Abstract
Background and purpose:
Intracellular pH (pHi) in heart is regulated by sarcolemmal H+-equivalent transporters such as Na+-H+ exchange (NHE) and Na+-HCO3 − cotransport (NBC). Inhibition of NBC influences pHi and can be cardioprotective in animal models of post-ischaemic reperfusion. Apart from a rabbit polyclonal NBC-antibody, a selective NBC inhibitor compound has not been studied. Compound S0859 (C29H24ClN3O3S) is a putative NBC inhibitor. Here, we provide the drug's chemical structure, test its potency and selectivity in ventricular cells and assess its suitability for experiments on cardiac contraction.
Experimental approach:
pHi recovery from intracellular acidosis was monitored using pH-epifluorescence (SNARF-fluorophore) in guinea pig, rat and rabbit isolated ventricular myocytes. Electrically evoked cell shortening (contraction) was measured optically. With CO2/HCO3 −-buffered superfusates containing 30 μM cariporide (to inhibit NHE), pHi recovery is mediated by NBC.
Key results:
S0859, an N-cyanosulphonamide compound, reversibly inhibited NBC-mediated pHi recovery (K i=1.7 μM, full inhibition at ∼30 μM). In HEPES-buffered superfusates, NHE-mediated pHi recovery was unaffected by 30 μM S0859. With CO2/HCO3 − buffer, pHi recovery from intracellular alkalosis (mediated by Cl−/HCO3 − and Cl−/OH− exchange) was also unaffected. Selective NBC-inhibition was not due to action on carbonic anhydrase (CA) enzymes, as 100 μM acetazolamide (a membrane-permeant CA-inhibitor) had no significant effect on NBC activity. pHi recovery from acidosis was associated with increased contractile-amplitude. The time course of recovery of pHi and contraction was slowed by S0859, confirming that NBC is a significant controller of contractility during acidosis.
Conclusions and implications:
Compound S0859 is a selective, high-affinity generic NBC inhibitor, potentially important for probing the transporter's functional role in heart and other tissues.
Keywords: Na+/HCO3− cotransport, selective NBC inhibitor, cardiac, pHi, heart, ventricular myocyte, N-cyanosulphonamide
Introduction
Intracellular pH (pHi) is a powerful modulator of excitation and contraction in the heart (Bountra and Vaughan-Jones, 1989; Orchard and Kentish, 1990; Orchard and Cingolani, 1994), and so must be controlled physiologically. As H+ ions are generated or consumed by metabolic reactions within cardiac cells, a sophisticated ion transport system compensates for displacements of cytoplasmic pHi, by moving H+ ions or their ionic equivalent (OH−, HCO3−) across the surface membrane, thereby maintaining pHi at a steady-state value of ∼7.2. These ionic movements are mediated by sarcolemmal ion transporters. Among these, Na+/H+ exchange (NHE) and Na+/HCO3− cotransport (NBC) execute H+-equivalent efflux (Je) (Leem et al., 1999), sometimes supplemented by an H+-lactate cotransporter (Poole et al., 1989), whereas Cl−/HCO3− exchange (AE) and Cl−/OH− (CHE) exchange are responsible for H+-equivalent influx (Leem and Vaughan-Jones, 1998b; Leem et al., 1999). The coupling of NHE and NBC to sarcolemmal Na+ transport is of particular interest, as H+-equivalent extrusion will modulate not only pHi but also [Na+]i and hence, through effects on sarcolemmal Na+/Ca2+ exchange, intracellular [Ca2+]. This link between pHi, [Na+]i and [Ca2+]i has been well documented during NHE activity, partly through the use of pharmacological NHE inhibitors like amiloride (Lazdunski et al., 1985; Bountra and Vaughan-Jones, 1989; Harrison et al., 1992) and its more selective congeners such as cariporide and zoniporide (Scholz et al., 1995; Knight et al., 2001; Masereel et al., 2003). The latter two compounds exhibit powerful cardioprotective properties in animal models of myocardial ischaemia/reperfusion (Scholz et al., 1993; Clements-Jewery et al., 2004) and may even attenuate or reverse features of myocardial hypertrophy (Yoshida and Karmazyn, 2000; Cingolani and Ennis, 2007), although their therapeutic potential in a clinical setting has so far proved inconclusive (for a review, see Avkiran and Marber, 2002).
Recent experimental work has suggested that inhibition of NBC may also afford some degree of cardioprotection during post-ischaemic reperfusion of animal hearts, and simulated ischaemia/reperfusion of isolated cardiomyocytes (Schafer et al., 2000; Khandoudi et al., 2001; Doggrell and Hancox, 2003). Inhibition of NBC, like NHE, will directly influence the regulation of pHi, and hence [Na+]i and [Ca2+]i. Studies so far have been based on the use of either a nonselective anion transport inhibitor, 4,4′-diisothiocyanostilbene 2,2′-disulphonic acid (DIDS, a stilbene compound) (Schafer et al., 2000), or a polyclonal antibody to inhibit cardiac NBC (Khandoudi et al., 2001). A simple compound with selective inhibitory action for NBC activity over other H+-equivalent transporters would be potentially useful, allowing one to explore NBC activity during pHi regulation in heart, and its role in controlling [Ca2+]i and contractility. As cardiac cells express at least three different NBC isoforms (Choi et al., 1999, 2000; Virkki et al., 2002) (but cf. Damkier et al., 2006), complete blockade of cardiac NBC activity would require the compound to exert pharmacological activity against all functionally active isoforms. S0859 is a putative NBC inhibitor compound (C29H24ClN3O3S) that has been used recently to block NBC activity in cardiac (Yamamoto et al., 2005) and epithelial (Schwab et al., 2005) tissues. However, this drug has not been fully characterized for its pharmacological potency and NBC-selectivity in wild-type cells, and has not been assessed for its general suitability in physiological experiments. In the present work, we reveal the chemical structure of S0859. We used H+-ion fluorophore measurements of pHi to examine the potency of the drug against NBC activity in mammalian ventricular myocytes. We measured its activity against the other principal H+-equivalent transporters in these cells and then explored its potential for use in physiological work. We used it to assess the influence of NBC activity on contraction of ventricular myocytes during intracellular acidosis. We conclude that S0859 is a selective and full cardiac NBC inhibitor, suitable for cellular experiments. Its potential use in other experimental situations, and as a therapeutic tool in clinical disease is discussed. An abstract of the present work has been published (Ch'en and Vaughan-Jones, 2001).
Methods
Solutions
HEPES-buffered Tyrode solution
Standard HEPES-buffered Tyrode solution contained (mM): NaCl 135, KCl 4.5, MgCl2 1, CaCl2 2, HEPES 20 and glucose 11. This was adjusted to pH 7.4 at 37 °C with 4 M NaOH.
CO2/HCO3−-buffered Tyrode solution
Standard CO2/HCO3−-buffered Tyrode solution (pH 7.4) had the same composition as HEPES-buffered Tyrode, but [NaCl] was reduced to 125 mM and HEPES was substituted with 22 mM NaHCO3. The solution pH was brought to 7.4 by bubbling with 5% CO2 (balanced in air) at 37 °C for at least 1 h prior to starting experiments.
Cl−-free CO2/HCO3−-buffered solutions contained (mM): sodium gluconate 125, potassium gluconate 4.5, magnesium gluconate 2, calcium gluconate 4, NaHCO3 22 and glucose 11. Solution pH was adjusted to 7.4 at 37 °C with 4 M NaOH. When 10–15 mM ammonium chloride was used, it was added directly to solutions without osmotic compensation. For alkali loading, 80 mM Na-acetate was added with appropriate osmotic compensation (reduction in [NaCl]).
Calibration solutions
Nigericin calibration solutions contained (mM): KCl 140, MgCl2 1, EGTA 0.5 and nigericin 0.01, buffered with one of the following organic buffers: 20 mM MES (pH 5.5), 20 mM PIPES (pH 6.5), 20 mM HEPES (pH 7.5) or 20 mM CAPSO (pH 9.5), and were adjusted to the correct pH with 4 M NaOH at 37 °C.
Chemicals
Cariporide (4-isopropyl-3-methylsulphonyl-benzoylguanidine (Hoe 642)) and S0859 were kindly provided by Sanofi-Aventis (formerly Hoechst AG, Hoechst, Germany). Collagenase was obtained from Boehringer (Mannheim, Germany), Blendzyme III from Roche (Burgess Hill, UK) and Decon 75 from Decon Laboratories Ltd (Sussex, UK). All other chemicals were obtained from Sigma (Poole, UK). Cariporide and S0859 were added as solids to perfusates shortly before use. Acetazolamide (ATZ) and dimethylamiloride were dissolved in a minimum volume of dimethylsulphoxide (less than 0.02% of the final volume) before being added to solutions.
Isolation of ventricular myocytes
The composition of cell isolation solutions and the detailed procedure were as described previously (Lagadic-Gossmann et al., 1992; Yamamoto et al., 2005). In brief, single ventricular myocytes were isolated from 350–450 g albino guinea pigs, 300 g Sprague–Dawley rats (killed by cervical dislocation) or 2.5–4.0 kg rabbits (killed by an i.v. injection of pentobarbitone, 50 mg kg−1) using enzymatic and mechanical dispersion. Guinea pig and rabbit hearts were digested with 0.7 and 1.0 mg ml−1 collagenase plus 0.04 and 0.1 mg ml−1 protease, respectively. Rat hearts were digested with 0.24 mg ml−1 Blendzyme III. The cells were suspended in HEPES-buffered Dulbecco's Modified Eagle's Medium and left at room temperature until use. Only rod-shaped myocytes showing calcium tolerance were used in this work.
Measurement of intracellular pH
Carboxy-seminapthorodafluor-1 (carboxy-SNARF-1) loading and calibration
The pHi of isolated ventricular myocytes was measured using the dual emission fluorophore carboxy-SNARF-1, loaded into cells as the acetoxymethyl ester. The full procedure for dye loading, measuring pHi and calibrating dye signals has been described previously (Buckler and Vaughan-Jones, 1990; Sun et al., 1996). Briefly, isolated myocytes were loaded for 8 min at room temperature with 10 μM carboxy-SNARF-1 acetoxymethyl ester. Carboxy-SNARF-1 fluorescence from individual cells was excited at 540±12 nm and measured simultaneously at 590±5 and 640±5 nm, with an inverted microscope converted for epifluorescence (Nikon Diaphot). Signals were digitized at 0.5 kHz using a CED 1401 digitiser (Cambridge, UK), and the emission ratio was calculated and converted to a pHi value using the pH ratiometric fluorescence equation. The pH calibration data for this equation were obtained in situ from individual cells using the nigericin (10 μM) technique (Thomas et al., 1979) and were averaged for more than 10 cells from at least three animals. To reduce potential contamination of the cell superfusion system with nigericin (Richmond and Vaughan-Jones, 1997), nigericin calibration was not performed after every experiment. Instead, calibration data were acquired routinely every 2 months, and were for the terms Rmax (maximum emission ratio at pH 5.5), Rmin (minimum ratio at pH 9.5) and F640, max/min (9.5/5.5 pH fluorescence ratio measured at 640 nm), measured at 37 °C. These most recently acquired pH calibration data were used as default values. The typical calibration curve parameter values were 1.623, 0.231 and 2.035, respectively. They predict a pKa for intracellular carboxy-SNARF-1 of 7.36.
Cleaning the superfusion system
The apparatus was thoroughly cleaned following a nigericin calibration. The superfusion lines were replaced and the superfusion chamber and switcher tap (Richmond and Vaughan-Jones, 1997) were dismantled and soaked in ethanol for several hours. This was followed by a soak for at least 12 h in 20% Decon 75 and then simmering in deionized water for several hours.
Measurement of cell shortening and intracellular pH
In some experiments, cell shortening and pHi were measured simultaneously. pHi was measured in cells loaded with carboxy-SNARF-1 according to the procedure above. Electrically evoked cell shortening was stimulated using a 70-V, 1-ms pulse delivered through platinum electrodes positioned within the superfusion chamber. Cell shortening was measured online using an edge-detection device (Crescent Technology, Sandy, UT, USA; Steadman et al., 1988), which follows the movement of the cell ends during contraction. For analysis, active shortening was captured using a sample and hold algorithm. Data were digitized at 250 Hz using the CED 1401.
Calculation of H+-equivalent fluxes
H+-equivalent fluxes were estimated as the product of the rate of pHi recovery (dpHi/dt) following an intracellular acid load, and the total intracellular buffering power (βtot). The intracellular acid load, which stimulates sarcolemmal H+-equivalent extrusion, was imposed by transiently superfusing a myocyte (usually 3–6 min) with NH4Cl (a procedure known as an ammonium prepulse). Intracellular acidification occurs when ammonium is removed from the superfusate. Measurements of pHi recovery typically commenced approximately 1 min after ammonium removal. The value of dpHi/dt was determined as the least squares regression line fitted to the initial change of calibrated pHi points sampled at 0.5 s intervals over a 0.5–1 min time period. The pHi associated with this recovery rate was taken to be the mid-point pHi of the linear best fit. Comparisons of H+-equivalent fluxes in the present work were made at a common mid-point pHi. The term βtot is the sum of intrinsic buffering power (βi) plus buffering power owing to CO2/HCO3− (βCO2). βi in guinea pig ventricular myocytes at 37 °C has been estimated (Leem et al., 1999) as
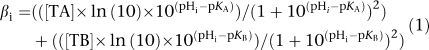 |
where [TA] and [TB] are the total concentration of buffer A (84.22 mM) and buffer B (29.38 mM), respectively. The pK values for buffers A and B are 6.03 and 7.57, respectively. The value for βCO2 was estimated using the Henderson–Hasselbalch equation as βCO2=2.303 × [HCO3−]i (Leem et al., 1999). This assumes the same pKa for CO2/HCO3− and the same solubility for CO2 on either side of the sarcolemma.
Results
Inhibition of cardiac Na+/H+ exchange
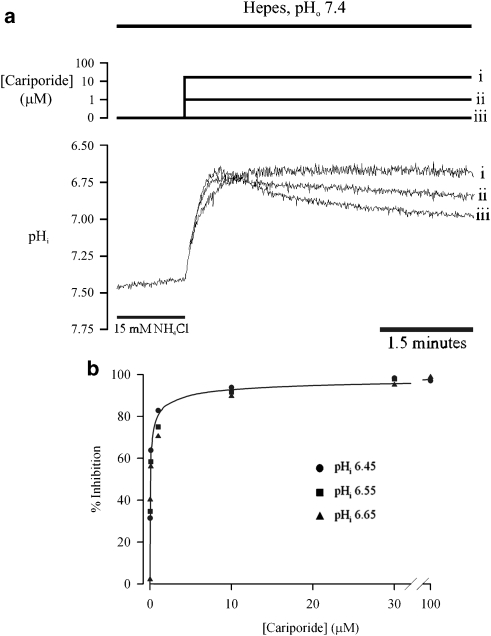
To examine the effect of S0859 on NBC activity, it was first necessary to inhibit the activity of NHE, as this normally operates in parallel with NBC. Therefore, we determined the potency of the NHE1 inhibitor, cariporide (Hoe 642), on the rate of pHi recovery following an ammonium prepulse in guinea pig myocytes superfused with HEPES-buffered solution. pHi usually acidified to 6.40–6.6, following ammonium removal (the longer the prepulse, the greater the subsequent acidification). Adjustment of the prepulse duration allowed flux measurements over a wide range of pHi. In the absence of CO2/HCO3 buffer, recovery is mediated by NHE activity alone (Lagadic-Gossmann et al., 1992; Leem et al., 1999). As shown in the representative traces of Figure 1a (superimposed from the same cell), while pHi recovered in the absence of cariporide (iii), it was significantly slowed by the addition of 1 μM cariporide (ii), and completely abolished at 30 μM (i). Figure 1b shows the effect of various cariporide concentrations on H+-efflux measured at three test pHi levels (6.45 •, 6.55 ▪ and 6.65 ▴), expressed as percentage inhibition of control activity. The inhibitory effect of cariporide was similar at all three pHi levels. The overall apparent Ki value for half-maximal inhibition was 54.5 nM, lower than that obtained previously for inhibition of NHE 1 in rat ventricular myocytes (150 nM at 37 °C; Hoshino and Avkiran, 2001), but of a similar order of magnitude. In subsequent experiments in the present study, requiring complete inhibition of NHE in the presence of 145 mM [Na+]o, 30 μM cariporide was used.
Figure 1.
Effect of cariporide. (a) Superimposed traces from the same cell of pHi recovery following a 15 mM ammonium prepulse in HEPES-buffered solution, pHo 7.4. As indicated by the logarithmic [cariporide] scale, the traces demonstrate the effect of (i) 30 μM, (ii) 1 μM and (iii) 0 μM cariporide on pHi recovery from an intracellular acid load; 30 μM was needed for complete inhibition. (b) Dose–response curves for cariporide inhibition of Na+/H+ exchange, measured at pHi levels of 6.45 (n=2–7), 6.55 (n=3–18) and 6.65 (n=3–33). s.e. bars are not shown for simplicity (s.e.m. for pHi 6.45: 1, 1, 1, 3, 1 and 16%; for pHi 6.55: 1, 1, 1, 1, 4, 8 and 19%, for pHi 6.65: 1, 1, 1, 1, 1, 7, 5 and 7%). pHi, intracellular pH.
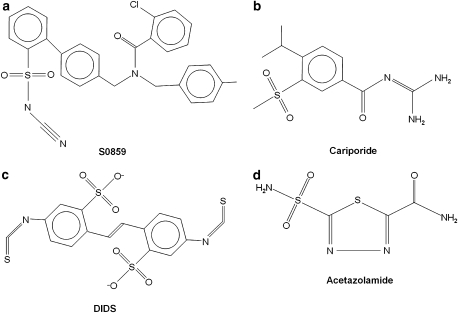
Structure of S0859
Figure 2a shows the chemical structure of S0859. The drug is an N-cyanosulphonamide compound, structurally unrelated to other inhibitors of cardiac pHi regulation such as cariporide (Figure 2b) and DIDS, a disulphonic stilbene inhibitor of anion transport (Figure 2c). To the extent that S0859 is a sulphonamide derivative, it is loosely related to the sulphonamide inhibitors of carbonic anhydrase (CA), represented by the drug ATZ (Figure 2d).
Figure 2.
Chemical structures of the inhibitor compounds. (a) S0859, (b) cariporide (4-isopropyl-3-methylsulphonyl-benzoylguanidine (Hoe 642)), (c) 4,4′-diisothiocyaniostilbene 2,2′-disulphonic acid (DIDS) and (d) acetazolamide (ATZ). S0859, C29H24ClN3O3S.
Effect of S0859 on Na+/HCO3− cotransport
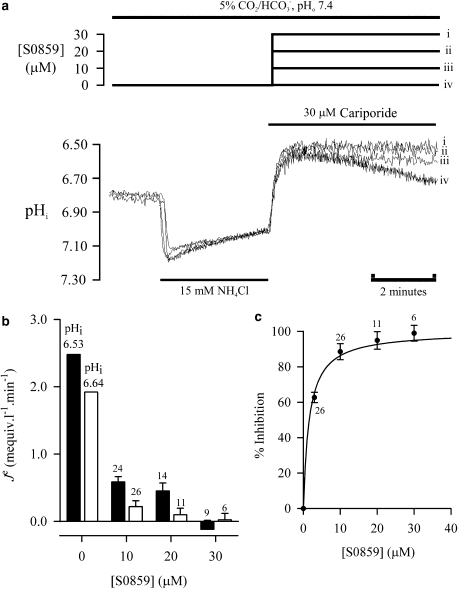
The effect of S0859 on pHi recovery via NBC was investigated in cells superfused with 5% CO2/HCO3−-buffered solutions (pHo 7.4) plus 30 μM cariporide (to inhibit NHE). Figure 3a shows a representative experiment, with superimposed traces from the same cell. Following ammonium removal, the cell was exposed to 30 μM cariporide plus (i) 30 μM, (ii) 20 μM, (iii) 10 μM or (iv) 0 μM S0859. Inspection of the traces reveals that NBC was inhibited in a dose-dependent manner with near-maximal inhibition at 30 μM. NBC inhibition by 15–30 μM S0859 was also observed in rat (n=8) and rabbit (n=5) ventricular myocytes. In five experiments (rabbit), inhibition was readily reversed, and the NBC activity during an intracellular acid load (induced by 10 mM ammonium chloride prepulse) resumed up to 2 min after drug washout.
Figure 3.
Effect of S0859 on Na+/HCO3− cotransport. (a) Superimposed traces from the same cell of pHi recovery following a 15 mM ammonium prepulse in 5% CO2/HCO3−-buffered solution, pHo 7.4. The traces demonstrate the effect of (i) 30 μM, (ii) 20 μM, (iii) 10 μM and (iv) 0 μM S0859 on pHi recovery from an intracellular acid load; 30 μM S0859 was needed for complete inhibition. (b) Histogram showing the effect of varying the concentration of S0859 on H+-equivalent efflux measured at pHi 6.53±0.01 and 6.64±0.01 (n, number shown above each column). (c) Mean dose–response curve for S0859 inhibition of Na+/HCO3− cotransport, measured at pHi 6.64±0.01 (n, number shown by each point). Dose for half-inhibition, Ki, is 1.6 μM. pHi, intracellular pH; S0859, C29H24ClN3O3S.
Figure 3b represents a collation of the results of experiments similar to that shown in Figure 3a and shows plots of NBC-mediated H-equivalent efflux measured at pHi 6.53±0.01 and 6.64±0.01 for the three concentrations of S0859 (adjusting the ammonium prepulse duration during experiments permitted the relevant pHi range to be accessed for these measurements). Control efflux values for NBC (that is, nil S0859), obtained previously (Leem et al., 1999), are also plotted. As expected, acid efflux was faster at more acidic pHi, consistent with the activation of NBC by reduced pHi (Leem et al., 1999). Increasing the concentration of S0859 from 0 μM to 10 μM–20 μM increased the extent of NBC inhibition at both pHi levels. At 30 μM, NBC activity was virtually zero. Figure 3c shows a plot of these data as a dose–response curve, expressed as the percentage inhibition of NBC-mediated H+-equivalent flux measured at pHi 6.64±0.01, giving an apparent Ki of 1.59 μM (95% confidence interval 1.11–2.06 μM).
Effect of S0859 on Na+/H+ exchange
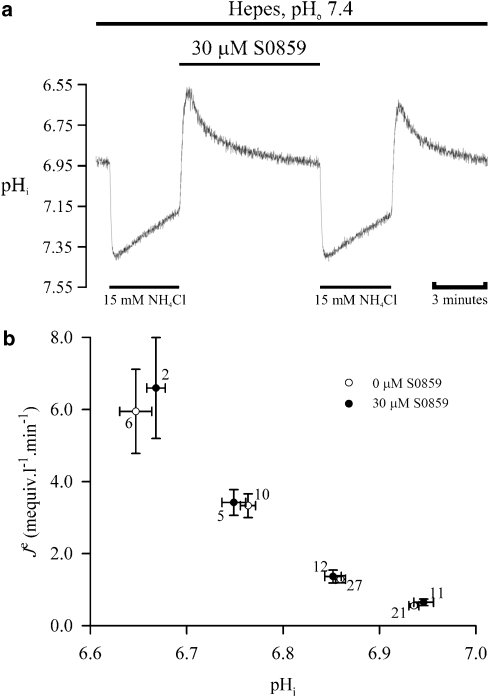
To assess the specificity of S0859 for NBC, the effect of the drug on other sarcolemmal H+-equivalent transporters was examined. To investigate possible interactions of the drug with NHE, pHi recovery from an intracellular acid load was measured in guinea pig myocytes superfused with HEPES-buffered Tyrode (nominally CO2/HCO3−-free) in the presence and absence of 30 μM S0859. A typical experiment is illustrated in Figure 4a, where two recoveries were explored in the same cell. Recovery is mediated by NHE activity (Lagadic-Gossmann et al., 1992; Leem et al., 1999; Yamamoto et al., 2005), and visual inspection indicated no significant effect of 30 μM S0859 on pHi recovery.
Figure 4.
Effect of S0859 on Na+/H+ exchange. (a) Recovery of pHi following an intracellular acid load (15 mM ammonium prepulse) in HEPES-buffered conditions, pHo 7.4, in the presence and subsequent absence of 30 μM S0859. (b) Plot of H+-equivalent efflux against pHi derived from pHi recoveries like those shown in (a) in normal Tyrode solution and in the presence of 30 μM S0859. pHi, intracellular pH; S0859, C29H24ClN3O3S.
Figure 4b represents the pooled data from several experiments similar to that shown in Figure 4a; the pHi is plotted against the rate of acid efflux obtained under control conditions and in the presence of 30 μM S0859. As expected, decreasing pHi increased the rate of acid efflux. Importantly, there was no significant effect of S0859 on this relationship when compared with control, thus indicating that 30 μM S0859 has no effect on NHE (P>0.05).
Effect of S0859 on Cl−/HCO3− exchange and Cl−/OH− exchange
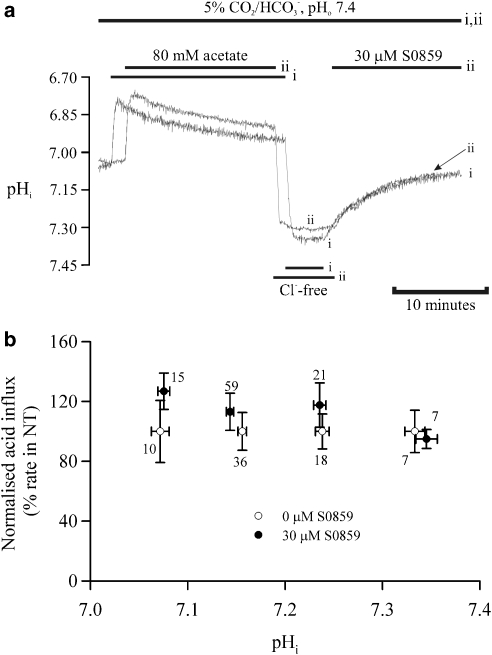
To determine whether S0859 affects acid-influx transport, the drug sensitivity of AE and CHE was examined. Guinea pig myocytes were superfused with 5% CO2/HCO3− solution (pHo 7.4) and an intracellular base load was induced with the weak acid prepulse technique (80 mM Na-acetate), during exposure to chloride-free superfusates (Cl− replaced by gluconate). The resulting high pHi was stable, showing no tendency to recover. Under Cl−-free conditions, both acid-influx transporters are inhibited (Leem and Vaughan-Jones, 1998b). Re-introduction of Cl−o then prompted an immediate pHi recovery, corresponding to the combined activation of AE and CHE. Figure 5a shows superimposed pHi traces from the same cell where chloride re-introduction was performed under (i) control conditions and (ii) in the presence of 30 μM S0859. The coincident traces during the slow recovery phase indicate that the combined H+-equivalent flux carried by AE and CHE is insensitive to 30 μM S0859. This is illustrated in Figure 5b where the pHi recovery rate in the presence of the drug is expressed as a percentage of that in control conditions; there was no significant difference between the two (P>0.05).
Figure 5.
Effect of S0859 on H+-equivalent loading. (a) Restoration of extracellular chloride, after a period of Cl−-free perfusion (replaced by gluconate), re-activates H+-equivalent influx transporters to mediate pHi recovery from an intracellular base load (induced by an 80-mM acetate prepulse). The traces show transporter-mediated acid loading in 5% CO2/HCO3−-buffered Tyrode solution (i) superimposed on a representative pHi recovery in the presence of 30 μM S0859, starting from a comparable pHi (ii). (b) Normalized pHi recovery from an acid load in the presence and absence of 30 μM S0859 over the pHi range 7.0–7.4 (n, number shown by each point). pHi, intracellular pH; S0859, C29H24ClN3O3S.
Is NBC inhibition by S0859 secondary to inhibition of carbonic anhydrase?
NBC activity has been proposed to be facilitated by certain isoforms of the enzyme CA, which may bind to intracellular- or extracellular-oriented sites on the transport protein (Alvarez et al., 2003; also see Lu et al., 2006). S0859 is a sulphonamide derivative, and some sulphonamides, such as ATZ, are well known CA inhibitors (Maren, 1976; Supuran et al., 2003). Therefore, we examined whether the ability of S0859 to inhibit NBC activity is secondary to an inhibition of CA. We tested for this possibility in two ways.
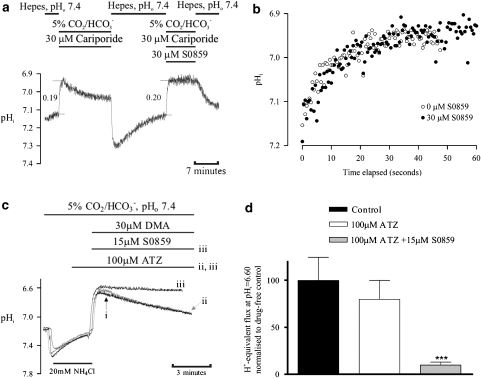
First, we checked whether the hydration of CO2 inside a guinea pig myocyte (a reaction catalysed by intracellular CA) was slowed by superfusion of S0859, as happens with ATZ (Leem and Vaughan-Jones, 1998a). Intracellular CO2 hydration, in effect, results in the generation of HCO3− and H+-ions, leading to a fall of pHi:
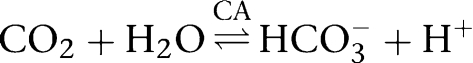 |
The first part of the experiment illustrated in Figure 6a shows the effect on pHi of switching the superfusate from a HEPES-buffered to a 5% CO2/HCO3−-buffered solution at constant pHo (=7.4). The immediate fall of pHi reflects the passive entry and hydration of CO2. Later on in the experiment, the same manoeuvre was repeated, but with 30 μM S0859 in the superfusate. The initial rate of fall of pHi was not different from that seen in the absence of the drug, as shown in Figure 6b. A similar result was observed in two further experiments, suggesting that intracellular CA is not inhibited by exposing a myocyte to 30 μM S0859.
Figure 6.
Effect of S0859 on carbonic anhydrase activity. (a) Effect of 30 μM S0859 on intracellular acid loading induced by application of 5% CO2/HCO3−. The magnitude of the acidosis is indicated (in pH units) beside the respective pHi traces. (b) Superimposed pHi traces of the 5% CO2/HCO3−-induced acid loading phase in the presence and absence of S0859 described in (a), shown on a faster time scale. (c) Superimposed traces of NBC-mediated recovery of pHi under control conditions (i, no ATZ added), in the presence of 100 μM ATZ (ii), and after addition of 15 μM S0859 in the presence of 100 μM ATZ (iii). (d) Histogram showing the effect of ATZ (n=26) and ATZ plus S0859 (n=3, P<0.001) on NBC-mediated H+-equivalent efflux (expressed as percentage of control, n=20) measured at pHi 6.60. pHi, intracellular pH; S0859, C29H24ClN3O3S.
Second, we maximally inhibited cardiac CA activity by exposing guinea pig myocytes to 100 μM ATZ. This is known to inhibit both intracellular and extracellular active sites of CA in cardiac tissue (Leem and Vaughan-Jones, 1998a). The experiment shown in Figure 6c shows that NBC-mediated pHi recovery from an intracellular acid load was not significantly affected by CA inhibition, as pHi recovery occurred at a similar rate in the presence and absence of ATZ (Figure 6d). In contrast, recovery rate was greatly inhibited (Figures 6c and d) when S0859 was present in the superfusate, indicating that NBC inhibition by the drug was independent of CA activity.
Assessing the influence of NBC on pHi and contractility using S0859
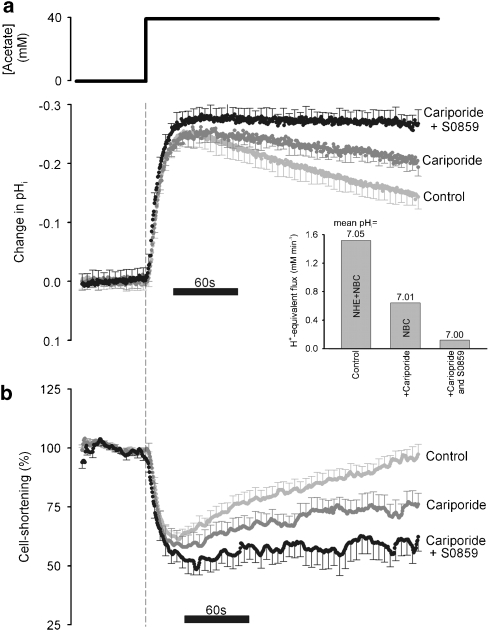
As S0859 appears selective for NBC among the pHi-regulatory transporters, we investigated its potential as a pharmacological tool for probing the role of NBC in modulating cardiac function. Figure 7 shows the effect of the drug on pHi regulation and on electrically evoked active cell shortening (contraction) of rat isolated ventricular myocytes. Figure 7a shows superimposed pHi time courses averaged from several experiments. pHi recovered from an intracellular acidosis imposed by superfusing 40 mM acetate (osmotically balanced solution). As described already, this recovery is known to be mediated by H+-equivalent extrusion through NHE and NBC (Lagadic-Gossmann et al., 1992; Leem et al., 1999; Yamamoto et al., 2005). Use of selective H+-equivalent transport inhibitors now clearly reveals this dual extrusion system (Figure 7a, see inset). Inhibiting NHE with cariporide slowed pHi recovery, whereas simultaneously inhibiting NBC with 10 μM S0859 terminated recovery almost entirely.
Figure 7.
Using S0859 to probe the role of NBC. Rat ventricular myocytes were electrically stimulated at 1 Hz (to trigger contraction) and exposed to an acute acid load by superfusion with 40 mM acetate. The intracellular pH (a) and cell shortening (b) were measured simultaneously. pHi, averaged for several experiments, is expressed as the change (ΔpHi) from the mean value recorded over a 1 min period before acetate addition. Cell shortening, averaged for several experiments, is expressed as a percentage of the mean control recorded over the 1 min period before acetate addition. For clarity, error bars are plotted for every tenth data point. Experiments were performed in the absence of drugs (to allow NHE- and NBC-mediated pHi recovery, n=18), in the presence of 30 μM cariporide (to allow NBC-mediated pHi recovery, n=10) and in the presence of 30 μM cariporide and 15 μM S0859 (to prevent NBC- and NHE-mediated pHi recovery, n=9). The acid displacement of pHi immediately after acetate exposure reduced cell shortening. The degree of pHi recovery during acetate exposure correlated with the recovery of cell shortening. The average H+-equivalent flux (inset) was calculated as the product of the slope of pHi recovery (measured from 40 s after acetate addition to the end of the trace) and the buffering capacity (βi+βCO2 estimated for the mean pHi (see Methods). The mean pHi, averaged during the pHi recovery period is stated in the inset above the histograms. Around 58% of the pHi recovery during the acetate-induced acid load was mediated by NHE activity and the remainder was predominantly NBC-mediated. The pHi recovery time courses mirrored the time courses of recovery of cell shortening. pHi, intracellular pH; S0859, C29H24ClN3O3S.
As cardiac contractility is exquisitely sensitive to changes of pHi (Bountra and Vaughan-Jones, 1989; Orchard and Kentish, 1990), it is of interest to note that the fractionation of pHi recovery by H+-equivalent transport inhibitors was mirrored by a similar fractionation in the amplitude of electrically evoked cell contraction. During superfusion of rat myocytes with acetate, in the absence of pharmacological inhibitors, the peak amplitude of shortening declined and then recovered, roughly in parallel with the recovery of pHi (Figures 7a and b). When acetate was added, the initial, rapid fall of pHi inhibited the contraction equally well in the presence and absence of S0859. Much of this effect is mediated via an inhibitory effect of intracellular H+ ions on the myofilaments and on Ca2+ binding to troponin C (Blanchard and Solaro, 1984). In the presence of cariporide, the time course of recovery of pHi and peak-shortening amplitude was slowed down (Figures 7a and b). In the presence of both cariporide and S0859, recovery of pHi and peak-shortening amplitude was virtually abolished. These data therefore confirm that, following intracellular acidosis, both NHE and NBC are important controllers of pHi and therefore, indirectly, of contraction. The data also show that, under control conditions before application of extracellular acetate, contractile amplitude in the presence of cariporide and S0859 was robust and stable, providing pHi was constant. The basic mechanisms of cardiac excitation and contraction are thus preserved in the presence of S0859.
The dose of S0859 used in experiments summarized in Figure 7 was 15 μM. This dose was selected as it provided ⩾90% inhibition of NBC activity (Figure 3c). We also tested a higher dose of 30 μM (n=10), but electrically paced cells seemed less stable at this dose, sometimes ceasing to respond to stimulation after a few minutes. Thus, for probing effects of S0859 on cell function, a dose of 15 μM was preferable.
Discussion
S0859, a selective NBC inhibitor
Although S0859 has been used recently to block NBC activity in cardiac and epithelial tissue (Schwab et al., 2005; Yamamoto et al., 2005), the present study is the first presentation of the drug's structure, its potency and its selectivity for NBC-inhibition. Inhibition of cardiac NBC by S0859 has been observed in ventricular myocytes from three mammalian species: guinea pig, rat and rabbit. The drug is an N-cyanosulphonamide compound, with little structural resemblance to typical NHE and anion transport inhibitors, such as cariporide and DIDS. The components of the molecule responsible for NBC inhibition are yet to be identified. S0859 shows an apparent extracellular Ki for cardiac NBC inhibition of 1.6 μM; near-total inhibition is achieved at about 30 μM (Figure 3c).
As described in Introduction, the normal control system for pHi in cardiac myocytes comprises at least four types of generic transporter. NHE and NBC extrude acid from the cell (by exporting H+ and importing HCO3−), whereas AE and CHE are H+-equivalent loading mechanisms (exporting HCO3− and OH−). These ion transport systems are similar to those found in many non-cardiac tissues. Although generic NHE in heart comprises only the NHE1 isoform (Slc9a1), several specific transporters are suggested to contribute to cardiac AE and CHE activity. These include protein products of the Slc4a gene family (for example, Slc4a1 and Slc4a3, also known as AE1 and AE3), which mediate AE, and products of the Slc26a gene family (Slc26a6 and Slc26a3), which may mediate both AE and CHE. Whatever the dominant H+-equivalent influx proteins in the ventricular myocyte, they are unaffected by S0859 at doses that completely inhibit generic NBC (30 μM). We have also shown that S0859 has no effect on acid-extruding NHE1. S0859 is thus specific for a particular gene family of bicarbonate transporters and shows no obvious pharmacological activity for other common pHi-regulatory membrane transporters. However, one transporter that was not screened in the present work is the H+-lactate cotransporter (product of Slc16 gene family, also known as the monocarboxylic acid transporter, MCT; Halestrap and Meredith, 2004), which contributes to pHi control during hypoxic and post-ischaemic episodes. At present, we cannot exclude an effect of S0859 on this latter carrier.
It is interesting that S0859 inhibited all cardiac NBC activity. At least three NBC isoforms have been detected in human and animal heart, at mRNA transcript and protein level, including electrogenic (for example, NBCe1 and NBCe2) (Choi et al., 1999; Virkki et al., 2002) and electroneutral (NBCn1) (Pushkin et al., 1999; Choi et al., 2000) isoforms. All three are products of the Slc4a gene family (Slc4a5, Slc4a4 and Slc4a7) (Romero et al., 2004). There is clear evidence for the functional activity of electrogenic NBC in ventricular tissue (Aiello et al., 1998), while results from a recent study suggest additional functional activity from an electroneutral NBC (Yamamoto et al., 2005). Thus, although S0859 appears to be useful as a generic NBC inhibitor in the cardiac cell, it may not be able to resolve functional contributions from specific NBC isoforms. In contrast, there are unpublished data (referred to in Schwab et al., 2005) that S0859 may be an NBCe1 (Slc4a4)-selective inhibitor.
Site of action of S0859
Carbonic anhydrase enzymes appear to facilitate NBC activity in heterologous cell systems when the transporter (NBCe1 or NBCe2) and CA protein are coexpressed (Alvarez et al., 2003; Becker and Deitmer, 2007), although this effect of CA remains controversial (Lu et al., 2006). The possibility therefore arises that inhibition of NBC by S0859 is via inhibition of CA. Although we do not exclude an effect of S0859 on CA activity, this cannot be the principal mechanism for NBC inhibition. This is because NBC activity in the isolated myocyte was unaffected by globally inhibiting CA with ATZ. S0859 must therefore inhibit NBC by a mechanism independent of CA activity. It thus seems likely that the site for NBC inhibition is on the transport molecule itself or on a closely associated non-CA molecule. The lack of effect of ATZ on NBC activity also suggests that the transporter is not significantly facilitated by CA in the isolated ventricular myocyte, at least not during a modest intracellular acidosis.
Pharmacological dissection of NBC activity
Characterizing the inhibitory action of S0859 on cardiac NBC required accurate measurement of NBC activity. This was estimated from pHi recovery rate following an acute intracellular acidosis. Recovery was measured in the presence of cariporide, to eliminate contributions from NHE activity, assuming that cariporide does not directly affect NBC. This assumption has previously been verified in guinea pig myocytes by a separate protocol, in which acid extrusion via NHE (obtained in HEPES-buffered conditions) was subtracted from the total acid extrusion obtained in CO2/HCO3−-buffered conditions; this yielded an NBC activity that was very similar to that obtained by pharmacological dissection of acid extrusion (CO2/HCO3−-buffered superfusates) using Hoe 694, a structural congener (and forerunner) of cariporide (Leem et al., 1999). Although not shown here, we have found that the above two protocols for estimating NBC flux also produce matching values in both rat and guinea pig ventricular myocytes, when using 30 μM cariporide rather than Hoe 694 to eliminate NHE (P Swietach and RD Vaughan-Jones, unpublished observations). Cariporide thus appears to have little or no potency against cardiac NBC at the dose used in the present study. The pharmacological dissection procedure should therefore have provided a reliable estimate of NBC activity.
S0859 as a tool to probe the functional effects of NBC
The selectivity of S0859 for NBC provides a means for exploring the importance of NBC to pHi regulation in the heart. This is illustrated by the effect of the drug on pHi recovery from an acid load in isolated myocytes (Figure 7a). Recovery can be fractionated into a cariporide-sensitive component mediated by NHE and an S0859-sensitive component mediated by NBC. The magnitude of each component is similar to that deduced previously when pHi recovery was compared in HEPES-buffered (no active NBC) and CO2/HCO3−-buffered (active NBC) superfusates (Yamamoto et al., 2005). The S0859-sensitive fraction is approximately 42% of total H+-equivalent extrusion, when measured at a pHi of approximately 7.00–7.05. This latter result is consistent with an important contribution of NBC to pHi regulation at levels close to steady state (pHi ∼7.2). More acidic pHi levels were not explored in the present work, but previous results have suggested that NHE is the more greatly activated transporter (Leem et al., 1999; Yamamoto et al., 2005), thus assuming the dominant role in H+-equivalent extrusion. Use of S0859 should now simplify such investigations of NHE versus NBC activity.
S0859 may be useful for elucidating the role of NBC in controlling contractility during physiological changes of pHi in the heart. At resting pHi, electrically evoked contraction of ventricular myocytes appears stable and reproducible in the presence of the drug, provided the dose does not exceed 15 μM. During the initial imposition of an intracellular acid load, the decrease in contraction along with the fall of pHi was similar in the presence and absence of cariporide and S0859 (see Figure 7b). However, the subsequent recovery of contractile amplitude was fastest in the absence of the drugs, as was the recovery of pHi, confirming that NBC and NHE modulate contractility, at least partly through their ability to modulate pHi. Sequential application of cariporide and S0859 indicates comparable contributions from NHE and NBC during this pHi recovery process. During intracellular acidosis, contraction can also be modulated by a rise of [Na+]i, induced by NHE- and NBC-mediated Na+-influx, which then raises [Ca2+]i through effects on sarcolemmal Na+/Ca2+ exchange (Bountra and Vaughan-Jones, 1989; Harrison et al., 1992; Yamamoto et al., 2005). We did not specifically investigate this Na+i-dependent mechanism, but use of S0859 may now help to unmask contributions from NBC.
Inhibitors of H+-equivalent extrusion in heart, notably NHE inhibitors such as cariporide and other derivatives of amiloride, have drawn considerable attention because of their cardioprotective properties in animal models of acute, cardiac ischaemia and reperfusion (Scholz et al., 1993; Xiao and Allen, 1999; Clements-Jewery et al., 2004; but cf. Avkiran and Marber, 2002), and their apparent ability to arrest or even reverse cardiac hypertrophy (Yoshida and Karmazyn, 2000). Cardioprotection and antihypertrophic properties have been attributed variously to inhibition of Na+ influx via NHE, and the maintenance of intracellular acidity. Inhibition of NBC ith DIDS, or with a polyclonal NBC-antibody, also appears to offer some degree of cardioprotection against acute ischaemia/reperfusion (Schafer et al., 2000; Khandoudi et al., 2001). Although such protection may again be related to changes of [Na+]i, pHi and [Ca2+]i, it may also be secondary to blockade of outward ionic current associated with electrogenic NBC activity (iNBC), which may directly influence cardiac electrical activity. A selective NBC inhibitor such as S0859 may therefore prove useful in assessing the transporter as a therapeutic target, in addition to NHE, in the management of ischaemic cardiac dysfunction and hypertrophy. Finally, given that S0859 is a general NBC inhibitor in cardiac cells, it may also prove useful for identifying NBC activity in other tissues, where several different types of transporter contribute to membrane HCO3−, OH− and H+ transport.
Acknowledgments
We thank Dr Heinz-Werner Kleemann of Sanofi-Aventis Deutschland GmbH for his most helpful advice and discussion during the course of this work. We also thank Kenneth W Spitzer for generously conducting the experiments on rabbit myocytes. The excellent technical assistance of Mrs Anna Skyrme is gratefully acknowledged. Cariporide and S0859 were kindly provided by Sanofi-Aventis. This work was funded by the British Heart Foundation.
Abbreviations
- AE
Cl−-HCO3− exchange
- ATZ
acetazolamide
- βCO2
buffering due to CO2
- βi
intrinsic buffering power
- βtot
total intracellular buffering power
- carboxy-SNARF-1
carboxy-seminapthorodafluor-1
- CHE
Cl−-OH− exchange
- DIDS
4,4′-diisothiocyanostilbene 2,2′-disulphonic acid
- Hoe 642
4-isopropyl-3-methylsulphonyl-benzoylguanidine
- Je
H+-equivalent efflux
- NBC
Na+/HCO3− cotransport
- NHE
Na+-H+ exchange
- S0859
C29H24ClN3O3S
Conflict of interest
The authors state no conflict of interest.
References
- Aiello EA, Petroff MG, Mattiazzi AR, Cingolani HE. Evidence for an electrogenic Na+-HCO3− symport in rat cardiac myocytes. J Physiol. 1998;512 Part 1:137–148. doi: 10.1111/j.1469-7793.1998.137bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez BV, Loiselle FB, Supuran CT, Schwartz GJ, Casey JR. Direct extracellular interaction between carbonic anhydrase IV and the human NBC1 sodium/bicarbonate co-transporter. Biochemistry. 2003;42:12321–12329. doi: 10.1021/bi0353124. [DOI] [PubMed] [Google Scholar]
- Avkiran M, Marber MS. Na+/H+ exchange inhibitors for cardioprotective therapy: progress, problems and prospects. J Am Coll Cardiol. 2002;39:747–753. doi: 10.1016/s0735-1097(02)01693-5. [DOI] [PubMed] [Google Scholar]
- Becker HM, Deitmer JW. Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3− cotransporter. J Biol Chem. 2007;282:13508–13521. doi: 10.1074/jbc.M700066200. [DOI] [PubMed] [Google Scholar]
- Blanchard EM, Solaro RJ. Inhibition of the activation and troponin calcium binding of dog cardiac myofibrils by acidic pH. Circ Res. 1984;55:382–391. doi: 10.1161/01.res.55.3.382. [DOI] [PubMed] [Google Scholar]
- Bountra C, Vaughan-Jones RD. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990;417:234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Ch'en FF, Vaughan-Jones RD.Effect of S0859, a putative Na+-HCO3− co-transport inhibitor, on intracellular pH regulation in the guinea-pig ventricular myocyte 2001Proceedings of the 34th International Congress of Physiological Sciences; New Zealand, ID no. 1495 (Abstract)
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC) Am J Physiol. 1999;276 3 Part 1:C576–C584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- Cingolani HE, Ennis IL. Sodium-hydrogen exchanger, cardiac overload, and myocardial hypertrophy. Circulation. 2007;115:1090–1100. doi: 10.1161/CIRCULATIONAHA.106.626929. [DOI] [PubMed] [Google Scholar]
- Clements-Jewery H, Sutherland FJ, Allen MC, Tracey WR, Avkiran M. Cardioprotective efficacy of zoniporide, a potent and selective inhibitor of Na+/H+ exchanger isoform 1, in an experimental model of cardiopulmonary bypass. Br J Pharmacol. 2004;142:57–66. doi: 10.1038/sj.bjp.0705749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damkier HH, Nielsen S, Praetorius J. An anti-NH2-terminal antibody localizes NBCn1 to heart endothelia and skeletal and vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;290:H172–H180. doi: 10.1152/ajpheart.00713.2005. [DOI] [PubMed] [Google Scholar]
- Doggrell SA, Hancox JC. Is timing everything? Therapeutic potential of modulators of cardiac Na+ transporters. Expert Opin Invest Drugs. 2003;12:1123–1142. doi: 10.1517/13543784.12.7.1123. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Frampton JE, McCall E, Boyett MR, Orchard CH. Contraction and intracellular Ca2+, Na+, and H+ during acidosis in rat ventricular myocytes. Am J Physiol. 1992;262 2 Part 1:C348–C357. doi: 10.1152/ajpcell.1992.262.2.C348. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Avkiran M. Effects of moderate hypothermia on sarcolemmal Na+/H+ exchanger activity and its inhibition by cariporide in cardiac ventricular myocytes. Br J Pharmacol. 2001;134:1587–1595. doi: 10.1038/sj.bjp.0704405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandoudi N, Albadine J, Robert P, Krief S, Berrebi-Bertrand I, Martin X, et al. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc Res. 2001;52:387–396. doi: 10.1016/s0008-6363(01)00430-8. [DOI] [PubMed] [Google Scholar]
- Knight DR, Smith AH, Flynn DM, MacAndrew JT, Ellery SS, Kong JX, et al. A novel sodium-hydrogen exchanger isoform-1 inhibitor, zoniporide, reduces ischemic myocardial injury in vitro and in vivo. J Pharmacol Exp Ther. 2001;297:254–259. [PubMed] [Google Scholar]
- Lagadic-Gossmann D, Buckler KJ, Vaughan-Jones RD. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol. 1992;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdunski M, Frelin C, Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J Mol Cell Cardiol. 1985;17:1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol. 1999;517 Part 1:159–180. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem CH, Vaughan-Jones RD. Out-of-equilibrium pH transients in the guinea-pig ventricular myocyte. J Physiol. 1998a;509 Part 2:471–485. doi: 10.1111/j.1469-7793.1998.471bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem CH, Vaughan-Jones RD. Sarcolemmal mechanisms for pHi recovery from alkalosis in the guinea-pig ventricular myocyte. J Physiol. 1998b;509 Part 2:487–496. doi: 10.1111/j.1469-7793.1998.487bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, et al. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J Biol Chem. 2006;281:19241–19250. doi: 10.1074/jbc.M602181200. [DOI] [PubMed] [Google Scholar]
- Maren TH. Relatons between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol. 1976;16:309–327. doi: 10.1146/annurev.pa.16.040176.001521. [DOI] [PubMed] [Google Scholar]
- Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na+/H+ exchanger. Eur J Med Chem. 2003;38:547–554. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- Orchard CH, Cingolani HE. Acidosis and arrhythmias in cardiac muscle. Cardiovasc Res. 1994;28:1312–1319. doi: 10.1093/cvr/28.9.1312. [DOI] [PubMed] [Google Scholar]
- Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990;258 6 Part 1:C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP, Price SJ, Levi AJ. The kinetics of transport of lactate and pyruvate into isolated cardiac myocytes from guinea pig. Kinetic evidence for the presence of a carrier distinct from that in erythrocytes and hepatocytes. Biochem J. 1989;264:409–418. doi: 10.1042/bj2640409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274:16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- Richmond PH, Vaughan-Jones RD. Assessment of evidence for K+-H+ exchange in isolated type-1 cells of neonatal rat carotid body. Pflugers Arch. 1997;434:429–437. doi: 10.1007/s004240050417. [DOI] [PubMed] [Google Scholar]
- Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflugers Arch. 2004;447:495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- Schafer C, Ladilov YV, Siegmund B, Piper HM. Importance of bicarbonate transport for protection of cardiomyocytes against reoxygenation injury. Am J Physiol Heart Circ Physiol. 2000;278:H1457–H1463. doi: 10.1152/ajpheart.2000.278.5.H1457. [DOI] [PubMed] [Google Scholar]
- Scholz W, Albus U, Counillon L, Gogelein H, Lang HJ, Linz W, et al. Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res. 1995;29:260–268. [PubMed] [Google Scholar]
- Scholz W, Albus U, Lang HJ, Linz W, Martorana PA, Englert HC, et al. Hoe 694, a new Na+/H+ exchange inhibitor and its effects in cardiac ischaemia. Br J Pharmacol. 1993;109:562–568. doi: 10.1111/j.1476-5381.1993.tb13607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A, Rossmann H, Klein M, Dieterich P, Gassner B, Neff C, et al. Functional role of Na+-HCO3− cotransport in migration of transformed renal epithelial cells. J Physiol. 2005;568 Part 2:445–458. doi: 10.1113/jphysiol.2005.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman BW, Moore KB, Spitzer KW, Bridge JHB. A video system for measuring motion in contracting heart cells. IEEE Trans Biomed Eng. 1988;35:264–267. doi: 10.1109/10.1375. [DOI] [PubMed] [Google Scholar]
- Sun B, Leem CH, Vaughan-Jones RD. Novel chloride-dependent acid loader in the guinea-pig ventricular myocyte: part of a dual acid-loading mechanism. J Physiol. 1996;495 Part 1:65–82. doi: 10.1113/jphysiol.1996.sp021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev. 2003;23:146–189. doi: 10.1002/med.10025. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO3− cotransporter (NBCe2) Am J Physiol Cell Physiol. 2002;282:C1278–C1289. doi: 10.1152/ajpcell.00589.2001. [DOI] [PubMed] [Google Scholar]
- Xiao XH, Allen DG. Role of Na+/H+ exchanger during ischemia and preconditioning in the isolated rat heart. Circ Res. 1999;85:723–730. doi: 10.1161/01.res.85.8.723. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Swietach P, Rossini A, Loh SH, Vaughan-Jones RD, Spitzer KW. Functional diversity of electrogenic Na+-HCO3− cotransport in ventricular myocytes from rat, rabbit and guinea pig. J Physiol. 2005;562 Part 2:455–475. doi: 10.1113/jphysiol.2004.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Karmazyn M. Na+/H+ exchange inhibition attenuates hypertrophy and heart failure in 1-wk postinfarction rat myocardium. Am J Physiol Heart Circ Physiol. 2000;278:H300–H304. doi: 10.1152/ajpheart.2000.278.1.H300. [DOI] [PubMed] [Google Scholar]