Abstract
Background and purpose:
Leukotriene B4 (LTB4), formed by the sequential actions of the 5-lipoxygenase (5-LO) and leukotriene A4 hydrolase (LTA4H), is a pro-inflammatory mediator implicated in the pathogenesis of inflammatory bowel disease. However, inhibitors of 5-LO have not proved to be consistent in their therapeutic efficacy in colitis. Another approach to inhibiting LTB4 synthesis is through the use of inhibitors of LTA4H, such as the novel, potent and selective compound, JNJ 26993135.
Experimental approach:
The effect of oral administration of JNJ 26993135 has been evaluated in a rat model of colitis provoked by colonic instillation of trinitrobenzenesulphonic acid (TNBS). The extent and severity of the macroscopic inflammatory response, the colonic levels of myeloperoxidase (MPO) and LTB4 and of the pro-inflammatory cytokines, tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) were measured.
Key results:
Oral administration of JNJ 26993135 (5, 15 and 30 mg kg−1, twice a day) dose-dependently reduced both the extent and intensity of the colonic inflammatory damage observed 3 days after TNBS challenge. JNJ 26993135 also dose-dependently reduced the elevated colonic levels of LTB4, as well as the inflammatory biomarkers, MPO, IL-6 and TNF-α. This dosing regimen was supported by the pharmacokinetic profile of JNJ 26993135, along with the demonstration of the inhibition of ex vivo production of LTB4 in whole blood following oral administration.
Conclusions and implications:
These results with JNJ 26993135 in the rat TNBS model support the role of LTB4 in colitis and the potential value of targeting LTA4H for the treatment of inflammatory bowel diseases.
Keywords: inflammatory bowel disease, colitis, LTA4H, leukotriene A4 hydrolase, leukotriene B4, 5-lipoxygenase, JNJ 26993135, tumour necrosis factor-α, interleukin-6, zileuton
Introduction
Among the local lipid mediators that have a potential role in inflammatory bowel diseases, leukotriene B4 (LTB4) is highly active in recruiting and activating a range of inflammatory cells including neutrophils, eosinophils and dendritic cells (Ford-Hutchinson et al., 1994; Murray et al., 2003; Shin et al., 2006). LTB4 also has chemotactic effects for CD4+ and CD8+ effector T cells (Ott et al., 2003; Tager et al., 2003), all such cells being considered to be involved in the pathogenesis of colitis (Boughton-Smith et al., 1988a, 1988b; Muller et al., 1998; Hogan and Rothenberg, 2004; Kristjansson et al., 2004; Hart et al., 2005; Nancey et al., 2006). Early work established that LTB4 can be found in the inflamed colonic tissue from both experimental models (Boughton-Smith et al., 1988a, 1988b; Rachmilewitz et al., 1989; Wallace and Keenan, 1990) and patients with colitis (Boughton-Smith et al., 1983; Sharon and Stenson, 1984; Lobos et al., 1987; Hawthorne et al., 1992). The precise role of LTB4 in the initiation and progression of pathological processes in gut diseases and its interaction with other inflammatory mediators however, is not fully clear.
Leukotriene B4 biosynthesis is initiated by the conversion of free arachidonic acid to an unstable epoxide intermediate, leukotriene A4 (LTA4) by actions of 5-lipoxygenase (5-LO) in the presence of the accessory 5-LO-activating protein, known as FLAP (Ford-Hutchinson et al., 1994). Subsequent hydrolysis of LTA4 by the enzyme LTA4 hydrolase (LTA4H) yields LTB4 (Haeggstrom, 2004; Rudberg et al., 2004). In addition to the development of leukotriene receptor antagonists, pharmacological approaches to modulate the production of LTB4 and other biologically active leukotrienes, such as leukotriene C4 (LTC4) and leukotriene D4 (LTD4), have previously focussed primarily on inhibiting the pivotal enzyme, 5-LO (Funk, 2005; Werz and Steinhilber, 2006).
One early 5-LO-inhibiting compound, zileuton, was active in experimental models of colitis such as the hapten model of colitis induced by trinitrobenzene sulphonic acid (TNBS) in rat (Zingarelli et al., 1993; Bertran et al., 1996), although this efficacy was variable (Zarif et al., 1996; Holma et al., 2001). Other agents such as an FLAP inhibitor, MK-886, have been reported to accelerate healing in chronic TNBS rat colitis (Wallace and Keenan, 1990). Although initial reports on the clinical efficacy of compounds acting on 5-LO were encouraging (Collawn et al., 1992; Rask-Madsen et al., 1992), more detailed clinical evaluation of zileuton, or of the FLAP inhibitor MK-591 suggested that overall, these agents failed to produce consistent rates of remission, maintenance of remission or other therapeutic benefits in ulcerative colitis patients (Hawkey et al., 1997; Roberts et al., 1997).
A different approach to attenuate the production of LTB4 has been through the inhibition of LTA4H (Haeggstrom, 2004). A series of inhibitors of LTA4H have been described with efficacy in vitro, while their anti-inflammatory profile in vivo has also been reported, although toxicity and pharmacokinetic issues of some of those early compounds were identified (Penning, 2001; Askonas et al., 2002; Kachur et al., 2002; Penning et al., 2002, 2003). Recent studies have shown the activity of a novel, highly selective and orally effective LTA4H inhibitor, JNJ 26993135, in a number of murine inflammatory models (Rao et al., 2007). JNJ 26993135 has been reported to inhibit the epoxide hydrolase activity of recombinant human LTA4H with an IC50 of 10 nM and has an IC50 of 340 nM for LTB4 production in murine whole blood (Rao et al., 2007). Following oral administration, it reduces acute ear and peritoneal inflammation and LTB4 production ex vivo in mouse blood (Rao et al., 2007).
In the present study, the ability of JNJ 26993135 to inhibit the ex vivo generation of LTB4 in blood following its oral administration, along with its pharmacokinetic profile, has been evaluated in rat. These findings support its study in a model of colitis. Thus, the effect of oral administration of JNJ 26993135 has been evaluated in the colitis provoked by colonic instillation of TNBS in rat over 3 days. This model is considered to exhibit many of the pharmacological, pathological, immunological and molecular aspects of clinical inflammatory bowel disease (Boughton-Smith et al., 1988a, 1988b; Morris et al., 1989; Torres et al., 1999; Sun et al., 2001; Whittle et al., 2003; Rivera et al., 2006). The actions of JNJ 26993135 in modifying the extent and severity of the macroscopic inflammatory response were determined, along with the effects on the colonic levels of LTB4 and of the neutrophil marker, myeloperoxidase (MPO), as well as on the pro-inflammatory cytokines, tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6), determined as inflammatory biomarkers.
Methods
The animal care and research protocols were reviewed by the University of Szeged and the Institutional Animal Care and Use Committee of Johnson and Johnson, and were in accordance with the guidelines of the United Kingdom Home Office guidance on the operation of the Animals (Scientific Procedures) Act 1986.
Inhibition of LTB4 production in blood ex vivo
To determine the activity of JNJ 26993135 on LTB4 production ex vivo in rat, the compound was prepared in 20% hydroxypropyl β cyclodextrin (HPCD) and administered in doses from 0.3 to 30 mg kg−1 orally to groups of three male Wistar rats (200–240 g). A vehicle control group that did not receive the compound and a baseline group whose blood was not stimulated were also included. At 4 h after dosing, blood was removed from a tail vein into heparin-containing tubes for both stimulation and subsequent assay of LTB4 and for the determination of the plasma concentrations of JNJ 26993135. Blood samples were diluted 1:1 in RPMI 1640 medium, after which 200 μl aliquots of the diluted blood were transferred to a microtiter plate. The calcium ionophore A23187 was added to samples (to give a final concentration of 20 μg ml−1) and incubated for 30 min to allow eicosanoid formation. The reaction was terminated by centrifugation (833 g, 10 min at 4 °C) to form a cell pellet, and the amount of LTB4 produced was assayed in the supernatants (diluted 5- to 15-fold) by enzyme immunoassay.
After preparation of plasma from the whole blood, liquid chromatography/mass spectrometry (LC/MS) analysis was used to quantitate the plasma levels of JNJ 26993135 relative to standard curves (Rao et al., 2007).
Evaluation of immunoassay of plasma LTB4
The cross-reactivity of the enzyme immunoassay used (Assay Designs-VWR, West Chester, PA, USA; catalogue no. 901-068) was for LTB4 (100%); 6-trans-12-epi-LTB4 (5.5%); 6-trans-LTB4 (4.9%); 12-epi-LTB4 (0.94%); and 20-OH-LTB4, PGE2, PGF2α, 20-COOH-LTB4, LTC4, LTD4, LTE4, 5(S)-HETE, 12(S)-HETE, 15(S)-HETE (<0.20%). In studies to evaluate the use of this immunoassay compared with HPLC/MS assay, each assay gave a comparable linear relationship (correlation r2=0.99) over the concentration range of LTB4 standards studied. However, the HPLC/MS assay was considerably less sensitive than commercial immunoassay kits available, with the lower limit of quantitation being 0.25 ng ml−1, compared with the lower limit of the immunoassay of 0.01 ng ml−1. Measurement of varying concentrations of LTB4 in eight plasma samples using both assays indicated a good correlation (r2=0.85) between the values determined by immunoassay and HPLC/MS.
Pharmacokinetic evaluation of JNJ 26993135
For the determination of the pharmacokinetic profile of JNJ 26993135, the compound, prepared in 20% HPCD, was administered i.v. (2 mg kg−1) or orally (10 mg kg−1) to groups of three male rats. At various time intervals, blood was removed from a tail vein into heparin-containing tubes and the plasma levels of JNJ 26993135 were determined by LC/MS analysis (Rao et al., 2007). The pharmacokinetic parameters Cmax (maximum plasma concentration), tmax (time of maximum plasma concentration), AUC 0–∞ (area under the plasma concentration time curve), t1/2 (half-life in plasma), CL/F (total clearance over bioavailable fraction), Vdss (volume of distribution at steady state) and F (bioavailable fraction) were determined from the data using Winonlin software (Pharsight, Mountain View, USA).
Induction of colitis
Male Wistar rats (200–240 g; n=64) were randomized before the commencement of the colitis study, housed at a temperature of 21–25 °C with a 12 h light cycle in groups of five animals and inspected and weighed every day. Food was only withdrawn overnight for 12 h prior to TNBS administration, and the rats were allowed free access to drinking water during all periods.
Under transient ether-induced anaesthesia, TNBS (10 mg in 0.25 ml of 50% ethanol, v v−1) was administered intrarectally through an 8 cm long soft plastic catheter (Boughton-Smith et al., 1988a, 1988b; Morris et al., 1989). The dose of TNBS used produced a reproducible yet not unduly severe colitis. The rats were allowed to recover with free access to food and drinking water. At the end of the experiment, 72 h after TNBS administration (that is on the morning of day 3), the animals were killed, the distal colon was exposed and the terminal 8 cm was dissected, photographed and stored appropriately for the subsequent analyses.
The primary parameters measured were the area of macroscopic injury and its severity score, the levels of MPO and LTB4, and the cytokines TNF-α and IL-6, in segments of the distal 8 cm of colon. In addition, the weight of the whole 8 cm colonic segment was measured as an indirect and nonspecific marker of oedema, while the body weight of the animals was determined each day of the study as an indicator of general health.
Experimental design for colitis model
JNJ 26993135 was prepared in the vehicle, HPCD and administered orally, twice daily (2 ml kg−1) commencing 24 h before TNBS administration, on the day of TNBS administration and on days 1 and 2 after challenge. The doses of JNJ 26993135 used were 5, 15 and 30 mg kg−1, p.o., twice a day (that is 10, 30 and 60 mg kg−1 day−1 total dose). This dose range was selected from and supported by studies on the pharmacokinetic profile of JNJ 26993135 and its effects on the plasma levels of LTB4 following ex vivo incubation of whole blood with the calcium ionophore, A23187, as described above. A further group of rats that was challenged with TNBS received the vehicle alone, twice a day (2 ml kg−1 p.o.), whereas another group had no challenge or drug treatment and was used for base-line measurements.
In a further study using the same dosing schedule, the actions of a single dose-level of JNJ 26993135 and of the 5-LO inhibitor, zileuton, were compared. For this study, JNJ 26993135 at a dose of 10 mg kg−1, p.o., twice a day (that is 20 mg kg−1 day−1 total dose) was selected from the previous dose–response curve, and zileuton at a dose of 30 mg kg−1, p.o., twice a day (that is 60 mg kg−1 day−1 total dose) was selected with reference to the available literature. Higher doses of zileuton were not evaluated, as hepatotoxicity has been reported at such doses (Beierschmitt et al., 2001).
Macroscopic analysis
The distal 8 cm portion of the colon (measured from the rectum) was removed, opened longitudinally and gently rinsed with ice-cold phosphate buffer (pH 7.4), blotted, weighed (Scaltec, Gottingen, Germany) and photographed (Samsung, Digimax 340, digital camera). The extent of macroscopically apparent inflammation, ulceration and tissue disruption was determined in a randomized manner from the colour images via computerized planimetry (Scion Image B4.02 version; Scion Corp.). The area of macroscopically visible mucosal involvement was calculated and expressed as the percentage of the total colonic segment area under study.
The tissue was then cut into longitudinal strips, each strip being 8 cm long and included the whole of the zone of injury. This tissue was weighed, processed and the resulting supernatant stored at −20 °C for the subsequent determination of MPO activity, protein levels, for the assay of LTB4 and for TNF-α and IL-6.
Macroscopic score
In addition to the quantitative measurement of area of damage, the degree of colonic damage was also assessed in a randomized blinded fashion using a Damage Score, utilizing a 1–10 scale that has been adapted from that used previously (Boughton-Smith et al., 1988a):
0 No damage.
1 One region of localized inflammation or thickening. No ulcers.
2 Linear ulceration, but no significant inflammation.
3 Linear ulceration with inflammation at one site.
4 Two or more sites of ulceration and/or inflammation. Ulcers present in at least one site.
5 Two or more sites of ulceration and inflammation with one major site of ulceration and inflammation extending 1 cm along the length of the colon.
6–10 Two or more sites of ulceration and inflammation with one major site of ulceration and inflammation extending 2, 3, 4, 5 or 6 cm along the length of the colon.
Myeloperoxidase activity
The 8 cm longitudinal strips of the colon were weighed and homogenized (Ultra Turrax, T25, 13 500 r.p.m., twice for 30 s; 250 mg colon per ml buffer) in ice-cold phosphate buffer (50 mM, pH 6.0), freeze-thawed three times and then centrifuged twice (each time at 15 000 g for 15 min at 4 °C). A 12 μl aliquot of the supernatant was then mixed with 280 μl phosphate buffer (50 mM, pH 6) containing 0.167 mg ml−1 of O-adenosine dihydrochloride and the reaction started with 10 μl of 0.03% hydrogen peroxide and assayed spectrophotometrically at 490 nm (Benchmark Microplate reader; Bio-Rad, Budapest, Hungary) after 90 s of shaking. MPO was expressed as mU per mg protein.
Colonic leukotriene B4 levels
The colonic tissue sample was minced with scissors and homogenized for 60 s in ice-cold Tris-HCl buffer (50 mM, pH 7.4), containing 100 mM NaCl, 1 mM CaCl2, 1 mg ml−1 D-glucose and 28 μM indomethacin. The homogenate was centrifuged twice at 15 000 g for 15 min at 4 °C. Homogenates were stored at −20 °C until use. The levels of LTB4 in the supernatant were assayed using immunoassay, as described above. The LTB4 values were expressed as pg per mg protein.
Colonic levels of cytokines
The colonic tissue samples were thawed, weighed and homogenized (Ultra-Turrax, T25, 2 × 30 s on ice; 250 mg colon per ml buffer) using a modified Greenburg buffer (300 mM NaCl, 15 mM Tris, 2 mM MgCl2, 2 mM Triton X-100, 20 ng ml−1 pepstatin A, 20 ng ml−1 leupeptin, 20 ng ml−1 aprotonin; pH 7.4). Tissue homogenates were lysed for 30 min on ice and then centrifuged twice (10 min, 14 000 g). The aliquots of the supernatant were stored at −20 °C until use.
The IL-6 protein levels in the colonic homogenate were determined using a two-site enzyme-linked immunosorbent assay for recombinant rat IL-6 according to the manufacturer's directions (R&D Systems Inc., Minneapolis, MN, USA; Quantikine rat IL-6 Elisa kit, catalogue no. R6000). Optical density was measured at 450 nm (wavelength correction was 540 nm). The mean minimum detectable level of rat IL-6 is stated to be 21 pg ml−1 for this kit. The IL-6 values were expressed as pg per mg protein.
The TNF-α levels were determined with quantitative enzyme-linked immunosorbent assay (R&D Systems Inc.; Quantikine rat TNF-alpha Elisa kit catalogue no. RTA00), using similar procedures as above, with the samples measured spectrophotometrically at 450 nm (Benchmark Microplate reader; Bio-Rad). This kit had minimum detection level of 5 pg ml−1 of rat TNF-α. The TNF-α values were expressed as pg per mg protein.
Protein determination
Using a commercial protein assay kit (Bio-Rad; catalogue no. 500–0002), aliquots (20 μl) of the diluted samples (25 × or 50 × with distilled water) were mixed with 980 μl distilled water and 200 μl Bradford reagent was added to each sample. After mixing and a 10 min incubation, the samples were assayed spectrophotometrically at 595 nm. Protein level was expressed as mg protein ml−1.
Data analysis and statistical evaluation
Results shown in the figures are expressed as mean±s.e.mean from (n) rats per experimental group. Each value used in these groups represents a single measurement for that parameter from each individual rat. For statistical comparisons, the two-tailed Student's t-test or ANOVA with the Bonferroni test were used, as appropriate. P<0.05 was taken as showing a significant difference between means. In the graphs, statistical comparison is made against the values for the TNBS-challenged HPCD vehicle group.
Drugs, reagents and materials
The investigational drug JNJ 26993135, 1-[4-(benzothiazol-2-yloxy)-benzyl]-piperidine-4-carboxylic acid (Rao et al., 2007), was synthesized in the laboratories of Johnson & Johnson Pharmaceutical Research & Development, LLC. Zileuton was obtained from the ChemPacific Corporation (Baltimore, Maryland, USA). These compounds were prepared freshly on each day of use in the vehicle, HPCD (Sigma Aldrich Chemical Company, St Louis, MO, USA). TNBS was obtained from Fluka Chemie AG (Buchs, Switzerland). All other compounds and assay reagents were from the Sigma Aldrich Chemical Company.
Results
Inhibition of LTB4 synthesis in blood ex vivo by JNJ 26993135
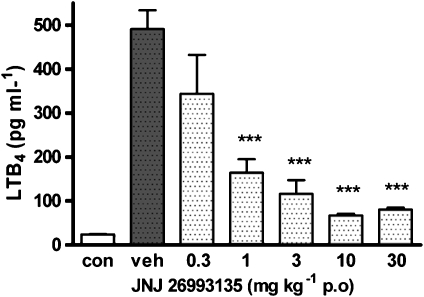
Incubation of blood taken from rats 4 h after oral administration of the vehicle (20% HPCD) with the ionophore A23187 (20 μg ml−1, 30 min) led to a significant (P<0.001) production of LTB4 in the plasma determined by immunoassay, compared with the non-stimulated control (Figure 1). Oral administration of JNJ 26993135 (0.3–30 mg kg−1) caused a dose-dependent reduction in this ex vivo-generated LTB4 production, which was significant (P<0.001) at doses of 1 mg kg−1 and above, as shown in Figure 1.
Figure 1.
Effects of oral administration of the vehicle (20% hydroxypropyl β cyclodextrin) or JNJ 26993135 (0.3–30 mg kg−1) on the ex vivo generation of leukotriene B4 (LTB4) in whole blood. Blood was collected 4 h after drug administration, incubated with A23187 (20 μg ml−1 for 30 min) and plasma LTB4 levels subsequently determined by immunoassay. The values of LTB4 in the plasma from the non-challenged control group (con) are also shown. Results are expressed as mean±s.e.mean (n=3 rats), where statistical significance is given as ***P<0.001 compared with the vehicle (veh) group.
The concentration of JNJ 26993135 in the plasma, 4 h following oral administration, increased proportionally with increasing doses. Thus, the mean plasma concentrations of JNJ 26993135 were 0.15±0.02, 1.0±0.5, 4.0±0.76, 7.7±0.89 and 33.5±2.45 μM with doses of 0.3, 1, 3, 10 and 30 mg kg−1, which inhibited the ionophore-stimulated ex vivo LTB4 production by 32, 70, 80, 91 and 88%, respectively (% inhibition data derived from Figure 1).
Pharmacokinetic profile of JNJ 26993135
The pharmacokinetic profile of JNJ 26993135 in rat was determined following the i.v. or oral administration of doses of 2 and 10 mg kg,−1 respectively. As shown in Table 1, the maximum plasma concentration of JNJ 26993135 was achieved 2 h following oral dosing, with excellent bioavailability (100%) and was eliminated with a half-life of 3.4 h. These measurements supported the selection of the dose range used for the colitis study in a twice-a-day administration regimen.
Table 1.
The pharmacokinetic profile of JNJ 26993135 following oral and intravenous administration in the rat
| Route | Dose (mg kg−1) | Cmax (μM) | tmax (h) | AUC(0–∞) (μM−1 h) | t1/2 (h) | CL/F (l h−1 kg−1) | Vdss (l kg−1) | F (%) |
|---|---|---|---|---|---|---|---|---|
| i.v. | 2 | 9.9±3.4 | NA | 21.5±1.5 | 3.1±0.2 | 0.24±0.01 | 1.1±0.07 | NA |
| p.o. | 10 | 13.6±1.21 | 2±0 | 110±5.7 | 3.4±0.1 | 0.24±0 | NA | 102 |
The pharmacokinetic parameters Cmax (maximum plasma concentration), tmax (time of maximum plasma concentration), AUC 0–∞ (area under the plasma concentration time curve), t1/2 (half-life in plasma), CL/F (total clearance over bioavailable fraction), Vdss (volume of distribution at steady state) and F (bioavailable fraction) are shown (NA=non-applicable measurement). Values are the mean±s.e.mean from groups of 3 rats following oral or intravenous administration of JNJ 26993135 in doses of 2 or 10 mg kg−1 respectively.
Effects of JNJ 26993135 on macroscopic colonic injury
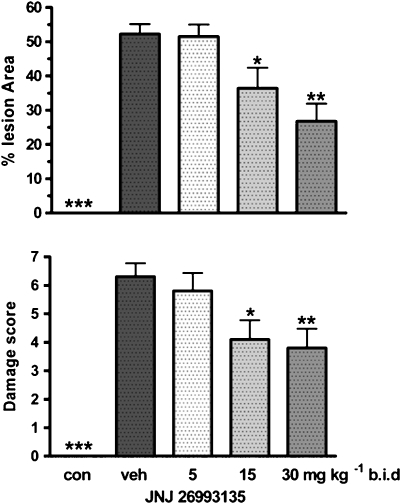
Intracolonic instillation of TNBS (10 mg) provoked areas of colonic injury, determined 3 days after challenge in the group of rats that had received the vehicle (HPCD) involving 52±4% (n=8) of the total colonic area of the segment studied (Figure 2). This macroscopic injury consisted of broad areas of haemorrhagic necrosis, with evidence of tissue inflammation and hyperaemia. There was no detectable macroscopic injury in the colons from the non-challenged group of rats receiving only saline p.o. (Figure 2).
Figure 2.
Effects of the vehicle (20% hydroxypropyl β cyclodextrin) or JNJ 26993135 in oral doses of 5, 15 and 30 mg kg−1, b.i.d., on the area of colonic injury, expressed as % of the total colonic area of the segment (upper panel), or the macroscopic score (1–10 scale) assigned to the colonic injury (lower panel). Measurements were taken 3 days after intracolonic challenge with trinitrobenzenesulphonic acid. The values from the non-challenged control group (con) are also shown. Results are expressed as mean±s.e.mean (n=8 rats), where statistical significance is given as *P<0.05, **P<0.01compared with the vehicle (veh) group.
Oral administration of JNJ 26993135 (5, 15 and 30 mg kg−1, b.i.d.) commencing 24 h prior to challenge, caused a dose-dependent reduction in the area of TNBS-induced colonic injury, being statistically significantly different from the damage in the challenged vehicle group at the intermediate and highest doses used (Figure 2).
When the severity of the colonic damage was evaluated using a 1–10 macroscopic score, comparable findings to those from the determination of damage area were seen. Thus, the score that was achieved following challenge in the vehicle group was dose-dependently reduced by treatment with JNJ 26993135 (5, 15 and 30 mg kg−1, p.o., b.i.d.) as shown in Figure 2.
Effects of JNJ 26993135 on body and colon weight
Following challenge with TNBS, there was a progressive fall in body weight over the 3-day treatment period in the challenged vehicle group, being significantly reduced to 93±2% (n=8; P<0.001) of the body weight prior to challenge on day 3. By contrast, the non-challenged group gained weight over the experimental period (to 115±2% of the pre-challenged weight n=8). Treatment with JNJ 26993135 (5, 15 and 30 mg kg−1, p.o., b.i.d.) dose-dependently attenuated the fall in body weight determined 3 days after TNBS challenge (to 94±1, 97±2 and 100±2% compared with the pre-challenged value with these doses respectively), this being significantly (P<0.05) different from the challenged vehicle group at the intermediate and highest doses used.
As an indirect index of inflammatory oedema in the colonic tissue, the weight of the colonic segments was determined at the end of the treatment period. The colonic weight in the challenged vehicle groups was significantly higher than that of the non-challenged colon for a comparable tissue segment (1.69±0.07 and 0.77±0.03 g, respectively, P<0.01). Treatment with JNJ 26993135 at the intermediate and higher doses significantly reduced the elevated colonic weight (to 1.35±0.02 and 1.41±0.05 g, respectively; P<0.05).
Effects of JNJ 26993135 on colonic MPO levels
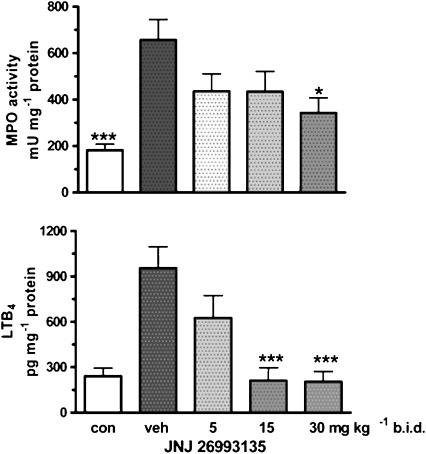
The level of MPO determined in the colonic tissue of rats that received TNBS challenge was substantially increased compared with that in the colons of unchallenged rats, as shown in Figure 3. Treatment with JNJ 26993135 (5, 15 and 30 mg kg−1, p.o., b.i.d.) caused a dose-dependent reduction in the TNBS-elevated MPO levels (Figure 3).
Figure 3.
Effects of the vehicle (20% hydroxypropyl β cyclodextrin) or JNJ 26993135 in oral doses of 5, 15 and 30 mg kg−1, b.i.d., on the colonic myeloperoxidase activity, expressed as mU per mg protein (upper panel) and colonic leukotriene B4 (LTB4) levels expressed as pg per mg protein (lower panel). Measurements were taken 3 days after intracolonic challenge with trinitrobenzenesulphonic acid. The values from the non-challenged control group (con) are also shown. Results are expressed as mean±s.e.mean (n=8 rats), where statistical significance is given as *P<0.05, ***P<0.001 compared with the vehicle (veh) group.
Effects of JNJ 26993135 on colonic LTB4 levels
The colonic levels of LTB4 were significantly elevated in the challenged vehicle group compared with those in the unchallenged group (Figure 3). This increase in colonic LTB4 levels was dose-dependently reduced by JNJ 26993135 (5, 15 and 30 mg kg−1, p.o., b.i.d.), as shown in Figure 3.
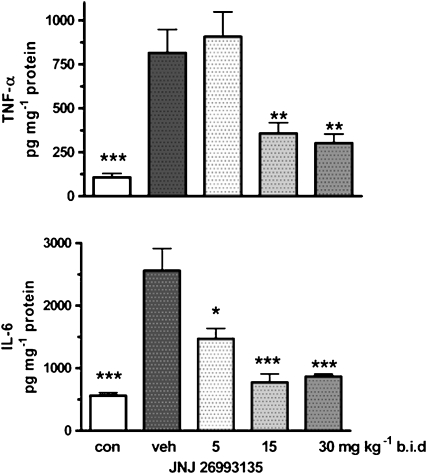
Effects of JNJ 26993135 on colonic TNF-α and IL-6 levels
The levels of TNF-α observed in the colonic tissue were significantly increased some fourfold in the group challenged with TNBS compared with those in the unchallenged rats, determined after 3 days as shown in Figure 4. These elevated TNF-α levels were reduced following administration of JNJ 26993135 (5, 15 and 30 mg kg−1, p.o., b.i.d.), as shown in Figure 4.
Figure 4.
Effects of the vehicle (20% hydroxypropyl β cyclodextrin) or JNJ 26993135 in oral doses of 5, 15 and 30 mg kg−1, b.i.d., on the colonic TNF-α activity, expressed as pg per mg protein (upper panel) and colonic IL-6 levels expressed as pg per mg protein (lower panel). Measurements were taken 3 days after intracolonic challenge with trinitrobenzenesulphonic acid. The values from the non-challenged control group (con) are also shown. Results are expressed as mean±s.e.mean (n=8 rats), where statistical significance is given as *P<0.05, **P<0.01, ***P<0.001 compared with the vehicle (veh) group.
The levels of IL-6 were likewise significantly increased some fourfold in the colonic tissue from the challenged group compared with those in the unchallenged control tissue, as shown in Figure 4. These IL-6 levels were reduced following administration of JNJ 26993135 (5, 15 and 30 mg kg−1, p.o., b.i.d.), being significant (P<0.05) at all doses.
Single dose-level comparison of JNJ 26993135 and zileuton
In a separate study, the effects of a single dose-level of JNJ 26993135 and zileuton were compared. JNJ 26993135 (10 mg kg−1, p.o., b.i.d.) significantly (P<0.05) reduced the macroscopic colonic damage from the level in HPCD vehicle-treated challenged rats (from 38±3 to 19±3% of total area measured; n=8), the increase in colonic weight (reduced from 1.5±0.9 to 1.1±0.07 g) and MPO (reduced from 588±152 to 287±38 mU per mg protein). The increases in the colonic tissue mediators following challenge were likewise substantially attenuated, with a significant (P<0.05) reduction in TNF-α (reduced from 986±205 to 455±79 pg per mg protein), IL-6 (reduced from 2846±373 to 1256±261 pg per mg protein) and LTB4 (reduced from 763±220 to 440±85 pg per mg protein).
Treatment with zileuton (30 mg kg−1, p.o., b.i.d.) significantly (P<0.05) reduced the macroscopic colonic damage compared with the HPCD control above (reduced from 38±3 to 21±4% of total area), the colonic weight (to 1.0±0.8 g) and the MPO levels (from 588±152 to 286±46 mU per mg protein). Colonic TNF-α levels were also reduced (from 986±205 to 382±57 pg per mg protein; P<0.05), whereas neither the IL-6 levels (from 2846±373 to 2145±316 pg per mg protein) nor the LTB4 levels (from 763±220 to 525±168 pg per mg protein respectively) were substantially reduced by zileuton in the dosage used.
Discussion
The present findings demonstrate the ability of the LTA4H inhibitor, JNJ 26993135, to reduce dose-dependently, both the extent and intensity of the rat colonic inflammatory damage observed 3 days after TNBS challenge. Following oral administration twice a day, a dosing regimen supported by the pharmacokinetic profile established in the current work, JNJ 26993135 also reduced the elevated levels of the biomarkers of colonic injury, the index of neutrophil infiltration, MPO and the cytokines, IL-6 and TNF-α.
As confirmed in the present study, increased colonic levels of LTB4 are associated with the colitis provoked by TNBS in rat (Boughton-Smith et al., 1988a; Rachmilewitz et al., 1989; Wallace and Keenan, 1990; Zingarelli et al., 1993; Holma et al., 2001; Camuesco et al., 2004; Gonzalez et al., 2004). In support of the mechanism by which JNJ 26993135 exerted its beneficial effects, the elevated colonic levels of LTB4 following challenge with TNBS observed in the current study were dose-dependently reduced. It is pertinent to note that in recent studies, selective antagonists for the histamine H4 receptor have also shown efficacy in this same model of colitis (Varga et al., 2005), as this receptor is considered to modulate LTB4 production and mast-cell-dependent recruitment of neutrophils (Takeshita et al., 2003; Zhang et al., 2007). Although it is possible that some contribution to this attenuation of the colonic levels of this pro-inflammatory leukotriene in the present study could have resulted from the general diminution of the extent of tissue injury, it seems more likely that the pharmacological effects observed with JNJ 26993135 reflected the primary mechanism of inhibition of LTB4 biosynthesis. Indeed, profound inhibition of ex vivo generation of LTB4 in blood stimulated by A23187 was observed in the present work 4 h following a single oral administration of JNJ 26993135, in doses effective in the colitis model.
Early studies on modulating LTB4 production by targeting the 5-LO enzyme have also demonstrated beneficial actions in experimental models of colitis, although the degree of efficacy of the various compounds varied (Wallace and Keenan, 1990; Zingarelli et al., 1993; Bertran et al., 1996; Zarif et al., 1996; Holma et al., 2001). In a direct single dose-level comparative study in the present work, the established 5-LO inhibitor, zileuton reduced the TNBS-induced macroscopic injury and the elevated MPO levels. However, it had less inhibitory activity on colonic cytokine and LTB4 production in the dose used than did JNJ 26993135. Higher doses of zileuton were not explored as they are known to alter liver function adversely in rat, even at the doses used in the current work (Beierschmitt et al., 2001) and hence limited conclusions can be drawn from this initial comparative study.
Clinical studies with either zileuton or the FLAP inhibitor, MK-591, did not support the further development of these 5-LO inhibitors for use in ulcerative colitis as they failed to affect the primary end points of accelerating or maintaining remission (Hawkey et al., 1997; Roberts et al., 1997). What remains unclear is whether the inconsistent efficacy reported, particularly in the clinic, reflected the nature of the particular compounds used, their pharmacokinetic properties, their broader pharmacological profile or the possibility that directly inhibiting the 5-LO enzyme is not an effective approach in this disease. Another theoretical possibility is that by inhibiting 5-LO and FLAP, the biosynthesis of the endogenous anti-inflammatory products, the lipoxins, could be concomitantly reduced, thereby attenuating the overall anti-inflammatory activity, as lipoxin A4 and lipoxin B4 can be formed by the action of either 5- or 15-lipoxygenase and from the leukotriene intermediate, LTA4 (Serhan and Savill, 2005; Chiang et al., 2006). A stable lipoxin A4 analogue had anti-inflammatory effects in colitis in mice (Gewirtz et al., 2002), and a lipoxin A4 analogue, ZK-192 also attenuated colitis in mice (Fiorucci et al., 2004). Specific inhibition of LTA4H should not similarly affect lipoxin biosynthesis. Indeed, in zymosan-induced peritonitis in mice, JNJ 26993135 inhibited the production of LTB4, whereas it did not reduce lipoxin A4 levels in the peritoneal lavage (Rao et al., 2007). However, it is not yet known whether colonic levels of lipoxins are altered in models of colitis as they have not yet been detected, whereas in colitic patients, only extremely low levels of ‘LXA' from colonic tissue have been reported (Mangino et al., 2006). Such actions on lipoxin production are thus unlikely to be the major mechanism of action of LTA4H inhibitors in rat colitis, which primarily is likely to reflect a diminution of the LTB4 production.
In the present study, the changes in colonic cytokines, TNF-α and IL-6, were measured as biomarkers for the degree of tissue inflammation, and JNJ 26993135 reduced their levels in the colonic tissue. Previous studies have shown that the colonic expression and levels of TNF-α and IL-6 are increased in TNBS-induced colitis (Sykes et al., 1999; Sun et al., 2001; Ten Hove et al., 2001; Maric et al., 2003; Villegas et al., 2003; Kitamura et al., 2004). An upregulation of soluble receptors to TNF-α and other cytokines such as IL-6 has been reported in colitic patients (Gustot et al., 2005; Raddatz et al., 2005). Clinical studies with antibodies targeting IL-6 receptors have shown such approaches to be of therapeutic benefit in initial trials (Ito, 2004), and there is ample clinical evidence of the efficacy of reducing TNF-α activity through the use of biological agents (Rutgeerts et al., 2006).
Although these cytokines are well recognized as inflammatory disease biomarkers, the present findings with JNJ 26993135 could also indicate a generalized reduction in the production of these potent pro-inflammatory mediators using LTA4H inhibitors. The promiscuous activity of LTB4 on the chemotaxis and activation of numerous cell types involved in the colitic processes suggests multiple potential points of intervention for LTA4H inhibitors, which could lead to a reduction of cytokine production and release. For example, it has been reported that LTB4 can enhance the production of TNF-α and IL-6 by macrophages (Gagnon et al., 1989; Thivierge and Rola-Pleszczynski, 1992; Stankova et al., 1993).
In earlier studies in inflammation models with inhibitors of the LTA4H enzyme, the compound SC-57461A was mentioned as having good efficacy when given orally twice a day over 8 weeks in reducing the spontaneous colitis observed in cotton-top tamarins, while also reducing the LTB4 levels in their rectal dialysates (Penning et al., 2002). As no actual data on the changes in colitis or in LTB4 levels with this compound were reported, it is difficult to assess its activity, whereas mild hepatic toxicity and the adipose accumulation of a metabolite apparently led to the discontinuation of the development of that particular compound (Penning et al., 2002). This pharmacological approach, however, is supported by the recent demonstration of an increase in LTA4H in colonic biopsies from patients with inflammatory bowel diseases (Jupp et al., 2007). The current data with the novel orally active agent, JNJ 26993135, in rat colitis now demonstrate the potential therapeutic efficacy of inhibiting LTA4H, and suggest that this target has utility in the development of new pharmacological agents for treating inflammatory bowel diseases.
Abbreviations
- 5-LO
5-lipoxygenase
- FLAP
5-lipoxygenase-activating protein
- IL-6
interleukin-6
- LT
leukotriene
- LTA4H
leukotriene A4 hydrolase
- LTB4
leukotriene B4
- TNBS
trinitrobenzene sulphonic acid
- TNF-α
tumour necrosis factor-α
Conflict of interest
This study was funded by Johnson and Johnson PRD, LLC., and the authors JPR, KAL, AMF and PJD are employees of that company. BJRW is supported by the William Harvey Research Foundation.
References
- Askonas LJ, Kachur JF, Villani-Price D, Liang CD, Russell MA, Smith WG. Pharmacological characterization of SC-57461A (3-[methyl[3-[4-(phenylmethyl)phenoxy]propyl]amino]propanoic acid HCl), a potent and selective inhibitor of leukotriene A(4) hydrolase I: in vitro studies. J Pharmacol Exp Ther. 2002;300:577–582. doi: 10.1124/jpet.300.2.577. [DOI] [PubMed] [Google Scholar]
- Beierschmitt WP, McNeish JD, Griffiths RJ, Nagahisa A, Nakane M, Amacher DE. Induction of hepatic microsomal drug-metabolizing enzymes by inhibitors of 5-lipoxygenase (5-LO): studies in rats and 5-LO knockout mice. Toxicol Sci. 2001;63:15–21. doi: 10.1093/toxsci/63.1.15. [DOI] [PubMed] [Google Scholar]
- Bertran X, Mane J, Fernandez-Banares F, Castella E, Bartoli R, Ojanguren I, et al. Intracolonic administration of zileuton, a selective 5-lipoxygenase inhibitor, accelerates healing in a rat model of chronic colitis. Gut. 1996;38:899–904. doi: 10.1136/gut.38.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton-Smith NK, Hawkey CJ, Whittle BJR. Biosynthesis of lipoxygenase and cyclo-oxygenase products from [14C]-arachidonic acid by human colonic mucosa. Gut. 1983;24:1176–1182. doi: 10.1136/gut.24.12.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton-Smith NK, Wallace JL, Morris GP, Whittle BJR. The effect of anti-inflammatory drugs on eicosanoid formation in a chronic model of inflammatory bowel disease in the rat. Br J Pharmacol. 1988a;94:65–72. doi: 10.1111/j.1476-5381.1988.tb11500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton-Smith NK, Wallace J, Whittle BJR. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988b;25:115–123. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- Camuesco D, Comalada M, Rodriguez-Cabezas ME, Nieto A, Lorente MD, Concha A, et al. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol. 2004;143:908–918. doi: 10.1038/sj.bjp.0705941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collawn C, Rubin P, Perez N, Bobadilla J, Cabrera G, Reyes E, et al. Phase II study of the safety and efficacy of a 5-lipoxygenase inhibitor in patients with ulcerative colitis. Am J Gastroenterol. 1992;87:342–346. [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Wallace JL, Mencarelli A, Distrutti E, Rizzo G, Farneti S, et al. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:15736–15741. doi: 10.1073/pnas.0404722101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson AW, Gresser M, Young RN. 5-Lipoxygenase. Annu Rev Biochem. 1994;63:383–417. doi: 10.1146/annurev.bi.63.070194.002123. [DOI] [PubMed] [Google Scholar]
- Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- Gagnon L, Filion LG, Dubois C, Rola-Pleszczynski M. Leukotrienes and macrophage activation: augmented cytotoxic activity and enhanced interleukin 1, tumor necrosis factor and hydrogen peroxide production. Agents Actions. 1989;26:141–147. doi: 10.1007/BF02126587. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, et al. Lipoxin A4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, de Medina FS, Martinez-Augustin O, Nieto A, Galvez J, Risco S, et al. Anti-inflammatory effect of diosmectite in hapten-induced colitis in the rat. Br J Pharmacol. 2004;141:951–960. doi: 10.1038/sj.bjp.0705710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustot T, Lemmers A, Louis E, Nicaise C, Quertinmont E, Belaiche J, et al. Profile of soluble cytokine receptors in Crohn's disease. Gut. 2005;54:488–495. doi: 10.1136/gut.2004.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeggstrom JZ. Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis. J Biol Chem. 2004;279:50639–50642. doi: 10.1074/jbc.R400027200. [DOI] [PubMed] [Google Scholar]
- Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Hawkey CJ, Dube LM, Rountree LV, Linnen PJ, Lancaster JF. A trial of zileuton versus mesalazine or placebo in the maintenance of remission of ulcerative colitis. The European Zileuton Study Group For Ulcerative Colitis. Gastroenterology. 1997;112:718–724. doi: 10.1053/gast.1997.v112.pm9041232. [DOI] [PubMed] [Google Scholar]
- Hawthorne AB, Boughton-Smith NK, Whittle BJR, Hawkey CJ. Colorectal leukotriene B4 synthesis in vitro in inflammatory bowel disease: inhibition by the selective 5-lipoxygenase inhibitor BWA4C. Gut. 1992;33:513–517. doi: 10.1136/gut.33.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan SP, Rothenberg ME. The eosinophil as a therapeutic target in gastrointestinal disease. Aliment Pharmacol Ther. 2004;20:1231–1240. doi: 10.1111/j.1365-2036.2004.02259.x. [DOI] [PubMed] [Google Scholar]
- Holma R, Salmenpera P, Riutta A, Virtanen I, Korpela R, Vapaatalo H. Acute effects of the cys-leukotriene-1 receptor antagonist, montelukast, on experimental colitis in rats. Eur J Pharmacol. 2001;429:309–318. doi: 10.1016/s0014-2999(01)01330-9. [DOI] [PubMed] [Google Scholar]
- Ito H. Novel therapy for Crohn's disease targeting IL-6 signalling. Expert Opin Ther Targets. 2004;8:287–294. doi: 10.1517/14728222.8.4.287. [DOI] [PubMed] [Google Scholar]
- Jupp J, Hillier K, Elliott DH, Fine DR, Bateman AC, Johnson PA, et al. Colonic expression of leukotriene-pathway enzymes in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:537–546. doi: 10.1002/ibd.20094. [DOI] [PubMed] [Google Scholar]
- Kachur JF, Askonas LJ, Villani-Price D, Ghoreishi-Haack N, Won-Kim S, Liang CD, et al. Pharmacological characterization of SC-57461A (3-[methyl[3-[4-(phenylmethyl)phenoxy]propyl]amino]propanoic acid HCl), a potent and selective inhibitor of leukotriene A(4) hydrolase II: in vivo studies. J Pharmacol Exp Ther. 2002;300:583–587. doi: 10.1124/jpet.300.2.583. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Nakamoto Y, Kaneko S, Mukaida N. Pivotal roles of interleukin-6 in transmural inflammation in murine T cell transfer colitis. J Leukoc Biol. 2004;76:1111–1117. doi: 10.1189/jlb.0604328. [DOI] [PubMed] [Google Scholar]
- Kristjansson G, Venge P, Wanders A, Loof L, Hallgren R. Clinical and subclinical intestinal inflammation assessed by the mucosal patch technique: studies of mucosal neutrophil and eosinophil activation in inflammatory bowel diseases and irritable bowel syndrome. Gut. 2004;53:1806–1812. doi: 10.1136/gut.2003.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobos EA, Sharon P, Stenson WF. Chemotactic activity in inflammatory bowel disease. Role of leukotriene B4. Dig Dis Sci. 1987;32:1380–1388. doi: 10.1007/BF01296664. [DOI] [PubMed] [Google Scholar]
- Mangino MJ, Brounts L, Harms B, Heise C. Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 2006;79:84–92. doi: 10.1016/j.prostaglandins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Maric I, Poljak L, Zoricic S, Bobinac D, Bosukonda D, Sampath KT, et al. Bone morphogenetic protein-7 reduces the severity of colon tissue damage and accelerates the healing of inflammatory bowel disease in rats. J Cell Physiol. 2003;196:258–264. doi: 10.1002/jcp.10275. [DOI] [PubMed] [Google Scholar]
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewzcuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Muller S, Lory J, Corazza N, Griffiths GM, Z'graggen K, Mazzucchelli L, et al. Activated CD4+ and CD8+ cytotoxic T cells are present in increased numbers in the intestinal mucosa from patients with active inflammatory bowel disease. Am J Pathol. 1998;152:261–268. [PMC free article] [PubMed] [Google Scholar]
- Murray J, Ward C, O'Flaherty JT, Dransfield I, Haslett C, Chilvers ER, et al. Role of leukotrienes in the regulation of human granulocyte behaviour: dissociation between agonist-induced activation and retardation of apoptosis. Br J Pharmacol. 2003;139:388–398. doi: 10.1038/sj.bjp.0705265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancey S, Holvoet S, Graber I, Joubert G, Philippe D, Martin S, et al. CD8+ cytotoxic T cells induce relapsing colitis in normal mice. Gastroenterology. 2006;131:485–496. doi: 10.1053/j.gastro.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Ott VL, Cambier JC, Kappler J, Marrack P, Swanson BJ. Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4. Nat Immunol. 2003;4:974–981. doi: 10.1038/ni971. [DOI] [PubMed] [Google Scholar]
- Penning TD. Inhibitors of leukotriene A4 (LTA4) hydrolase as potential anti-inflammatory agents. Curr Pharm Des. 2001;7:163–179. doi: 10.2174/1381612013398248. [DOI] [PubMed] [Google Scholar]
- Penning TD, Chandrakumar NS, Desai BN, Djuric SW, Gasiecki AF, Malecha JW, et al. Synthesis of imidazopyridines and purines as potent inhibitors of leukotriene A4 hydrolase. Bioorg Med Chem Lett. 2003;13:1137–1139. doi: 10.1016/s0960-894x(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Penning TD, Russell MA, Chen BB, Chen HY, Liang CD, Mahoney MW, et al. Synthesis of potent leukotriene A(4) hydrolase inhibitors. Identification of 3-[methyl[3-[4-phenylmethyl) phenoxy] propyl] amino] propanoic acid. J Med Chem. 2002;45:3482–3490. doi: 10.1021/jm0200916. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D, Simon PL, Schwartz LW, Griswold DE, Fondacaro JD, Wasserman MA. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989;97:326–337. doi: 10.1016/0016-5085(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Raddatz D, Bockemuhl M, Ramadori G. Quantitative measurement of cytokine mRNA in inflammatory bowel disease: relation to clinical and endoscopic activity and outcome. Eur J Gastroenterol Hepatol. 2005;17:547–557. doi: 10.1097/00042737-200505000-00012. [DOI] [PubMed] [Google Scholar]
- Rao N, Dunford PJ, Xue X, Lundeen KA, Coles F, Riley JP, et al. Anti-inflammatory activity of a potent, selective leukotriene A4 hydrolase inhibitor, in comparison to the 5-lipoxygenase inhibitor, zileuton. J Pharmacol Exp Ther. 2007;321:1154–1160. doi: 10.1124/jpet.106.115436. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen J, Bukhave K, Laursen LS, Lauritsen K.5-Lipoxygenase inhibitors for the treatment of inflammatory bowel disease Agents Actions 1992. Spec No:C37–46 [PubMed]
- Rivera E, Flores I, Rivera E, Appleyard CB. Molecular profiling of a rat model of colitis: validation of known inflammatory genes and identification of novel disease-associated targets. Inflamm Bowel Dis. 2006;12:950–966. doi: 10.1097/01.mib.0000231575.11678.8c. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Simon TJ, Berlin RG, Haggitt RC, Snyder ES, Stenson WF, et al. Leukotrienes in ulcerative colitis: results of a multicenter trial of a leukotriene biosynthesis inhibitor, MK-591. Gastroenterology. 1997;112:725–732. doi: 10.1053/gast.1997.v112.pm9041233. [DOI] [PubMed] [Google Scholar]
- Rudberg PC, Tholander F, Andberg M, Thunnissen MM, Haeggstrom JZ. Leukotriene A4 hydrolase: identification of a common carboxylate recognition site for the epoxide hydrolase and aminopeptidase substrates. J Biol Chem. 2004;279:27376–27382. doi: 10.1074/jbc.M401031200. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Van Assche G, Vermeire S. Review article: Infliximab therapy for inflammatory bowel disease -seven years on. Aliment Pharmacol Ther. 2006;23:451–463. doi: 10.1111/j.1365-2036.2006.02786.x. [DOI] [PubMed] [Google Scholar]
- Sharon P, Stenson WF. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984;86:453–460. [PubMed] [Google Scholar]
- Shin EH, Lee HY, Bae YS. Leukotriene B4 stimulates human monocyte-derived dendritic cell chemotaxis. Biochem Biophys Res Commun. 2006;348:606–611. doi: 10.1016/j.bbrc.2006.07.084. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Stankova J, Dupuis G, Gagnon N, Thivierge M, Turcotte S, Rola-Pleszczynski M. Priming of human monocytes with leukotriene B4 enhances their sensitivity in IL-2 driven tumor necrosis factor-alpha production. Transcriptional and post-transcriptional upregulation of IL-2 receptors. J Immunol. 1993;150:4041–4051. [PubMed] [Google Scholar]
- Sun FF, Lai PS, Yue G, Yin K, Nagele RG, Tong DM, et al. Pattern of cytokine and adhesion molecule mRNA in hapten-induced relapsing colon inflammation in the rat. Inflammation. 2001;25:33–45. doi: 10.1023/a:1007023611478. [DOI] [PubMed] [Google Scholar]
- Sykes AP, Bhogal R, Brampton C, Chander C, Whelan C, Parsons ME, et al. The effect of an inhibitor of matrix metalloproteinases on colonic inflammation in a trinitrobenzenesulphonic acid rat model of inflammatory bowel disease. Aliment Pharmacol Ther. 1999;13:1535–1542. doi: 10.1046/j.1365-2036.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Sakai K, Bacon KB, Gantner F. Critical role of histamine H4 receptor in leukotriene B4 production and mast cell-dependent neutrophil recruitment induced by zymosan in vivo. J Pharmacol Exp Ther. 2003;307:1072–1078. doi: 10.1124/jpet.103.057489. [DOI] [PubMed] [Google Scholar]
- Ten Hove T, Corbaz A, Amitai H, Aloni S, Belzer I, Graber P, et al. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-alpha production in mice. Gastroenterology. 2001;121:1372–1379. doi: 10.1053/gast.2001.29579. [DOI] [PubMed] [Google Scholar]
- Thivierge M, Rola-Pleszczynski M. Platelet-activating factor enhances interleukin-6 production by alveolar macrophages. J Allergy Clin Immunol. 1992;90:796–802. doi: 10.1016/0091-6749(92)90104-a. [DOI] [PubMed] [Google Scholar]
- Torres MI, Garcia-Martin M, Fernandez MI, Nieto N, Gil A, Rios A. Experimental colitis induced by trinitrobenzenesulfonic acid: an ultrastructural and histochemical study. Dig Dis Sci. 1999;44:2523–2529. doi: 10.1023/a:1026651408998. [DOI] [PubMed] [Google Scholar]
- Varga C, Horvath K, Berko A, Thurmond RL, Dunford PJ, Whittle BJR. Inhibitory effects of histamine H4 receptor antagonists on experimental colitis in the rat. Eur J Pharmacol. 2005;522:130–138. doi: 10.1016/j.ejphar.2005.08.045. [DOI] [PubMed] [Google Scholar]
- Villegas I, La Casa C, Orjales A, Alarcon de la Lastra C. Effects of dosmalfate, a new cytoprotective agent, on acute and chronic trinitrobenzene sulphonic acid-induced colitis in rats. Eur J Pharmacol. 2003;460:209–218. doi: 10.1016/s0014-2999(02)02949-7. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Keenan CM. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990;258:G527–G534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- Werz O, Steinhilber D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol Ther. 2006;112:701–718. doi: 10.1016/j.pharmthera.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Whittle BJR, Cavicchi M, Lamarque D.Assessment of anticolitic drugs in the trinitrobenzene sulfonic acid (TNBS) rat model of inflammatory bowel disease Inflammation Protocols; Methods In Molecular Biology 2003Humana Press: New Jersey; 209–222.In: Winyard PG, Willoughby DA (eds).vol. 225 [DOI] [PubMed] [Google Scholar]
- Zarif A, Eiznhamer D, Callaghan C, Doria MI, Broutman L, Keshavarzian A. The effect of a selective 5-lipoxygenase inhibitor, zileuton, on tissue damage in acute colonic inflammation in rats. Inflammation. 1996;20:217–227. doi: 10.1007/BF01488200. [DOI] [PubMed] [Google Scholar]
- Zhang M, Thurmond RL, Dunford PJ. The histamine H(4) receptor: a novel modulator of inflammatory and immune disorders. Pharmacol Ther. 2007;113:594–606. doi: 10.1016/j.pharmthera.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Zingarelli B, Squadrito F, Graziani P, Camerini R, Caputi P. Effects of zileuton, a new 5-lipoxygenase inhibitor, in experimentally induced colitis in rats. Agents Actions. 1993;39:150–156. doi: 10.1007/BF01998968. [DOI] [PubMed] [Google Scholar]