Abstract
Background and purpose:
Pulmonary veins are the most important focus for the generation of atrial fibrillation. Abnormal calcium homeostasis with ryanodine receptor dysfunction may underlie the arrhythmogenic activity in pulmonary veins. The preferential ryanodine receptor stabilizer (K201) possesses antiarrhythmic effects through calcium regulation. The purpose of this study was to investigate the effects of K201 on the arrhythmogenic activity and calcium regulation of pulmonary vein cardiomyocytes.
Experimental approach:
The ionic currents and intracellular calcium were studied in isolated single cardiomyocytes from rabbit pulmonary vein before and after the administration of K201, by the whole-cell patch clamp and indo-1 fluorimetric ratio techniques.
Key results:
K201 (0.1, 0.3, 1 μM) reduced the firing rates in pulmonary vein cardiomyocytes, decreased the amplitudes of the delayed afterdepolarizations and prolonged the action potential duration. K201 decreased the L-type calcium currents, Na+/Ca2+ exchanger currents, transient inward currents and calcium transients. K201 (1 μM, but not 0.1 μM or 0.3 μM) also reduced the sarcoplasmic reticulum calcium content. Moreover, both the pretreatment and administration of K201 (0.3 μM) decreased the isoprenaline (10 nM)-induced arrhythmogenesis in pulmonary veins.
Conclusions and implications:
K201 reduced the arrhythmogenic activity of pulmonary vein cardiomyocytes and attenuated the arrhythmogenicity induced by isoprenaline. These findings may reveal the anti-arrhythmic potential of K201.
Keywords: atrial fibrillation, calcium handling, pulmonary vein, ryanodine receptor, triggered activity
Introduction
Atrial fibrillation is the most common cardiac arrhythmia seen in clinical practice and induces cardiac dysfunction and strokes. Alterations in the intracellular calcium handling play an important role in the genesis of atrial fibrillation (Schotten et al., 2002; Hove-Madsen et al., 2004). Calcium release through the Ca2+-induced Ca2+ release by ryanodine receptors (RyRs) is essential for cardiac function. The RyR activity is regulated by channel-stabilizing proteins and a dysfunction of the RyRs would induce a calcium leak during diastole. Previous studies have shown that diastolic leaking due to RyR dysfunction generates triggered activity with the genesis of delayed afterdepolarizations (DADs) (Marx et al., 2000; Wehrens et al., 2003). Patients with atrial fibrillation have been found to have an increase in the RyR opening probability due to protein kinase A hyperphosphorylation (Vest et al., 2005). Moreover, a previous study reported the increased susceptibility to atrial fibrillation in FKBP12.6-deficient mice due to an enhanced diastolic sarcoplasmic reticulum (SR) calcium leak (Sood et al., 2006). Those findings indicated the importance of the RyRs in the pathophysiology of atrial fibrillation.
Pulmonary veins have been known to be important sources of ectopic beats with the initiation of paroxysmal atrial fibrillation and the foci of ectopic atrial tachycardia (Walsh et al., 1992; Haissaguerre et al., 1998; Chen et al., 1999). Other studies also have suggested that pulmonary veins play a role in the maintenance of atrial fibrillation (Pappone et al., 2000; Oral et al., 2006). Previous studies have indicated that an abnormal calcium regulation may contribute to the high arrhythmogenic activity of pulmonary vein cardiomyocytes (Chen et al., 2001, 2004; Honjo et al., 2003; Chou et al., 2005; Patterson et al., 2005; Wongcharoen et al., 2006). The T- and L-type calcium currents (ICa−L) and Na+–Ca2+ exchanger (NCX) currents have been demonstrated to regulate the electrical activity in the pulmonary veins (Chen et al., 2002, 2006; Patterson et al., 2005; Wongcharoen et al., 2006). Moreover, abnormal RyRs play a role in the arrhythmogenic activity of pulmonary veins through the enhancement of a calcium leak (Honjo et al., 2003). Pulmonary veins have a lower negative membrane potential and smaller inward rectifier potassium currents; therefore, a small calcium entry may easily induce pulmonary vein automaticity and triggered activity (Chen et al., 2001; Melnyk et al., 2005).
K201 (JTV519), a preferential RyR stabilizer, (4-[3-(4-benzylpiperidin-1-yl) propionyl]-7-methoxy-2,3,4,5,-tetrahydro-1,4-benzothiazepine monohydrochloride) has a cardioprotective effect against calcium overload (Kaneko, 1994). K201 prevents cardiac arrhythmias caused by RyR dysfunction-induced DADs (Yano et al., 2003; Wehrens et al., 2004) and may also reduce the micro-calcium transient in infarcted Purkinje cells (Boyden et al., 2004). Previous studies have shown that K201 may decrease experimental atrial fibrillation (Nakaya et al., 2000; Kumagai et al., 2003). In addition to its action as a RyR stabilizer, K201 was found to reduce the ICa−L and inward and delayed rectifier potassium currents (Kaneko, 1994; Kimura et al., 1999; Ito et al., 2000, Nakaya et al., 2000). All of these effects may have the potential for treating atrial fibrillation. Because pulmonary veins play a pivotal role in the genesis of atrial fibrillation and have a high arrhythmogenic activity due to abnormal calcium handling, K201 could reduce their arrhythmogenic activity though its regulation of calcium homeostasis. The purpose of the present study was to investigate the effects of K201 on the arrhythmogenic activity and calcium handling in the cardiomyocytes from pulmonary veins.
Methods
Isolation of pulmonary vein cardiomyocytes
Cardiomyocytes from rabbit pulmonary veins were enzymically dissociated by the previously described procedure (Chen et al., 2006). Cardiomyocytes with pacemaker activity were identified by the presence of constant spontaneous beating during perfusion with Tyrode's solution (B1; see Table 1 for composition of all solutions used in these experiments). The cells were allowed to stabilize in the bath for at least 30 min before the experiments.
Table 1.
Extracellular (bath) and intracellular (pipette) solutions used in this study
|
Pipette solutions, mM |
Bath solutions, mM |
|||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | B1 | B2 | B3 | |
| NaCl | 20 | 137 | 140 | |||
| CaCl2 | 1.75 | 1.8 | 1.8 | 2 | ||
| MgCl2 | 1 | 1 | 0.4 | 0.5 | 0.5 | 1 |
| KCl | 20 | 5.4 | ||||
| CsCl | 130 | 110 | 5.4 | |||
| TEA Cl | 20 | 137 | ||||
| Mg2ATP | 5 | 5 | 5 | |||
| LiGTP | 0.1 | |||||
| NaGTP | 0.1 | |||||
| K aspartate | 110 | |||||
| Na2 phosphocreatine | 5 | 5 | ||||
| EGTA | 0.5 | 10 | ||||
| BAPTA | 5 | |||||
| HEPES | 10 | 10 | 10 | 10 | 10 | 5 |
| Glucose | 5 | 10 | 10 | 10 | ||
| Niflumic acid | 0.1 | |||||
| Nitrendipine | 0.01 | |||||
| Strophanthidin | 0.01 | |||||
| pH | 7.2 | 7.2 | 7.25 | 7.4 | 7.4 | 7.4 |
| (KOH) | (CsOH) | (CsOH) | (NaOH) | (NaOH) | (NaOH) | |
TEA Cl indicates tetraethylammonium chloride.
Electrophysiological and pharmacological study
A whole-cell patch-clamp was performed on the cardiomyocytes before and after the administration of K201 using an Axopatch 1D amplifier (Axon Instruments, CA, USA) at 35±1 °C. K201 was a kind gift from the Aetas Pharm Co. (Tokyo, Japan). Borosilicate glass electrodes (OD, 1.8 mm) were used, with tip resistances of 3–5 MΩ. Before the formation of the membrane–pipette seal, the tip potentials were zeroed in solution B1. The junction potentials (9 mV) measured from the differences between the bath (B1) and pipette (P1) solutions were corrected for the action potential (AP) recordings. The APs were recorded in the current–clamp mode.
The ionic currents were measured in the voltage–clamp mode in the pulmonary vein cardiomyocytes with pacemaker activity (Chen et al., 2004). A small hyperpolarizing step from a holding potential of −50 mV to a testing potential of −55 mV for 80 ms was delivered at the beginning of each experiment. The area under the capacitive currents was divided by the applied voltage step to obtain the total cell capacitance. Normally, 60–80% series resistance (Rs) was electronically compensated. The AP measurements were started at 5 min after the cell rupture. The 50% (APD50) and 90% (APD90) of the AP duration (elicited by 1 Hz electrical stimulation) were measured in pulmonary vein cardiomyocytes without spontaneous activity. The DADs were measured during electrical stimulation (1 Hz) and defined as the presence of a spontaneous depolarization of the impulse after full repolarization had occurred. The APs and transient inward currents were recorded in the pipette solution P1. ICa−L and NCX currents were recorded in the pipette solutions P2 and P3, respectively. The AP, transient inward currents and NCX currents were measured by ruptured patch-clamp, and the ICa−L was measured by means of perforated patch-clamp with amphotericin B. Voltage command pulses were generated by a 12-bit digital-to-analog converter controlled by pCLAMP software (Axon Instruments). The recordings were low-pass filtered at half the sampling frequency. Data were sampled at rates varying from 2 to 25 kHz.
The ICa−L was measured as an inward current during depolarization from a holding potential of −50 mV to testing potentials ranging from −40 to +60 mV in 10-mV steps for 300 ms at a frequency of 0.1 Hz in the bath solution B2.
Transient inward currents were induced at clamped potentials from −40 to +40 mV for a duration of 3 s and then repolarized to −40 mV. The amplitude of the transient inward current was measured as the difference between the peak of the transient current and the mean of the current just before and after the transient current (Chen et al., 2001) in the bath solution B1.
The NCX current was elicited by depolarizing pulses between −100 and +100 mV from a holding potential of −40 mV for 300 ms at a frequency of 0.1 Hz. The amplitudes of the NCX current were measured as 10 mM nickel-sensitive currents in the bath solution B3.
Measurement of intracellular calcium
The intracellular calcium concentration ([Ca2+]i) was recorded using a fluorimetric ratio technique (indo-1 fluorescence) in cardiomyocytes with pacemaker activity. The fluorescent indicator indo-1 was loaded by incubating the myocytes at room temperature for 20–30 min with 25 μM of indo-1/AM (Sigma Chemical, St Louis, MO, USA). The cardiomyocytes were then perfused with the solution B1 at 35±1 °C for at least 20 min to wash out the extracellular indicator and to allow for the intracellular de-esterification of the indo-1. The background and cell autofluorescence were cancelled out by zeroing the output of the photomultiplier tubes using cells without indo-1 loading. The experiments were performed at 35±1°C.
An UV light of 360 nm with a monochromator was used for the excitation of the indo-1 from a xenon arc lamp controlled by the microfluorimetry system (OSP100-CA, Olympus, Tokyo, Japan) and the excitation light beam was directed into an inverted microscope (IX-70:Olympus). The emitted fluorescence signals from the indo-1/AM loaded myocytes were digitized at 200 Hz. The ratio of the fluorescence emission at 410 and 485 nm (R410/485) was used as the index of the [Ca2+]i. This approach avoided uncertainties from the calibration of the fluorescent Ca2+ indicators. The [Ca2+]i transient, peak systolic [Ca2+]i, diastolic [Ca2+]i and decay portion of the [Ca2+]i transient (τCa) were measured during a 2 Hz field stimulation with 10 ms twice-threshold strength square-wave pulses. The τCa was determined by a monoexpoential least-squares fit. The [Ca2+]i transient was calculated from the difference of the peak systolic [Ca2+]i and diastolic [Ca2+]i. The fluorescence ratio data were processed and stored in a computer using software (OSP-SFCA; Olympus). The SR Ca2+ content was estimated by adding 20 mM of caffeine after electrical stimulation at 2 Hz for at least 30 s. The total SR Ca2+ content was measured from the peak amplitude of the caffeine-induced [Ca2+]i transients.
Statistical analysis
Continuous variables are expressed as mean±s.e.mean. The differences before and after the administration of K201 were analysed by a paired t-test. Nominal variables were compared by a Chi2 analysis with Yates correction or Fisher's exact test. Values of P<0.05 were considered to be statistically significant.
Results
Effect of K201 on the electrical activity of pulmonary vein cardiomyocytes
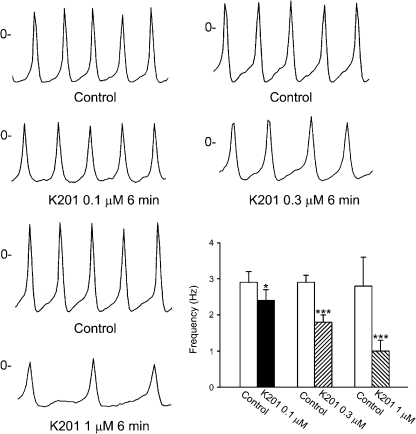
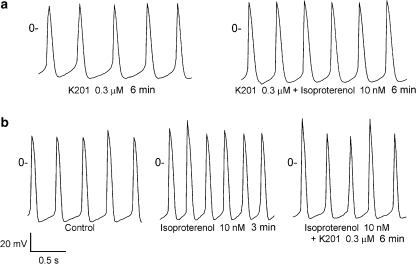
K201 had rapid effects on pulmonary vein electrical activity and reached a steady state in 3–5 min. Figure 1 shows examples of the effect of K201 on the spontaneous activity. In the cardiomyocytes with pacemaker activity, K201 (0.1, 0.3 and 1 μM) decreased the firing rates by 21±5, 35±6% and 66±9%, respectively (Figure 1). In addition, in the presence of K201 (0.3 μM, Figure 2a), isoprenaline (10 nM) increased the firing rates to a lesser extent (from 2.1±0.4 to 2.5±0.5 Hz, 21±4% acceleration, n=6) than those without the pretreatment with K201 (2.7±0.4–3.9±0.7 Hz, 51±12% acceleration, n=6, Figure 2b). After isoprenaline (10 nM) had increased the spontaneous activity (Figure 2b), addition of K201 (0.3 μM) decreased the firing rates by 17±5% (from 3.9±0.7 to 3.3±0.7 Hz, n=6, P<0.05, Figure 2b). This effect of K201 was less than it had been in the absence of isoprenaline.
Figure 1.
Tracings and the average data of the spontaneous activity of pulmonary vein cardiomyocytes before and after the administration of different concentrations of K201. *P<0.05, ***P<0.005 versus the control cardiomyocytes.
Figure 2.
Effect of K201 on the increased spontaneous activity of pulmonary vein cardiomyocytes induced by isoprenaline. (a) In the presence of K201 (0.3 μM), isoprenaline (10 nM) increased the pulmonary vein firing rates to a lesser extent than that in the absence of isoprenaline. (b) After isoprenaline induced arrhythmogenesis, K201 reduced the firing rates from 2.9 to 2.7 Hz.
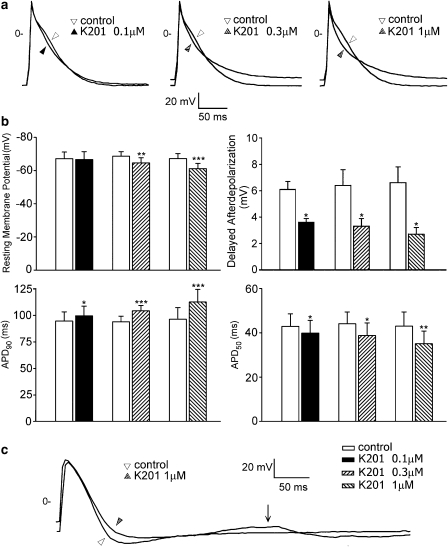
In the pulmonary vein cardiomyocytes without pacemaker activity, K201 (0.1, 0.3 and 1 μM) increased the APD90 by 8±2, 11±2 and 18±4%, respectively (Figure 3a) and shortened the APD50 by 7±2, 13±5 and 19±4%, respectively. K201 decreased the amplitudes of the APs at concentrations of 0.3 and 1 μM and positively shifted the resting membrane potential at all concentrations used. Figure 3b shows the mean values of the AP parameters before and after the administration of K201. Moreover, as shown in Figure 3c, in the cardiomyocytes with DADs, K201 reduced the amplitude of the DADs by 41±9% at 0.1 μM (n=6), by 48±8% at 0.3 μM (n=7) and by 59±7% at 1 μM (n=6).
Figure 3.
Action potentials of the pulmonary vein cardiomyocytes without spontaneous activity before and after the administration of different concentrations of K201. (a) Superimposed action potentials were elicited by electrical stimulation (1 Hz). (b) Average data of the action potential parameters (0.1 μM, n=8; 0.3 μM, n=9; 1 μM, n=7) and delayed afterdepolarizations (DADs), 0.1 μM, n=6; 0.3 μM, n=7; 1 μM, n=6) before and after the administration of K201. (c) An example of K201 (1 μM) reducing the amplitude of a DAD (arrow). *P<0.05, **P<0.01, ***P<0.005 versus the control cardiomyocytes.
Effect of K201 on ICa−L, NCX and transient inward currents of pulmonary vein cardiomyocytes
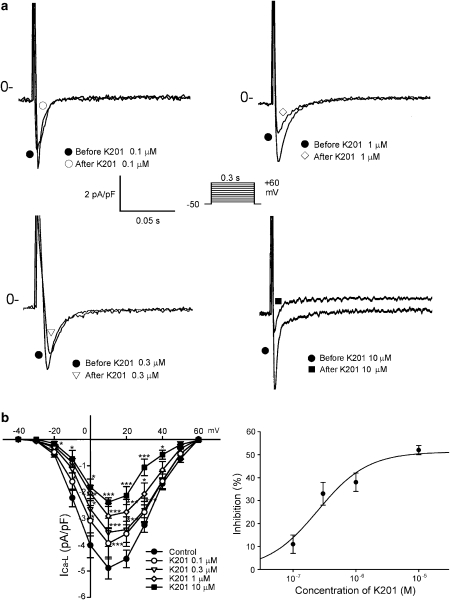
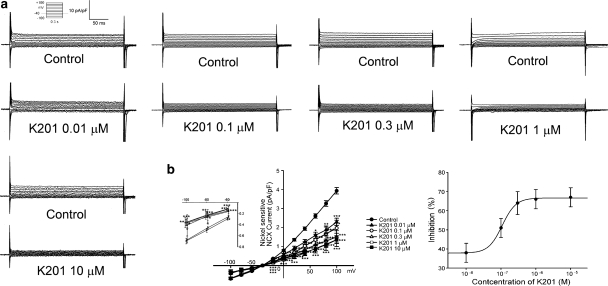
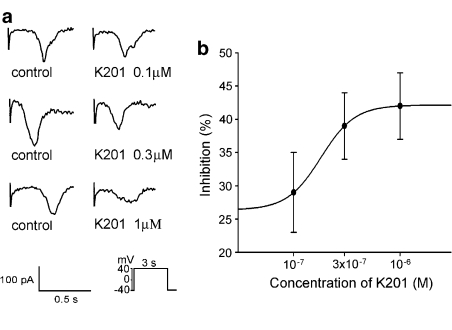
Figure 4 shows the tracings and I–V relationship of the different concentrations of K201 on ICa−L in the cardiomyocytes. K201 (0.1, 0.3, 1 and 10 μM) decreased the peak ICa−L by 11±4, 33±5, 38±4 and 52±2%, respectively. The IC50 was 7.2 μM with a Hill coefficient of 1.1. K201 (0.01, 0.1, 0.3, 1 and 10 μM) also decreased the peak reverse mode of the nickel-sensitive NCX currents by 38±5, 51±5, 64±6, 66±5 and 67±5%, respectively (Figure 5). The IC50 was 0.095 μM with a Hill coefficient of 2.2. In addition, K201 (0.01, 0.1, 0.3, 1 and 10 μM) decreased the peak forward mode of the nickel-sensitive NCX currents by 14±12, 32±9, 35±9, 43±10 and 45±11% respectively (Figure 5). Thus, K201 did not show significant concentration-dependent effects on NCX at 0.1, 0.3, 1 and 10 μM. Moreover, K201 (0.1, 0.3 and 1 μM) decreased the transient inward currents in the pulmonary vein cardiomyocytes by 29±6, 39±5 and 42±5%, respectively (Figure 6).
Figure 4.
Effects of K201 on the L-type calcium currents (ICa−L) in pulmonary vein cardiomyocytes. (a) and (b) the examples of current traces elicited from −50 to +10 mV and the I–V relationship of ICa−L before and after the administration of K201 (0.1 μM, n=10; 0.3 μM, n=8; 1 μM, n=9; 10 μM, n=7) in the cardiomyocytes. *P<0.05, ***P<0.005 versus the control cardiomyocytes. The inset in the current trace shows the clamp protocol. The concentration–inhibition relationship was fitted by the Hill equation, Inhibition %=A/(1+(IC50/[C])h), IC50 is the concentration of chemical [C] inducing a half-maximum response, and h is the Hill coefficient.
Figure 5.
Effects of K201 on Na+–Ca2+ exchanger (NCX) currents in pulmonary vein cardiomyocytes. (a) and (b) the examples of current traces of nickel-sensitive NCX before and after the administration of K201 (0.01 μM, n=7; 0.1 μM, n=9; 0.3 μM, n=7; 1 μM, n=12, 10 μM, n=5) to cardiomyocytes. Insets of current traces show the various clamp protocols. *P<0.05, **P<0.01, ***P<0.005 versus the control cells. Concentration–inhibition relationship was fitted by the Hill equation in Figure 4.
Figure 6.
Effects of K201 on the transient inward currents. (a) The tracings of the transient inward currents before and after the administration of different concentrations of K201 (0.1, 0.3 and 1 μM) in pulmonary vein cardiomyocytes. (b) The average of transient inward currents before and after K201 (0.1 μM, n=19; 0.3 μM, n=15; 1 μM, n=23). The inset in the current traces shows the clamp protocol. Concentration–inhibition relationship was fitted by the Hill equation in Figure 4 with a Hill coefficient of 2.7.
Effect of K201 on the intracellular calcium of the pulmonary vein cardiomyocytes
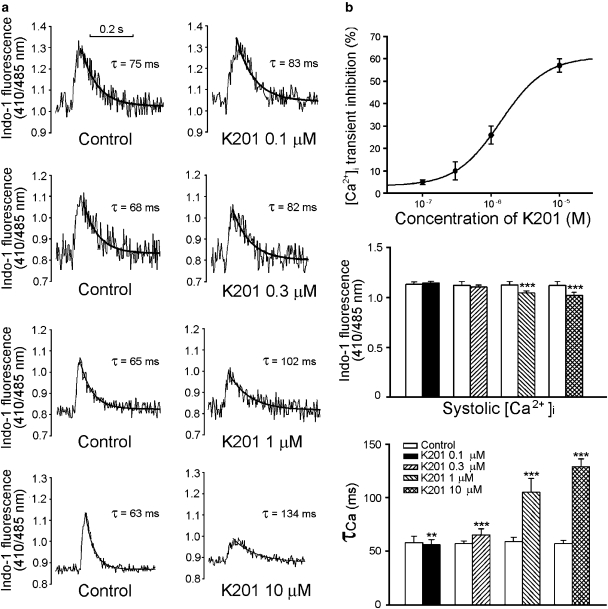
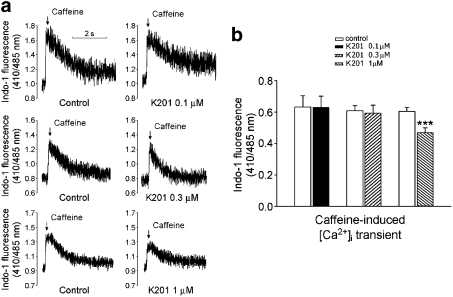
Figure 7a shows the recordings of ([Ca2+]i) before and after the administration of K201. K201 (0.1, 0.3, 1 and 10 μM) decreased the [Ca2+]i transient by 5±1, 10±4, 26±4 and 57±3%, respectively (Figure 7b). The IC50 was 4.1 μM with a Hill coefficient of 1.3. In addition, K201 (1 and 10 μM) significantly prolonged the decay time of the [Ca2+]i transient. Further, as shown in Figure 8a, K201 at a concentration of 1 μM (but not at 0.1 or 0.3 μM) decreased the SR Ca2+ content by 23±3% (Figure 8b).
Figure 7.
Effects of K201 on the intracellular calcium concentrations ([Ca2+]i) in pulmonary vein cardiomyocytes. (a) and (b) The tracings and average data of the [Ca2+]i transient before and after different concentrations of K201 (0.1 μM, n=18; 0.3 μM, n=14; 1 μM, n=15; 10 μM, n=9) to cardiomyocytes. **P<0.01, ***P<0.005 versus the control cells. Concentration–inhibition relationship was fitted by the Hill equation in Figure 4.
Figure 8.
Effects of K201 on the caffeine (20 mM)-induced Ca2+ transients. (a) and (b) The tracings and average data of the SR Ca2+ content (0.1 μM, n=13; 0.3 μM, n=12; 1 μM, n=14). ***P<0.005 versus the control cardiomyocytes.
Comparisons between K201 and verapamil
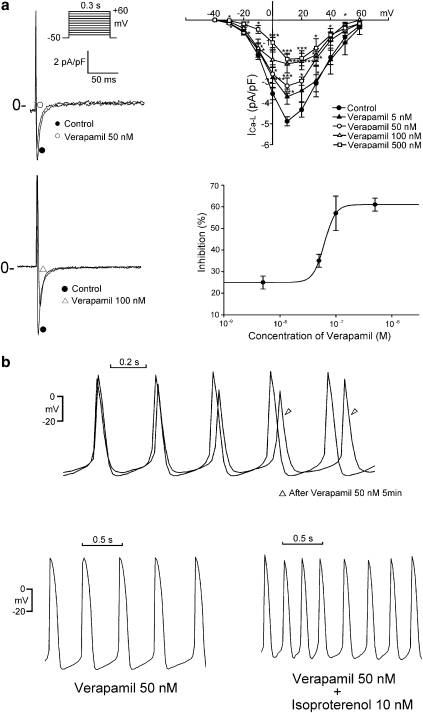
In order to evaluate the contribution of inhibition of the ICa−L induced by K201 to the reduction in the spontaneous activity of pulmonary vein cardiomyocytes, the ICa−L inhibitor, verapamil, was also applied to these cells. Verapamil dose-dependently (5, 50, 100 and 500 nM) reduced the peak ICa−L (Figure 9a). The IC50 was 0.075 μM with a Hill coefficient of 4.4. Verapamil (50 and 100 nM) reduced the pulmonary vein spontaneous activity by 19±6% (from 2.7±0.3 to 2.3±0.3 Hz, n=11, Figure 9b) and 52±8% (from 2.9±0.4 to 1.4±0.3 Hz, n=5), respectively. Although the effects of verapamil (50 nM) on the peak ICa−L were comparable to those of K201 (0.3 μM), reduction of spontaneous activity by verapamil (50 nM; 19±6%) was less than that induced by K201 (0.3 μM; 35±6%). Moreover, as shown in Figure 9b, in the presence of verapamil (50 nM), isoprenaline (10 nM) increased spontaneous activity of pulmonary vein cardiomyocytes by 42±9% (from 2.2±0.4 to 3.3±0.3 Hz, n=5), which was greater than the effect of isoprenaline in the presence of K201 (0.3 μM; (21±4%)).
Figure 9.
Effect of verapamil on ICa−L and spontaneous activity in pulmonary vein cardiomyocytes. (a) Examples of current traces elicited from −50 to +10 mV and the I–V relationship of ICa−L before and after the administration of verapamil (5 nM, n=8, 50 nM, n=6; 100 nM, n=6, 500 nM, n=8). Concentration–inhibition relationship was fitted by the Hill equation in Figure 4. (b) Example of verapamil (50 nM) decreasing the beating rate from 2.9 to 2.7 Hz in a cardiomyocyte. In the presence of verapamil (50 nM), isoprenaline (100 nM) increases the beating rate from 2.5 to 4 Hz in a pulmonary vein cardiomyocyte. *P<0.05, ***P<0.005 versus the control cells. The inset in the current trace shows the clamp protocol.
Discussion and conclusions
Effects of K201 on electrical activity of the pulmonary vein cardiomyocytes
Pulmonary veins have a high arrhythmogenic activity due to an abnormal calcium regulation with the genesis of triggered activity and transient inward currents (Chen et al., 2001, 2002, 2004, 2006; Honjo et al., 2003; Chou et al., 2005; Patterson et al., 2005; Wongcharoen et al., 2006). Previous studies have shown that a spontaneous intracellular calcium release may result in the triggered activity of pulmonary veins (Chou et al., 2005). The inhibition of arrhythmogenesis in pulmonary veins through the inhibition of NCX currents further suggests the importance of calcium regulation in this arrhythmogenesis (Chen et al., 2002, 2006; Patterson et al., 2005). A low concentration of ryanodine could induce arrhythmogenic activity in pulmonary veins but not in atrial cells, suggesting a potential role of RyRs in pulmonary vein arrhythmogenesis (Honjo et al., 2003). The RyR stabilizer, K201, reduced calcium overload-induced arrhythmias during ischaemia and reperfusion injuries (Ito et al., 2000). In this study, K201 was found to reduce the pulmonary vein arrhythmogenic activity through the suppression of pulmonary vein firing rates, DADs and transient inward currents. DADs are oscillations in the plasma membrane potentials after the completion of the AP and have been proposed to be caused by a diastolic Ca2+ release from the SR with the genesis of transient inward currents. Therefore, K201 may reduce DADs in the pulmonary vein cardiomyocytes through the stabilization of the RyRs allowing the reduction in the diastolic calcium leak. Moreover, the inhibitory effect of K201 on NCX may also contribute in part to the decrease in DADs and firing rates of these cardiomyocytes. Although K201 also reduces ICa−L, when compared to the known ICa−L blocker, verapamil, the greater inhibitory effects of K201 on the spontaneous activity pulmonary vein cardiomyocytes suggests that the K201 may reduce arrhythmogenesis through multiple ionic effects.
K201 has been demonstrated to regulate intracellular calcium in isolated failing SRs (Yano et al., 2003); nevertheless, knowledge about the effects of K201 on the calcium transient and SR calcium content in whole cells is still limited. Ca2+ entry via the NCX currents (reverse mode of the NCX currents) has been shown to contribute to intracellular calcium transients and the SR calcium content (Litwin et al., 1998). Therefore, the decrease in the [Ca2+]i transient by K201 shown in this study may arise from the effects of a reduction in ICa−L and NCX currents with stabilization of the RyR. K201 was also found to delay the decay of the [Ca2+]i transient. This effect could be caused by the inhibitory effects of K201 on NCX currents. Further, we found that K201 at concentrations of 0.1 and 0.3 μM did not change the SR Ca2+ content, but did decrease the SR Ca2+ content at a concentration of 1 μM. The decrease in the calcium content caused by a higher concentration of K201 may be due to the effects of K201 on ICa−L and NCX currents. In addition, it is possible that K201 at high concentrations may block the RyR channels and thus reduce the caffeine-induced calcium release (Hunt et al., 2007). The SR Ca2+ content may determine the spontaneous diastolic Ca2+ release and influence the firing rate of the pacemaker cells (Maltsev et al., 2004). As the Ca2+ content of the SR becomes reduced, there would no longer be a diastolic release of Ca2+, which would result in a decrease in DADs and further contribute to the decrease in the arrhythmogenesis in pulmonary vein cardiomyocytes. Moreover, blocking of potassium channels by K201 also has to be taken into account, since the firing rates of cardiomyocytes are probably related to repolarization force (Irisawa et al., 1993). However, the major targets of K201 and to what extent those mechanisms play a role in the antiarrhythmic effects of K201 are not clear. The non-specific actions of K201 on several ion currents may result in a greater antiarrhythmic potential. Moreover, the wide range of Hill coefficients and percentage block of different processes suggest that in some instances the K201 does not act directly on the target being assayed.
It has been shown that K201 increases the AP duration in ventricular muscle (Kumagai et al., 2003; Boyden et al., 2004). Our results showed that K201 prolonged the APD90 at quite low concentrations in pulmonary vein cardiomyocytes, which may arise from its inhibitory effects on potassium channels (Nakaya et al., 2000). The effect of K201 on the prolongation of the AP duration may prevent the genesis of micro re-entry in these cardiomyocytes, which has been proposed to be one of the mechanisms of arrhythmogenicity in this tissue (Chou et al., 2005). All of these findings may contribute to the observed antiarrhythmic effects of K201 on pericarditis-induced atrial fibrillation or experimental atrial fibrillation in guinea-pig hearts (Nakaya et al., 2000; Kumagai et al., 2003). K201 has been shown to decrease inward rectifier potassium currents (Nakaya et al., 2000). Since inward rectifier potassium currents play a pivotal role in determining the resting membrane potential, the drop in the resting membrane potential by high concentrations of K201 may be caused by its inhibitory effects on the inward rectifier potassium currents. The decrease in the resting membrane potentials caused by K201 may reduce the depolarizing threshold; thus calcium entry may easily trigger pulmonary vein arrhythmogenesis. Nevertheless, this arrhythmogenic effect may be prevented by the decrease in the calcium transients caused by K201.
Role of K201 on isoprenaline-induced arrhythmogenicity
Isoprenaline plays an important role in the pathophysiology of atrial fibrillation and also increases the arrhythmogenic activity in pulmonary veins with an enhancement of their firing rates and triggered activity (Chen et al., 2001). K201 has been demonstrated to reduce the calcium leak due to hyperphosphorylation of the RyR, induced by β-adrenoceptor agonists. In this study, we demonstrated that pretreatment with K201 reduced the arrhythmogenic activity of isoprenaline in pulmonary vein cardiomyocytes. In addition, the administration of K201 decreased isoprenaline-accelerated firing rates. These findings suggest that K201 not only prevents, but also reduces the arrhythmogenic effects of isoprenaline and further demonstrates the antiarrhythmic potential of K201 in treating atrial fibrillation. The beneficial effects of K201 may be caused by reducing the calcium leak from the hyperphosphorylation of RyR (see above) or a reduction in the intracellular Ca2+ concentration. Moreover, the antiarrhythmic potential of K201 was attenuated in the presence of isoprenaline, a result compatible with the earlier findings that activation of β1-adrenoceptors reduces the sensitivity of cardiomyocytes to calcium channel blockers (Legssyer et al., 1997).
In conclusion, K201 was shown to regulate calcium homeostasis, to decrease electrical activity in pulmonary vein cardiomyocytes and to attenuate the isoprenaline-induced arrhythmogenesis in these cells. These findings underline the antiarrhythmic potential of K201.
Acknowledgments
The present work was supported by the Topnotch Stroke Research Center Grant, Ministry of Education and Grants NSC 94-2314-B-075-093, NSC 94-2314-B-010-056, NSC 94-2314-B-010-053, NSC 95-2314-B-016-015, NSC 95-2314-B-038-026, VGH 94-204, VGH 94-005, VGH 94-206, VGH 94-009, SKH-TMU-95-04 from Shin Kong Wu Ho-Su Memorial Hospital.
Abbreviations
- AP
action potential
- APD50
50% of the AP duration
- APD90
90% of the AP duration
- [Ca2+]i
intracellular calcium
- DAD
delayed afterdepolarization
- ICa−L
L-type calcium current
- NCX
Na+-Ca2+ exchanger
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
Conflict of interest
The authors state no conflict of interest.
References
- Boyden PA, Dun W, Barbhaiya C, Ter Keurs HE. 2APB- and JTV519 (K201)-sensitive micro Ca2+ waves in arrhythmogenic Purkinje cells that survive in infarcted canine heart. Heart Rhythm. 2004;1:218–226. doi: 10.1016/j.hrthm.2004.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–1886. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- Chen YC, Chen SA, Chen YJ, Tai CT, Chan P, Lin CI. T-type calcium current in electrical activity of cardiomyocytes isolated from rabbit pulmonary vein. J Cardiovasc Electrophysiol. 2004;15:567–571. doi: 10.1046/j.1540-8167.2004.03399.x. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Chen SA, Chen YC, Yeh HI, Chan P, Chang MS, et al. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: implication in initiation of atrial fibrillation. Circulation. 2001;104:2849–2854. doi: 10.1161/hc4801.099736. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Chen SA, Chen YC, Yeh HI, hang MS, Lin CI. Electrophysiology of single cardiomyocytes isolated from rabbit pulmonary veins: implication in initiation of focal atrial fibrillation. Basic Res Cardiol. 2002;97:26–34. doi: 10.1007/s395-002-8384-6. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Chen YC, Tai CT, Yeh HI, Lin CI, Chen SA. Angiotensin II and angiotensin II receptor blocker modulate the arrhythmogenic activity of pulmonary vein. Br J Pharmacol. 2006;147:12–22. doi: 10.1038/sj.bjp.0706445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CC, Nihei M, Zhou S, Tan A, Kawase A, Macias ES, et al. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–2897. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- Honjo H, Boyett MR, Niwa R, Inada S, Yamamoto M, Mitsui K, et al. Pacing-induced spontaneous activity in myocardial sleeves of pulmonary veins after treatment with ryanodine. Circulation. 2003;107:1937–1943. doi: 10.1161/01.CIR.0000062645.38670.BD. [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez Font E, Aris A, et al. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, Chen K, et al. K201 (JTV519) suppresses spontaneous Ca2+ release and [3H] ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem J. 2007;404:431–438. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Ito K, Shigematsu S, Sato T, Abe T, Li Y, Arita M. JTV-519, a novel cardioprotective agent, improves the contractile recovery after ischaemia–reperfusion in coronary perfused guinea-pig ventricular muscles. Br J Pharmacol. 2000;130:767–776. doi: 10.1038/sj.bjp.0703373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Dev Res. 1994;33:429–438. [Google Scholar]
- Kimura J, Kawahara M, Sakai E, Yatabe J, Nakanishi H. Effects of a novel cardioprotective drug, JTV-519, on membrane currents of guinea pig ventricular myocytes. Jpn J Pharmacol. 1999;79:275–281. doi: 10.1254/jjp.79.275. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Nakashima H, Gondo N, Saku K. Antiarrhythmic effects of JTV-519, a novel cardioprotective drug, on atrial fibrillation/flutter in a canine sterile pericarditis model. J Cardiovasc Electrophysiol. 2003;14:880–884. doi: 10.1046/j.1540-8167.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- Legssyer AK, Hove-Madsen L, Hoerter J, Fischmeister R. Sympathetic modulation of the effect of nifedipine on myocardial contraction and Ca current in the rat. J Mol Cell Cardiol. 1997;29:579–591. doi: 10.1006/jmcc.1996.0301. [DOI] [PubMed] [Google Scholar]
- Litwin SE, Li J, Bridge JH. Na–Ca exchange and the trigger for sarcoplasmic reticulum Ca release: studies in adult rabbit ventricular myocytes. Biophys J. 1998;75:359–371. doi: 10.1016/S0006-3495(98)77520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Vinogradova TM, Bogdanov KY, Lakatta EG, Stern MD. Diastolic calcium release controls the beating rate of rabbit sinoatrial node cells: numerical modeling of the coupling process. Biophys J. 2004;86:2596–2605. doi: 10.1016/S0006-3495(04)74314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Melnyk P, Ehrlich JR, Pourrier M, Villeneuve L, Cha TJ, Nattel S. Comparison of ion channel distribution and expression in cardiomyocytes of canine pulmonary veins versus left atrium. Cardiovasc Res. 2005;65:104–116. doi: 10.1016/j.cardiores.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Nakaya H, Furusawa Y, Ogura T, Tamagawa M, Uemura H. Inhibitory effects of JTV-519, a novel cardioprotective drug, on potassium currents and experimental atrial fibrillation in guinea-pig hearts. Br J Pharmacol. 2000;131:1363–1372. doi: 10.1038/sj.bjp.0703713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, Jr, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Eng J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Schotten U, Greiser M, Benke D, Buerkel K, Ehrenteidt B, Stellbrink C, et al. Atrial fibrillation-induced atrial contractile dysfunction: a tachycardiomyopathy of a different sort. Cardiovasc Res. 2002;53:192–201. doi: 10.1016/s0008-6363(01)00453-9. [DOI] [PubMed] [Google Scholar]
- Sood S, Desai P, Wehrens XH.Increased susceptibility to atrial fibrillation in FKBP12.6 deficient mice Circulation 2006114II–199.(abstract) [Google Scholar]
- Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- Walsh EP, Saul JP, Hulse JE, Rhodes LA, Hordof AJ, Mayer JE, et al. Transcatheter ablation of ectopic atrial tachycardia in young patients using radiofrequency current. Circulation. 1992;86:1138–1146. doi: 10.1161/01.cir.86.4.1138. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- Wongcharoen W, Chen YC, Chen YJ, Chang CM, Yeh HI, Lin CI, et al. Effects of a Na+/Ca2+ exchanger inhibitor on pulmonary vein electrical activity and ouabain-induced arrhythmogenicity. Cardiovasc Res. 2006;70:497–508. doi: 10.1016/j.cardiores.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]