Abstract
Background and purpose:
This study investigated the α1β2δ isoform of the GABAA receptor that is presumably expressed in the forebrain. The functional and pharmacological properties of this receptor combination are largely unknown.
Experimental approach:
We expressed α1β2δ GABAA receptors in Xenopus laevis oocytes. GABA-activated currents, in the presence and absence of modulators, were recorded using the two-electrode voltage clamp technique.
Key results:
The α1β2δ isoform of the GABAA receptor exhibited an extremely small GABA-mediated current. Tracazolate increased the current amplitude evoked by a half-maximal concentration (EC50) of GABA by 59-fold. The maximum current was increased 23-fold in the presence of a saturating GABA concentration. Concomitant with the increase in the maximum, was a 4-fold decrease in the EC50. Finally, a mutation in the second transmembrane domain of the δ subunit that increases receptor efficacy (L286S), eliminated the increase in the maximum GABA-activated current. The endogenous neurosteroid, tetrahydrodeoxycorticosterone (THDOC), also decreased the EC50 and increased the maximum current amplitude, although to a lesser degree than that of tracazolate.
Conclusions and implications:
Taken all together, these findings indicate that the small GABA-mediated currents in the absence of the modulator are due to a low efficacy for activation. In the absence of modulators, α1β2δ GABA receptors would be effectively silent and therefore contribute little to inhibition in the CNS. In the presence of tracazolate or endogenous neurosteroids however, this particular receptor isoform could exert a profound inhibitory influence on neuronal activity.
Keywords: α1β2δ GABAA receptors, tracazolate, neurosteroid THDOC
Introduction
GABA is the major inhibitory neurotransmitter in the CNS. The GABA type A receptor (GABAA receptor) contains an integral chloride ion pore that can be gated (opened) by GABA and modulated by a variety of pharmacologically and clinically important drugs. GABAA receptors are composed of five subunits that can belong to several different subunit classes. So far, 19 different subunits have been identified in the mammalian brain; α1–6, β1–4, γ1–3, δ, ɛ, θ, ρ1–3 (Barnard et al., 1998). Depending on the subunit composition, these receptors exhibit distinct electrophysiological and pharmacological properties (Sieghart, 1995; Sanna et al., 1997; Hevers and Luddens, 1998; Belelli et al., 2002; Sieghart and Sperk, 2002; Yang et al., 2005; Orser, 2006).
The δ-subunit is one of the GABAA receptor subunits with a highly specific regional and subcellular distribution. It is abundant in cerebellar and dentate gyrus granule cells, some cortical areas, thalamic relay nuclei and the striatum (Benke et al., 1991; Laurie et al., 1992; Wisden et al., 1992; Fritschy and Mohler, 1995; Sperk et al., 1997; Pirker et al., 2000; Peng et al., 2002). GABA receptors containing αβδ-subunits have been localized to extra- and peri-synaptic membranes, exhibit a high sensitivity to GABA, show little desensitization and are believed to be one of the primary mediators of tonic inhibition. This tonic inhibition increases neuronal membrane conductance thereby modifying the spatial and temporal integration of excitatory signals (Brickley et al., 2001; Macdonald et al., 2004). It has been proposed that δ-subunits assemble with α4-subunits in the forebrain and α6-subunits in the cerebellum to form functional receptors. More recently, an association of δ with α1 has been proposed and this particular combination may be a target for the actions of ethanol in the brain (Glykys et al., 2007).
GABAA receptors containing αβδ subunits are modulated by many clinically important drugs, such as anxiolytics, anticonvulsants and sedative/hypnotics (Sundstrom-Poromaa et al., 2002; Wohlfarth et al., 2002; Wallner et al., 2003; Storustovu and Ebert, 2006; Winsky-Sommerer et al., 2007). Pyrazolopyridines (that is, tracazolate (4-(butylamino)-1-ethyl-6-methyl-1H-pyrazolo(3,4-β)pyridine-5-carboxylic acid ethyl ester), etazolate and cartazolate) are one such class of compounds that represent a chemically unique group of non-sedative anxiolytic agents (Barnes et al., 1983; Patel et al., 1985; Young et al., 1987; Thompson et al., 2002). Tracazolate demonstrates a wider separation between sedative and therapeutic doses than do benzodiazepines and appears to cause fewer adverse interactions than the benzodiazepines in combination with barbiturates and alcohol. Tracazolate can potentiate or inhibit recombinant GABAA receptor function depending on subunit combination. It has been shown that the nature of the third subunit (γ1–3, δ or ɛ) within the receptor complex is critical in determining the functional response of tracazolate (Thompson et al., 2002).
Neurosteroids (including pregnanolone and allopregnanolone) are also powerful modulators of αβδ-containing GABAA receptors (Adkins et al., 2001; Brown et al., 2002; Wohlfarth et al., 2002). Tetrahydrodeoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one) (THDOC), a metabolite of deoxycorticosterone (Crawley et al., 1986; Bitran et al., 1991; Kokate et al., 1994; Lambert et al., 1995; Reddy, 2003), increases the maximal GABA-evoked current several fold and converts αβδ GABAA receptor responses to highly desensitizing, slowly deactivating currents (Bianchi et al., 2002). This latter result would appear to be in agreement with a behavioural study using δ-knockout mice, which demonstrated that a global deletion of the δ-subunit of the GABAA receptor resulted in a decrease in the sensitivity of mice to the sedative/hypnotic, anxiolytic and proabsence effects of neuroactive steroids (Mihalek et al., 1999; Spigelman et al., 2002).
α1β2δ receptors, although presumed to exist (Mody, 2004), have not been well investigated. This study was designed to investigate the functional characteristics of α1β2δ GABAA receptors and the nature of its modulation by tracazolate and neurosteroids. Here we show that this GABAA receptor combination exhibits an extremely low efficacy for GABA-mediated activation. However, tracazolate and THDOC both increase the maximal current amplitude even in the presence of saturating GABA concentrations. We propose that these receptors, owing to their low efficacy, would be essentially silent, but upon exposure to neurosteroids or compounds, such as tracazolate, could exert a profound inhibition in the brain.
Materials and methods
cDNA cloning and in vitro transcription
Wild-type rat GABA receptor subunits α1, β2, γ2 and δ were cloned into the pGEMHE vector (Liman et al., 1992). The cDNA for each construct was linearized by NheI digestion. The cDNAs were then transcribed and capped using standard in vitro transcription techniques (mMACHINE T7 kit; Ambion, Austin, TX, USA). cRNAs were purified and resuspended in diethyl pyrocarbonate-treated water.
Site-directed mutagenesis
The mutant δ L286S was made by the QuikChange method of site-directed mutagenesis using the KOD DNA Polymerase Kit (Novagen, Darmstadt, Germany). Successful mutagenesis was verified by sequencing.
Oocyte preparation and receptor expression
All procedures involving the Xenopus were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio. Xenopus laevis (Xenopus I, Ann Arbor, MI, USA) were anaesthetized by MS-222, and oocytes were surgically removed. The oocytes were placed in a solution consisting of 85.5 mM NaCl, 2.5 mM KCl, 5 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na2HPO4, 50 U ml−1 penicillin and 50 mg ml−1 streptomycin, pH 7.5. Oocytes were dispersed in this solution without Ca2+, but in the presence of 0.25% Collagenase A (Roche Diagnostics, Indianapolis, IN, USA). After isolation, Stage VI oocytes were thoroughly rinsed and maintained at 16 °C in the above-mentioned solution with added Ca2+. Micropipettes for injecting cRNA were pulled on a Sutter P87 horizontal puller and the tips were cut with forceps to 30–40 μm in diameter. To match the concentrations, cRNAs were electrophoresed each at several dilutions in a 1% agarose gel. Based on relative intensity of the bands, and in some cases measurement with a spectrophotometer, the cRNA concentrations were readjusted to be equal. cRNAs were injected into oocytes with a Nanoject microinjection system (Drummond Scientific) at a total volume of 50 nl. We injected oocytes with the following cRNA ratios: α1:β2:H2O (1:1:8), α1:β2:γ2 (1:1:8) and α1:β2:δ (1:1:8). The concentrations of cRNAs for α1 and β2 were 0.01–0.02 μg μl−1 and for δ cRNA, the concentration was 0.08–0.16 μg μl−1. We injected an eightfold excess of δ-encoding or γ2-encoding cRNAs to ensure that a majority of the expressed receptor complexes contained all three subunits.
Voltage-clamp experiments
Two-electrode voltage-clamp procedures were used for current recording 2 or 3 days after cRNA injection. The oocyte was placed in a small volume chamber with a continuous perfusion system, as described previously (Amin and Weiss, 1994). The flow rate in this chamber is ≈120 μl s−1. The δ-containing receptors show very little desensitization, but the γ-containing receptors, at high agonist concentrations, desensitize rapidly. Given this rapid desensitization and the slow flow rate in the chamber, the peak of the GABA-activated currents will be attenuated. In all cases, current amplitudes were measured at the peak. The normal extracellular recording solution (OR2) contained 92.5 mM NaCl, 2.5 mM KCl, 5 mM HEPES, 1 mM CaCl2 and 1 mM MgCl2, pH 7.5, adjusted with NaOH. Recording microelectrodes were fabricated from thin-walled glass micropipettes (A-M Systems, Carlsborg, WA, USA) on a Sutter P-87 horizontal puller (Sutter, Novato, CA, USA) and filled with 3 M KCl (resistances were 1–3 MΩ). The oocytes were voltage clamped at −70 mV using a GeneClamp 500B (Axon Instruments, Foster City, CA, USA). On-line digitization of the signal at 20 Hz was carried out with the ITC-16 data acquisition board (Instrutech, Long Island, NY, USA) and Igor software (Wavemetrics, Lake Oswego, OR, USA). We applied GABA for 15–20 s for α1β2 GABAA receptors and for 20 s for α1β2δ GABAA receptors. The duration of co-application of GABA with tracazolate or THDOC was 40 s, slightly longer than with GABA alone as the modulation had slower kinetics than the activation. In comparing the modulation of α1β2 and α1β2δ receptors, we used 3 μM GABA to activate the receptors. In earlier experiments, this GABA concentration was approximately the EC50 for both receptor subtypes. When all the data were pooled after the modulation studies, the EC50s for the two combinations diverged, so that 3 μM GABA represented a retrospective fractional activation of 0.67 and 0.34 for α1β2 and α1β2δ. This difference should be taken into account when comparing the fractional potentiation. However, our primary purpose for comparing the two receptor combinations was to confirm that the δ-subunit was indeed a functional component of the recombinant α1β2δ receptors. Temperature-dependent experiments were performed using the heater/cooler apparatus N 640352, model CL-100 and N 640353, model SC-20 (Warner Instruments, Hamden, CT, USA).
Data analysis
The dose–response relationship of the GABA-induced current in recombinant GABAA receptors was least squares fit to the following equation:
where the GABA-induced current (I) is a function of the GABA concentration, EC50 is the GABA concentration required for inducing a half-maximal current, nH is the Hill coefficient, and Imax is the maximum current. The maximum current was then used to normalize the dose–response curve for each individual oocyte. For activation, the average of the normalized currents for each GABA concentration was used to plot the data. All the data are presented as mean±s.e.m. For the reported parameters such as EC50s and Hill coefficients, the reported mean±s.e.m. represents the averages of the individual fits.
Drugs and solutions
GABA (Calbiochem, San Diego, CA, USA) stock solution (100 mM) was prepared daily from the solid. Tracazolate and THDOC, both obtained from Sigma, were prepared in DMSO at 100 mM and frozen in aliquots. The working concentrations were freshly prepared from the stock solution immediately before use.
Results
Small currents in oocytes expressing α1β2δ GABAA receptors
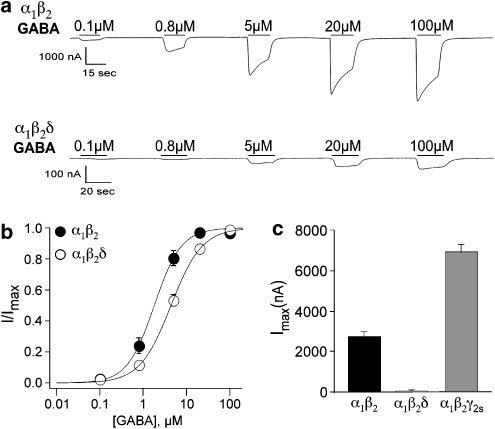
Xenopus laevis oocytes were injected with cRNA encoding for either α1β2- or α1β2δ-subunits. We expressed α1β2 receptors for the sake of comparison with α1β2δ receptors in order to ensure that the δ-subunit was being expressed. After 1–3 days, the oocytes were voltage clamped and exposed to increasing concentrations of GABA to record GABA-mediated currents and assess the sensitivity for activation. Figure 1a shows representative currents induced by application of different GABA concentrations to α1β2 and α1β2δ receptor combinations. Although roughly equivalent amounts of cRNA were injected in the two cases, the maximum GABA-mediated current amplitude (Imax) for α1β2δ was 50-fold less than that of α1β2; 55±5 nA (n=39) versus 2740±310 nA (n=23; Figure 1c). In addition, α1β2δ receptors exhibited a lower sensitivity to GABA, with the GABA concentration required for half-maximal activation (EC50) being about twice that for α1β2 receptors (Figure 1b; Table 1). Although we are unable to rule out the possibility of a sub-population of α1β2 receptors along with α1β2δ, this difference in EC50 confirms that the δ-subunit was indeed being incorporated into the receptor. We also compared the maximal current amplitude of α1β2δ with that of the prototypical α1β2γ2s GABAA receptor combination. In this case, the maximum GABA-mediated current in response to a saturating concentration of GABA was 126-fold higher for α1β2γ2s as compared to α1β2δ (Figure 1c).
Figure 1.
Representative recordings of currents in response to increasing concentrations of GABA on α1β2 and α1β2δ receptors. (a) GABA-mediated currents from oocytes expressing α1β2 and α1β2δ receptors are shown. (b) Concentration–response curves for GABA on oocytes expressing α1β2 and α1β2δ receptors. The continuous line is the best fit of the Hill equation yielding EC50s of 1.8±0.1 (n=16) and 4.4±0.1 (n=20) for α1β2 and α1β2δ, respectively. All currents were normalized to the maximal GABA-induced current in the same oocyte. (c) Bar graphs plotting the maximum currents of α1β2 (Imax=2736±306, n=23) and α1β2δ (Imax=55±5, n=39) GABAA receptors and α1β2γ2s (Imax=6949±354, n=42). Although similar amounts of cRNA were injected, the currents were much larger in the α1β2- and α1β2γ2s-expressing oocytes.
Table 1.
Effects of tracazolate (3–20 μM) on EC50s and Hill coefficients of GABAA receptor combinations
| Combination | EC50 (μM) | Hill | n | EC50 (μM) +3 μMTrac | Hill | n | EC50 (μM) +10 μMTrac | Hill | n | EC50 (μM) +20 μMTrac | Hill | n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α1β2 | 1.8±0.1 | 1.4±0.1 | 16 | 0.4±0.1* | 1.1±0.7 | 11 | 0.2±0.1* | 0.6±0.2 | 10 | 0.1±0.0* | 1.3±0.3 | 6 |
| α1β2δ | 4.4±0.1 | 1.2±0.0 | 20 | 2.8±0.6* | 1.0±0.1 | 11 | 1.0±0.3* | 0.9±0.2 | 10 | 0.4±0.1* | 1.8±0.3 | 6 |
| α1β2δ(9′L/S) | 2.8±0.1 | 1.3±0.1 | 20 | ND | ND | ND | 0.4±0.0 | 1.4±0.2 | 10 | ND | ND | ND |
ND, not determined. *Significantly different from values without tracazolate; P<0.02.
Tracazolate increases the maximum current amplitude of α1β2δ-containing GABAA receptors
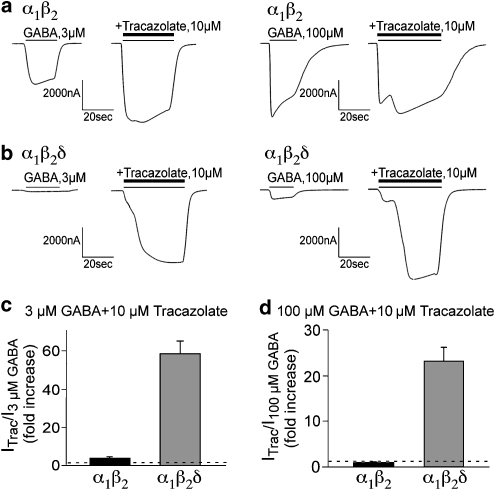
It has previously been shown that tracazolate, a non-benzodiazepine anxiolytic, modulates GABA receptors containing the δ-subunit (Thompson et al., 2002). We therefore tested if tracazolate can modulate the GABA-mediated activation of α1β2δ receptors. Oocytes expressing α1β2- and α1β2δ-subunits were tested with 3 and 100 μM (saturating) GABA without and with the co-application of tracazolate (10 μM). Neither receptor combination was directly activated by 10 μM tracazolate alone. The GABA-gated currents for α1β2 and α1β2δ are shown in Figures 2a and b, respectively. The fold increases in the peak current amplitudes imparted by tracazolate are plotted in Figures 2c and d. Note the inflection in the currents when GABA and tracazolate are applied simultaneously (obvious in subsequent figures as well). The likely interpretation for this inflection is differences in the activation/modulation rates for GABA and tracazolate. More specifically, the activation by GABA precedes in time the modulation by tracazolate. This is supported by the observation that the inflection is not present when we preincubate oocytes with tracazolate and then apply GABA plus tracazolate (data not shown). For α1β2 receptors, tracazolate increased the peak current amplitude in response to 3 μM GABA by 3.9±0.8-fold (n=13) (Figure 2c). The current in response to 100 μM GABA was not potentiated by tracazolate (n=54; Figure 2d). In contrast to these observations for α1β2, we observed a profound potentiation of the GABA-mediated current amplitude by tracazolate at both the lower and higher GABA concentrations for the α1β2δ receptor. Tracazolate increased the peak current amplitude in response to 3 or 100 μM GABA by almost 60-fold (n=14, P<0.001; Figure 2c) and about 20-fold (n=54, P<0.00001; Figure 2d), respectively. Despite the low level of GABA-mediated activation described in Figure 1, α1β2δ receptors are indeed highly expressed on the cell surface. These findings suggest that α1β2δ receptors are of extremely low efficacy for GABA-mediated activation (that is, GABA behaves as a weak partial agonist). We will provide additional support for this in a later section.
Figure 2.
The action of tracazolate on the peak current amplitude from oocytes expressing α1β2 and α1β2δ GABAA receptors. (a) α1β2 receptor currents at 3 μM (left) and 100 μM GABA (right) in the absence and presence of 10 μM tracazolate. (b) α1β2δ receptor currents at 3 μM (left) and 100 μM GABA (right) in the absence and presence of 10 μM tracazolate. (c) The fold increase by tracazolate is shown for 3 μM GABA for α1β2 and α1β2δ. Although a modest 3.9±0.8-fold (n=13) increase was observed for α1β2, the α1β2δ currents were increased by 58.7±6.3-fold (n=14). (d) The fold increase by tracazolate is shown with a saturating concentration of GABA (100 μM) for α1β2 and α1β2δ. α1β2 receptors show no potentiation (1.0±0.1-fold, n=54), whereas the α1β2δ currents were increased by 23.4±3.0-fold (n=54).
Tracazolate enhances the sensitivity of α1β2δ-containing GABAA receptors to GABA
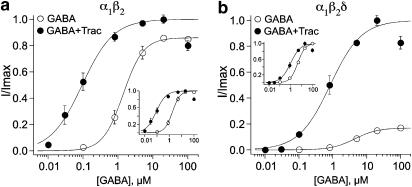
To probe further into the mechanism of action of tracazolate, GABA concentration–response curves were constructed in the absence and presence of 10 μM tracazolate on oocytes expressing α1β2 and α1β2δ GABAA receptors (Figures 3a and b, respectively). α1β2-Containing receptors were ninefold more sensitive to GABA in the presence of tracazolate (Figure 3a; EC50 values in Table 1). α1β2δ-Containing receptors were fourfold more sensitive to GABA in the presence of 10 μM tracazolate (Figure 3b; Table 1). The insets in Figures 3a and b show normalized α1β2 and α1β2δ dose–response curves so the increase in GABA sensitivity is more easily visualized. We obtained qualitatively similar results for both receptor combinations in the presence of 3 and 20 μM tracazolate (Table 1). Thus, although both receptor combinations demonstrate an increase in sensitivity to GABA with tracazolate, the profound increase in the maximum requires the presence of the δ-subunit.
Figure 3.
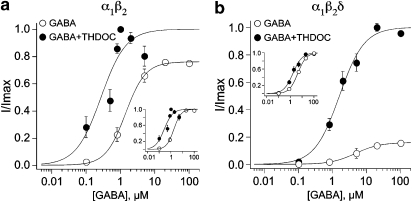
Tracazolate increases the sensitivity of α1β2 and α1β2δ GABAA receptors. (a) Concentration–response curves for GABA were obtained in the absence and presence of 10 μM tracazolate for oocytes expressing α1β2 receptors. Very little change in the maximum current was observed, although 10 μM tracazolate decreased the EC50 from 1.8±0.1 μM (n=16) to 0.2±0.1 μM (n=10). The inset shows the normalized dose–response curves in order to facilitate comparison of the EC50s. (b) Concentration–response curves for GABA were obtained in the absence and presence of 10 μM tracazolate for oocytes expressing α1β2δ receptors. The increase in the maximum and the decrease in EC50 in the presence of 10 μM tracazolate (from 4.4±0.1 μM, n=20 to 1.0±0.1 μM, n=10) is obvious when the dose–response relationships were normalized and replotted (inset). The error bars for the open circles in (b) are smaller than the symbols.
THDOC potentiates GABA receptors comprised of α1β2δ-subunits
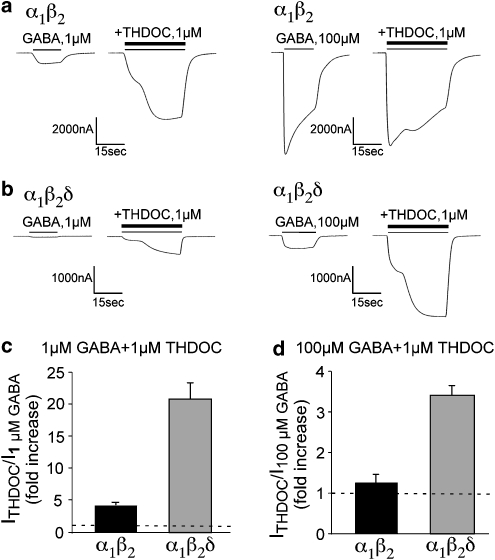
It is known that neurosteroids potentiate GABA receptors containing the δ-subunit (Brown et al., 2002; Wohlfarth et al., 2002; Bianchi and Macdonald, 2003). Oocytes expressing α1β2 or α1β2δ GABAA receptors were tested with 1 and 100 μM GABA without and with 1 μM THDOC. Neither receptor combination was directly activated by 1 μM THDOC alone. THDOC potentiated both α1β2 (Figure 4a) and α1β2δ (Figure 4b) currents elicited by low (1 μM) GABA concentrations. However, as evident in Figure 4c, the mean enhancement was significantly larger for α1β2δ GABAA receptors than for α1β2 receptors (P<0.0005). With a saturating concentration of GABA (100μM), THDOC enhanced α1β2δ currents 3.4±0.2-fold (n=12), whereas α1β2 currents exhibited less enhancement (Figure 4d; n=16 P<0.05). For α1β2δ receptors, THDOC modulation produced substantially larger currents than the maximal currents produced by GABA alone.
Figure 4.
The actions of THDOC (1 μM) on the peak current amplitude for oocytes expressing α1β2 and α1β2δ GABAA receptors. (a) α1β2 receptor currents at 1 μM (left) and 100 μM GABA (right) in the absence and presence of 1 μM THDOC. (b) α1β2δ receptor currents at 1 μM (left) and 100 μM GABA (right) in the absence and presence of 1 μM THDOC. (c) The fold increase by THDOC is shown for 1 μM GABA for α1β2 and α1β2δ. α1β2 receptor currents were increased fourfold in the presence of 1 μM GABA in comparison to the 20-fold increase for α1β2δ. (d) The fold increase by THDOC is shown for a saturating concentration of GABA (100 μM) for α1β2 and α1β2δ. α1β2 exhibited a modest 1.25-fold increase (n=16), whereas the α1β2δ currents were increased by 3.4±0.2-fold (n=15).
To further investigate the actions of THDOC, dose–response curves for GABA were constructed in the absence and presence of 1 μM THDOC on oocytes expressing α1β2 or α1β2δ GABAA receptors (Figures 5a and b, respectively). THDOC produced a leftward shift in the GABA dose–response curves for both α1β2 and α1β2δ receptors. As seen in Figure 5a, α1β2-containing receptors were sixfold more sensitive to GABA in the presence of THDOC (EC50=0.3±0.1 μM, n=4) than without THDOC (EC50=1.8±0.1 μM, n=16; P<0.00005). α1β2δ-Containing receptors were 2.6-fold more sensitive to GABA in the presence of THDOC with EC50s of 4.4±0.1 μM (n=20) and 1.7±0.2 μM (n=5; P<0.005) without and with THDOC, respectively.
Figure 5.
THDOC increased the sensitivity of the GABAA receptors. (a) Concentration–response curves for GABA were obtained in the absence and presence of 1 μM THDOC for oocytes expressing α1β2 receptors. THDOC decreased the EC50 from 1.8±0.1 μM (n=16) to 0.3±0.1 μM (n=4). The inset shows the data normalized to facilitate the comparison of the shift in sensitivity. (b) Concentration–response curves for GABA were obtained in the absence (open circles) and presence (filled circles) of 1 μM THDOC for oocytes expressing α1β2δ receptors. The profound increase in the maximum amplitude was observed, and the decrease in EC50 imparted by 1 μM THDOC (from 4.4±0.1 μM, n=20 to 1.7±0.2 μM, n=5) was obvious when the dose–response relationships were normalized and replotted (inset).
Low efficacy was not due to the temperature dependence of α1β2δ-containing GABAA receptors
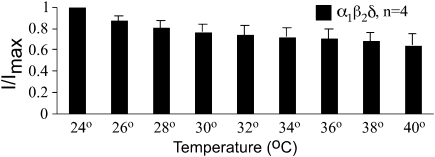
We considered the possibility that the low efficacy of α1β2δ GABA receptors might be due to the lower recording temperature (≈24 °C). Perhaps at physiological temperatures where these receptors normally exist, the efficacy is higher. For example, if the opening rate were highly temperature dependent and increased with increasing temperature, this could account for the observed small currents and proposed low efficacy. To test this possibility, we recorded GABA-evoked currents in oocytes expressing α1β2δ receptors across a range of temperatures. For these experiments, we used saturating concentrations of GABA (100 μM). As shown in Figure 6 (a plot of the maximum current as a function of recording temperature), we actually observed a slight decrease in the maximum current amplitude at higher temperatures. In these acute experiments, it is unlikely that temperature was affecting the translation or surface expression of the receptor. A more likely explanation is that this decrease in current amplitude is due to an increase in receptor desensitization. Nevertheless, the low efficacy seems to be an inherent property of this receptor combination and not a consequence of the recording temperature.
Figure 6.
Low efficacy of α1β2δ receptors is not due to the low recording temperatures. Maximal currents (100 μM GABA) were recorded from oocytes expressing α1β2δ receptors and each of four oocytes was recorded across a range of temperatures. There was a slight decrease in the maximal current as temperature was increased, opposite to what would be expected if the low efficacy were due to the low recording temperatures.
Additional evidence for low efficacy
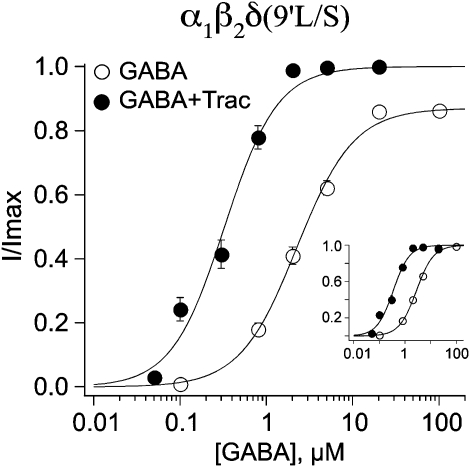
We previously employed the mutation of a conserved leucine in the second transmembrane domain to determine the stoichiometry of the α1β2γ2 GABAA receptor (Chang and Weiss, 1999). Subsequent studies demonstrated that the increase in sensitivity to GABA imparted by this mutation was due to an enhanced gating or efficacy (Chang et al., 1996; Bianchi and Macdonald, 2001). Indeed, when we introduced this mutation into the δ subunit (L286S), we noted a profound increase in the amplitude of GABA-mediated currents (L286S; 4547±184 nA; n=39) compared with the wild-type α1β2δ receptor (Figure 1c; 55±5 nA; n=39), suggesting that this mutation increased receptor efficacy. Figure 7 shows the GABA dose–response relationships before and after the addition of 10 μM tracazolate. Although the increase in sensitivity imparted by tracazolate was still present (Table 1), the maximum currents were hardly affected (1.4±0.0-fold increase for L286S versus 1.0±0.1-fold increase for wild type).
Figure 7.
The 9′ leucine mutation (L286S) in the δ-subunit occludes the tracazolate-mediated increase in maximal amplitude. The mutant δ-subunit was co-expressed in oocytes with wild-type α1 and β2. Dose–response relationships were constructed in the absence and presence of 10 μM tracazolate. Although the leftward shift was still observed, the dramatic increase in the maximum current was absent. The EC50s were 2.8±0.1 (n=20) and 0.4±0.0 (n=10) in the absence and presence of tracazolate, respectively.
Discussion and conclusions
α1β2δ as a native receptor combination
Assembly of different combinations of GABA receptor subunits in different brain regions contributes to the diversity of GABA-mediated inhibition in the CNS (Macdonald and Olsen, 1994). The specific functional properties of δ-subunit-containing GABAA receptors, such as high sensitivity to GABA and low desensitization, allow them to be activated by the micromolar levels of GABA constantly present in the extracellular space (Lerma et al., 1986; Saxena and Macdonald, 1996; Stell et al., 2003; Mtchedlishvili and Kapur, 2006). In this regard, they can exert a tonic inhibitory influence. δ-Containing isoforms of GABAA receptors also play a particularly important role in the regulation of inhibition in the CNS by endogenous neurosteroids as well as behavioral responses to certain sedative/hypnotic and anxiolytic drugs (Mihalek et al., 1999; Peng et al., 2002; Wohlfarth et al., 2002; Bianchi and Macdonald, 2003; Wallner et al., 2003; Dibbens et al., 2004).
The δ-subunit has been assumed to be predominately co-expressed with α4- and/or α6-subunits, whereas the α1-subunit has been assumed to be co-expressed with γ2. There have been reports, however, demonstrating that α1, β2 and δ subunits may be co-expressed in the same neurons (Mertens et al., 1993; Pirker et al., 2000; Mangan et al., 2005). One study, using quantitative immunoblotting, has suggested the presence of an α1α6β2δ receptor combination in rat cerebellum (Thompson et al., 1996). An α6 knockout, however, found no evidence for the existence of α1βxδ receptors (Jones et al., 1997). More recently, α4 knockout studies have shown that α1- and δ-subunits are co-expressed in hippocampal interneurons and may form functional receptors with the β2-subunit. It was suggested that this receptor is a target for low concentrations of ethanol (Glykys et al., 2007). Furthermore, changes in the expression of the δ-subunit, such as with progesterone exposure, could regulate the levels of these δ-containing receptors at opportune times (Mostallino et al., 2006).
In terms of methodology, the most accepted approach to detect native subunit combinations is immunoprecipitation. Although this method may be appropriate for abundant populations of receptors, it may not be sensitive enough to discriminate minor receptor subtypes. Nevertheless, even if the α1β2δ combination is present as a minor GABAA receptor subtype in the adult CNS (McKernan and Whiting, 1996), given the strong potentiation reported here, this receptor could play an important role in the modulation of inhibition in the CNS via neurosteroids and modulators, such as tracazolate.
We compared the function and modulation of α1β2δ receptors to that of α1β2, primarily to demonstrate that the δ-subunit was indeed incorporated into the functional receptor. There is evidence, however, that α1β2 may be expressed in hippocampal pyramidal neurons and account for up to 10% of the total extrasynaptic receptor pool (Mortensen and Smart, 2006). If so, the differential modulation of this combination by tracazolate and THDOC should be considered when evaluating the physiological actions of these modulators.
α1β2δ is a low-efficacy receptor
The α1β2δ receptor combination, when exogenously expressed, demonstrated extremely small GABA-activated currents. These currents were some 50-fold less than that of α1β2 and 126-fold less than that of α1β2γ2s with comparable amounts of injected cRNA. However, both tracazolate and THDOC produced a dramatic increase in the current amplitude for α1β2δ receptors, even at saturating GABA concentrations. Furthermore, the action of these compounds on receptor sensitivity (EC50) was minimal. In its simplest interpretation, these observations imply that the modulators are not altering receptor affinity, but rather modulating receptor gating. Furthermore, a mutation (L286S) in the δ-subunit that increased the efficacy of GABA and restored the current amplitude to levels comparable to that of α1β2γ2s prevented the increase in amplitude mediated by tracazolate. Taken together, these findings indicate that the α1β2δ receptor combination is likely to be a low-efficacy receptor. Other δ-containing receptors have been deemed low efficacy, such as α1β1δ (Thompson et al., 2002) and α1β3δ (Wohlfarth et al., 2002; Feng and Macdonald, 2004a, 2004b). Thus, it appears that for αβδ isoforms in general, GABA behaves as a partial agonist (Adkins et al., 2001; Brown et al., 2002). The most detailed single-channel analysis to date on δ-containing recombinant GABA receptors (α1β3δ) indicated a low open channel probability and mean open time, as well as one less open state than that of α1β3γ2L (Fisher and Macdonald, 1997; Akk et al., 2004a). We also observed small currents in response to GABA and profound modulation by tracazolate for α1β2δ receptors and no tracazolate-mediated potentiation of α1β2γ2s receptors when these two combinations were expressed in HEK293 cells (unpublished observations). Thus, the low efficacy of α1β2δ receptors is not a consequence of the oocyte expression system.
Even at saturating concentrations of GABA, owing to this low efficacy, the α1β2δ receptor would be essentially silent. Upon exposure to THDOC or tracazolate, however, this receptor combination could exert a profound inhibitory influence on excitability in the CNS. This provides a unique mechanism for recruiting inhibition without the cost of receptor trafficking to the surface or the activation of intracellular signaling cascades. Although we observed an increase in current amplitude by tracazolate and THDOC for α1β2 and α1β2γ2, this was only observed at low GABA concentrations and was due to a leftward displacement in the dose–response relationship. At saturating GABA concentrations, there was no increase in the maximum amplitude and a similar result has been obtained for α1β3γ2 receptors (Thompson et al., 2002). Given that GABA concentrations in the synaptic cleft are likely to far exceed saturating levels (Wardell et al., 2006), this provides a mechanism by which these modulators can selectively target δ-containing, extrasynaptic, tonic receptors.
Mechanism of modulation by tracazolate and THDOC
In the present study, 10 μM tracazolate imparted a 59-fold increase in the GABA-mediated current amplitude at low GABA concentrations (3 μM) and a 23-fold increase in the current amplitude at saturating GABA concentrations (100 μM). Other δ-containing receptors exhibit a strong potentiation to tracazolate, although less than that observed here for α1β2δ. For α2β3δ (Yang et al., 2005) and α1β1δ (Thompson et al., 2002) receptor combinations, tracazolate potentiated the maximum current approximately threefold.
Thompson et al. (2002) determined that the particular isoform of the β subunit is important for the actions of tracazolate on receptors. More specifically, tracazolate exhibits a higher sensitivity on α1β3γ2 receptors in comparison with α1β1γ2. Interestingly, whether tracazolate potentiates or inhibits the receptor depends upon the nature of the third subunit (γ1–3, δ or ɛ). The presence of a γ2 or δ subunit imparts a potentiation by tracazolate, the presence of an ɛ-subunit imparts an inhibition, and the presence of γ1 or γ2 can impart an intermediate profile with potentiation or inhibition depending on the concentrations of agonist and modulator. This is similar to what we observed here for the α1β2 combination in the absence of a third subunit. Given our data and that of Thompson et al., it is clear that for the α1βxδ combination, the sensitivity of modulation by tracazolate seems to be dependent on the β subunit present.
Thompson et al. (2002) have observed that under conditions where the receptor rapidly entered the desensitized state (for example, α1β1-3ɛ or high concentrations of GABA on α1β3γ2s), the functional response to tracazolate was inhibition, whereas in conditions with little desensitization (for example, α1β1δ or low GABA concentration on α1β3γ2s) the functional response was potentiation. They propose that tracazolate displays higher affinity for the desensitized state than for the agonist bound state of the receptor. This mirrors our findings where we observed potentiation in α1β2δ, a combination that displays modest desensitization, and inhibition in α1β2γ2 at high agonist concentrations where a strong desensitization was evident. Although the hypothesis is certainly tempting, the inability to separate activation from desensitization makes a causal relationship difficult to confirm.
We also compared the actions of the endogenous neuroactive steroid THDOC on GABA receptors comprised of α1β2- and α1β2δ-subunits. As was true for tracazolate, we observed a greater enhancement for the ternary complex, with increases in current amplitude of 20.7- and 3.4-fold for α1β2δ at an EC50 and saturating concentration of GABA, respectively.
The list of neuroactive steroids is long, and therefore, reconciliation between studies employing the various compounds on different GABA receptor subunit combinations can be difficult. What is clear is that there is strong evidence that neurosteroids have multiple sites of action on the GABA receptor (Akk et al., 2001; Morris and Amin, 2004; Ueno et al., 2004; Akk et al., 2004b; Wardell et al., 2006; Hosie et al., 2007). Recently, two domains have been identified and suggested to represent neurosteroid binding sites (Hosie et al., 2006). One binding domain is within the α-subunit, whereas the second binding domain is at the α-β-subunit interface. These two domains are presumed to represent sites for modulation and activation, respectively. Because the neurosteroids are hydrophobic, the neurosteroid binding site(s) is likely to include a region of the GABAA receptor polypeptide embedded within the membrane, although access to the site appears to be extracellular, as intracellular application of the neurosteroid is ineffective (Twyman and Macdonald, 1992). More recent evidence from patch clamp studies in which the neurosteroids were applied through various compartments, as well as imaging studies with a fluorescent neurosteroid analogue, indicates interaction with sites embedded in the membrane that can be accessed by lateral membrane diffusion (Akk et al., 2004b). Recent imaging studies indicate, however, that GABA-active steroids can accumulate both in the plasma membrane and the intracellular space (Akk et al., 2005).
Although single-channel kinetic analyses have been carried out on GABA receptors to gain further insight into the modulatory mechanism, a definitive model is still lacking (Akk et al., 2004b). Our macroscopic data agree with the studies of Bianchi and Macdonald (2003) that conclude a shift from low to high efficacy, similar to that assumed for tracazolate discussed earlier. In its simplest form, a low-efficacy receptor can be modelled by a kinetic scheme with a slow channel-opening rate. In this scheme, the resulting low open-channel probability (Popen) would underlie the small macroscopic GABA-mediated maximum currents. Although a quantitative validation of this mechanism would require a detailed single-channel analysis to derive the relevant rate constants, an increase in the opening rate by tracazolate could impart the observed profound increase in the maximum current, with a modest decrease in the EC50 (Figure 3b of Amin and Weiss, 1993).
Although not specifically examined in the present study, neurosteroids have been shown to directly activate the receptor, albeit with low efficacy compared to GABA. This adds neurosteroids to the growing list of GABA receptor modulators that both activate and potentiate the receptor (Rusch et al., 2004; Campo-Soria et al., 2006). As for other modulators (diazepam, flurazepam, zolpidem and etomidate), perhaps an allosteric model can serve to unify the activation and modulation processes.
In summary, the α1β2δ GABA receptor subunit combination can be strongly modulated by the anxiolytic drug tracazolate and the endogenous neurosteroid THDOC. As this receptor combination is likely to be expressed in the forebrain (Dibbens et al., 2004) and cerebellum (Poltl et al., 2003), our findings suggest a major contribution of these receptors to the control of normal tonic inhibition and a potential for its therapeutic regulation.
Acknowledgments
This study was funded, in part, by National Institutes of Health Grants NS 36195 and NS 35291.
Abbreviations
- GABAA receptor
GABA type A receptor
- THDOC
tetrahydrodeoxycorticosterone (3α,21-dihydroxy-5α-pregnan-20-one)
- Tracazolate
(4-(butylamino)-1-ethyl-6-methyl-1H-pyrazolo(3,4-β)pyridine-5-carboxylic acid ethyl ester)
Conflict of interest
The authors state no conflict of interest.
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, et al. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α subunit. J Physiol. 2001;532:673–684. doi: 10.1111/j.1469-7793.2001.0673e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Activation of GABAA receptors containing the α4 subunit by GABA and pentobarbital. J Physiol. 2004a;556:387–399. doi: 10.1113/jphysiol.2003.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol. 2004b;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, et al. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin JA, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Amin J, Weiss DS. Homomeric ρ1 GABA channels: activation properties and domains. Receptors Channels. 1994;2:227–236. [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Barnes DM, White WF, Dichter MA. Etazolate (SQ20009): electrophysiology and effects on [3H]flunitrazepam binding in cultured cortical neurons. J Neurosci. 1983;3:762–772. doi: 10.1523/JNEUROSCI.03-04-00762.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Benke D, Mertens S, Trzeciak A, Gillessen D, Mohler H. Identification and immunohistochemical mapping of GABAA receptor subtypes containing the δ-subunit in rat brain. FEBS Lett. 1991;283:145–149. doi: 10.1016/0014-5793(91)80573-l. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the δ subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Mutation of the 9′ leucine in the GABAA receptor γ2L subunit produces an apparent decrease in desensitization by stabilizing open states without altering desensitized states. Neuropharmacology. 2001;41:737–744. doi: 10.1016/s0028-3908(01)00132-0. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3 α-hydroxy-5α [β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo-Soria C, Chang Y, Weiss DS. Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol. 2006;148:984–990. doi: 10.1038/sj.bjp.0706796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Weiss DS. Allosteric activation mechanism of the α1β2γ2 γ-aminobutyric acid type A receptor revealed by mutation of the conserved M2 leucine. Biophys J. 1999;77:2542–2551. doi: 10.1016/s0006-3495(99)77089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Glowa JR, Majewska MD, Paul SM. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 1986;398:382–385. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, et al. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 2004;13:1315–1319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Macdonald RL. Multiple actions of propofol on αβγ and αβδ GABAA receptors. Mol Pharmacol. 2004a;66:1517–1524. doi: 10.1124/mol.104.003426. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Macdonald RL. Proton modulation of α1β3δ GABAA receptor channel gating and desensitization. J Neurophysiol. 2004b;92:1577–1585. doi: 10.1152/jn.00285.2004. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. Single channel properties of recombinant GABAA receptors containing γ2 or delta subtypes expressed with α 1 and β 3 subtypes in mouse L929 cells. J Physiol. 1997;505 Part 2:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABAA receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, et al. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Gallagher MJ, Feng HJ, Kang J. GABAA receptor epilepsy mutations. Biochem Pharmacol. 2004;68:1497–1506. doi: 10.1016/j.bcp.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Mangan PS, Sun C, Carpenter M, Goodkin HP, Sieghart W, Kapur J. Cultured hippocampal pyramidal neurons express two kinds of GABAA receptors. Mol Pharmacol. 2005;67:775–788. doi: 10.1124/mol.104.007385. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mertens S, Benke D, Mohler H. GABAA receptor populations with novel subunit combinations and drug binding profiles identified in brain by α5- and δ-subunit-specific immunopurification. J Biol Chem. 1993;268:5965–5973. [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, et al. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I. Another “tonic” in the realm of epilepsy. Epilepsy Curr. 2004;4:248–249. doi: 10.1111/j.1535-7597.2004.46011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KD, Amin J. Insight into the mechanism of action of neuroactive steroids. Mol Pharmacol. 2004;66:56–69. doi: 10.1124/mol.66.1.56. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostallino MC, Mura ML, Maciocco E, Murru L, Sanna E, Biggio G. Changes in expression of the δ subunit of the GABAA receptor and in receptor function induced by progesterone exposure and withdrawal. J Neurochem. 2006;99:321–332. doi: 10.1111/j.1471-4159.2006.04055.x. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Orser BA. Extrasynaptic GABAA receptors are critical targets for sedative–hypnotic drugs. J Clin Sleep Med. 2006;2:S12–S18. [PubMed] [Google Scholar]
- Patel JB, Malick JB, Salama AI, Goldberg ME. Pharmacology of pyrazolopyridines. Pharmacol Biochem Behav. 1985;23:675–680. doi: 10.1016/0091-3057(85)90436-8. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, et al. GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Poltl A, Hauer B, Fuchs K, Tretter V, Sieghart W. Subunit composition and quantitative importance of GABAA receptor subtypes in the cerebellum of mouse and rat. J Neurochem. 2003;87:1444–1455. doi: 10.1046/j.1471-4159.2003.02135.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions. Trends Pharmacol Sci. 2003;24:103–106. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Rusch D, Zhong H, Forman SA. Gating allosterism at a single class of etomidate sites on α1β2γ2L GABAA receptors accounts for both direct activation and agonist modulation. J Biol Chem. 2004;279:20982–20992. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- Sanna E, Murgia A, Casula A, Biggio G. Differential subunit dependence of the actions of the general anesthetics alphaxalone and etomidate at γ-aminobutyric acid type A receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1997;51:484–490. [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar γ-aminobutyric acidA receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia. 2002;43 Suppl 5:3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: Functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, Pollard S, Stephenson FA. Developmental regulation of expression of GABAA receptor α1 and α6 subunits in cultured rat cerebellar granule cells. Neuropharmacology. 1996;35:1337–1346. doi: 10.1016/s0028-3908(96)00114-1. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Wingrove PB, Connelly L, Whiting PJ, Wafford KA. Tracazolate reveals a novel type of allosteric interaction with recombinant γ-aminobutyric acidA receptors. Mol Pharmacol. 2002;61:861–869. doi: 10.1124/mol.61.4.861. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Tsutsui M, Toyohira Y, Minami K, Yanagihara N. Sites of positive allosteric modulation by neurosteroids on ionotropic γ-aminobutyric acid receptor subunits. FEBS Lett. 2004;566:213–217. doi: 10.1016/j.febslet.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardell B, Marik PS, Piper D, Rutar T, Jorgensen EM, Bamber BA. Residues in the first transmembrane domain of the Caenorhabditis elegans GABAA receptor confer sensitivity to the neurosteroid pregnenolone sulfate. Br J Pharmacol. 2006;148:162–172. doi: 10.1038/sj.bjp.0706719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABAA δ-subunit-containing receptors. Eur J Neurosci. 2007;25:1893–1899. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Jones BL, Henderson LP. Role of the α subunit in the modulation of GABAA receptors by anabolic androgenic steroids. Neuropharmacology. 2005;49:300–316. doi: 10.1016/j.neuropharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Young R, Urbancic A, Emrey TA, Hall PC, Metcalf G. Behavioral effects of several new anxiolytics and putative anxiolytics. Eur J Pharmacol. 1987;143:361–371. doi: 10.1016/0014-2999(87)90460-2. [DOI] [PubMed] [Google Scholar]