Abstract
Background and purpose:
Intact endothelium plays a pivotal role in post-ischaemic angiogenesis. It is a phenomenon finely tuned by activation and inhibition of several endothelial receptors. The presence of α1-adrenoceptors on the endothelium suggests that these receptors may participate in regenerative phenomena by regulating the responses of endothelial cells involved in neo-angiogenesis.
Experimental approach:
We evaluated the expression of the subtypes of the α1-adrenoceptor in isolated endothelial cells harvested from Wistar-Kyoto (WKY) rats. We explored the possibility these α1-adrenoceptors may influence the pro-angiogenic phenotype of endothelial cells in vitro. In vivo, we used a model of hindlimb ischaemia in WKY rats, to assess the effects of α1 adrenoceptor agonist or antagonist on angiogenesis in the ischaemic hindlimb by laser Doppler blood flow measurements, digital angiographies, hindlimb perfusion with dyed beads and histological evaluation.
Key results:
In vitro, pharmacological antagonism of α1-adrenoceptors in endothelial cells from WKY rats by doxazosin enhanced, while stimulation of these adrenoceptors with phenylephrine, inhibited endothelial cell proliferation and DNA synthesis, ERK and retinoblastoma protein (Rb) phosphorylation, cell migration and tubule formation. In vivo, we found increased α1-adrenoceptor density in the ischaemic hindlimb, compared to non-ischaemic hindlimb, suggesting an enhanced α1-adrenoceptor tone in the ischaemic tissue. Treatment with doxazosin (0.06 mg kg−1 day−1 for 14 days) did not alter systemic blood pressure but enhanced neo-angiogenesis in the ischaemic hindlimb, as measured by all our assays.
Conclusions:
Our findings support the hypothesis that the α1-adrenoceptors in endothelial cells provide a negative regulation of angiogenesis.
Keywords: endothelium, receptors, vascular biology, pharmacology, angiogenesis
Introduction
Angiogenesis is considered an important feature of a viable endothelium. Its mechanism entails specific, composite and coordinated sequences (Papetti and Herman, 2002) of several cellular and molecular processes, intimately regulated by the endothelial cells (Carmeliet, 2000). Proliferation, cell migration and tubule formation by endothelial cells represent the first steps in angiogenesis, leading to the sprouting of immature sinusoidals around which a more complex capillary will develop (Kanda et al., 2004). The connection between angiogenesis and endothelial cells is so close that angiogenesis is now considered to be an aspect of endothelial function and several models of endothelial dysfunction show impaired angiogenesis (le Noble et al., 1998; Martin et al., 2003). Although cytokines such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor are considered the most important mediators of neo-angiogenesis, a growing body of molecular partners have been shown to regulate this phenomenon at different levels.
The sympathetic nervous system is a central mechanism in the control of vascular biology. Catecholamines activate α1-adrenoceptors localized on vascular smooth muscle cells, thus increasing peripheral vascular tone and vascular resistance (Guarino et al., 1996; Guimaraes and Moura, 2001; Liggett, 2006). The presence of α1-adrenoceptors on endothelial cells has been long postulated, based on physiological vasodilatation responses (Tuttle and Falcone, 2001; McKee et al., 2003). So far, there are no detailed investigations into the specific biological actions of α1-adrenoceptors on neo-angiogenesis, although α1-adrenoceptor blockade may enhance neo-angiogenesis (Fulgenzi et al., 1998). Nevertheless, the underlying mechanisms have not been extensively investigated in vitro and mostly ascribed to increased circumferential wall stress levels (Franke et al., 1984). In fact, antagonism of α1-adrenoceptors has been considered to be analogous to the action of vasodilators, such as dipyridamole (Picano and Michelassi, 1997), adenosine (Dusseau et al., 1986) or prostaglandins (Koller et al., 1995). Some authors have used high doses of α1-adrenoceptor antagonists to induce neo-angiogenesis through a massive vasodilatation, even in the absence of natural stimulants of neo-angiogenesis such as exercise or chronic ischaemia (Dawson and Hudlicka, 1989; Hudlicka, 1998; Zhou et al., 1998). These studies, though, all lack an exploration of the role of α1-adrenoceptors on the pro-angiogenic phenotype of the endothelium, in a context where there is no haemodynamic perturbation.
Our investigation starts from the hypothesis that α1-adrenoceptors negatively regulate the pro-angiogenic phenotype of endothelial cells and therefore inhibit neo-angiogenesis. We looked for expression of α1-adrenoceptor subtypes in endothelial cells and then we evaluated the in vitro effects of α1-adrenoceptor blockade and stimulation with doxazosin and phenylephrine, respectively, on relevant signalling and biological responses in endothelial cells (Zou et al., 2006). Moreover, we performed in vivo experiments using low doses of doxazosin, without effect on systemic blood pressure, to confirm that chronic α1-adrenoceptor blockade enhances ischaemia-induced neo-angiogenesis, independently of vasodilatation.
Materials and methods
In vitro studies
Aortic endothelial cells harvested from Wistar-Kyoto (WKY) rats were cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, Milano, Italy) as previously described (Lembo et al., 1997) and validated (Iaccarino et al., 2002, 2004). All experiments were performed in triplicate with cells between passages 5 and 8.
In vitro hypoxia
Hypoxia was induced overnight in a medium saturated at 1 atm with 95% N2 and 5% CO2, as previously described (Morisco et al., 2007) and containing (mM) concentrations of 116 NaCl, 5.4 KCl, 0.8 MgSO4, 26.2 NaHCO3, 1 NaH2PO4, 1.8 CaCl2 and 0.01 glycine, and 0.001 (% w/v) phenol red using a hypoxia chamber (temperature: 37 °C; atmosphere: 5% CO2 and 95% N2). The pH, PO2 and PCO2 of the medium was 7.36±0.2, 45.3±1.2, 35.3±0.8 mm Hg, and 7.32±0.9, 32.6±1.1 and 37.9±2.1 mm Hg, before and at the end of hypoxia, respectively.
Reverse transcriptase-PCR
Total RNA was extracted and isolated from endothelial cells or rat hearts by use of TRIzol reagent kit (Invitrogen, San Giuiliano Milanese, Milano, Italy). RNA was then reverse transcribed into cDNA using Moloney murine leukemia virus Reverse Transcriptase (Stratagene) by standard methods (Iaccarino et al., 1998); cDNA samples were then used as templates for the PCR amplification using the pairs of specific primers reported in Table 1. Glyceraldehyde-3-phosphate dehydrogenase expression was used as loading and integrity control. PCR amplification was performed as previously described (Iaccarino et al., 2004; Lanni et al., 2007).
Table 1.
Primers used for amplification of rat α1-adrenoceptor subtypes
| Subtype | Forward | Reverse | Expected band size (bp) |
|---|---|---|---|
| α1A | 5′-GTGAACATTTCCAAGGCCAT | 5′-GGTCGATGGAGATGATGCAG | ∼300 |
| α1B | 5′-ACTTCACTGGCCCCAACCAG | 5′-TACTGCAGAGAGTAGCGCAC | ∼388 |
| α1D | 5′-ACCTGCAGACCGTCACCAACTA | 5′-GGTGCAGAGGCTGAGGA | ∼190 |
Cell proliferation assay
Endothelial cells were seeded at a density of 10 000 per well in six-well plates, serum starved, pre-incubated overnight with doxazosin or phenylephrine (10−8–10−6 M) and then stimulated with 5% fetal bovine serum (FBS) (Iaccarino et al., 1999). Cell number was measured at 24 h after stimulation as previously described (Iaccarino et al., 2005).
DNA synthesis
Endothelial cells were serum-starved for 24 h and then incubated in DMEM with [3H]thymidine and 5% FBS. After 24 h, [3H]thymidine incorporation was assessed as previously described (Iaccarino et al., 1999).
Migration assay
Cellular migration was measured using a wounding assay (Galasso et al., 2006). A grid pattern was drawn on the underside of six-well plates before endothelial cells were plated on them to serve as landmarks for the start of the migration period. Endothelial cells were grown to confluency and allowed to remain so for a further 24 h. Cultures were then starved for 12 h with DMEM without FBS. A cell scraper was used to wipe away the cell monolayer on one side of the start line that had been drawn on the bottom of the plate. The cells were challenged with FBS (5%) with or without doxazosin (10−7 M) or phenylephrine (107 M). Images were captured with a fluorescence digital microscope (Zeiss) at × 10 magnification 12 h after incubation with the assistance of the landmarks drawn on the underside of the plate. Several fields of view were captured per well, and experiments were repeated three times. Migration was quantified by measuring the number of the cell migrated into the scraped area (Rocnik et al., 2006).
Matrigel assay
The formation of network-like structures by endothelial cells on Matrigel (BD Biosciences, Buccinasco, Milano, Italy) was performed as previously described (Galasso et al., 2006). The 12-well multidishes were coated with growth factor-reduced Matrigel (10 mg ml−1; Becton Dickinson, Bedford, MA, USA) according to the manufacturer's instructions. Endothelial cells (4 × 104) were plated and incubated at 37 °C for 24 h in 500 μl of DMEM medium. Tubule formation was defined as a structure exhibiting a length four times its width. Network formation was observed using an inverted phase-contrast microscope (Zeiss). Representative fields were taken, and the average of the total number of complete tubes formed by cells was counted in 15 random fields by three independent investigators (GG, GS and MC).
In vivo study design
All animal procedures were in accordance with University guidelines for research in animals. We studied two groups of healthy WKY rats: doxazosin treated (pumps filled with doxazosin, dissolved in 0.002% ascorbic acid, 0.06 mg per kg per day; n=14); sham treated (vehicle only: 0.002% ascorbic acid, used as control; n=7). See the experimental protocol depicted in Figure 1.
Figure 1.
Scheme of the in vivo experimental protocol.
Experimental animals and surgical procedures
Experiments were carried out with 12-week-old normotensive WKY male rats (n=21), weighing 240–310 g, which had access to food and water ad libitum. Animals were allowed to acclimatize for 3–4 days prior to the start of treatments. The model of unilateral hindlimb ischaemia was prepared as described previously (Takeshita et al., 1994; Lee et al., 2003). Briefly, anaesthesia was performed with an intramuscular injection of a mixture of tiletamine (50 mg per kg) and zolazepam (50 mg per kg); the right common femoral artery was exposed, isolated and permanently ligated using two non-reabsorbable sutures (5-0 silk; Ethicon); then, it was excised between the two sutures, after the emergence from the inguinal ligament. Afterwards, a small pocket was created by spreading apart connective tissue as far as the peritoneum; in this pouch, we implanted a mini-osmotic pump (Alzet Model 2002), filled to deliver over a period of 14 days. Finally, the wound was closed in layers.
Laser Doppler perfusion analysis
We measured hindlimb blood flow by means of laser Doppler (laser Doppler blood flow; Perimed Italy, Cuggiono, Milano, Italy) at six time points: before and after surgery (data not shown) and on postoperative days 3, 7, 10 and 14 (Galasso et al., 2006). Excess hair was removed by commercial depilatory cream from the lower limbs, and rats were put on a heating pad at 37 °C to minimize temperature variations. However, to account for other variables such as ambient light and temperature, calculated perfusion was expressed as a ratio of ischaemic to non-ischaemic hindlimb. For each time point described, we performed three consecutive measurements over the same region of interest. Variability between measurements was 3±1%. Finally, the average perfusions of the ischaemic and non-ischaemic limb were calculated on the basis of coloured histogram pixels (Murohara et al., 1998).
Blood pressure measurement
At 7 and 12 days after surgery, in three rats per group we measured invasive blood pressure as previously described (Iaccarino et al., 2001a). Briefly, rats were anaesthetized as above, and a polyethylene catheter (PE-10) was inserted into the external carotid artery. The catheter was heparin-filled (100 mU ml−1) and exteriorized subcutaneously in the interscapular area. After surgery, animals were housed in single cages and allowed to recover. Arterial pressure was measured in conscious freely moving rats. The arterial catheter was connected to a low-volume pressure transducer connected to a computer for analysis of the blood pressure record (Powerlab; ADI Instruments). Arterial blood pressure and heart rate were measured in each animal for 30 min, daily over the next 3 days. Heart rate was calculated from the arterial pressure records. For each rat, the average of the measurements performed during the 3 days was considered.
Digital angiographies and blood flow determination
These experimental procedures were performed as described previously (Iaccarino et al., 2005). Briefly, on day 14, animals were anaesthetized and a catheter was inserted into the left common carotid and advanced to the abdominal aorta right before the iliac bifurcation. Blood flow was assessed by digital angiographies of the ischaemic and non-ischaemic hindlimb after injection of nitroglycerine (20 μg) to induce maximal vasodilatation. We counted the number of cineangiographic frames (TIMI frame count, TFC) as the contrast medium advanced to the dorsal paw artery (Gibson et al., 1996). We also used dyed beads to evaluate blood flow by injection of 6 × 105 yellow dyed beads (Triton Technologies) through the catheter previously introduced. Animals were killed with a lethal dose of pentobarbital. Samples of the gastrocnemius muscle (520–880 mg) were collected and frozen with liquid nitrogen. Next, samples were homogenized and digested, the beads were collected and suspended in dimethyl formamide. The release of dye was assessed by light absorption at a wavelength of 448 nm. Data are expressed as the ratio of dye extracted from ischaemic to that extracted from non-ischaemic muscle (Iaccarino et al., 2005).
Histology
Tissue specimens (tibialis anterior muscle) were dissected and immediately fixed by immersion in phosphate-buffered saline (0.01 M, pH 7.2–7.4)/formalin for at least 12 h. They were then treated as previously described (Iaccarino et al., 2005) and processed for histochemistry to count the number of capillary blood vessels per examined area, so as to evaluate capillary density. Final values are expressed as mean capillary number/muscle fibre.
Radioligand binding assay
Receptor binding on muscular membranes was performed, partially modifying a previously described technique (Iaccarino et al., 2001a, 2001b, 2005). Briefly, reactions were conducted in triplicate, in a volume of 200 μl of binding buffer (150 mM NaCl, 5 mM EDTA, 50 mM Tris-HCl, pH 7.4) containing protease inhibitors, using the α1-adrenoceptor selective antagonist [125I]HEAT (125iodo-2-[beta-(4-hydroxyphenyl)-ethyl-amino-methyl]-tetralone, 250 000 c.p.m.; PerkinElmer Italia, Monza, Milano, Italy) and the non-selective β-adrenoceptor antagonist ligand [125iodo]cyanopindolol ([125I]CYP). Nonspecific binding was determined by the addition of prazosin (5 × 10−6 M) for α1-adrenoceptors (Iaccarino et al., 2001b) or ICI 118 551, a selective β2-adrenoceptor antagonist (3 × 10−7 M) (Gong et al., 2002) for β2-adrenoceptors. After incubation in a shaking water bath at 37 °C for 60 min, unbound radioactivity was separated from membrane-bound radioactivity by vacuum filtration through glass-fibre filters (Iaccarino et al., 1998). After extensive ice-cold washing (50 mM Tris buffer), bound radioactivity remaining on the filters was assessed on a gamma counter and receptor density, expressed in picomoles, was normalized to milligrams of membrane proteins.
Immunoblot analysis
Muscles or endothelial cells were homogenized in lysis buffer at 4 °C as described (Akhter et al., 1998; Iaccarino et al., 2004, 2005). Insoluble materials were removed by centrifugation at 20 000 g for 15 min. Equal amounts of soluble proteins were electrophoresed by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane filters (Amersham Biosciences). Serine-tyrosine phosphorylated ERK1/2 (extracellular signal regulated kinase; Cell Signaling Technology, Danvers, MA, USA), pRb (retinoblastoma), total ERK (Santa Cruz Biotechnology, Santa Cruz, CA, USA), pAkt (Santa Cruz Biotechnology), total Akt (Santa Cruz Biotechnology) were visualized with specific antibodies, anti-rabbit and anti-goat horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) and standard chemiluminescence (Pierce) on autoradiographic films. Autoradiographies were then digitalized and densitometry quantification performed using dedicated software (ImageQuaNT; Molecular Dynamics).
Systemic levels of VEGF, used as marker of the ischaemic insult (Seko et al., 1997; Iaccarino et al., 2005), were determined in non-ischaemic hindlimb muscle samples by immunoprecipitation (Akhter et al., 1998; Iaccarino et al., 1999) of VEGF (protein A/G+agarose beads conjugated with a rabbit polyclonal antibody raised against VEGF (Santa Cruz Biotechnology) visualized by a goat polyclonal IgG (Santa Cruz Biotechnology).
Experiments were performed in triplicate to ensure reproducibility. Data are presented as arbitrary densitometry units after normalization for the total corresponding protein or actin as internal control.
Data presentation and statistical analysis
Values are presented as mean±s.e.mean. For normally distributed values, the Student's t-test was used, otherwise the non-parametric Mann–Whitney U-test was applied; two-way ANOVA was performed to compare the different parameters among the groups. A significance level of P<0.05 was assumed for all statistical evaluations. Statistics were computed with GraphPad Prism Software (San Diego, CA, USA).
Results
Endothelial expression of α1-adrenoceptor subtypes
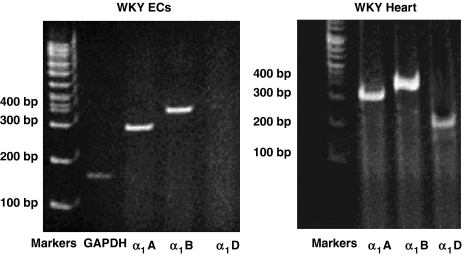
Reverse transcriptase-PCR showed that α1A- and α1B-adrenoceptors but not the α1D subtype were expressed in cultured rat aorta endothelial cells. As a control, we used cDNA prepared from rat hearts, which show the presence of the three isoforms of the α1-adrenoceptor (Figure 2). After hypoxia, α1A-adrenoceptor gene expression (as measured by reverse transcriptase-PCR) was upregulated (0.90±0.06 vs 0.60±0.07; densitometric units normalized by actin expression (CDU); P<0.05, ANOVA), whereas there was no significant increase in the expression of the α1B-adrenoceptor gene (0.40±0.03 vs 0.35±0.02, NS).
Figure 2.
Expression of α-adrenoceptor subtypes in Wistar-Kyoto (WKY) endothelial cells and WKY heart by reverse transcriptase-PCR. The figure shows that α1A- and α1B-adrenoceptors but not the α1D subtype were expressed in cultured rat aorta endothelial cells.
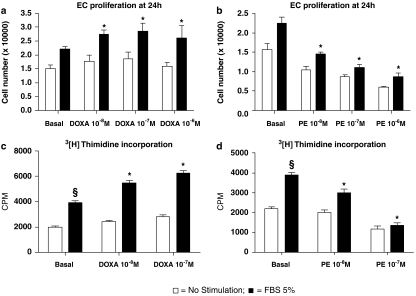
Effects of doxazosin and phenylephrine on endothelial cell proliferation
To evaluate the effects of α1-adrenoceptors on the proliferative phenotype, we studied endothelial cells in active proliferation induced by the mitogenic agent, FBS. Antagonism of α1-adrenoceptors with doxazosin alone did not change endothelial cell number, but chronic exposure (24 h) to 10−7 M doxazosin enhanced endothelial cell proliferation to FBS (FBS: +45±4.1% vs doxazosin+FBS: +89.4±7.1%, P<0.05; Figure 3a). Similar results were obtained by measuring DNA synthesis, when doxazosin increased the [3H]thymidine incorporation following FBS (Figure 3c).
Figure 3.
In vitro effects of doxazosin (DOXA; a, c) and phenylephrine (PE; b, d) on endothelial cell biology. All experiments depicted in this figure were performed from three to five times in duplicate. Role of increasing doses of doxazosin (a) and phenylephrine (b) on fetal bovine serum (FBS)-induced cell proliferation. Given alone, doxazosin did not affect endothelial cell proliferation. However, chronic incubation (24 h) with doxazosin enhanced endothelial cell proliferation in response to the mitogenic stimulus, FBS (5%; 24 h) (*P<0.05 vs basal+FBS) with a peak effect at a concentration of 10−7 M doxazosin. In contrast, chronic incubation with phenylephrine reduced endothelial cell number and decreased proliferation (*P<0.05 vs basal+FBS). DNA synthesis assayed by [3H]thymidine incorporation. FBS (5%, 24 h) increased DNA synthesis (§P<0.03 vs basal); and this response was augmented by doxazosin (c) and reduced by phenylephrine (d) treatment (24 h) (*P<0.05 vs basal+FBS).
Opposing effects were obtained after chronic stimulation of endothelial α1-adrenoceptors with an agonist, 10−7 M phenylephrine. Chronic exposure (24 h) to phenylephrine antagonized FBS-induced endothelial cell proliferation (FBS: +49±3.7% vs phenylephrine+FBS: −13.1%±2.2%, P<0.05; Figure 3b). Also, phenylephrine did not increase FBS induced [3H]thymidine incorporation and antagonized DNA synthesis (Figure 3d).
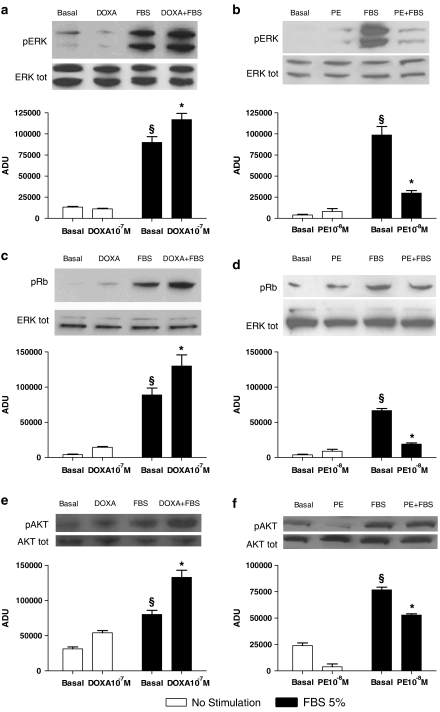
Effects of doxazosin and phenylephrine on endothelial cell signal transduction
Consistent with the results on cell proliferation, doxazosin treatment did not stimulate per se the mitogen-activated protein ERK, but pre-incubation with this agent resulted in an enhancement of FBS-induced ERK activation (P<0.05; Figure 4a). Similar effects were observed when the phosphorylation of retinoblastoma (Rb) protein, a check point for cell proliferation (Deshpande et al., 2005) was measured: doxazosin did not induce phosphorylation of Rb by itself, but enhanced phosphorylation after FBS stimulation (P<0.05; Figure 4c). Here also, phenylephrine produced effects opposite to those produced by doxazosin. Chronic phenylephrine exposure inhibited FBS-induced phosphorylation of ERK and Rb (P<0.05; Figures 4b and d). Similarly to Erk, doxazosin and phenylephrine show reciprocal effects on FBS induced AKT activation (Figures 4e and f).
Figure 4.
In vitro effects of doxazosin (DOXA) and phenylephrine (PE) on endothelial cell signal transduction. (a, b) Extracellular signal regulated kinase (ERK)/mitogen-activated protein kinase activation: western blot of activated (phosphorylated: pERK) ERK1/2 after stimulation with fetal bovine serum (FBS). Equal amounts of proteins were confirmed via blotting for total ERK. Representative blots are presented in the inset. Densitometric analysis (bar graph) shows that FBS stimulation caused ERK activation (§P<0.05 vs basal). Doxazosin alone did not increase ERK phosphorylation but significantly improved FBS-induced ERK activation (a). Phenylephrine 10−8 M pre-incubation (24 h) did not change ERK activation but attenuated responses to FBS (b) (*P<0.05 vs basal+FBS; ANOVA; n=3–5 experiments, repeated in triplicate). (c, d) Progression in cell cycle evaluated by retinoblastoma phosphorylation (pRb). This protein regulates cell cycle progression through the restriction point within the G1 phase. After 12 h of stimulation with FBS. Rb was phosphorylated, as assessed by western blot (§P<0.05 vs basal). Densitometric analysis shows that doxazosin pre-incubation (10−7 M, 24 h) enhanced Rb activation after FBS (c), whereas phenylephrine (10−8 M, 24 h) reduced this response (d) (*P<0.05 vs basal+FBS). Equal amounts of proteins were verified by blotting for total ERK; n=3, repeated in duplicate. (e, f) Akt activation (pAkt) after FBS stimulation. FBS induced phosphorylation of Akt, doxazosin alone did not activate Akt, but hastened FBS activation (e). Conversely, phenylephrine (10−8 M, 24 h) decreased FBS-induced activation of Akt (f) (*P<0.05 vs basal+FBS; ANOVA; n=3–5 experiments, repeated in triplicate). Equal amounts of proteins were verified by blotting for total Akt. ADU indicates arbitrary densitometry units, after correction for total protein content; representative blots are presented in the inset.
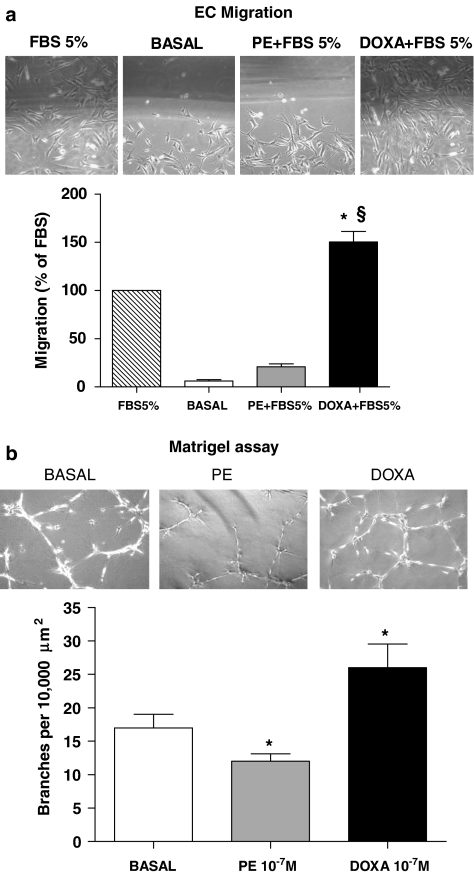
Doxazosin stimulates endothelial cell migration and vascular tube formation
Angiogenesis requires migration of endothelial cells to the sites of new capillary formation in ischaemic tissues, and cellular migration in vitro is an indicator of the angiogenic potential of an agent. Therefore, we determined the effect of doxazosin and phenylephrine on the migration of endothelial cells using a cell monolayer-wounding assay performed in the presence of DMEM with and without 5% FBS. As expected, endothelial cells cultured in the presence of DMEM+5% FBS displayed a greater capacity to migrate into the wounded area at 12 h following wounding of the cell monolayer (Figure 5a). With doxazosin pretreatment, the ability of endothelial cells to migrate into the wounded area was enhanced; whereas treatment with phenylephrine resulted in an inhibition of FBS-induced cell migration. Furthermore, we investigated the ability of doxazosin to enhance vascular network formation in vitro. We plated endothelial cells on Matrigel matrix, which induces network organization of the endothelial cells. As represented in Figure 5b, culture of endothelial cell on a Matrigel matrix revealed that the total number of network projections per microscopic field was significantly higher when cells were cultured in the presence of doxazosin compared with endothelial cells cultured with DMEM only. Taken together, our in vitro results illustrate the ability of doxazosin to regulate migration and the formation of vascular structures by endothelial cells. Once again, phenylephrine inhibited this pro-angiogenic property of endothelial cells.
Figure 5.
Cellular migration and vascular network formation. (a) Endothelial cell migration was measured 12 h after plating using a wounding assay. Migration of confluent endothelial cells was measured after the cell monolayer was partially wiped away. Photomicrographs show cells migrating into the wounded area. The area of the migrating cells was measured in several fields of view and is shown in the graph below. Data are presented as percent of migration with respect to fetal bovine serum (FBS) alone (*P<0.05 vs FBS 5%; §P<0.01 vs basal). (b) Endothelial cell network formation in vitro. Representative phase contrast photomicrographs of endothelial cells are shown plated on Matrigel in control conditions, in the presence of doxazosin 10−7 M or phenylephrine 10−7 M. Microscopy revealed numbers of network projections (branches) formed in each group after 12 h of incubation (*P<0.05 vs basal). It is interesting to note that phenylephrine modifies cell refraction, which is probably due to the favourable effects of phenylephrine on apoptosis. Data are presented as mean±s.e.
Effects of doxazosin during chronic ischaemia in vivo
Blood pressure measurements
To transpose our findings to an in vivo situation, we explored α1-adrenoceptor in the rat ischaemic hindlimb. Ischaemia is known to cause increased sympathetic discharge, with stimulation of both α- and β-adrenoceptors. We aimed to antagonize the α1-adrenoceptor activation through chronic infusion of doxazosin at low dosages, to rule out the possibility that changes in haemodynamics could influence the adaptative response to ischaemia. We measured blood pressure invasively in rats at days 7 and 12, and direct measurements of arterial blood pressure showed no significant differences in treated and not treated rats (mean arterial pressure: 7 days: doxazosin: 85±2.7 mm Hg; sham: 85±1.8 mm Hg; 12 days: doxazosin: 84±3.1 mm Hg; sham: 85±2.4 mm Hg; all differences are not significant).
α1-Adrenoceptor density
Chronic ischaemia resulted in an increase of α1–adrenoceptor density (from 22.3±4.4 to 39.2±2.9 pmol mg−1 of protein), suggesting a role of α1-adrenoceptors in the adaptative response of the ischaemic muscle. Moreover, α1-adrenoceptor blockade with doxazosin for 14 days resulted in the expected upregulation of α1-adrenoceptor density (96±14.8 pmol mg−1 of protein; P<0.05 vs untreated ischaemic hindlimb), thus indicating an effective α1-adrenoceptor blockade by the low dosage of this agent, despite the lack of effect on blood pressure and vascular resistance.
β-Adrenoceptor binding in ischaemic hindlimb
In our previous publication (Iaccarino et al., 2005), we proposed that endothelial β2-adrenoceptors, which are downregulated in chronic ischaemia, contributed to neo-angiogenesis driven by the sympathetic system. We therefore explored the effect of α1-adrenoceptor blockade on β2-adrenoceptor density in the ischaemic hindlimb. As expected, β-adrenoceptor density downregulates during chronic ischaemia, but α1-adrenoceptor blockade with doxazosin restored normal β-adrenoceptor density and in particular increased expression of the β2-adrenoceptor (Figures 6a and b). This result suggested a contra-regulation of α1- and β2-adrenoceptors during ischaemia.
Figure 6.
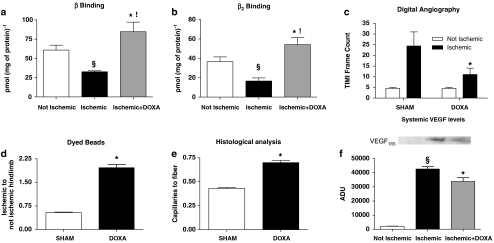
Increased neo-angiogenic responses by doxazosin (DOXA) treatment during chronic ischaemia in vivo. (a, b) β-Adrenoceptor density in rat hindlimbs. Total β-adrenoceptor (e) and β2-adrenoceptor (f) density were analysed in rat muscles from the ischaemic and non-ischaemic hindlimbs. We observed a reduction in both β-adrenoceptor and β2-adrenoceptor density within the ischaemic hindlimb (§P<0.03 vs not ischaemic). Rats receiving doxazosin in chronic infusion showed a significant upregulation of β- and β2-adrenoceptors (*P<0.02 vs ischaemic; !P<0.05 vs non-ischaemic). (c) TIMI frame count (TFC) of digital angiographies. This technique allows a better assessment of the deep vascular tree, which in the laser Doppler analysis is mostly affected by the cutaneous circulation. After 14 days of chronic ischaemia, digital angiographies showed a reduced number of TFCs in ischaemic hindlimbs treated with doxazosin, compared with sham rats (*P<0.05); the smaller TFC is indicative of improved blood perfusion. (d) Dyed beads dilution assay, where doxazosin treatment increased blood flow in ischaemic hindlimb, with respect to controls. Data shown are the dyed beads contained per milligram of hindlimb muscle tissue, expressed as the ratio between the ischaemic and non-ischaemic muscle (*P<0.05). (e) Histological analysis of capillaries in the rat tibialis anterior muscle. Compared with sham hindlimb, doxazosin increased capillary density, evaluated as number of capillaries corrected for number of muscle fibres, in the ischaemic tissue (*P<0.05). (f) Systemic levels of vascular endothelial growth factor (VEGF) in the non-ischaemic contralateral muscle. Fourteen days after femoral artery resection, we evaluated VEGF levels in the contralateral hindlimbs by western blots, as an indicator of systemic VEGF. Using muscle of rats that were not subjected to femoral artery resection as the non ischaemic reference (not ischaemic), we found that ischaemia caused an increase in systemic levels of VEFG (§P<0.01 vs non-ischaemic). Doxazosin treatment leads to a limitation of the ischaemic insult and consequently to a reduction of systemic levels of VEGF after 14 days (*P<0.05 vs ischaemic) (n=3, repeated in duplicate; a representative blot is presented in the inset; ADU: arbitrary densitometry units).
Ischaemic hindlimb perfusion
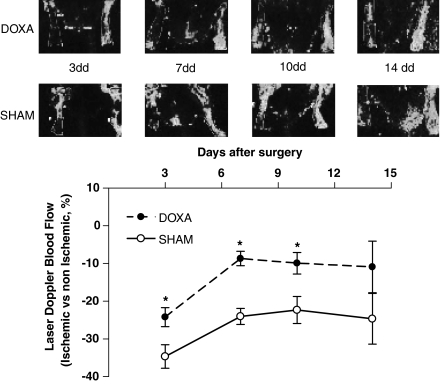
Laser Doppler analysis (Figure 7) showed impairment in ischaemic hindlimb perfusion compared with the contralateral hindlimb. Interestingly, concomitant infusion of doxazosin improved blood flow in the ischaemic limb (P<0.05, repeated measurements ANOVA). This effect was confirmed by the analysis of digital angiographies (Figure 6c; Supplementary Movies 1 and 2) performed on day 14, showing an improvement in hindlimb perfusion of the doxazosin-treated rats (doxazosin: 11±3.6; sham: 24±6.3; number of TFC; P<0.05, ANOVA). No changes were observed in terms of perfusion in the contralateral, non-ischaemic, hindlimb.
Figure 7.
Laser Doppler analysis. Determination of laser Doppler blood flow on postoperative days 3, 7, 10, 14 shows a deficit in ischaemic hindlimb perfusion, compared with the contralateral hindlimb, that is significantly attenuated in doxazosin as compared with sham rats (*P<0.05, repeated measurements, ANOVA; laser Doppler blood flow data are expressed as percent of ischaemic to non-ischaemic limb).
Another evaluation of regional blood flow was performed by infusion of dyed microspheres (Figure 6d), which confirmed the beneficial effects of doxazosin on ischaemic hindlimb blood flow.
Histology
Data on capillary density (Figure 6e) derived from histological analysis of the tibialis anterior muscle also showed the benefits of doxazosin treatment. Capillary density decreased with ischaemia, but the density in doxazosin-treated ischaemic muscle was identical to that in non-ischaemic muscles, after 14 days of treatment.
Systemic VEGF levels
Chronic ischaemia leads to increased circulating VEGF levels. Once the ischaemic insult is removed, VEGF levels return to the basal values. In our model, a reduction of VEGF levels indicated a reduction in the ischaemic insult. We therefore measured VEGF165 by western blot, in the contralateral, non-ischaemic hindlimb, as it is related to the circulating levels of this cytokine. Indeed, serum and non-ischaemic muscle contents of VEGF are closely related (Iaccarino et al., 2005). As indicated in Figure 6f, systemic levels of VEGF were reduced in the doxazosin-treated rats compared with the sham group, suggesting that ischaemia in the experimental hindlimb was significantly reduced after doxazosin (Seko et al., 1997).
Discussion
Our report shows for the first time that endothelial α1-adrenoceptors downregulate ischaemic angiogenesis through a direct action on the pro-angiogenic responses of endothelial cells. So far, studies on the role of α1-adrenoceptor blockade on angiogenesis proposed mainly a haemodynamic mechanism to explain the improved blood flow in animal models of chronic ischaemia and did not explore the role of endothelial cells. Previous papers have shown that high doses of α1-adrenoceptor antagonists (approximately 5 mg per kg per day vs therapeutic doses (Ben-Dov et al., 2006) of 0.06 mg per kg per day, tested for clinical practice) may have pro-angiogenic effects (Dawson and Hudlicka, 1989; Price and Skalak, 1996; Fulgenzi et al., 1998; Zhou et al., 1998). Similar findings have been obtained with other vasodilators (Dusseau et al., 1986; Koller et al., 1995; Picano and Michelassi, 1997) and attributed directly to their haemodynamic effects (Franke et al., 1984; Cooke and Losordo, 2002). In several papers, Hudlicka's group has used high doses of the α1-adrenoceptor blocker prazosin to induce angiogenesis, even in the absence of ischaemia (Dawson and Hudlicka, 1989), and has proposed an initial involvement of endothelial cells (Hudlicka, 1998; Carmeliet, 2000). However, the published literature does not allow us to determine which part of the neo-angiogenesis after high doses of α1 blockers is due to vasodilatation and which (if any) is due to the direct inhibition of endothelial cell α1-adrenoceptors. Our paper is the first to provide the evidence that α1-adrenoceptor blockade favours angiogenesis, independently of vasodilatation. To support this statement, we provide two sets of evidence, gathered in vitro and in vivo.
In vitro, the absence of any haemodynamic component allows a better assessment of the biological properties of endothelial α1-adrenoceptors. It is well established that endothelial cells are the key modulator of angiogenesis (Carmeliet, 2000; Augustin, 2001). In this study, in vitro, chronic α1-adrenoceptor stimulation inhibited and chronic α1-adrenoceptor blockade enhanced endothelial cell proliferation to the mitogenic stimulus, 5% FBS. This mitogen was chosen because it is a nonspecific stimulator of cell proliferation acting through multiple intracellular pathways. Therefore, the effect of α1-adrenoceptors cannot be attributed to the inhibition of a single signal transduction pathway, but rather it is a phenomenon that involves all of endothelial cell biology. Doxazosin and phenylephrine not only interfered with endothelial cell proliferation, DNA synthesis and molecular activation of ERK and Rb in response to FBS but also affected endothelial cell migration and vascular tube formation in Matrigel cultures. These in vitro data are in good agreement with previous results (Alexandrov et al., 1998; Yamauchi et al., 2001), showing, in different tissues, a regulatory role of α1-adrenoceptors on cell proliferation. Further studies will be necessary to identify the intracellular signal transduction pathways leading to α1-adrenoceptor-mediated inhibition of neo-angiogenesis. To follow up our in vitro experiments, we chose to treat rats with a low dose of doxazosin, which was a fraction of dosages used in previous studies (Dawson and Hudlicka, 1989; Zhou et al., 1998). Although blood pressure was not different between doxazosin-treated and sham rats, doxazosin enhanced angiogenesis induced by chronic ischaemia. In this situation, the pro-angiogenic action of doxazosin cannot be explained by a haemodynamic mechanism (Benning and Kyprianou, 2002) and is probably attributable to the cellular effects of α1-adrenoceptor blockade.
Neo-angiogenesis has long been known to be a highly ordered multistep molecular process under tight regulation by endothelial cells (Papetti and Herman, 2002) and closely associated with endothelial cell proliferation and migration and to the capability of these cells to modulate the levels of VEGF, the most important cytokine system involved in the formation of new vessels (Carmeliet, 2000). A series of biological, chemical, hormonal effectors can interfere with this process. Our data support the notion that α1-adrenoceptor should also be ranked among these agents. We have recently demonstrated that the β2-adrenoceptors participate in angiogenesis, by enhancing endothelial cell proliferation and survival (Iaccarino et al., 2002; Ciccarelli et al., 2007). The present work adds the α1-adrenoceptor to the list of factors influencing angiogenesis, and magnifies the role of endogenous catecholamines, the neurotransmitter agonists at adrenoceptors, in the regenerative response to chronic ischaemia. We hypothesize that α1- and β2-adrenoceptors mediate opposite effects on neo-angiogenesis, comparable to their regulation of the vascular tone. In particular, the α1-adrenoceptor is inhibitory, whereas the β2-adrenoceptor is stimulant to neo-angiogenesis. Interestingly, in ischaemia, the α1-adrenoceptors are upregulated, thus causing a predominance of α1-adrenoceptor signalling over that of β2-adrenoceptors, which is downregulated. Furthermore, in conditions such as hypertension, where the α1-adrenoceptor tone is higher than that of the β2-adrenoceptors, there is also an impairment in neo-angiogenesis (Emanueli et al., 2001; Iaccarino et al., 2005). It is interesting to note that in the ischaemic hindlimb, α1-adrenoceptor blockade resulted in a normalization of β2-adrenoceptor density together with improved neo-angiogenesis. Whether this association of events is mechanistic or just incidental is the object of ongoing experiments. α1-Adrenoceptor upregulation, in particular, might be a regulatory mechanism aimed at preventing excessive angiogenesis. This upregulation might be triggered by ischaemia, through regulatory sequences within the gene promoter, which have been demonstrated for both the α1A- and α1B-adrenoceptor (Eckhart et al., 1997; Michelotti et al., 2003).
In summary, α1-adrenoceptors appear to play a critical role in endothelial cells and this finding adds a new dimension to the intricate network of signals triggered by the adrenoceptor system (Liggett, 2006). Our results do not offer a molecular definition of the regulation of angiogenesis by α1-adrenoceptors, which could be further investigated in knock-out models. On the other hand, the pharmacological approach of our study provides the background for evaluating the clinical implications of α1-adrenoceptors in ischaemia in patients.
External data objects
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- ERK
extracellular signal regulated kinase
- FBS
fetal bovine serum
- HRP
horseradish peroxidase
- Rb
retinoblastoma protein
- TFC
TIMI frame count
- VEGF
vascular endothelial growth factor
- WKY
Wistar-Kyoto
Conflict of interest
Guido Iaccarino is the recipient of the Doxazosin International Award in 2004.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor–Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- Alexandrov A, Keffel S, Goepel M, Michel MC. Stimulation of alpha1A-adrenoceptors in rat-1 cells inhibits extracellular signal-regulated kinase by activating p38 mitogen-activated protein kinase. Mol Pharmacol. 1998;54:755–760. doi: 10.1124/mol.54.5.755. [DOI] [PubMed] [Google Scholar]
- Augustin HG. Tubes, branches, and pillars: the many ways of forming a new vasculature. Circ Res. 2001;89:645–647. [PubMed] [Google Scholar]
- Ben-Dov IZ, Ben-Arie L, Mekler J, Bursztyn M. How should patients treated with alpha-blockers be followed? Insights from an ambulatory blood pressure monitoring database. J Hypertens. 2006;24:861–865. doi: 10.1097/01.hjh.0000222755.69358.72. [DOI] [PubMed] [Google Scholar]
- Benning CM, Kyprianou N. Quinazoline-derived alpha1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an alpha1-adrenoceptor-independent action. Cancer Res. 2002;62:597–602. [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Ciccarelli M, Cipolletta E, Santulli G, Campanile A, Pumiglia K, Cervero P, et al. Endothelial beta2 adrenergic signaling to AKT: role of Gi and SRC. Cell Signal. 2007;19:1949–1955. doi: 10.1016/j.cellsig.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Losordo DW. Nitric oxide and angiogenesis. Circulation. 2002;105:2133–2135. doi: 10.1161/01.cir.0000014928.45119.73. [DOI] [PubMed] [Google Scholar]
- Dawson JM, Hudlicka O. The effects of long term administration of prazosin on the microcirculation in skeletal muscles. Cardiovasc Res. 1989;23:913–920. doi: 10.1093/cvr/23.11.913. [DOI] [PubMed] [Google Scholar]
- Deshpande A, Sicinski P, Hinds PW. Cyclins and cdks in development and cancer: a perspective. Oncogene. 2005;24:2909–2915. doi: 10.1038/sj.onc.1208618. [DOI] [PubMed] [Google Scholar]
- Dusseau JW, Hutchins PM, Malbasa DS. Stimulation of angiogenesis by adenosine on the chick chorioallantoic membrane. Circ Res. 1986;59:163–170. doi: 10.1161/01.res.59.2.163. [DOI] [PubMed] [Google Scholar]
- Eckhart AD, Yang N, Xin X, Faber JE. Characterization of the alpha1B-adrenergic receptor gene promoter region and hypoxia regulatory elements in vascular smooth muscle. Proc Natl Acad Sci USA. 1997;94:9487–9492. doi: 10.1073/pnas.94.17.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanueli C, Salis MB, Stacca T, Gaspa L, Chao J, Chao L, et al. Rescue of impaired angiogenesis in spontaneously hypertensive rats by intramuscular human tissue kallikrein gene transfer. Hypertension. 2001;38:136–141. doi: 10.1161/01.hyp.38.1.136. [DOI] [PubMed] [Google Scholar]
- Franke RP, Grafe M, Schnittler H, Seiffge D, Mittermayer C, Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984;307:648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- Fulgenzi G, Graciotti L, Collis MG, Hudlicka O. The effect of alpha 1 adrenoceptor antagonist prazosin on capillary supply, blood flow and performance in a rat model of chronic muscle ischaemia. Eur J Vasc Endovasc Surg. 1998;16:71–77. doi: 10.1016/s1078-5884(98)80095-6. [DOI] [PubMed] [Google Scholar]
- Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, et al. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CM, Cannon CP, Daley WL, Dodge JT, Jr, Alexander B, Jr, Marble SJ, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- Gong H, Sun H, Koch WJ, Rau T, Eschenhagen T, Ravens U, et al. Specific beta(2)AR blocker ICI 118 551 actively decreases contraction through a G(i)-coupled form of the beta(2)AR in myocytes from failing human heart. Circulation. 2002;105:2497–2503. doi: 10.1161/01.cir.0000017187.61348.95. [DOI] [PubMed] [Google Scholar]
- Guarino RD, Perez DM, Piascik MT. Recent advances in the molecular pharmacology of the alpha 1-adrenergic receptors. Cell Signal. 1996;8:323–333. doi: 10.1016/0898-6568(96)00066-6. [DOI] [PubMed] [Google Scholar]
- Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- Hudlicka O. Is physiological angiogenesis in skeletal muscle regulated by changes in microcirculation. Microcirculation. 1998;5:5–23. [PubMed] [Google Scholar]
- Iaccarino G, Barbato E, Cipolleta E, Esposito A, Fiorillo A, Koch WJ, et al. Cardiac betaARK1 upregulation induced by chronic salt deprivation in rats. Hypertension. 2001a;38:255–260. doi: 10.1161/01.hyp.38.2.255. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Ciccarelli M, Sorriento D, Cipolletta E, Cerullo V, Iovino GL, et al. AKT participates in endothelial dysfunction in hypertension. Circulation. 2004;109:2587–2593. doi: 10.1161/01.CIR.0000129768.35536.FA. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Ciccarelli M, Sorriento D, Galasso G, Campanile A, Santulli G, et al. Ischemic neoangiogenesis enhanced by beta2-adrenergic receptor overexpression: a novel role for the endothelial adrenergic system. Circ Res. 2005;97:1182–1189. doi: 10.1161/01.RES.0000191541.06788.bb. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Cipolletta E, Fiorillo A, Annecchiarico M, Ciccarelli M, Cimini V, et al. Beta(2)-adrenergic receptor gene delivery to the endothelium corrects impaired adrenergic vasorelaxation in hypertension. Circulation. 2002;106:349–355. doi: 10.1161/01.cir.0000022690.55143.56. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Keys JR, Rapacciuolo A, Shotwell KF, Lefkowitz RJ, Rockman HA, et al. Regulation of myocardial betaARK1 expression in catecholamine-induced cardiac hypertrophy in transgenic mice overexpressing alpha1B-adrenergic receptors. J Am Coll Cardiol. 2001b;38:534–540. doi: 10.1016/s0735-1097(01)01396-1. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Smithwick LA, Lefkowitz RJ, Koch WJ. Targeting Gbeta gamma signaling in arterial vascular smooth muscle proliferation: a novel strategy to limit restenosis. Proc Natl Acad Sci USA. 1999;96:3945–3950. doi: 10.1073/pnas.96.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- Kanda S, Miyata Y, Kanetake H. Role of focal adhesion formation in migration and morphogenesis of endothelial cells. Cell Signal. 2004;16:1273–1281. doi: 10.1016/j.cellsig.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Koller A, Huang A, Sun D, Kaley G. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles. Role of endothelial nitric oxide and prostaglandins. Circ Res. 1995;76:544–550. doi: 10.1161/01.res.76.4.544. [DOI] [PubMed] [Google Scholar]
- Lanni F, Santulli G, Izzo R, Rubattu S, Zanda B, Volpe M, et al. The Pl(A1/A2) polymorphism of glycoprotein IIIa and cerebrovascular events in hypertension: increased risk of ischemic stroke in high-risk patients. J Hypertens. 2007;25:551–556. doi: 10.1097/HJH.0b013e328013cd67. [DOI] [PubMed] [Google Scholar]
- le Noble FA, Stassen FR, Hacking WJ, Struijker Boudier HA. Angiogenesis and hypertension. J Hypertens. 1998;16:1563–1572. doi: 10.1097/00004872-199816110-00001. [DOI] [PubMed] [Google Scholar]
- Lee EW, Michalkiewicz M, Kitlinska J, Kalezic I, Switalska H, Yoo P, et al. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J Clin Invest. 2003;111:1853–1862. doi: 10.1172/JCI16929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo G, Iaccarino G, Vecchione C, Barbato E, Morisco C, Monti F, et al. Insulin enhances endothelial alpha2-adrenergic vasorelaxation by a pertussis toxin mechanism. Hypertension. 1997;30:1128–1134. doi: 10.1161/01.hyp.30.5.1128. [DOI] [PubMed] [Google Scholar]
- Liggett SB. Cardiac 7-transmembrane-spanning domain receptor portfolios: diversify, diversify, diversify. J Clin Invest. 2006;116:875–877. doi: 10.1172/JCI28234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- McKee AP, Van Riper DA, Davison CA, Singer HA. Gender-dependent modulation of alpha 1-adrenergic responses in rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2003;284:H1737–H1743. doi: 10.1152/ajpheart.00779.2002. [DOI] [PubMed] [Google Scholar]
- Michelotti GA, Bauman MJ, Smith MP, Schwinn DA. Cloning and characterization of the rat alpha 1a-adrenergic receptor gene promoter. Demonstration of cell specificity and regulation by hypoxia. J Biol Chem. 2003;278:8693–8705. doi: 10.1074/jbc.M211986200. [DOI] [PubMed] [Google Scholar]
- Morisco C, Marrone C, Trimarco V, Crispo S, Monti MG, Sadoshima J, et al. Insulin resistance affects the cytoprotective effect of insulin in cardiomyocytes through an impairment of MAPK phosphatase-1 expression. Cardiovasc Res. 2007;76:453–464. doi: 10.1016/j.cardiores.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–C970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- Picano E, Michelassi C. Chronic oral dipyridamole as a ‘novel' antianginal drug: the collateral hypothesis. Cardiovasc Res. 1997;33:666–670. doi: 10.1016/s0008-6363(96)00262-3. [DOI] [PubMed] [Google Scholar]
- Price RJ, Skalak TC. Chronic alpha 1-adrenergic blockade stimulates terminal and arcade arteriolar development. Am J Physiol. 1996;271:H752–H759. doi: 10.1152/ajpheart.1996.271.2.H752. [DOI] [PubMed] [Google Scholar]
- Rocnik EF, Liu P, Sato K, Walsh K, Vaziri C. The novel SPARC family member SMOC-2 potentiates angiogenic growth factor activity. J Biol Chem. 2006;281:22855–22864. doi: 10.1074/jbc.M513463200. [DOI] [PubMed] [Google Scholar]
- Seko Y, Imai Y, Suzuki S, Kamijukkoku S, Hayasaki K, Sakomura Y, et al. Serum levels of vascular endothelial growth factor in patients with acute myocardial infarction undergoing reperfusion therapy. Clin Sci (Lond) 1997;92:453–454. doi: 10.1042/cs0920453. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle JL, Falcone JC. Nitric oxide release during alpha1-adrenoceptor-mediated constriction of arterioles. Am J Physiol Heart Circ Physiol. 2001;281:H873–H881. doi: 10.1152/ajpheart.2001.281.2.H873. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Itoh H, Shinoura H, Miyamoto Y, Hirasawa A, Kaziro Y, et al. Involvement of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase in alpha1B-adrenergic receptor/Galphaq-induced inhibition of cell proliferation. Biochem Biophys Res Commun. 2001;281:1019–1023. doi: 10.1006/bbrc.2001.4472. [DOI] [PubMed] [Google Scholar]
- Zhou A, Egginton S, Hudlicka O, Brown MD. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with alpha1-antagonist prazosin. Cell Tissue Res. 1998;293:293–303. doi: 10.1007/s004410051121. [DOI] [PubMed] [Google Scholar]
- Zou MX, Roy AA, Zhao Q, Kirshenbaum LA, Karmazyn M, Chidiac P. RGS2 is upregulated by and attenuates the hypertrophic effect of alpha1-adrenergic activation in cultured ventricular myocytes. Cell Signal. 2006;18:1655–1663. doi: 10.1016/j.cellsig.2006.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.