Abstract
Background and purpose:
To analyse the influence of hypertension in the modulation induced by inducible NOS (iNOS)-derived NO and superoxide anion (O2 •−) of vasoconstrictor responses and the sources of O2 •− implicated.
Experimental approach:
Vascular reactivity experiments were performed in segments of aorta from normotensive, Wistar Kyoto (WKY) and spontaneously hypertensive rats (SHR); protein and mRNA expressions were respectively measured by western blot and quantitative reverse transcription-polymerase chain reaction and O2 •− production was evaluated by ethidium fluorescence.
Key results:
The contractile responses to phenylephrine (1 nM–30 μM) and 5-hydroxytryptamine (0.1–100 μM) were greater in aortic segments from SHR than WKY. The selective iNOS inhibitor, 1400W (10 μM), increased the phenylephrine contraction only in WKY segments; however, iNOS protein and mRNA expressions were greater in aorta from SHR than WKY. Superoxide dismutase (SOD, 150 U ml−1) reduced phenylephrine and 5-hydroxytryptamine responses only in aorta from SHR; the NAD(P)H oxidase inhibitor apocynin (0.3 mM) decreased phenylephrine and 5-hydroxytryptamine responses more in vessels from SHR than WKY. Co-incubation with SOD plus 1400W potentiated the phenylephrine and 5-hydroxytryptamine responses more in segments from SHR than WKY. O2 •− production was greater in aorta from SHR than WKY; apocynin abolished this difference.
Conclusions and implications:
Increased O2 •− formation from NADP(H) oxidase in vessels from hypertensive rats contributes to the vasoconstrictor responses and counteract the increase of NO from iNOS and the consequent modulation of these responses.
Keywords: iNOS, superoxide anion, NAD(P)H oxidase, aorta, hypertension
Introduction
Hypertension is associated with elevated levels of circulating proinflammatory cytokines (Savoia and Schiffrin, 2006; Vaziri and Rodríguez-Iturbe, 2006), which may alter the vascular expression of enzymes like inducible nitric oxide synthase (iNOS) and modify the regulation of vascular tone during this pathology. Indeed, increased vascular iNOS activity and/or protein expression (Chou et al., 1998; Vaziri et al., 1998; Briones et al., 2000, 2002a) have been described in hypertension. The role of iNOS-derived NO in vasoconstrictor and endothelium-dependent vasodilator responses has been previously analysed by our group and others in lipopolysaccharide or interleukin-1β-stimulated arteries (Hernanz et al., 2003; Vo et al., 2005; Jiménez-Altayó et al., 2006). However, the participation of iNOS-derived NO in vasoconstrictor responses in unstimulated vessels is not well studied.
Oxidative stress can affect vascular reactivity by different mechanisms. Reactive oxygen species function as second messengers, activating numerous signalling molecules and play an important role in vascular physiopathology (Paravicini and Touyz, 2006; Vaziri and Rodríguez-Iturbe, 2006). Several sources of superoxide anion (O2•−) within vessels have been described. Among them, xanthine oxidase, uncoupled NOS and COX can produce O2•− in different conditions. However, at the vascular level it is well established that nicotinamide adenine dinucleotide (phosphate) (NAD(P)H) oxidase, present in all three vessel layers (Griendling et al., 1994; Mohazzab et al., 1994; Chamseddine and Miller, 2003), is the main source of O2•− (Paravicini and Touyz, 2006; Vaziri and Rodríguez-Iturbe, 2006). An increase of O2•− production has been observed in human and different experimental models of hypertension, including spontaneously hypertensive rats (SHR) (Bouloumie et al., 1997; McIntyre et al., 1999; Cai and Harrison, 2000; Griendling et al., 2000; Lassegue and Griendling, 2004). More specifically, the enhanced O2•− generation in hypertension is a known result of the activation of vascular NAD(P)H oxidase (Zalba et al., 2001; Cruzado et al., 2005).
The mechanisms whereby increased O2•− production might contribute to high blood pressure are currently under active investigation. However, it is also well known that by interacting with NO, O2•− forms peroxynitrite, thus decreasing NO availability for smooth muscle relaxation. Hypertension is associated with changes in vascular responses, such as impairment of endothelium-dependent vasodilator responses or enhancement of vasoconstrictor response to different agonists (Marín, 1993; Marín and Rodríguez-Martínez, 1997; Mattei et al., 1997). Several studies have analysed the relationship between increased O2•− production and the impairment of endothelium-dependent relaxation in hypertension (for review see Touyz, 2004; Feletou and Vanhoutte, 2006). However, the O2•− contribution to the altered vasoconstrictor responses in hypertension as well as its relationship with the iNOS-derived NO is less studied. The present study was performed to analyse how hypertension might alter the role of O2•− in the vasoconstrictor responses to phenylephrine, the sources of this O2•− and its relationship with iNOS-derived NO.
Methods
Animals
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publications no. 85-23, revised 1996) and complies with the current Spanish and European laws (RD 223/88 MAPA and 609/86). Six-month-old male normotensive Wistar Kyoto (WKY) and SHR rats were used. Systolic BP was recorded with an automatic sphygmomanometer using a tail-cuff method device placed on the tail of pretrained rats, which had spent 1 h in a warm chamber at 37 °C, and were restrained. Measurements of BP were repeated at least three times, and the average systolic BP was calculated: 131.1±2.8 for WKY, n=13, and 189.7±1.1 mm Hg for SHR, n=6 (P<0.05). Rats were killed by decapitation and the thoracic aorta was removed and placed in Krebs–Henseleit solution (in mM: 115 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4.7H2O, 2.5 CaCl2, 1.2 KH2PO4, 11.1 glucose and 0.01 Na2EDTA) at 4 °C.
Vascular reactivity experiments
Vascular reactivity was studied in aortic segments by isometric tension recording, as described previously (Álvarez et al., 2005). Briefly, two parallel stainless steel pins were introduced through the lumen of the segments: one was fixed to the organ bath wall and the other one was connected to a force transducer (Grass FT03C, Quincy, MA, USA), which in turn was connected to a Grass model TD polygraph. Segments were incubated in an organ bath containing 5 ml of Krebs–Henseleit solution at 37 °C, continuously bubbled with a 95% O2–5% CO2 mixture (pH 7.4). An optimal resting tension of 1.5 g was applied to all aortic segments. This tension was adjusted every 15 min during a 60-min equilibration period before adding drugs. Aortic segments were initially exposed to 75 mM KCl to test their functional integrity. The presence of endothelium was confirmed by the effect of 10 μM ACh (Sigma Chemical Co., St Louis, MO, USA) on segments contracted with phenylephrine (Sigma Chemical) at a concentration that produces close to 50% of the contraction induced by KCl. After 60 min of washout, concentration–response curves to phenylephrine or 5-hydroxytryptamine (Sigma Chemical) were performed. A single concentration-dependent curve was performed in each segment. The effects of superoxide dismutase (SOD; Sigma Chemical), catalase (Sigma Chemical), the NAD(P)H oxidase inhibitor apocynin (Sigma Chemical), the xanthine oxidase inhibitor allopurinol (Research Biochemicals Incorporated, Natick, MA, USA) and/or the selective iNOS inhibitor N-(3-(aminomethyl)benzyl)acetamidine hydrochloride (1400W; Calbiochem, San Diego, CA, USA) were investigated by their addition 30 min before phenylephrine or 5-hydroxytryptamine. Some experiments were performed in the presence of the protein synthesis inhibitor dexamethasone (Sigma Chemical), which was added at the time the aorta was first placed in the Krebs–Henseleit solution. In another set of experiments, endothelium was mechanically removed by rubbing the intimal surface.
Western blot analysis
iNOS expression was analysed in aortic segments from WKY and SHR in either basal conditions (immediately after dissection of the artery from the animal) or in segments under similar conditions of reactivity experiments. Segments were frozen in liquid nitrogen and stored at −70 °C until analysis.
Segments were homogenized in ice-cold Tris-EDTA buffer (in mM: Tris-50, EDTA-1.0, pH 7.4). Homogenates (30 μg protein per lane) and prestained molecular SDS-PAGE standards (Bio-Rad Laboratories, Hercules, CA, USA) were electrophoretically separated on a 7.5% SDS-PAGE and then transferred to polyvinyl difluoride membranes overnight at 4 °C, using a Mini Trans-Blot Cell System (Bio-Rad) containing 25 mM Tris, 190 mM glycine, 20% methanol and 0.05% SDS. Mouse macrophages were used for iNOS positive controls. Then, the membrane was blocked for 60 min at room temperature in Tris-buffered solution (10 mM Tris, 100 mM NaCl, 0.1% Tween 20) with 5% powdered non-fat milk. Next, the membrane was incubated for 1 h at room temperature with mouse monoclonal antibody for iNOS (1:10 000; Transduction Laboratories, Lexington, UK). After washing, the membrane was incubated with anti-mouse IgG antibody (1:2000) conjugated to horseradish peroxidase (Transduction Laboratories). The membrane was thoroughly washed and the immunocomplexes were detected using an enhanced horseradish peroxidase/luminol chemiluminescence system (ECL Plus, GE Healthcare, Little Chalfont, UK) and subjected to autoradiography (Hyperfilm ECL, GE Healthcare). Signals on the immunoblot were quantified with an NIH Image V1.56 computer program. The same membrane was used to determine α-actin expression, and the content of the latter was used to correct iNOS expression in each sample by means of a monoclonal antibody anti-α-actin (1:3 000 000; Sigma Chemical).
Results are expressed as the ratio between signals on the immunoblot corresponding to iNOS and to α-actin. To compare the results of protein expression within the same experiment and with other experiments, we assigned a value of 1 to the ratio in arteries from WKY and used that value to calculate the relative density of the other bands in the same gel.
RT-PCR real-time assay
iNOS mRNA was determined in aortic segments in similar conditions to those used for protein expression determination. Total RNA was obtained using TRIzol (Invitrogen Life Technologies, Philadelphia, PA, USA). A total of 1 μg of DNAse I-treated RNA was reverse transcribed into cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) in a 50 μl reaction. PCR was performed in duplicate for each sample using 0.5 μl of cDNA as a template for iNOS, 1 × TaqMan Universal PCR Master Mix (Applied Biosystems) and 10 × of Taqman Gene Expression Assays (Applied Biosystems, Rn00561646_m1) in a 20 μl reaction. Quantitative RT-PCR was carried out in an ABI PRISM 7000 Sequence Detection System (Applied Biosystems) using the following conditions: 2 min 50 °C, 10 min 95 °C followed by 40 cycles of 15 s 95 °C and 1 min 60 °C. As a normalizing internal control we amplified β2 microglobulin (Rn00560865_m1). To calculate the relative index of gene expression, we employed the 2−ΔΔCt method (Livak and Schmittgen, 2001) using WKY samples as calibrator.
In situ detection of vascular O2•− production
The oxidative fluorescent dye dihydroethidium was used to evaluate O2•− production in situ, as described previously (Jiménez-Altayó et al., 2006). Hydroethidine freely permeates cells and, in the presence of O2•−, is oxidized to ethidium bromide, which is trapped by intercalation with DNA. Ethidium bromide is excited at 546 nm and has an emission spectrum of 610 nm. Frozen tissue segments from control and apocynin (0.3 mM, 30 min)-incubated arteries were cut into 10-μm thick sections and placed on a glass slide. Serial sections were equilibrated under identical conditions for 30 min at 37 °C in Krebs-HEPES buffer (in mM: NaCl 130, KCl 5.6, CaCl2 2, MgCl2 0.24, HEPES 8.3, glucose 11, pH 7.4). Fresh buffer containing dihydroethidium (2 μM) was applied topically onto each tissue section, cover-slipped and incubated for 30 min in a light-protected humidified chamber at 37 °C, and then viewed in a fluorescent laser scanning confocal microscope (Leica TCS SP2 equipped with a krypton/argon laser, × 40 objective), using the same imaging settings in each case. Fluorescence was detected with a 568 nm longpass filter. Elastin autofluorescence was used to delimit the different layers of the vascular wall and was visualized by excitation at 488 nm and detection at 535 nm. For fluorescence quantification, four rings per animal were sampled for each experimental condition and averaged. The mean fluorescence densities in the target region were calculated.
Data analysis and statistics
Vasoconstrictor responses induced by phenylephrine or 5-hydroxytryptamine were expressed as a percentage of the tone generated by 75 mM KCl. The maximum response (Emax) and EC50 values were calculated by a non-linear regression analysis of each individual concentration–response curve using GraphPad Prism Software (San Diego, CA, USA).
Results are expressed as mean±s.e.m. of the number of rats indicated; differences were analysed using Student's t-test, one-way or two-way ANOVA followed by a Bonferroni test. A P-value below 0.05 was considered significant.
Results
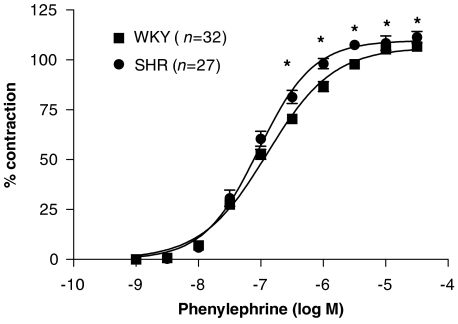
Phenylephrine (1 nM–30 μM) induced concentration-dependent contraction in aortic segments that was slightly greater in SHR than in WKY (Figure 1), as reported previously (Álvarez et al., 2005, 2007). 5-hydroxytryptamine (0.1–100 μM) also induced concentration-dependent contractions greater (P<0.05) in SHR (Emax: 153.8±4.9% of the tone generated by 75 mM KCl; EC50: 2.26±0.31 μM; n=14) than in WKY (Emax: 123.8±6.9%; EC50: 4.23±0.54 μM; n=9). The mean values for KCl contraction in aorta were 3100±39 and 2913±49 mg for WKY (n=42) and SHR (n=42), respectively (P<0.05).
Figure 1.
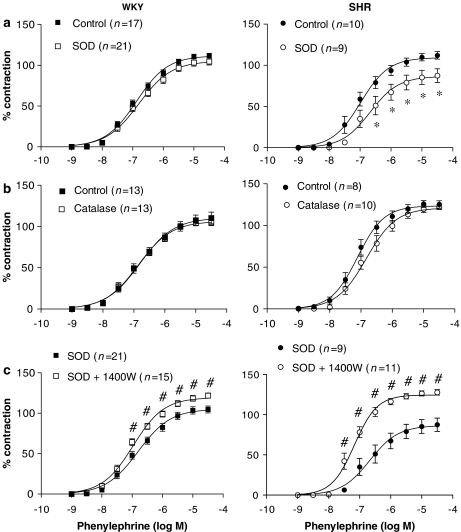
Concentration–response curve to phenylephrine in intact aortic segments from normotensive (WKY) and hypertensive (SHR). Results are expressed as a percentage of the response to 75 mM KCl for the number of animals indicated in parentheses. ANOVA (two-way): *P<0.05 vs control. WKY, Wistar Kyoto rat; SHR, spontaneously hypertensive rat.
Participation of iNOS-derived NO in vasoconstrictor responses
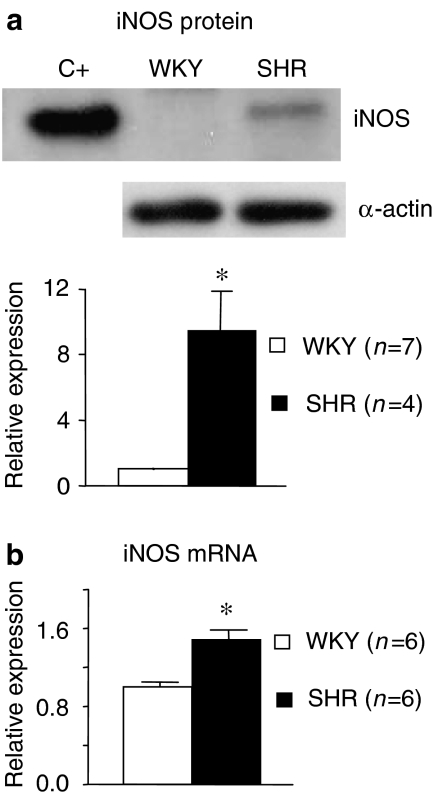
In basal conditions, neither iNOS protein nor mRNA expression could be detected in aortic segments from either WKY or SHR (results not shown). In aortic segments from both strains under similar conditions of reactivity experiments, iNOS protein and mRNA expressions were detected, and they were greater in arteries from hypertensive than from normotensive animals (Figure 2). iNOS protein expression was abolished in arteries from both strains when the endothelial layer was removed or when the arteries had been incubated with dexamethasone (1 μM) (results not shown).
Figure 2.
(a) Representative western blot and densitometric analysis for the inducible isoform of nitric oxide synthase (iNOS) in aortic segments from WKY and SHR; mouse macrophages were used for iNOS positive controls (C+). (b) Quantitative RT-PCR assessment of iNOS mRNA expression in the same segments. Results are expressed as the relative expression of protein or mRNA in SHR compared to WKY. ANOVA (one-way): *P<0.05. The number of animals are indicated in parentheses. WKY, Wistar Kyoto rat; SHR, spontaneously hypertensive rat.
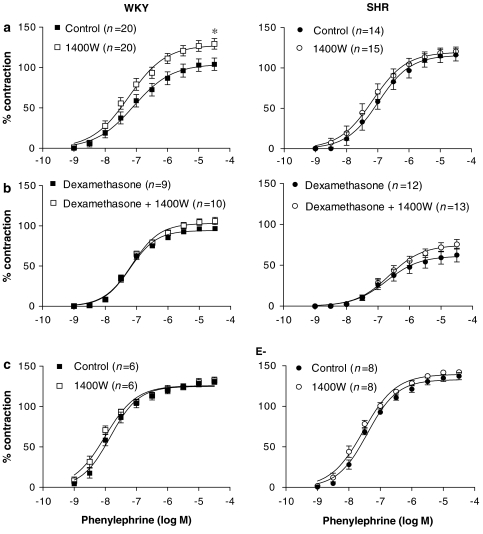
The selective iNOS inhibitor 1400W (10 μM) increased the vasoconstrictor response induced by phenylephrine in aorta from WKY, but did not modify it in aorta from SHR (Figure 3a). The effect of 1400W was abolished by co-incubation with dexamethasone (4 μM) (Figure 3b) as well as by endothelium removal (Figure 3c). 1400W did not affect 5-hydroxytryptamine responses in segments from both strains (results not shown).
Figure 3.
Effect of 1400W (10 μM) on the concentration–response curve to phenylephrine in: (a) intact aortic segments from WKY and SHR, (b) segments incubated with dexamethasone (1 μM) and (c) endothelium-denuded segments (E−). Results are expressed as a percentage of the response to 75 mM KCl for the number of animals indicated in parentheses. ANOVA (two-way): *P<0.05 vs control. WKY, Wistar Kyoto rat; SHR, spontaneously hypertensive rat.
Participation of reactive oxygen species on vasoconstrictor responses
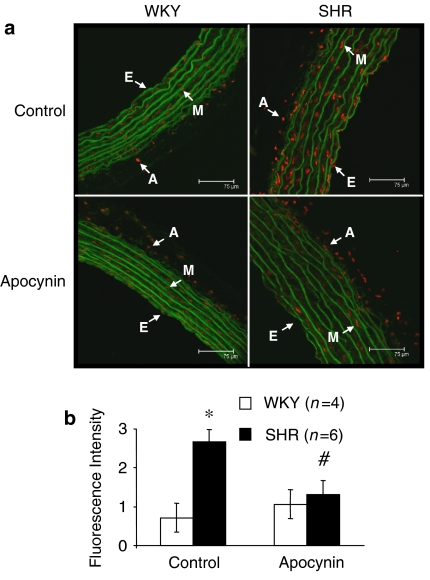
Production of O2•− was greater in aortic segments from hypertensive than normotensive animals (Figure 4). Preincubation of vessels with apocynin (0.3 mM) decreased O2•− production in segments from hypertensive animals (Figure 4), confirming the participation of NAD(P)H oxidase on O2•− production in this strain.
Figure 4.
(a) Representative fluorescent photomicrographs and (b) quantitative analysis of confocal microscopic sections of aortic segments from WKY and SHR incubated without (control) or with apocynin (0.3 mM). Vessels were labelled with the oxidative dye dihydroethidium, which produces a red fluorescence when oxidized to ethidium bromide by superoxide anion (O2•−). Natural autofluorescence of elastin was used to delimit the different layers of the vascular wall (appears green). Image size 375 × 375 μm. Images were captured with a fluorescence confocal microscope (× 40 oil immersion objective, zoom 1). E=endothelial layer; M=media layer; A=adventitial layer. *P<0.05 vs WKY, #P<0.05 vs control by Student's t-test. WKY, Wistar Kyoto rat; SHR, spontaneously hypertensive rat.
To study the contribution of O2•− in the response to phenylephrine, experiments were performed in the presence of SOD, which dismutates O2•− to hydrogen peroxide (H2O2). SOD (150 U ml−1) reduced the phenylephrine contraction only in aorta from SHR (Figure 5a). The participation of H2O2 in response to phenylephrine was analysed in experiments performed in the presence of catalase, which transforms two molecules of H2O2 to water and oxygen. Catalase (1000 U ml−1) did not modify the response to phenylephrine in either strain (Figure 5b). To exclude the possibility that the inhibitory effect observed with SOD could be due to the H2O2 produced by the added SOD, segments were preincubated with SOD (150 U ml−1) plus catalase (1000 U ml−1). Co-incubation of SOD with catalase did not modify the observed inhibitory effect of SOD in segments from hypertensive animals (data not shown).
Figure 5.
Effect of (a) superoxide dismutase (SOD; 150 U ml−1) and (b) catalase (1000 U ml−1) on the concentration–response curve to phenylephrine in aortic segments from SHR and WKY. (c) Effect of 1400W (10 μM) on the concentration–response curve to phenylephrine in aortic segments from WKY and SHR treated with SOD. Results are expressed as a percentage of the response to 75 mM KCl for the number of animals indicated in parentheses. ANOVA (two-way): *P<0.05 vs control; #P<0.05 vs SOD. SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
The decreased contractile response to phenylephrine in the presence of SOD observed in SHR could be to due to the increased bioavailability of iNOS-derived NO. To test this hypothesis, we analysed the effect of 1400W on phenylephrine responses in arteries incubated with SOD. In these conditions, the contractile response to phenylephrine was potentiated and this potentiation was greater in SHR than in WKY (Figure 5c).
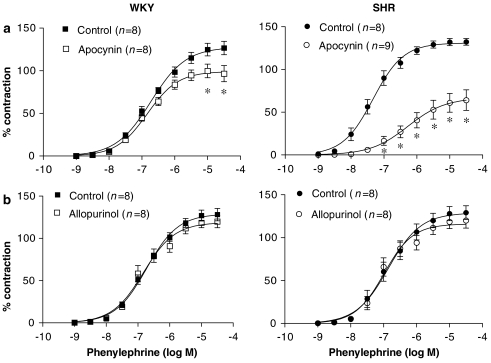
To study if NAD(P)H oxidase and/or xanthine oxidase were the sources for the O2•− involved in the phenylephrine responses, the arteries were preincubated with apocynin or allopurinol, respective inhibitors of these enzymes. Apocynin (0.3 mM), but not allopurinol (0.3 mM), inhibited the contractile response to phenylephrine in both strains, and this effect was greater in aortic segments from SHR than in WKY (Figure 6).
Figure 6.
Effect of (a) apocynin (0.3 mM) and (b) allopurinol (0.3 mM) on the concentration–response curve to phenylephrine in aortic segments from WKY and SHR. Results are expressed as a percentage of the response to 75 mM KCl for the number of animals indicated in parentheses. ANOVA (two-way): *P<0.05 vs control. WKY, Wistar Kyoto rat; SHR, spontaneously hypertensive rat.
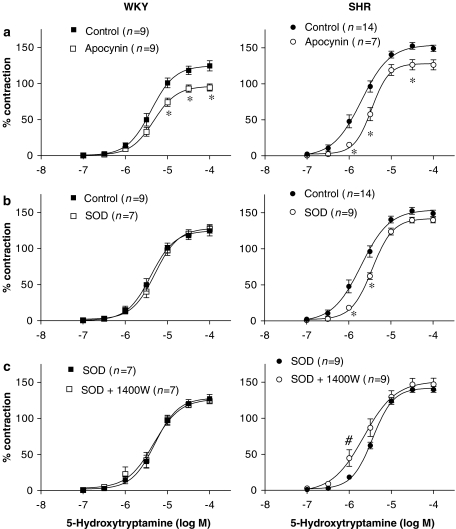
To assess if the greater participation of O2•− on vasoconstrictor responses observed in aorta from hypertensive rats was specific for phenylephrine or other agonists were similarly affected, we analysed the effect of SOD and apocynin on vasoconstrictor response to 5-hydroxytryptamine. Figure 7a shows that apocynin inhibited 5-hydroxytryptamine response in aorta from both strains. This inhibitory effect was greater in SHR than in WKY, as shown by the modification of both Emax (control: 153.8±4.9%; apocynin: 127.4±6.9%, P<0.05) and EC50 (control: 2.26±0.31; apocynin: 3.43±0.35 μM, P<0.05) observed in this strain; in contrast, in WKY, apocynin only diminished Emax (control: 123.8±6.9%; apocynin: 96.7±5.0%, P<0.05), without changes in EC50 (control: 4.23±0.54; apocynin: 5.20±0.77 μM, P<0.05). Moreover, SOD reduced 5-hydroxytryptamine response only in aorta from SHR (Figure 7b). The decrease of the contractile response to 5-hydroxytryptamine in the presence of SOD observed in SHR could be also due to the increased bioavailability of iNOS-derived NO because, in these conditions, the contractile response to 5-hydroxytryptamine was potentiated by 1400W only in this strain (Figure 7c).
Figure 7.
Effect of (a) apocynin (0.3 mM) and (b) superoxide dismutase (SOD; 150 U ml−1) on the concentration–response curve to 5-hydroxytryptamine in aortic segments from SHR and WKY. (c) Effect of 1400W (10 μM) on the concentration–response curve to 5-hydroxytryptamine in aortic segments from WKY and SHR treated with SOD. Results are expressed as a percentage of the response to 75 mM KCl for the number of animals indicated in parentheses. ANOVA (two-way): *P<0.05 vs control; #P<0.05 vs SOD. SHR, spontaneously hypertensive rat; WKY, Wistar Kyoto rat.
Discussion
Hypertension is associated with increased activity and/or expression of iNOS (Wu et al., 1996; Chou et al., 1998; Vaziri et al., 1998; Briones et al., 2000, 2002a) as well as increased production of O2•− (Kerr et al., 1999; Zalba et al., 2001) in different vascular beds; these changes might contribute to the alterations in vascular tone occurring in this pathology. The main results of the present study suggest that the increased production of O2•− derived from NAD(P)H oxidase, observed in aorta from hypertensive rats, counteracts the enhanced production of NO derived from iNOS, occurring in hypertension, and the modulation exerted by NO of vasoconstrictor responses.
Expression of iNOS protein and mRNA was detected in aortic segments after incubation in the organ bath but not under basal conditions, and these expressions were greater in segments from SHR than WKY, as already described (Wu et al., 1996; Chou et al., 1998; Vaziri et al., 1998; Briones et al., 2002a). The observed iNOS expression might be due, at least partially, to low levels of endotoxin in the incubation medium (Rees et al., 1990; Alonso et al., 1998). The fact that dexamethasone prevented this expression supports this possibility. However, we cannot discount the possibility that iNOS was present in freshly excised aorta but at levels too low to detect and that incubation in the organ bath merely increased iNOS levels. In any case, our results suggest that aorta from SHR might be more susceptible to upregulation of iNOS. Hypertension has been considered a chronic inflammatory disease with elevated vascular and plasma levels of proinflammatory cytokines (Savoia and Schiffrin, 2006; Vaziri and Rodríguez-Iturbe, 2006). It is then possible that the increased proinflammatory cytokines would increase the in vivo expression of inducible enzymes, such as iNOS. The physiological relevance of the increased iNOS expression is unknown. Conflicting results regarding the role of iNOS in the control of blood pressure are reported. Thus, iNOS does not seem to play a significant role in preventing DOCA-salt-induced hypertension (Sun et al., 2005). However, Hong et al. (2000) demonstrated that the iNOS inhibitor aminoguanidine decreased blood pressure in SHR. Furthermore, overexpression of iNOS in the rostral ventrolateral medulla increases urinary noradrenaline excretion and blood pressure via activation of the sympathetic nervous system, which is mediated by an increase in oxidative stress (Kimura et al., 2005).
The modulation induced by iNOS-derived NO of the phenylephrine contraction, but not of 5-hydroxytryptamine, in aorta from normotensive rats was demonstrated by the increased phenylephrine response observed in the presence of the selective iNOS inhibitor 1400W. In addition, this effect was abolished by dexamethasone, demonstrating that the effect of 1400W was selective for iNOS. Other investigators have also described modulation by iNOS-derived NO of vasoconstrictor responses to adrenergic agonists in different vascular beds after incubation with lipopolysaccharide (Briones et al., 2000; O'Brien et al., 2001). Surprisingly, 1400W had no effect on segments from SHR despite the greater iNOS expression we observed in this strain. iNOS is expressed in the three layers of the vascular wall (Zhang et al., 1999; Hernanz et al., 2003, 2004; Briones et al., 2005). The fact that endothelium removal abolished the effect of 1400W suggests that endothelial iNOS is responsible for the NO production that modulates the phenylephrine responses in aortic segments.
Reactive oxygen species, such as O2•− and H2O2, modulate vascular tone and their increase has been involved in the vascular alterations associated to hypertension. Both oxidative stress and hypertension seem to constitute a self-perpetuating cycle in which the initiating factor of this vicious cycle varies in different forms of hypertension (Vaziri and Rodríguez-Iturbe, 2006). O2•− induces vasoconstriction through different mechanisms; among them, it is well established that O2•− reacts with and inactivates NO, producing the peroxynitrite anion, ONOO−. Also, the addition of O2•−-generating systems to organ baths produces contraction in aorta and cerebral arteries (Shen et al., 2000; Hernanz et al., 2003), confirming that O2•− acts as a vasoconstrictor factor. Several authors have demonstrated the involvement of O2•− in vasoconstrictor responses to 5-HT (Srivastava et al., 2002) or to adrenoceptor agonists (Srivastava et al., 1998; Briones et al., 2002b). However, no direct modulation of contractile responses by O2•− has been reported by others (Girouard and de Champlain, 2004). The fact that SOD diminished the contractile response to phenylephrine and 5-hydroxytryptamine only in aortic segments from hypertensive rats indicates the involvement of O2•− in this strain and agrees with the increased O2•− production widely reported in hypertension (Kerr et al., 1999; Zalba et al., 2001; Paravicini and Touyz, 2006; Vaziri and Rodríguez-Iturbe, 2006; present results). In agreement, major modulation of contractile responses by O2•− in vessels from hypertensive subjects has been described (Püntmann et al., 2005). On the other hand, H2O2 has dual effects at vascular level, since it can produce both vasoconstriction and vasodilation (Ardanaz and Pagano, 2006). Nevertheless, its participation in the responses to vasoconstrictor agents is contradictory. Thus, Girouard and de Champlain (2004) and Thakali et al. (2005) have proposed that endogenous H2O2 is not involved in the contractions, respectively, induced by phenylephrine and endothelin-1. However, other studies have shown that catalase reduces the contractile response to noradrenaline (Srivastava et al., 1998). In our study, catalase affected neither phenylephrine responses nor the effect of SOD, thus excluding H2O2 involvement from this response.
As mentioned above, 1400W increased the contractile response to phenylephrine only in aorta from WKY, suggesting a greater modulation of contractile responses by iNOS-derived NO in segments from normotensive rats despite the smaller iNOS expression found in this strain. It is possible that the increased O2•− observed in SHR might react with iNOS-derived NO, thereby decreasing NO bioavailability, and thus explaining the lack of effect of 1400W in this strain. The fact that the combination of SOD plus 1400W increased the phenylephrine response more in SHR than WKY confirms this hypothesis and correlates with the higher iNOS expression observed in SHR vessels.
It is well established that NAD(P)H oxidase is the main source of O2•− at vascular level (Paravicini and Touyz, 2006; Vaziri and Rodríguez-Iturbe, 2006). Thus, the suggestion that NAD(P)H oxidase plays an important role in the control of the vascular tone is now evident (Souza et al., 2001). Apocynin, an inhibitor of NAD(P)H oxidase, diminished the vasoconstrictor responses to phenylephrine more in aorta from SHR than in WKY, whereas allopurinol, a xanthine oxidase inhibitor, did not modify the phenylephrine response in either strain. In agreement, Miyagawa et al. (2007) recently found that apocynin, but not allopurinol, reduced noradrenaline responses in rat femoral arteries from SHR but not in WKY. Moreover, apocynin reduced O2•− production in aorta from hypertensive rats. The vasoconstrictor responses induced by 5-hydroxytryptamine were also decreased by apocynin only in SHR, although the magnitude of inhibition was greater for the α-adrenoceptor agonist. This suggests different degree of modulation of the contractile responses by O2•− depending on the vasoconstrictor used, but confirms the greater role of O2•− in vessels from hypertensive rats. Taken together, these results point to NAD(P)H oxidase as responsible for the increased O2•− production in vessels from hypertensive animals. In this sense, increased activity and expression of NAD(P)H oxidase with hypertension has been reported (Zalba et al., 2001; Cruzado et al., 2005; Vaziri and Ni, 2005). NAD(P)H oxidase activation is also involved in the contraction induced by angiotensin II in radial arteries from hypertensive patients (Püntmann et al., 2005) as well as in the spontaneous tone observed in aorta from DOCA-salt hypertensive rats (Ghosh et al., 2004) and SHR (Lodi et al., 2006). However, we cannot exclude the possible involvement of other sources of O2•−. For instance, O2•− production in aorta from SHR was reduced by NG-nitro-L-arginine methyl ester, indicating O2•− generation from uncoupled endothelial NOS in hypertension (Li et al., 2006).
In conclusion, our results demonstrate that hypertension increases iNOS expression but decreases the bioavailability and the modulation elicited by iNOS-derived NO of contractile responses in aorta as a result of the increased O2•− production from NAD(P)H oxidase. Although similar results were not found in mesenteric resistance vessels (unpublished results) and the results obtained in aorta might not to be relevant to blood pressure regulation, the findings found here would contribute to the explanation of the altered vasoconstrictor responses observed in different vessels, associated with hypertension.
Acknowledgments
We are grateful to Dr Carmen Fernández-Criado for the care of the animals and Carol F Warren for linguistic assistance. This study was supported by grants from CYCIT (SAF 2006-02376), FISS (PI041917 and Red RECAVA, RD06/0014/0011) and Fundación Mutua Madrileña.
Abbreviations
- 1400W
N-(3-(aminomethyl)benzyl)acetamidine hydrochloride
- Emax
maximum response
- iNOS
inducible nitric oxide synthase
- NAD(P)H
nicotinamide adenine dinucleotide (phosphate)
- O2•−
superoxide anion
- SHR
spontaneously hypertensive rat
- SOD
superoxide dismutase
- WKY
Wistar Kyoto rat
Conflict of interest
The authors state no conflict of interest.
References
- Alonso MJ, Rodríguez-Martínez MA, Martínez-Orgado J, Marín J, Salaices M. The L-arginine inhibition of rat middle cerebral artery contractile responses is mediated by inducible nitric oxide synthase. J Auton Pharmacol. 1998;18:105–113. doi: 10.1046/j.1365-2680.1998.1820105.x. [DOI] [PubMed] [Google Scholar]
- Álvarez Y, Briones AM, Balfagón G, Alonso MJ, Salaices M. Hypertension increases the participation of vasoconstrictor prostanoids from cyclooxygenase-2 in phenylephrine responses. J Hypertens. 2005;23:767–777. doi: 10.1097/01.hjh.0000163145.12707.63. [DOI] [PubMed] [Google Scholar]
- Álvarez Y, Pérez-Girón JV, Hernanz R, Briones AM, García-Redondo A, Beltrán A, et al. Losartan reduces the increased participation of COX-2 derived products in vascular responses of hypertensive rats. J Pharmacol Exp Ther. 2007;321:381–388. doi: 10.1124/jpet.106.115287. [DOI] [PubMed] [Google Scholar]
- Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- Bouloumie A, Bauersachs J, Linz W, Scholkens BA, Wiemer G, Fleming I, et al. Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension. 1997;30:934–941. doi: 10.1161/01.hyp.30.4.934. [DOI] [PubMed] [Google Scholar]
- Briones AM, Alonso MJ, Hernanz R, Miguel M, Salaices M. Alterations of the nitric oxide pathway in cerebral arteries from spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2002a;39:378–388. doi: 10.1097/00005344-200203000-00009. [DOI] [PubMed] [Google Scholar]
- Briones AM, Alonso MJ, Hernanz R, Tovar S, Vila E, Salaices M. Hypertension alters the participation of contractile prostanoids and superoxide anions in lipopolysaccharide effects on small mesenteric arteries. Life Sci. 2002b;71:1997–2014. doi: 10.1016/s0024-3205(02)01967-7. [DOI] [PubMed] [Google Scholar]
- Briones AM, Alonso MJ, Marín J, Balfagón G, Salaices M. Influence of hypertension on nitric oxide synthase expression and vascular effects of lipopolysaccharide in rat mesenteric arteries. Br J Pharmacol. 2000;131:185–194. doi: 10.1038/sj.bjp.0703552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones AM, Salaices M, Vila E. Ageing alters the production of nitric oxide and prostanoids after IL-1β exposure in mesenteric resistance arteries. Mech Ageing Dev. 2005;126:710–721. doi: 10.1016/j.mad.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Chamseddine AH, Miller FJ., Jr Gp91phox contributes to NADPH oxidase activity in aortic fibroblasts but not smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H2284–H2289. doi: 10.1152/ajpheart.00459.2003. [DOI] [PubMed] [Google Scholar]
- Chou TC, Yen MH, Li CY, Ding YA. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension. 1998;31:643–648. doi: 10.1161/01.hyp.31.2.643. [DOI] [PubMed] [Google Scholar]
- Cruzado MC, Risler NR, Miatello RM, Yao G, Schiffrin EL, Touyz RM. Vascular smooth muscle cell NAD(P)H oxidase activity during the development of hypertension: effect of angiotensin II and role of insulin like growth factor-1 receptor transactivation. Am J Hypertens. 2005;18:81–87. doi: 10.1016/j.amjhyper.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Wang HD, McNeill JR. Role of oxidative stress and nitric oxide in regulation of spontaneous tone in aorta of DOCA-salt hypertensive rats. Br J Pharmacol. 2004;141:562–573. doi: 10.1038/sj.bjp.0705557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, de Champlain J. Inhibitory effect of melatonin on alpha1-adrenergic-induced vasoconstriction in mesenteric beds of spontaneously hypertensive rats. Am J Hypertens. 2004;17:339–346. doi: 10.1016/j.amjhyper.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Hernanz R, Alonso MJ, Briones AM, Vila E, Simonsen U, Salaices M. Mechanisms involved in the early increase of serotonin contraction evoked by endotoxin in rat middle cerebral arteries. Br J Pharmacol. 2003;140:671–680. doi: 10.1038/sj.bjp.0705501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernanz R, Alonso MJ, Zibrandtsen H, Álvarez Y, Salaices M, Simonsen U. Measurements of nitric oxide concentration and hyporeactivity in rat superior mesenteric artery exposed to endotoxin. Cardiovasc Res. 2004;62:202–211. doi: 10.1016/j.cardiores.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Hong HJ, Loh SH, Yen MH. Suppression of the development of hypertension by the inhibitor of inducible nitric oxide synthase. Br J Pharmacol. 2000;131:631–637. doi: 10.1038/sj.bjp.0703603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Altayó F, Briones AM, Giraldo J, Planas AM, Salaices M, Vila E. Increased superoxide anion production by interleukin-1β impairs nitric oxide-mediated relaxation in resistance arteries. J Pharmacol Exp Ther. 2006;316:42–52. doi: 10.1124/jpet.105.088435. [DOI] [PubMed] [Google Scholar]
- Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension. 1999;33:1353–1358. doi: 10.1161/01.hyp.33.6.1353. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Hirooka Y, Sagara Y, Ito K, Kishi T, Shimokawa H, et al. Overexpression of inducible nitric oxide synthase in rostral ventrolateral medulla causes hypertension and sympathoexcitation via an increase in oxidative stress. Circ Res. 2005;96:252–260. doi: 10.1161/01.RES.0000152965.75127.9d. [DOI] [PubMed] [Google Scholar]
- Lassegue B, Griendling KK. Reactive oxygen species in hypertension; an update. Am J Hypertens. 2004;17:852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Li H, Witte K, August M, Brausch I, Godtel-Armbrust U, Habermeier A, et al. Reversal of endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive rats. J Am Coll Cardiol. 2006;47:2536–2544. doi: 10.1016/j.jacc.2006.01.071. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lodi F, Cogolludo A, Duarte J, Moreno L, Coviello A, Peral De Bruno M, et al. Increased NADPH oxidase activity mediates spontaneous aortic tone in genetically hypertensive rats. Eur J Pharmacol. 2006;21:97–103. doi: 10.1016/j.ejphar.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Marín J. Mechanisms involved in the increased vascular resistance in hypertension. J Auton Pharmacol. 1993;13:127–176. doi: 10.1111/j.1474-8673.1993.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Marín J, Rodríguez-Martínez MA. Role of vascular nitric oxide in physiological and pathological conditions. Pharmacol Ther. 1997;75:111–134. doi: 10.1016/s0163-7258(97)00051-x. [DOI] [PubMed] [Google Scholar]
- Mattei P, Virdis A, Ghiadoni L, Taddei S, Salvetti A. Endothelial function in hypertension. J Nephrol. 1997;10:192–197. [PubMed] [Google Scholar]
- McIntyre M, Bohr DF, Dominiczak AF. Endothelial function in hypertension: the role of superoxide anion. Hypertension. 1999;34:539–545. doi: 10.1161/01.hyp.34.4.539. [DOI] [PubMed] [Google Scholar]
- Miyagawa K, Ohashi M, Yamashita S, Kojima M, Sato K, Ueda R, et al. Increased oxidative stress impairs endothelial modulation of contractions in arteries from spontaneously hypertensive rats. J Hypertens. 2007;25:415–421. doi: 10.1097/HJH.0b013e3280115b96. [DOI] [PubMed] [Google Scholar]
- Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol. 1994;266:H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- O'Brien AJ, Wilson AJ, Sibbald R, Singer M, Clapp LH. Temporal variation in endotoxin-induced vascular hyporeactivity in a rat mesenteric artery organ culture model. Br J Pharmacol. 2001;133:351–360. doi: 10.1038/sj.bjp.0704079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Püntmann VO, Hussain MB, Mayr M, Xu Q, Singer DR. Role of oxidative stress in angiotensin-II mediated contraction of human conduit arteries in patients with cardiovascular disease. Vasc Pharmacol. 2005;43:277–282. doi: 10.1016/j.vph.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Rees DD, Cellek S, Palmer RM, Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990;173:541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- Shen JZ, Zheng XF, Kwan CY. Differential contractile actions of reactive oxygen species on rat aorta: selective activation of ATP receptor by H2O2. Life Sci. 2000;14:PL291–PL296. doi: 10.1016/s0024-3205(00)00539-7. [DOI] [PubMed] [Google Scholar]
- Souza HP, Laurindo FR, Ziegelstein RC, Berlowitz CO, Zweier JL. Vascular NAD(P)H oxidase is distinct from the phagocytic enzyme and modulates vascular reactivity control. Am J Physiol Heart Circ Physiol. 2001;280:H658–H667. doi: 10.1152/ajpheart.2001.280.2.H658. [DOI] [PubMed] [Google Scholar]
- Srivastava P, Hegde LG, Patnaik GK, Dikshit M. Role of endothelial-derived reactive oxygen species and nitric oxide in norepinephrine-induced rat aortic ring contractions. Pharmacol Res. 1998;38:265–274. doi: 10.1006/phrs.1998.0357. [DOI] [PubMed] [Google Scholar]
- Srivastava P, Rajanikanth M, Raghavan SA, Dikshit M. Role of endogenous reactive oxygen derived species and cyclooxygenase mediators in 5-hydroxytryptamine-induced contractions in rat aorta: relationship to nitric oxide. Pharmacol Res. 2002;45:375–382. doi: 10.1006/phrs.2001.0859. [DOI] [PubMed] [Google Scholar]
- Sun Y, Carretero OA, Xu J, Rhaleb NE, Wang F, Lin C, et al. Lack of inducible NO synthase reduces oxidative stress and enhances cardiac response to isoproterenol in mice with deoxycorticosterone acetate-salt hypertension. Hypertension. 2005;46:1355–1361. doi: 10.1161/01.HYP.0000192651.06674.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakali K, Demel SL, Fink GD, Watts SW. Endothelin-1-induced contraction in veins is independent of hydrogen peroxide. Am J Physiol Heart Circ Physiol. 2005;289:H1115–H1122. doi: 10.1152/ajpheart.00086.2005. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance. Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Ni Z. Expression of NOX-I, gp91phox, p47phox and P67phox in the aorta segments above and below coarctation. Biochim Biophys Acta. 2005;25:321–327. doi: 10.1016/j.bbagen.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Ni Z, Oveisi F. Upregulation of renal and vascular nitric oxide synthase in young spontaneously hypertensive rats. Hypertension. 1998;31:1248–1254. doi: 10.1161/01.hyp.31.6.1248. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:582–593. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- Vo PA, Lad B, Tomlinson JA, Francis S, Ahluwalia A. Autoregulatory role of endothelium-derived nitric oxide (NO) on lipopolysaccharide-induced vascular inducible NO synthase expression and function. J Biol Chem. 2005;280:7236–7243. doi: 10.1074/jbc.M411317200. [DOI] [PubMed] [Google Scholar]
- Wu CC, Hong HJ, Chou TZ, Ding YA, Yen MH. Evidence for inducible nitric oxide synthase in spontaneously hypertensive rats. Biochem Biophys Res Commun. 1996;228:459–466. doi: 10.1006/bbrc.1996.1682. [DOI] [PubMed] [Google Scholar]
- Zalba G, Beaumont FJ, San José G, Fortuño A, Fortuño MA, Díez J. Is the balance between nitric oxide and superoxide altered in spontaneously hypertensive rats with endothelial dysfunction. Nephrol Dial Transplant. 2001;16:2–5. doi: 10.1093/ndt/16.suppl_1.2. [DOI] [PubMed] [Google Scholar]
- Zhang H, Du Y, Cohen RA, Chobanian A, Brecher P. Adventitia as a source of inducible nitric oxide synthase in the rat aorta. Am J Hypertens. 1999;12:467–475. doi: 10.1016/s0895-7061(98)00271-4. [DOI] [PubMed] [Google Scholar]