pig | Gastric fundus | Partially reduced by nifedipine | Petkov and Boev, 1996a | |
| Cat | Gastric fundus | Partially reduced by nifedipine | Petkov and Boev, 1996b | |
| Canine | Pulmonary arterial smooth muscle cells | Unaffected by nifedipine reduced by tyrosine kinase inhibitors | Contraction and increased [Ca2+]i | Doi et al., 2000; Wilson et al., 2002 |
| Renal arterial smooth muscle cells | Unaffected by nisoldipine reduced by tyrosine kinase inhibitors | Contraction and increased [Ca2+]i | Wilson et al., 2002 | |
| Rabbit | Pulmonary arterial smooth muscle cells | Unaffected by nifedipine | Increased [Ca2+]i | Kang et al., 2003 |
| Carotid artery smooth muscle | Increased [Ca2+]i | Kawanabe et al., 2002 | ||
| Pial artery | Contraction and increased [Ca2+]i | Flemming et al., 2002, 2003 | ||
| Choroidal artery | Abolished by nifedipine | Increased [Ca2+ ]i | Curtis and Scholfield, 2001 | |
| Human | Lower oesophageal sphinctor smooth muscle | Increased [Ca2+ ]i | Wang et al., 2003 | |
| Bronchus | Cortijo et al., 1997 | |||
| Porcine | Airway smooth muscle | Increased [Ca2+ ]i | Ay, 2004 | |
| Swine | Renal artery | Increased [Ca2+ ]i | Utz et al., 1999 | |
| Prostate cell line | Increased [Ca2+ ]i | Thebault et al., 2005 |
Abbreviation: SERCA, sarcoplasmic/endoplasmic reticulum Ca2+ ATPases.
A model for SOCE
Several mechanisms for activation of SOCE have been proposed (Berridge, 1995; Parekh and Penner, 1997; Gibson et al., 1998; Putney, 2001; Bolotina, 2004). Smani et al. (2004) presented a simple model that may explain how Ca2+ influx factor (CIF, produced upon depletion of Ca2+ stores) activates SOCCs using mouse aortic smooth muscle cells. After CIF activation of SOCCs, CIF induces displacement of inhibitory calmodulin (CaM) from Ca2+-independent phospholipase (iPLA2), a key event leading to activation of iPLA2 and generation of lysophospholipids; the latter, in turn, activate SOCCs in a plasma membrane—delimited manner. Upon refilling of the stores and termination of CIF production, CaM rebinds to iPLA2 to resume its inhibition and so terminating the activity of SOCCs and Ca2+ entry (Smani et al., 2004). Studies by Trepakova et al. (2001) identified native CIF in support of this hypothesis. A novel 3-pS Ca2+-conducting channel that is activated by 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) and thapsigargin causes passive depletion of intracellular Ca2+ stores, and this channel is likely to be a native store-operated channel in VSMCs. The 3-pS channels are activated in inside-out membrane patches from smooth muscle cells immediately upon application of CIF extracted from mutant yeast cell lines (Trepakova et al., 2001). The existence of CIF that is produced by depleted stores and the idea that it may trigger activation of SOCCs was proposed more than a decade ago. However, the molecular structure of CIF has yet to be defined even though it is reportedly stable and can be partly purified (Kim et al., 1995). Moreover, functional studies demonstrate that the plasma membrane Na+/Ca2+ exchanger (NCX) is also involved in the regulation of vascular Ca2+ homeostasis by contributing to SOCE (Arnon et al., 2000). Evidence for an important role of NCX in SOCE came from a study showing that NCX is functionally expressed in cultured VSMCs from the human pulmonary artery and that Ca2+ entry via the reverse mode of NCX participates in store depletion-mediated elevation in [Ca2+]cyt. Thus, blockade of NCX in its reverse mode may serve as a potential therapeutic approach for the management of pulmonary hypertension (Zhang et al., 2005a). The functional evidence for a positive role of NCX in Ca2+ filling is also supported by immunoblotting and immunfluorescence studies where NCX is expressed in cultured arterial myocytes (Juhaszova et al., 1994). Besides, expression of NCX1 (mainly NCX1.3) is detected in coronary artery smooth muscle cells (Slodzinski et al., 1995). Furthermore, nitric oxide (NO), which induces vascular relaxation by accelerating SERCA-dependent refilling of Ca2+ stores, would be expected to blunt CIF production and so terminate the activity of SOCCs and Ca2+ influx (Cohen et al., 1999).
Members of the canonical transient receptor potential family (TRPC), particularly TRPC1, are involved in SOCE in VSMCs (Golovina et al., 2001; Xu and Beech, 2001; Sweeney et al., 2002a; Bergdahl et al., 2005). Xu et al. (2006) suggested that TRPC5 is another component of SOCC. Studies on non-vascular cells have implicated that additional TRPC family members exist in association with SOCE, including TRPC3 (Liu et al., 2000; Zagranichnaya et al., 2005), TRPC4 (Hofmann et al., 2002; Strubing et al., 2003) and TRPC7 (Zagranichnaya et al., 2005). In addition, TRPC1 may also be linked to TRPP2 (polycystin-2) Ca2+ permeable channels (Tsiokas et al., 1999; Giamarchi et al., 2006). More recently, Roos et al. (2005) have demonstrated that a single membrane-spanning protein termed STIM1 (stromal-interacting molecule 1) plays an essential role in the activation of SOCCs. The STIM1 protein serves as a sensor of Ca2+ within the stores (Roos et al., 2005). Other studies provide strong evidence showing that Orai1 (the Greek mythological characters the Orai, which are the keepers of the gates of heaven) is a pore subunit of the store-operated Ca2+ release-activated Ca2+ channels (Feske et al., 2006; Prakriya et al., 2006). Soboloff et al. (2006) revealed a powerful gain in the SOCC function that is dependent on the presence of both STIM1 and Orai1 (Soboloff et al., 2005). STIM1 may interact with TRPC1 and is involved in SOCE (Lopez et al., 2006). Jackson (2006) suggested that given the similarities between Ca2+ handling in platelets and VSMCs, it is likely that STIM1 and possibly Orai1 as well take part in SOCE in VSMCs and human airway myocytes (Jackson, 2006; Peel et al., 2006).
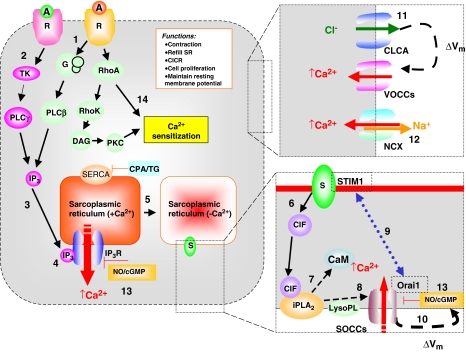
Based on these reports, we propose a model for SOCE in smooth muscle excitation/contraction coupling, which takes into account that, in many cases, the responses to SERCA inhibitors and to IP3-generating receptor agonists, both have VOCC-dependent and VOCC-independent components (Figure 1). In addition, the contractile response to the SERCA inhibitors is more variable than that to receptor agonists. The release of Ca2+ from intracellular stores in response to Ins(1,4,5)P3 has two effects. Firstly, there is a rise in [Ca2+]i—possibly amplified by Ca2+-induced Ca2+ release from ryanodine-sensitive stores—which activates Ca2+-dependent Cl− channels, thus producing membrane depolarization that promotes Ca2+ entry via VOCCs. Wayman et al. (1996a) described that Ca2+ store depletion activates a biphasic inward current in mouse anoccygeous smooth muscle cells. An initial transient current upon the release of Ca2+ from the SR is due to activation of Ca2+-dependent Cl− channels (Wayman et al., 1996a). Secondly, depleted Ca2+ stores generate CIF, which diffuses to the plasma membrane. A cascade of plasma-membrane-delimited reactions in which CIF displaces inhibitory CaM from the membrane-bound Ca2+-independent iPLA2 leading to iPLA2 activation and the generation of lysophospholipids that in turn stimulate SOCCs (Smani et al., 2004). Activation of SOCE via SOCCs is responsible for sustaining the contraction and refilling the stores upon removal of the agonist. On the other hand, activation of protein kinase C (PKC) by diacylglycerol following receptor stimulation may play an additional role in the sensitization of the contractile apparatus to Ca2+, further amplifying the response to receptor agonists. Besides, receptor agonists can also induce Ca2+ sensitization via Rho-associated kinase pathways (Ghisdal et al., 2003). The SERCA inhibitors, which deplete the stores and cause SOCE, fail to activate these sensitizing processes, thus explaining the observed low magnitude of contractions produced by CPA or thapsigargin, as compared with receptor agonists. Moreover, in pulmonary arteries and spleen, the cellular response activated by capacitative calcium entry in smooth muscle is reduced by tyrosine kinase inhibitors, suggesting a phosphorylation step via tyrosine kinase in the SOCE pathway. Finally, NO was found to modulate the SOCC activity via a guanosine-3′,5′-cyclicmonophosphate (cGMP)-dependent mechanism (Clementi and Meldolesi, 1997; Bolotina, 1999).
Figure 1.
A model for excitation/contraction coupling in a tonic smooth muscle cell in which sustained contraction involves Ca2+ entry through both VOCCs and SOCCs. (1) Physiologically, SOCE is initiated either by stimulation of receptors that couple through heterotrimeric GTP-binding protein (G proteins) to activate phospholipase Cβ (PLCβ) or (2) by stimulation of receptors that couple through tyrosine phosphorylation to activate PLCγ (Parekh and Penner, 1997; Patterson et al., 2002). This results in breakdown of phosphoinositide and production of IP3. (3) This second messenger activates IP3 receptors, which are ligand-gated Ca2+ channels located in the SR. The resulting release of Ca2+ into the cytoplasm causes a transient increase in [Ca2+]i, whereas (4) emptying of Ca2+ stores generates a retrograde signal that activates SOCCs in the plasma membrane, which are responsible for the sustained increase in [Ca2+]i after the initial Ca2+ transient. (5) Depleted Ca2+ stores generate a key messenger molecule called Ca2+ influx factor (CIF), which diffuses to the plasma membrane. (6) A cascade of plasma-membrane-delimited reactions in which (7) CIF displaces inhibitory CaM from the membrane-bound iPLA2, leading to iPLA2 activation and the generation of lysophospholipids (8) that in turn activate SOCCs. Ca2+ release from the SR causes the sensor (i.e., Ca2+-sensing STIM1 protein) to aggregate in areas close to the plasma membrane and to interact with SOCC (i.e., Orai1), which is believed to be the store-operated channel (9). On the other hand, (10) SOCC may also provide direct depolarization, independently of Ca2+ -activated Cl− channel VOCCs are opened by membrane depolarization due to initial Ca2+ -release from SR, which stimulates a Ca2+ -activated Cl− channel (11). (12) The plasma membrane NCX is involved in the regulation of Ca2+ homeostasis in blood vessels by contributing to Ca2+ entry. Finally, (13) NO/cGMP inhibits SOCCs, possibly by enhanced re-filling of the Ca2+ -stores. (14) Ca2+ sensitization might occur via agonist-induced activation of either the small G protein RhoA/Rho-associated kinase (Rho K) pathway or protein kinase C (PKC). A, agonist; CICR, Ca2+ -induced Ca2+ release; CIF, calcium influx factor; CLCA, Ca2+-activated Cl− channel; iPLA2, Ca2+-independent phospholipase A2; CaM, calmodulin; CPA, cyclopiazonic acid; DAG, diacylglycerol; G, GTP binding proteins; cGMP, guanosine-3′,5′-cyclicmonophosphate; IP3, inositol-1,4,5-trisphosphate; NCX, Na+/Ca2+ exchanger; NO, nitric oxide; PKC, protein kinase C; PLC, phospholipase C; R, receptor; S, putative Ca2+ sensor; SOCC, store-operated Ca2+ channel; STIM1, stromal-interacting molecule 1; TG, thapsigorgin; TK, tyrosine kinase; Vm, membrane potential; VOCC; voltage-operated Ca2+ channel.
Pharmacological inhibition of SOCE
A recent review by Putney (2001) discussed a number of drugs that possess inhibitory activity against SOCCs, but in most cases with less than optimal specificity. The SOCE inhibitors include cations (lanthanides, Gd3+ and divalent cations), P450 inhibitors (econazole, miconazole, clotrimazole and ketoconazole), cyclooxygenae inhibitors (niflumic acid, flufenamic acid and tenidap), lipoxygenase inhibitors (nordihydroguaiaretic acid and eicosatetraynoic acid), putative channel blockers ((SK&F 96365, SC38249, LU52396, L-651, 582, tetrandrine, 2-Aminoethyl diphenylborinate) and mechanism-based inhibitors (U73122 (phospholipase C inhibitor) and wortmannin (phosphatidylinositol kinase inhibitor)) (Putney, 2001). The simplest and most dependable SOCE inhibitors are Ca2+ mimics, for example, divalent cations, and the potent trivalent lanthanides.
There has been considerable progress in our understanding of the pharmacological profile of SOCCs in VSMCs. Flemming et al. (2003) characterized the pharmacological properties of store-operated channels in VSMCs of rabbit pial arterioles with the use of various SOCC inhibitors (Flemming et al., 2003) and demonstrated that SOCE is inhibited by Gd3+ in a concentration dependent manner (IC50=101 nM). The inhibitory effect of other inhibitors in the same study includes: 10 μM La3+ (70% inhibition), 75 μM 2-Aminoethyl diphenylborinate (66% inhibition), 100 μM Ni2+ (57% inhibition), 10 μM wortmannin (76% inhibition) and 100 μM capsaicin (12% inhibition). Drugs that are ineffective include: 1 μM nifedipine, 10 μM SK&F96365, 10 μM LOE908, 10–100 μM ruthenium red, 100 μM sulindac, 0.5 mM streptomycin and a 1:10 000 dilution of Grammostolla spatula venom (Flemming et al., 2003). On the contrary, Wayman et al. (1996a) reported that SOCCs in anococcygeus smooth muscle cells are insensitive to Gd3+ or La3+ at concentrations of up to 400 μM (Wayman et al., 1996a). SOCCs in VSMCs of canine renal arteries show some degree of sensitivity to 100 μM Gd3+, while those in pulmonary arterial smooth muscle are resistant to Gd3+ (Wilson et al., 2002). SOCCs of rat intrapulmonary arteries are sensitive to 1 μM La3+ and these channels in main pulmonary arteries are blocked by La3+ only at much higher concentrations (>100 μM) (Robertson et al., 2000; Ng and Gurney, 2001). The SOCCs of the anococcygeus and ileal smooth muscles can be inhibited by 10 μM SK&F96365 (Wayman et al., 1996a; Zholos et al., 2000; Ng and Gurney, 2001).
A recent study showed that diethylstilbestrol (DES), a synthetic estrogenic agonist, elicits a rapid and reversible block of SOCCs in rat basophilic leukaemia cells, aortic smooth muscle and human platelets. DES also inhibits whole-cell Ca2+ release-activated Ca2+ currents and thapsigargin-induced capacitative calcium entry (Zakharov et al., 2004). In contrast, trans-stilbene, a close structural analog of DES that lacks hydroxyl and ethyl groups, had no effect on the Ca2+ release-activated Ca2+ current and on SOCE. Thus, DES is proposed to be an effective inhibitor of SOCCs in a diversity of cell types (Zakharov et al., 2004). Brueggemann et al. (2006) also reported the pharmacological characteristics of a store-operated current, including its sensitivity to DES, 2-Aminoethyl diphenylborinate or micromolar Gd3+, and compared the effects of these inhibitors on thapsigargin- or [Arg8]-vasopressin-activated SOCE in rat mesenteric artery VSMCs using fura-2 (Brueggemann et al., 2006).
Other important roles played by SOCE in response to hormones and neurotransmitters
SOCCs and VSMC proliferation
Considerable existing evidence supports an important role for Ca2+ in cell proliferation, where activation of SOCCs is thought to participate in the process. VSMC proliferation normally occurs during the development and progression of hypertension. Pulmonary vascular medial hypertrophy due to VSMC proliferation contributes to the increased pulmonary vascular resistance in patients with pulmonary hypertension. A rise in [Ca2+]cyt promotes the growth of pulmonary artery VSMCs. Resting [Ca2+]cyt, intracellular stored [Ca2+], SOCE and store-operated Ca2+ currents are greater in proliferating human pulmonary artery VSMCs than in growth-arrested cells (Sweeney et al., 2002a). In cells treated with an antisense oligonucleotide specifically designed to cleave TRPC1 mRNA (resulting in reduced mRNA and protein expression of TRPC1), the amplitudes of the store depletion-activated currents (ISOC) and SOCE elicited by passive depletion of Ca2+ stores are reduced. Importantly, there is a 50% reduction in the growth rate of these cells, indicating that TRPC1 may encode a SOCC that plays a critical role in VSMC proliferation of the pulmonary artery by regulating SOCE-associated changes in [Ca2+]cyt (Sweeney et al., 2002b).
Spontaneous SOCC activity
In addition to SOCC stimulation by store depletion, spontaneous channel activity has been also recorded in unstimulated smooth muscle cells. In freshly isolated rabbit portal vein myocytes, approximately 45% of outside-out patches contain spontaneous single-channel currents with a unitary conductance 23 pS, which have similar properties as to those of channel currents evoked by noradrenaline and the diacylglycerol analogue 1-oleoyl-2-acetyo-sn-glycerol (Albert and Large, 2001). The molecular identity of the channel is unknown. However, it is becoming increasingly evident that there are several similarities between these channels and the TRP and TRPL (transient receptor potential-like) channels previously described in Drosophilia photoreceptors (Harteneck et al., 2000). Firstly, in rabbit portal vein smooth muscle, the non-selective cation channels are activated by diacylglycerol in a PKC-independent manner (Helliwell and Large, 1997). The mammalian homologue hTRPC6, hTRPC3 and mouse TRPC7 are non-selective cation channels that are activated by diacylglycerol independently of PKC (Hofmann et al., 1999; Okada et al., 1999). Secondly, the relative permeability of some of these channels to divalent cations is similar. Thirdly, in the absence of activators, channels open and close spontaneously in native venous myocytes and in cells expressing TRP and TRPL channels (Hofmann et al., 1999). Lastly, the probability of channel opening is greatly increased at positive potential (Chyb et al., 1999; Hofmann et al., 1999).
Using whole-cell, perforated-patch recording method, Bae et al. (1999) described a basal non-selective cation current in freshly dispersed rabbit pulmonary artery myocytes and concluded that the non-selective cation conductance is a component of the resting membrane potential (Bae et al., 1999). Spontaneous SOCC activity could be one reason for the resting membrane potentials (−60 and −45 mV) of VSMCs being significantly less negative than the K+ equilibrium potential (EK, about −85 mV, Nelson and Quayle, 1995; Kuriyama et al., 1998). Albert et al. (2003) suggested that in rabbit ear artery myocytes, there exists a constitutively active Ca2+-permeable cation channel that is regulated by external Ca2+ ions and suppressed by the tonic PKC activity. Such a constitutively active Ca2+-permeable cation current may contribute to the resting membrane conductance and basal Ca2+ influx in the arteries (Albert et al., 2003).
Molecular identity of SOCCs in VSMC
Emerging evidence links SOCE to TRP channels. It has long been known that smooth muscle contraction can occur independently of changes in the membrane potential; what is less clear is the nature of the Ca2+ permeation pathway that is stimulated without voltage activation. In this regard, ROCCs activated by ligand–receptor interaction and SOCCs are thought to be principal modes of voltage-independent Ca2+ entry. It is possible that ROCCs and SOCCs may be closely related members of the TRP channel family (McFadzean and Gibson, 2002). The TRP channel proteins were first identified in the Drosophila melanogaster fruit fly where a mutation led to visual defects due to defects in the Ca2+ influx pathway. There have since been a large number of TRP channel proteins identified and these can be classified into three categories. They all have six transmembrane domains and are non-selective ion channels.
TRPC, where C stands for classical or canonical due to the highest homology with the molecular identity of TRP channels in Drosophila. There are at least seven members (TRPC 1–7) of this subfamily, with TRPC1 being the most abundant in vascular tissue. TRPC2 is a pseudogene in man. However, the expression of the various isoforms of TRPC channels in the vasculature is likely to be highly species and vascular bed dependent. There is evidence suggesting that the ROC may in fact be a TRPC6 and possibly TRPC1 channel (Xu and Beech, 2001; Inoue, 2005). It is likely that ROC and SOC channels are made up of heteromeric combinations of various TRPC proteins.
TRPV, where V stands for vanilloid, as these channels (TRPV 1–6) are closely related to the vanilloid receptor.
TRPM, where M stands for melastatin (a tumor suppressor).
Although TRP channels are widely studied for their roles in ion conductance, it is also clear that they have several other principal functions as manifested by their sensory activities in perception of temperature, taste, pH, chemical stimuli (for example capsaicin) and osmolarity (Inoue, 2005).
Multiple homologues of TRPC proteins are expressed in VSMCs (Beech, 2005). When expressed heterologously, TRP channels generally form functional entities that exhibit electrophysiological properties characteristic of non-selective cation channels. There is growing support for the involvement of the TRPC1–7 family in the formation of Ca2+-permeable non-selective cation channels in VSMCs (Table 2). Several studies demonstrate that TRPC mRNA and TRPC proteins are expressed in several smooth muscle preparations. In rat pulmonary artery VSMCs, reverse transcription-PCR analysis revealed the expression of TRPC1, TRPC3, TRPC4, TRPC5 and TRPC6, while mRNA immunostaining identified proteins for TRPC1, TRPC3, TRPC4 and TRPC6 (Ng and Gurney, 2001). Moreover, reverse transcription-PCR and western blotting performed on RNA and protein isolated from distal intrapulmonary arteries and main pulmonary artery VSMCs revealed both mRNA and protein expression for TRPC1, TRPC4 and TRPC6, but not for TRPC2, TRPC3, TRPC5 or TRPC7 (Wang et al., 2003). In a number of murine and canine smooth muscle cell preparations, mRNA for TRPC4, TRPC6 and TRPC7 is detected but with no detection of mRNA for TRPC1, TRPC2 and TRPC5. In rat renal resistance arteries and aorta, mRNA and protein are probed for TRPC1, TRPC3, TRPC4, TRPC5 and TRPC6, while mRNA for TRPC2 and TRPC7 is undetectable (Facemire et al., 2004). Furthermore, in situ hybridization yielded strong labeling of TRPC1, TRPC3, TRPC4, TRPC5 and TRPC6 in endothelial and VSMCs of human coronary and cerebral arteries. TRPC7 is only expressed in endothelial cells but not in the underlying VSMCs. Results from immunohistochemical staining are in consistence with those from in situ hybridization (Yip et al., 2004).
Table 2.
Detection of TRPC in smooth muscle of various tissues
| Tissue | TRPC1a | TRPC2 | TRPC3 | TRPC4a | TRPC5a | TRPC6 | TRPC7 | References |
|---|---|---|---|---|---|---|---|---|
| Aorta | + | + | + | + | + | + | Facemire et al., 2004 | |
| A7r5 | + | + | + | Brueggemann et al., 2006 | ||||
| Rat mesentery artery | + | + | + | Brueggemann et al., 2006 | ||||
| Rat renal resistance artery | + | + | + | + | + | Facemire et al., 2004 | ||
| Rat intralobar pulmonary arteries | + | + | + | Lin et al., 2004 | ||||
| Rat cerebral arteries | + | + | + | + | + | Flemming et al., 2003 | ||
| Rabbit portal vein myocyte | + | Albert and Large, 2003 | ||||||
| Human lower oesophageal sphinctor smooth muscle | + | + | + | + | + | Wang et al., 2003 | ||
| Human coronary & cerebral artery | + | + | + | + | + | Yip et al., 2004 | ||
| Human pulmonary artery | + | Golovina et al., 2001 | ||||||
| Human internal mammary artery | + | + | Bergdahl et al., 2005 | |||||
| Pig trachea smooth muscle | + | + | + | Ay et al., 2004 | ||||
| Murine & canine smooth muscle | + | + | + | Walker et al., 2001 | ||||
| Rat prostate smooth muscle cell line | + | + | Thebault et al., 2005 | |||||
| Lamb fetal pulmonary smooth muscle cells | + | + | + | + | Resnik et al., 2007 |
Abbreviation: TRPC, transient receptor potential family.
Remark: ‘+'-expression.
Empty cells refers to ‘particular TRPC is not expressed in the tissue'.
Indicates that particular TRPC may involve SOCC activity. TRPC2 is a pseudogene in man.
Some studies favour a role for TRPC proteins in smooth muscle function. For example, TRPC1 partially mediates SOCE in smooth muscle (Inoue et al., 2001). In arterioles, the application of an antibody against an extracellular epitope of TRPC1 (T1E3) reduces the thapsigargin-induced reduction in [Ca2+] by 25%, suggesting that part of thapsigargin-evoked SOCC activity is likely to be mediated by TRPC1 (Xu and Beech, 2001). A T1E3 antibody was also found to cause a 50% reduction in the SOCE-mediated contraction of rat cerebral arteries (Bergdahl et al., 2005), thus, supporting the notion that additional TRPC subunits are likely to play a positive role in thapsigargin-induced activation of SOCCs. More recently, Xu et al. (2006) showed that E3-targeted externally acting anti-TRPC5 blocking antibody (T5E3) suppressed Ca2+ entry in arterioles only after activation in store-operated mechanism triggered by thapsigargin in the absence of extracellular Ca2+, while T5E3 pre-adsorbed to its antigenic peptide had no effect. Collectively, these findings suggest that Ca2+ entry caused by passive store-depletion in arteriolar VSMCs may involve TRPC1 and TRPC5 (Xu et al., 2006).
Golovina et al. (2001) provided evidence for an upregulation of SOCC activity in proliferating VSMCs, suggesting that the increased TRPC1 mRNA may underlie SOCC-dependent rises in [Ca2+]i during VSMC proliferation. In addition, they also demonstrated that human pulmonary artery myocytes treated with antisense oligonucleotides to cleave mRNA for TRPC1 have a low expression of TRPC1, a reduced amplitude of CPA-evoked currents and a decreased cell growth rate (Golovina et al., 2001). The phenylephrine- and CPA-evoked non-selective cation channel activation mediating tonic constrictions in rabbit vena cava is associated with oscillations of [Ca2+]i generated by SOCE that may be specifically encoded by genes for TRPC1 (Liu et al., 2000; Lee et al., 2001). However, there is not always such a clear association between the expression of mRNA or channel proteins and their physiological significance in native cells. Despite a prominent role for TRPC1 as the main candidate for SOCCs in smooth muscle, other evidence showed that TRPC4 may be also related to SOCCs. The mRNA for TRPC4 was found to be the most abundant among all TRPCs detected in murine and canine smooth muscle cells (Walker et al., 2001). In addition, other studies also support a positive role of TRPC6 in mediating noradrenaline-evoked cation current (Icat, ROC) in VSMCs (Inoue et al., 2001).
The newly identified STIMs may mediate SOCE. Roos et al. (2005) proposed that STIM1 is an essential and conserved component of SOCC. STIM1 contains a functional EF-hand domain for Ca2+ binding and can act as a Ca2+ sensor to monitor the Ca2+-loading levels inside the stores (reviewed by Draber and Draberova, 2005; Putney, 2007). After store depletion, STIM1 is transolocated to the plasma membrane to activate SOCCs by the following proposed mechanisms: (i) interaction with a putative TRP pore-forming subunit; (ii) activation of Ca2+ entry by means of conformational coupling to its coiled domain and (iii) assembly with additional STIM1 monomers and other components to form a unique functional Ca2+ channel (Liou et al., 2005; Zhang et al., 2005b; Spassova et al., 2006).
Pathophysiological importance of SOCE
SOCE and cardiovascular diseases
Some cardiovascular diseases are specifically associated with a failure or malfunction of SOCE. The Ca2+-handling capability of the SR is defective in pulmonary hypertension and hyperglycaemia.
Prolonged exposure to alveolar hypoxia causes pulmonary hypertension with profound vascular remodelling and alterations in the Ca2+ homeostasis in pulmonary artery VSMCs (Shimoda et al., 2000). Several studies provide evidence that pulmonary hypertension (including chronic hypoxic pulmonary hypertension and chronic intrauterine pulmonary hypertension) is related to SOCE. Firstly, store-operated channels of pulmonary artery VSMCs are upregulated by chronic hypoxia, and increased SOCC activity contributes to the enhanced vascular tone in hypoxic pulmonary hypertension. Small interfering RNA knockdown of TRPC1 and TRPC6 specifically inhibits the thapsigargin- and 1-oleoyl-2-acetyo-sn-glycerol-induced cation entry. Removal of extracellular Ca2+ or inhibition of SOCE by La3+ and SKF-96365 prevent the elevated levels of [Ca2+]i in pulmonary artery VSMCs and inhibit the augmented vascular tone in pulmonary arteries of chronic hypoxic rats. In contrast, nifedipine has a negligible effect (Lin et al., 2004). Secondly, hypoxia-related pulmonary vasoconstriction requires SOCE in isolated rat lungs, since the enhanced vascular tone can be inhibited by SK&F-96365, NiCl2 or LaCl3 (Weigand et al., 2005). Thirdly, the platelet-derived growth factor-mediated proliferation of pulmonary arterial smooth muscle cells is associated with c-Jun/STAT3-induced upregulation of the TRPC6 expression. The resultant increase in SOCE raises [Ca2+]i, which facilitates the return of Ca2+ to the SR and augments pulmonary artery VSMC growth (Yu et al., 2003). Lastly, chronic intrauterine pulmonary hypertension increases SOCE and acute normoxia normally diminishes SOCE in fetal lamb pulmonary artery VSMCs. Under normoxic conditions, the expression of TRPC1, 3, 5 and 6 is greater in normotensive than hypertensive VSMCs (Resnik et al., 2007). The SOCCs may thus represent an additional target for therapeutic intervention.
Hyperglycaemia also attenuates SOCE in VSMCs following depletion of intracellular Ca2+ stores (Rivera et al., 1995); the underlying mechanisms may be related to altered PKC activity as PKC-dependent phosphorylation was thought to be contributory to the inactivation of SOCE (Parekh and Penner, 1995). Reduced SOCE in the retinal arterioles from streptozotocin-treated rats may also be related to the channel modulation by PKCβ (Curtis et al., 2003).
Organ culture and SOCE
Blood vessel preparations kept in organ culture are viable for several days and maintain their contractility with little evidence of a significant change in phenotype from the contractile to the synthetic state when vessels are cultured in the absence of supplementary growth factors (Hellstrand, 1998; Lindqvist et al., 1999). On the other hand, organ culture of human saphenous vein and porcine aorta leads to the formation of a neointima, a lesion that is characteristic of restenosis after angioplasty (Soyombo et al., 1990; Koo and Gotlieb, 1991). The synthetic phenotype of smooth muscle cells is of importance in clinical situations where growth and proliferation of VSMCs, such as neointima formation, is part of the atherosclerotic process (Ross, 1993).
Since the distinction between contractile and synthetic smooth muscle phenotypes was made largely based upon ultrastructure (Owen, 1995; Thyberg et al., 1996), the issue arises as to what roles alterations of ion channel properties and intracellular Ca2+ stores may play in this process. Changes in Ca2+ handling capacity in intact arteries, similar to those observed in cultured VSMCs are likely to influence cell excitability and SOCC pharmacology, making SOCC a potential target for the prevention or even treatment of hypertension and atherosclerosis.
In rat basilar and tail arteries, the intracellular Ca2+ release upon depletion of SR stores is increased after 3–4 days in serum-free organ culture (Dreja et al., 2001), which is not associated with any increase in basal [Ca2+]i. The voltage-dependent Ca2+ currents are reduced but SOCE is augmented in organ-cultured basilar arteries. SOCE is enhanced and vascular contractility is maintained in rat cerebral arteries kept in culture for several days. Under these conditions, there is also a 50% increase in the nifedipine-insensitive currents that are activated by store depletion. The mRNA levels for TRPC1 and TRPC6 increase significantly after organ culture, and a polyclonal TRPC1 antibody against an extracellular epitope (T1E3 antibody) inhibits contractility by 50% (Bergdahl et al., 2005). In segments of the human internal mammary, artery kept in organ culture for 24 h and then exposed to balloon dilatation in vitro, followed by further culture for 48 h, the mRNA expression of TRPC1 and TRPC6 mRNA is higher as compared with the undilated segments (Bergdahl et al., 2005), suggesting that organ culture or mechanical injury could impact on the plasticity of TRPC and cellular Ca2+ handling in blood vessels.
NO and SOCE
The endothelial production of NO accounts for endothelium-dependent vasodilatation in response to dilator agonists. Several mechanisms have been proposed to explain NO-dependent modulation of [Ca2+]i in VSMCs, which is the primary regulator of vascular tone. NO affects voltage-dependent activation of smooth muscle L-type Ca2+ channels either directly or indirectly through opening K+ channels and ensuing hyperpolarization (Robertson et al., 1993; Archer et al., 1994; Bolotina et al., 1994). While other studies indicate that NO is capable of modulating the activity of voltage-independent SOCCs. NO induces a rapid decrease in [Ca2+]i by accelerating sequestration of Ca2+ into intracellular stores via SERCA; as a result, refilled Ca2+ stores inhibit SOCE and reduce vascular tone (Cohen et al., 1999). NO-mediated inhibition of SOCCs and ROCCs is impaired in chronic hypoxia-induced pulmonary hypertension (Jernigan et al., 2006). Considering that a rise in [Ca2+]i is the major stimulus for contraction, gene expression and proliferation of VSMCs, such impaired NO signalling may have important implications in the regulation of not only pulmonary vascular tone but also arterial wall remodelling; both are jointly involved in the development of pulmonary hypertension associated with chronic hypoxia. In contrast, excessive NO interferes with the release of Ca2+ from thapsigargin-sensitive stores and reduces SOCE into VSMCs subsequent to depletion of Ca2+ stores in the mesenteric vascular bed of bile duct-ligated rats (a model of liver cirrhosis). This mechanism may mediate the reduced pressor response reported in cirrhosis (Atucha et al., 2005). It appears that NO modulates the SOCC activity via a cGMP-dependent pathway because sodium nitroprusside, which releases intracellular cGMP by activating guanylyl cyclase, was found to inhibit non-selective cation currents activated by store depletion in the mouse anococcygeus smooth muscle cells (Wayman et al., 1996b). Moreover, sodium nitroprusside, S-nitroso-N-acetyl-DL-penicillamine and 8-bromo-cGMP inhibit a Ca2+-permeable, non-selective cation current activated by endothelin-1 in rat aortic smooth muscle cells (Minowa et al., 1997). Therefore, regulation of the agonist-activated SOCE is another mechanism responsible for NO-mediated vasodilatation. More importantly, the Ca2+-permeable non-selective cation channels are important targets for nitrovasodilators.
Conclusions
Capacitative Ca2+ entry via SOCCs, along with VOCCs and ROCCs, plays an important role in the regulation of smooth muscle tone. The relative contribution of SOCE to excitation/contraction coupling depends on the smooth muscle type and appears to be greatest in tonic smooth muscle. However, many questions remain unanswered and need full resolution. The most important areas of future research are (i) identification of selective activators/inhibitors that act directly on SOCCs for further pharmacological characterization of these channels and their potential therapeutic value; (ii) elucidation of cellular mechanisms that link depletion of the SR to the opening of SOCCs in the plasma membrane and (iii) elucidation of a complete molecular structure, a crucial step towards full understanding of the channel function. It is important to determine not only the expression of TRP proteins in VSMCs but also their tendency to heteromultimerise in native cells. SOCCs in arterioles have a distinct pharmacological profile. Knowledge of this profile provides support for the hypothesis that there exist multiple types of SOCCs in smooth muscle and will facilitate comparisons with heterologously expressed genes that encode putative subunits of SOCCs. The results presented in this review favour the proposal that arteriolar SOCCs are likely to be TRPC1 and TRPC5. Finally, are there mechanisms of store-operated entry involving additional means of activation (excluding Stim1) and other store-operated channels (excluding Orais)? The existence of distinct SOCCs in VSMCs may aid in developing target-specific novel therapeutic drugs.
Acknowledgments
We are thankful to Professors JW Jr Putney, J Brayden and AK Grover for their helpful comments and suggestions on this paper. This study was funded by Research Grants Council of Hong Kong SAR (CUHK 4362/04M), CUHK Li Ka Shing Institute of Health Sciences, CUHK Focused Investment Scheme, and the Canadian Heart and Stroke Foundation (IL). LMY and FPL were supported by these grants.
Abbreviations
- 8-bromo-cyclic GMP
8-bromo-guanosine-3′,5′-cyclicmonophosphate
- CaM
calmodulin
- CIF
calcium influx factor
- CPA
cyclopiazonic acid
- DAG
diacylglycerol
- DES
diethylstilbestrol
- IP3
inositol 1,4,5-trisphosphate
- NO
nitric oxide
- ROCC
receptor-operated Ca2+ channels
- SERCA
sarcoplasmic/endoplasmic reticulum Ca2+ ATPases
- SOCC
store-operated Ca2+ channel
- SOCE
store-operated calcium entry
- SR
sarcoplasmic reticulum
- STIM1
stromal-interacting molecule 1
- TRP
transient receptor potential
- VOCC
voltage-operated Ca2+ channel
- VSMCs
vascular smooth muscle cells
Conflict of interest
The authors state no conflict of interest.
References
- Albert AP, Large WA. The effect of external divalent cations on spontaneous non-selective cation currents in rabbit portal vein myocytes. J Physiol. 2001;536:409–420. doi: 10.1111/j.1469-7793.2001.0409c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Large WA. Synergism between inositol phosphates and diacylglycerol on native TRPC6-like channels in rabbit portal vein myocytes. J Physiol. 2003;552:789–795. doi: 10.1113/jphysiol.2003.052977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert AP, Piper AS, Large WA. Properties of a constitutively active Ca2+-permeable non-selective cation channel in rabbit ear artery myocytes. J Physiol. 2003;549:143–156. doi: 10.1113/jphysiol.2002.038190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon A, Hamlyn JM, Blaustein MP. Na+ entry via store-operated channels modulates Ca2+ signaling in arterial myocytes. Am J Physiol Cell Physiol. 2000;278:C163–C173. doi: 10.1152/ajpcell.2000.278.1.C163. [DOI] [PubMed] [Google Scholar]
- Atucha NM, Nadal FJ, Alcaraz A, Iyu D, Ortiz MC, Garcia-Estan J. Reduced capacitative calcium entry in the mesenteric vascular bed of bile duct-ligated rats. Eur J Pharmacol. 2005;525:117–122. doi: 10.1016/j.ejphar.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L909–L917. doi: 10.1152/ajplung.00317.2003. [DOI] [PubMed] [Google Scholar]
- Bae YM, Park MK, Lee SH, Ho WK, Earm YE. Contribution of Ca2+-activated K+ channels and non-selective cation channels to membrane potential of pulmonary arterial smooth muscle cells of the rabbit. J Physiol. 1999;514:747–758. doi: 10.1111/j.1469-7793.1999.747ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ. Emerging functions of 10 types of TRP cationic channel in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2005;32:597–603. doi: 10.1111/j.1440-1681.2005.04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergdahl A, Gomez MF, Wihlborg AK, Erlinge D, Eyjolfson A, Xu SZ, et al. Plasticity of TRPC expression in arterial smooth muscle: correlation with store-operated Ca2+ entry. Am J Physiol Cell Physiol. 2005;288:C872–C880. doi: 10.1152/ajpcell.00334.2004. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Capacitative calcium entry. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotina VM. Mechanism of nitric oxide-induced vasodilatation. Refilling of intracellular stores by sarcoplasmic reticulum Ca2+-ATPase and inhibition of store-operated Ca2+ influx. Circ Res. 1999;84:210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Brueggemann LI, Markun DR, Henderson KK, Cribbs LL, Byron KL. Pharmacological and electrophysiological characterization of store-operated currents and capacitative Ca2+ entry in vascular smooth muscle cells. J Pharmacol Exp Ther. 2006;317:488–499. doi: 10.1124/jpet.105.095067. [DOI] [PubMed] [Google Scholar]
- Burt RP, Chapple CR, Marshall I. The role of capacitative Ca2+ influx in the alpha 1B-adrenoceptor-mediated contraction to phenylephrine of the rat spleen. Br J Pharmacol. 1995;116:2327–2333. doi: 10.1111/j.1476-5381.1995.tb15073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron K, Taylor CW. Vasopressin stimulation of Ca2+ mobilization, two bivalent cation entry pathways and Ca2+ efflux in A7r5 rat smooth muscle cells. J Physiol. 1995;485:455–468. doi: 10.1113/jphysiol.1995.sp020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells R, Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- Cioffi DL, Wu S, Alexeyev M, Goodman SR, Zhu MX, Stevens T. Activation of the endothelial store-operated ISOC Ca2+ channel requires interaction of protein 4.1 with TRPC4. Circ Res. 2005;97:1164–1172. doi: 10.1161/01.RES.0000193597.65217.00. [DOI] [PubMed] [Google Scholar]
- Clementi E, Meldolesi J. The cross-talk between nitric oxide and Ca2+: a story with a complex past and a promising future. Trends Pharmacol Sci. 1997;18:266–269. doi: 10.1016/s0165-6147(97)01087-0. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Trepakova ES, et al. Nitric oxide inhibits capacitative cation influx in human platelets by promoting sarcoplasmic/endoplasmic reticulum Ca2+-ATPase-dependent refilling of Ca2+ stores. Circ Res. 1999;84:201–209. doi: 10.1161/01.res.84.2.201. [DOI] [PubMed] [Google Scholar]
- Cortijo J, Villagrasa V, Marti-Cabrera M, Villar V, Moreau J, Advenier C, et al. The spasmogenic effects of vanadate in human isolated bronchus. Br J Pharmacol. 1997;121:1339–1349. doi: 10.1038/sj.bjp.0701277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis TM, Major EH, Trimble ER, Scholfield CN. Diabetes-induced activation of protein kinase C inhibits store-operated Ca2+ uptake in rat retinal microvascular smooth muscle. Diabetologia. 2003;46:1252–1259. doi: 10.1007/s00125-003-1178-5. [DOI] [PubMed] [Google Scholar]
- Curtis TM, Scholfield CN. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J Physiol. 2001;532:609–623. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi S, Damron DS, Ogawa K, Tanaka S, Horibe M, Murray PA. K+ channel inhibition, calcium signaling, and vasomotor tone in canine pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2000;279:L242–L251. doi: 10.1152/ajplung.2000.279.2.L242. [DOI] [PubMed] [Google Scholar]
- Draber P, Draberova L. Lifting the fog in store-operated Ca2+ entry. Trends Immunol. 2005;26:621–624. doi: 10.1016/j.it.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dreja K, Bergdahl A, Hellstrand P. Increased store-operated Ca2+ entry into contractile vascular smooth muscle following organ culture. J Vasc Res. 2001;38:324–331. doi: 10.1159/000051063. [DOI] [PubMed] [Google Scholar]
- Facemire CS, Mohler PJ, Arendshorst WJ. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol. 2004;286:F546–F551. doi: 10.1152/ajprenal.00338.2003. [DOI] [PubMed] [Google Scholar]
- Fasolato C, Nilius B. Store depletion triggers the calcium release-activated calcium current (ICRAC) in macrovascular endothelial cells: a comparison with Jurkat and embryonic kidney cell lines. Pflugers Arch. 1998;436:69–74. doi: 10.1007/s004240050605. [DOI] [PubMed] [Google Scholar]
- Fellner SK, Arendshorst WJ. Capacitative calcium entry in smooth muscle cells from preglomerular vessels. Am J Physiol. 1999;277:F533–F542. doi: 10.1152/ajprenal.1999.277.4.F533. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Flemming R, Cheong A, Dedman AM, Beech DJ. Discrete store-operated calcium influx into an intracellular compartment in rabbit arteriolar smooth muscle. J Physiol. 2002;543:455–464. doi: 10.1113/jphysiol.2002.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming R, Xu SZ, Beech DJ. Pharmacological profile of store-operated channels in cerebral arteriolar smooth muscle cells. Br J Pharmacol. 2003;139:955–965. doi: 10.1038/sj.bjp.0705327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, et al. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Ghisdal P, Vandenberg G, Morel N, Roux M, Crest M, Honore E, et al. Rho-dependent kinase is involved in agonist-activated calcium entry in rat arteries. J Physiol. 2003;551:855–887. doi: 10.1113/jphysiol.2003.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarchi A, Padilla F, Coste B, Raoux M, Crest M, Honore E, et al. The versatile nature of the calcium-permeable cation channel TRPP2. EMBO Rep. 2006;7:787–793. doi: 10.1038/sj.embor.7400745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A, McFadzean I, Tucker JF, Wayman C. Variable potency of nitrergic-nitrovasodilator relaxations of the mouse anococcygeus against different forms of induced tone. Br J Pharmacol. 1994;113:1494–1500. doi: 10.1111/j.1476-5381.1994.tb17165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A, McFadzean I, Wallace P, Wayman CP. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends Pharmacol Sci. 1998;19:266–269. doi: 10.1016/s0165-6147(98)01222-x. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, et al. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Gonzalez De La Fuente P, Savineau JP, Marthan R. Control of pulmonary vascular smooth muscle tone by sarcoplasmic reticulum Ca2+ pump blockers: thapsigargin and cyclopiazonic acid. Pflugers Arch. 1995;429:617–624. doi: 10.1007/BF00373982. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- Helliwell RM, Large WA. Alpha 1-adrenoceptor activation of a non-selective cation current in rabbit portal vein by 1,2-diacyl-sn-glycerol. J Physiol. 1997;499:417–428. doi: 10.1113/jphysiol.1997.sp021938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrand P. Long-term effects of intracellular calcium and growth factors on excitation and contraction in smooth muscle. Acta Physiol Scand. 1998;164:637–644. doi: 10.1111/j.1365-201x.1998.tb10707.x. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Schaefer M, Schultz G, Gudermann GT. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Huang Y, Leung FP, Yao XQ, Yung LM.Store-operated Ca2+ entry in vascular smooth muscle Acta Pharmacologica Sinica 2006. The 15th World Congress of Pharmacology. Meeting abstract, S20.3
- Inoue R. TRP channels as a newly emerging non-voltage-gated Ca2+ entry channel superfamily. Curr Pharm Design. 2005;11:1899–1914. doi: 10.2174/1381612054021079. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoute H, Hara Y, Shimizu S, Naitoh S, et al. The transient receptor potential protein homologue TRP6 is the essential component of vascular a1-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Iwamuro Y, Zhang XF, Okamoto Y, Miwa S, Masaki T. Characterization of voltage-independent Ca2+ channels activated by endothelin-1. Nippon Yakurigaku Zasshi. 1999;1:96P–102P. doi: 10.1254/fpj.114.supplement_96. [DOI] [PubMed] [Google Scholar]
- Iwasawa K, Nakajima T, Hazama H, Goto A, Shin WS, Toyo-oka T, et al. Effects of extracellular pH on receptor-mediated Ca2+ influx in A7r5 rat smooth muscle cells: involvement of two different types of channel. J Physiol. 1997;503:237–251. doi: 10.1111/j.1469-7793.1997.237bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WF. Vascular smooth muscle store-operated Ca2+ channels: what a TRP. Am J Physiol Heart Circ Physiol. 2006;291:H2592–H2594. doi: 10.1152/ajpheart.00869.2006. [DOI] [PubMed] [Google Scholar]
- Jernigan NL, Broughton BR, Walker BR, Resta TC. Impaired NO-dependent inhibition of store- and receptor-operated calcium entry in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2006;290:L517–L525. doi: 10.1152/ajplung.00308.2004. [DOI] [PubMed] [Google Scholar]
- Juhaszova M, Ambesi A, Lindenmayer GE, Bloch RJ, Blaustein MP. Na+-Ca2+ exchanger in arteries: identification by immunoblotting and immunofluorescence microscopy. Am J Physiol. 1994;266:C234–C242. doi: 10.1152/ajpcell.1994.266.1.C234. [DOI] [PubMed] [Google Scholar]
- Kang TM, Park MK, Uhm DY. Effects of hypoxia and mitochondrial inhibition on the capacitative calcium entry in rabbit pulmonary arterial smooth muscle cells. Life Sci. 2003;72:1467–1469. doi: 10.1016/s0024-3205(02)02441-4. [DOI] [PubMed] [Google Scholar]
- Kawanabe Y, Hashimoto N, Masaki T. Ca2+ channels involved in endothelin-induced mitogenic response in carotid artery vascular smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C330–C337. doi: 10.1152/ajpcell.00227.2001. [DOI] [PubMed] [Google Scholar]
- Kim HY, Thomas D, Hanley MR. Chromatographic resolution of an intracellular calcium influx factor from thapsigargin-activated Jurkat cells. Evidence for multiple activities influencing calcium elevation in Xenopus oocytes. J Biol Chem. 1995;270:9706–9708. doi: 10.1074/jbc.270.17.9706. [DOI] [PubMed] [Google Scholar]
- Koo EW, Gotlieb AI. Neointimal formation in the porcine aortic organ culture. I. Cellular dynamics over 1 month. Lab Invest. 1991;64:743–753. [PubMed] [Google Scholar]
- Kuriyama H, Kitamura K, Itoh T, Inoue R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol Rev. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]
- Kwan CY, Chaudhary R, Zheng XF, Ni J, Lee RM. Effects of sarcoplasmic reticulum calcium pump inhibitors on vascular smooth muscle. Hypertension. 1994;23:I156–I160. doi: 10.1161/01.hyp.23.1_suppl.i156. [DOI] [PubMed] [Google Scholar]
- Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev. 2004;56:439–513. doi: 10.1124/pr.56.4.1. [DOI] [PubMed] [Google Scholar]
- Lee CH, Poburko D, Sahota P, Sanhu J, Reuhlmann DO, van Breemen C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J Phyiol. 2001;435:641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, et al. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Nordstrom I, Malmqvist U, Nordenfelt P, Hellstrand P. Long-term effects of Ca2+ on structure and contractility of vascular smooth muscle. Am J Physiol. 1999;277:C64–C73. doi: 10.1152/ajpcell.1999.277.1.C64. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang W, Singh B, Lockwich T, Jadlowiec J, O'Connell B, et al. Trp1, a candidate protein for the store-operated Ca2+ influx mechanism in salivary gland cells. J Biol Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed hTRPC1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- Low AM, Loke JC, Kwan CY, Daniel EE. Sensitivity to protein kinase C inhibitors of nicardipine-insensitive component of high K+ contracture in rat and guinea-pig aorta. Br J Pharmacol. 1994;112:604–610. doi: 10.1111/j.1476-5381.1994.tb13117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadzean I, Gibson A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br J Pharmacol. 2002;135:1–13. doi: 10.1038/sj.bjp.0704468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowa T, Miwa S, Kobayashi S, Enoki T, Zhang XF, Komuro T, et al. Inhibitory effect of nitrovasodilators and cyclic GMP on ET-1-activated Ca2+-permeable nonselective cation channel in rat aortic smooth muscle cells. Br J Pharmacol. 1997;120:1536–1544. doi: 10.1038/sj.bjp.0701059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro DD, Wendt IR. Effects of cyclopiazonic acid on [Ca2+]i and contraction in rat urinary bladder smooth muscle. Cell Calcium. 1994;15:369–380. doi: 10.1016/0143-4160(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res. 2001;89:923–929. doi: 10.1161/hh2201.100315. [DOI] [PubMed] [Google Scholar]
- Noguera MA, Ivorra MD, Chulia S, D'Ocon P. Capacitative Ca2+ entry associated with alpha1-adrenoceptors in rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:83–89. doi: 10.1007/pl00005033. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Asano M, Ito K, Uyama Y, Imaizumi Y, Watanabe M. Potent vasoconstrictor actions of cyclopiazonic acid and thapsigargin on femoral arteries from spontaneously hypertensive rats. Br J Pharmacol. 1997;120:65–73. doi: 10.1038/sj.bjp.0700857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Kawai K, Ito S, Nakazato Y. Ca2+ entry activated by emptying of intracellular Ca2+ stores in ileal smooth muscle of the rat. Br J Pharmacol. 1995;114:1165–1170. doi: 10.1111/j.1476-5381.1995.tb13329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike M, Gericke M, Droogmans G, Nilius B. Calcium entry activated by store depletion in human umbilical vein endothelial cells. Cell Calcium. 1994;16:367–376. doi: 10.1016/0143-4160(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- Owen RA, Molon-Noblot S, Hubert MF, Kindt MV, Keenan KP, Eydelloth RS. The morphology of juxtaglomerular cell hyperplasia and hypertrophy in normotensive rats and monkeys given an angiotensin II receptor antagonist. Toxicol Pathol. 1995;23:606–619. doi: 10.1177/019262339502300319. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Activation and inactivation mechanisms of capacitative calcium influx. Proc Natl Acad Sci USA. 1995;92:7907–7911. doi: 10.1073/pnas.92.17.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store-depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Ford DL, Hurt KJ, Bae SS, Suh PG, et al. Phospholipase C-γ is required for agonist-induced Ca2+ entry. Cell. 2002;111:529–541. doi: 10.1016/s0092-8674(02)01045-0. [DOI] [PubMed] [Google Scholar]
- Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res. 2006;7:119. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov GV, Boev KK. Cyclopiazonic acid-induced changes in contractile activity of smooth muscle strips isolated from cat and guinea-pig stomach. Eur J Pharmacol. 1996a;318:109–115. doi: 10.1016/s0014-2999(96)00764-9. [DOI] [PubMed] [Google Scholar]
- Petkov GV, Boev KK. The role of sarcoplasmic reticulum and sarcoplasmic reticulum Ca2+-ATPase in the smooth muscle tone of the cat gastric fundus. Pflugers Arch. 1996b;431:928–935. doi: 10.1007/s004240050087. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr TRP, inositol 1,4,5 trisphosphate receptors, and capacitative calcium entry. Proc Natl Acad Sci USA. 1999b;96:14669–14671. doi: 10.1073/pnas.96.26.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr Pharmacology of capacitative calcium entry. Mol Interv. 2001;1:84–94. [PubMed] [Google Scholar]
- Putney JW., Jr New molecular players in capacitative Ca2+ entry. J Cell Sci. 2007;120:1959–1965. doi: 10.1242/jcs.03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW, Jr, McKay RR. Capacitative calcium entry channels. Bioessays. 1999a;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Resnik ER, Keck M, Sukovich DJ, Herron JM, Cornfield DN. Chronic intrauterine pulmonary hypertension increases capacitative calcium entry in fetal pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L953–L959. doi: 10.1152/ajplung.00327.2006. [DOI] [PubMed] [Google Scholar]
- Rivera AA, White CR, Guest LL, Elton TS, Marchase RB. Hyperglycemia alters cytoplasmic Ca2+ responses to capacitative Ca2+ influx in rat aortic smooth muscle cells. Am J Physiol. 1995;269:C1482–C1488. doi: 10.1152/ajpcell.1995.269.6.C1482. [DOI] [PubMed] [Google Scholar]
- Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993;265:C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Robertson TP, Hague D, Aaronson PI, Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sekiguchi F, Shimamura K, Akashi M, Sunano S. Effects of cyclopiazonic acid and thapsigargin on electromechanical activities and intracellular Ca2+ in smooth muscle of carotid artery of hypertensive rats. Br J Pharmacol. 1996;118:857–864. doi: 10.1111/j.1476-5381.1996.tb15478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda LA, Sham JS, Sylvester JT. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res. 2000;49:549–560. [PubMed] [Google Scholar]
- Slodzinski MK, Juhaszova M, Blaustein MP. Antisense inhibition of Na+/Ca2+ exchange in primary cultured arterial myocytes. Am J Physiol. 1995;269:C1340–C1345. doi: 10.1152/ajpcell.1995.269.5.C1340. [DOI] [PubMed] [Google Scholar]
- Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- Snetkov VA, Aaronson PI, Ward JP, Knock GA, Robertson TP. Capacitative calcium entry as a pulmonary specific vasoconstrictor mechanism in small muscular arteries of the rat. Br J Pharmacol. 2003;140:97–106. doi: 10.1038/sj.bjp.0705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem. 2005;280:39786–39794. doi: 10.1074/jbc.M506064200. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Soyombo AA, Angelini GD, Bryan AJ, Jasani B, Newby AC. Intimal proliferation in an organ culture of human saphenous vein. Am J Pathol. 1990;137:1401–1410. [PMC free article] [PubMed] [Google Scholar]
- Spassova MA, Soboloff J, He LP, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,2,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, et al. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol. 2002a;92:1594–1602. doi: 10.1152/japplphysiol.00722.2001. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan XJ. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002b;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- Tepel M, Ruess C, Mehring N, Neusser M, Zidek W. Effect of inhibition of sarcoplasmic Ca2+-ATPase on vasoconstriction and cytosolic Ca2+ in aortic smooth muscle from spontaneously hypertensive and normotensive rats. Clin Exp Hypertens. 1994;16:493–506. doi: 10.3109/10641969409067958. [DOI] [PubMed] [Google Scholar]
- Thebault S, Zholos A, Enfissi A, Slomianny C, Dewailly E, Roudbaraki M, et al. Receptor-operated Ca2+ entry mediated by TRPC3/TRPC6 proteins in rat prostate smooth muscle (PS1) cell line. J Cell Physiol. 2005;204:320–328. doi: 10.1002/jcp.20301. [DOI] [PubMed] [Google Scholar]
- Thyberg J. Differentiated properties and proliferation of arterial smooth muscle cells in culture. Int Rev Cytol. 1996;169:183–265. doi: 10.1016/s0074-7696(08)61987-7. [DOI] [PubMed] [Google Scholar]
- Tosun M, Paul RJ, Rapoport RM. Coupling of store-operated Ca2+ entry to contraction in rat aorta. J Pharmacol Exp Ther. 1998;285:759–766. [PubMed] [Google Scholar]
- Trepakova ES, Gericke M, Hirakawa Ym, Weisbrod RM, Cohen RA, Bolotina VM. Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J Biol Chem. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci USA. 1999;96:3934–3939. doi: 10.1073/pnas.96.7.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz J, Eckert R, Trautwein W. Changes of intracellular calcium concentrations by phenylephrine in renal arterial smooth muscle cells. Pflugers Arch. 1999;438:725–731. doi: 10.1007/s004249900091. [DOI] [PubMed] [Google Scholar]
- Walker RL, Hume JR, Horowitz B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am J Physiol Cell Physiol. 2001;280:C1184–C1192. doi: 10.1152/ajpcell.2001.280.5.C1184. [DOI] [PubMed] [Google Scholar]
- Wallace P, Ayman S, McFadzean I, Gibson A. Thapsigargin-induced tone and capacitative calcium influx in mouse anococcygeus smooth muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:368–375. doi: 10.1007/s002109900100. [DOI] [PubMed] [Google Scholar]
- Wang J, Laurier LG, Sims SM, Preiksaitis HG. Enhanced capacitative calcium entry and TRPC channel gene expression in human LES smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1074–G1083. doi: 10.1152/ajpgi.00227.2002. [DOI] [PubMed] [Google Scholar]
- Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L848–L858. doi: 10.1152/ajplung.00319.2003. [DOI] [PubMed] [Google Scholar]
- Wayman CP, McFadzean I, Gibson A, Tucker JF. Two distinct membrane currents activated by cyclopiazonic acidinduced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br J Pharmacol. 1996a;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman CP, McFadzean I, Gibson A, Tucker JF. Inhibition by sodium nitroprusside of a calcium store depletion-activated non-selective cation current in smooth muscle cells of the mouse anococcygeus. Br J Pharmacol. 1996b;118:2001–2008. doi: 10.1111/j.1476-5381.1996.tb15636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol. 2005;289:L5–L13. doi: 10.1152/ajplung.00044.2005. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Mason HS, Smith GD, Nicholson N, Johnston L, Janiak R, et al. Comparative capacitative calcium entry mechanisms in canine pulmonary and renal arterial smooth muscle cells. J Physiol. 2002;543:917–931. doi: 10.1113/jphysiol.2002.021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Boulay G, Flemming R, Beech DJ. E3-targeted anti-TRPC5 antibody inhibits store-operated calcium-entry in freshly isolated pial arterioles. Am J Physiol Heart Circ Physiol. 2006;291:H2653–H2659. doi: 10.1152/ajpheart.00495.2006. [DOI] [PubMed] [Google Scholar]
- Xuan YT, Glass PS. Propofol regulation of calcium entry pathways in cultured A10 and rat aortic smooth muscle cells. Br J Pharmacol. 1996;117:5–12. doi: 10.1111/j.1476-5381.1996.tb15147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan YT, Wang OL, Whorton AR. Thapsigargin stimulates Ca2+ entry in vascular smooth muscle cells: nicardipine-sensitive and -insensitive pathways. Am J Physiol. 1992;262:C1258–C1265. doi: 10.1152/ajpcell.1992.262.5.C1258. [DOI] [PubMed] [Google Scholar]
- Yip H, Chan WY, Leung PC, Kwan HY, Liu C, Huang Y, et al. Expression of TRPC homologs in endothelial cells and smooth muscle layers of human arteries. Histochem Cell Biol. 2004;122:553–561. doi: 10.1007/s00418-004-0720-y. [DOI] [PubMed] [Google Scholar]
- Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, et al. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003;284:C316–C330. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–29568. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- Zakharov SI, Smani T, Dobrydneva Y, Monje F, Fichandler C, Blackmore PF, et al. Diethylstilbestrol is a potent inhibitor of store-operated channels and capacitative Ca2+ influx. Mol Pharmacol. 2004;66:702–707. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yuan JX, Barrett KE, Dong H. Role of Na+/Ca2+ exchange in regulating cytosolic Ca2+ in cultured human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2005a;288:C245–C252. doi: 10.1152/ajpcell.00411.2004. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005b;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV, Tsytsyura YD, Philyppov IB, Shuba MF, Bolton TB. Voltage-dependent inhibition of the muscarinic cationic current in guinea-pig ileal cells by SK&F 96365. Br J Pharmacol. 2000;129:695–702. doi: 10.1038/sj.bjp.0703115. [DOI] [PMC free article] [PubMed] [Google Scholar]