Abstract
Background and purpose:
Enterohepatic recirculation (EHC) is a common pharmacokinetic phenomenon that has been poorly modelled in animals. The presence of EHC leads to the appearance of multiple peaks in the concentration-time profile and increased exposure, which may have implications for drug effect and extrapolation across species. The aim of this investigation was to develop a population pharmacokinetic model for diclofenac and rofecoxib that describes EHC and to assess its consequence for the pharmacodynamics of both drugs.
Experimental approach:
The pharmacokinetics of diclofenac and rofecoxib was characterized in male rats following intravenous, intraperitoneal and oral administration. Blood samples were collected at pre-defined time points after dosing to determine plasma concentrations over time. A parametric approach using nonlinear mixed effects modelling was applied to describe EHC, whilst simulations were used to evaluate its impact on PGE2 inhibition.
Key results:
For diclofenac, EHC was described by a compartmental model with periodic transfer rate and metabolite formation rate. For rofecoxib, EHC modelling required a conversion compartment with first-order recycling rate and lag time. Based on model predictions, EHC causes an increase of 95% in the systemic exposure to diclofenac and of 15% in the exposure to rofecoxib. In addition, EHC prolongs the inhibition of PGE2 and increases the duration of the anti-inflammatory effect (24 h for rofecoxib 10 mg kg−1) without affecting maximum inhibition.
Conclusions and implications:
Our findings show the relevance of exploring EHC in a quantitative manner to accurately interpret pharmacodynamic findings in vivo, in particular when scaling across species.
Keywords: enterohepatic recirculation, pharmacokinetics, PK–PD modelling, COX-2 inhibitors, rofecoxib, diclofenac, NONMEM
Introduction
Diclofenac and rofecoxib belong to the family of non-steroidal anti-inflammatory drugs or cyclo-oxygenase (COX) inhibitors. COX inhibitors are effective anti-inflammatory, antipyretic and analgesic agents, which are commonly used in the treatment of acute and chronic pain, rheumatoid arthritis and osteoarthritis. They act by inhibiting COX activity and consequently the formation of pro-inflammatory mediators such as prostaglandins (PGs) and thromboxanes (Vane, 1971). Since the early 1990s, it has been generally accepted that COX exists in two isoforms. COX-1 is a housekeeping enzyme responsible for modulating physiological events and is present in most tissues including the stomach, kidney and platelets, whereas COX-2 is highly induced in various cells by pro-inflammatory stimuli, mitogens and cytokines. In view of the IC80 ratio obtained from the human whole blood assay, diclofenac is a non-selective COX inhibitor, whereas rofecoxib is a highly selective COX-2 inhibitor (Warner and Mitchell, 2004).
Some COX inhibitors such as diclofenac and rofecoxib are subject to enterohepatic recirculation (EHC) in animals (Peris-Ribera et al., 1991; Eeckhoudt et al., 1997; Ogiso et al., 1997; Priymenko et al., 1998; Warner et al., 1999; Baillie et al., 2001). However, published reports have been of a descriptive nature, which restricts the extrapolation of findings to different experimental conditions. For instance, Baillie et al. (2001) refer to a secondary peak concentration following an i.v. bolus of rofecoxib in rats (2 mg kg−1 i.v. bolus), with Tmax at 10 h after drug administration, whereas Peris-Ribera et al. (1991) have reported the same phenomenon after p.o. and i.v. administration of diclofenac, with Tmax values varying between 2 and 4 h. In contrast to non-compartmental analysis, a model-based approach enables further characterization of the influence of EHC on pharmacokinetic (PK) parameters such as bioavailability, clearance and terminal half-life. Moreover, integrated PK–pharmacodynamic (PK–PD) modelling can be used to investigate whether EHC-related changes in PK may consequently influence PD effects or increase the risk of side effects, such as gastrointestinal toxicity (Reuter et al., 1997; Seitz and Boelsterli, 1998). In fact, it has been hypothesized that COX inhibitors that are not subject to EHC are less likely to produce intestinal damage (Reuter et al., 1997).
Different methodologies have been developed to describe EHC in animals and humans (Fukuyama et al., 1994; Alvarez-Bujidos et al., 1998; Ploeger et al., 2000; Ezzet et al., 2001; Schaiquevich et al., 2002; Moriwaki et al., 2003). Of particular interest is the review by Roberts et al. (2002) which provides a detailed evaluation of EHC concepts and models. They conclude that classical compartmental models with recycling loops are only suitable in the analysis of concentration–time profiles with a single secondary peak without large inter- and intra-individual variability. Moreover, rich sampling of PK data is required as input for the description of EHC, including data from portal and systemic concentrations (Moriwaki et al., 2003) or from bile duct-cannulated animals (Fukuyama et al., 1994). An alternative to empirical models is the use of physiologically based PK models. In fact, such an approach was proposed by Ploeger et al. (2000) to describe the kinetics of biliary excretion of glycyrrhizic acid. Also in this situation, the identification of relevant sources of variability remains limited. Noisy data with irregular patterns cannot be described by those models without a hierarchical component for random effects to which inter-individual variability can be assigned.
In early drug development, it is essential to accurately estimate drug clearance and concentration–effect relationships; as such information is required to subsequently predict the appropriate clinical dose range in humans. In most pre-clinical studies, drugs are initially administered as i.v. bolus and followed by p.o. dosing in a consecutive experiment. Usually, EHC and metabolite formation rates are ignored and no specific experiments are conducted to characterize their role on systemic kinetics. To date, despite the availability of nonlinear mixed effect models for EHC in humans, no efforts have been made to describe EHC in pre-clinical species that capture the appearance of multiple peaks and account for large inter-individual variability without the need for additional experiments. Therefore, the aim of this investigation was to develop and compare the performance of different PK models of EHC for diclofenac and rofecoxib in rats. The impact of variable metabolite formation rate due to EHC was explored with diclofenac by extending model parameterization. In addition to the use of standard dummy compartments describing drug transfer across the gastrointestinal tract, we have assessed the suitability of the recycling model proposed by Wajima et al. (2002) and the semiparametric model based on constrained longitudinal spline (CLS) by Fattinger and Verotta (1995), Park et al. (1997) and Wajima et al. (2002). Furthermore, simulations were performed to evaluate the influence of EHC on the inhibition of PGE2, a biomarker for the PD of diclofenac and rofecoxib on COX-2 activity. Besides the advantages of nonlinear mixed effects, the proposed approach may warrant a reduction in the number of animals required for the investigation of EHC in pre-clinical species.
Materials and methods
All animal procedures were approved by the Ethical Committee on Animal Experimentation of the University of Leiden and by the GSK Ethical Committee on Animal Experimentation.
The experimental part of this investigation consisted of two separate studies. One study was performed in The Netherlands for the assessment of the concentration–time profiles after i.v. administration of rofecoxib and i.p. administration of diclofenac and rofecoxib (study A). The second study was performed in the UK for the assessment of the concentration–time profiles after i.v. and p.o. administration of diclofenac and rofecoxib (study B). Data obtained from the different experiments were combined for the characterization of EHC in rats by nonlinear mixed effects modelling.
Animals and surgical procedures
Study A
Experiments were performed on male Sprague–Dawley rats (Charles River BV, Maastricht, The Netherlands) weighing 331±4 g (mean±s.d., n=6) for diclofenac and 320±7 g (mean±s.d., n=10) for rofecoxib. The animals were housed in standard plastic cages (six per cage before surgery and individually after surgery) with a normal 12-h day–night schedule (lights on 0700 hours) and at a temperature of 21 °C. The animals had access to standard laboratory chow (RMH-TM; Hope Farms, Woerden, The Netherlands) and acidified water ad libitum.
Three days before the start of the experiment, indwelling pyrogen-free cannulae (polythene; 14 cm, 0.52 mm i.d., 0.96 mm o.d.) were implanted into the right jugular vein for infusion of rofecoxib and in the right femoral artery (polythene; 4 cm, 0.28 mm i.d., 0.61 mm o.d.+20 cm 0.58 mm i.d., 0.96 mm o.d.) for serial blood sampling. The arterial cannula was filled with heparinized 25% (w/v) polyvinylpyrrolidone (Brocacef, Maarssen, The Netherlands) in saline (NaCl, 0.9%). Animals receiving i.p. drug administration were implanted with only an arterial cannula. The surgical procedures were performed under anaesthesia with 0.1 mg kg−1 i.m. dose of medetomidine hydrochloride (Domitor; Pfizer, Capelle a/d Ijssel, The Netherlands) and 1 mg kg−1 subcutaneous dose of ketamine base (Ketalar; Parke-Davis, Hoofddorp, The Netherlands).
Study B
Experiments were performed on male Sprague–Dawley rats (Harlan, UK) weighing 297±11 g (mean±s.d., n=3) for diclofenac and 245±4 g (mean±s.d., n=3) for rofecoxib. The animals were housed in standard plastic cages (six per cage before surgery and individually after surgery) with a normal 12-h day–night schedule (lights on 0700 hours) and at a temperature of 21 °C. The animals had access to standard laboratory chow and acidified water ad libitum.
Three days before the start of the experiment, indwelling pyrogen-free cannulae (polythene, 14 cm, 0.52 mm i.d., 0.96 mm o.d.) were implanted into the right jugular vein for infusion of rofecoxib or diclofenac and in the right femoral artery (polythene, 4 cm, 0.28 mm i.d., 0.61 mm o.d. +20 cm 0.58 mm i.d., 0.96 mm o.d.) for serial blood sampling. The surgical procedures were performed under anaesthesia with isoflurane (Fancy, Poole, UK).
The experiment was carried out on two study days with a wash-out period at least two days (>48 h) between treatments. Drugs were administered intravenously on the first study day and orally on the second study day.
Drugs and dosages
For study A, rofecoxib was extracted from Vioxx tablets (12.5 mg Vioxx; Merck, Kirkland Quebec, Canada). Vioxx tablets were crushed to fine power, suspended in 50 ml of HPLC grade ethyl acetate, shaken for 5 min and filtered. Residues were then crystallized from acetonitrile after evaporating the solvent in vacuum. During the extraction process, rofecoxib was protected from direct light to prevent degradation. The purity (97%) of rofecoxib was compared in triplicate to a pure sample of rofecoxib by LC-MSMS. Rofecoxib was dissolved in dimethylsulphoxide at concentrations of 10 mg kg−1 for i.p. administration and 10 and 5.95 mg kg−1 for i.v. administration. For study B, rofecoxib was synthesized by Medicinal Chemistry (GlaxoSmithKline, Harlow, UK). Rofecoxib was dissolved in 5% glucose containing 10% (v/v) dimethylsulphoxide and 10% (w/v) Epcapsin HPB (0.05 mg kg−1) and administered intravenously at a dose of 0.5 mg kg−1 in 60 min. Rofecoxib in 1% methylcellulose was administered orally at a dose of 2 mg kg−1. For study A, diclofenac was obtained from Sigma-Aldrich BV (Zwijndrecht, The Netherlands) and administered as an i.p. bolus dose of 2 mg kg−1. For study B, diclofenac was obtained from Sigma-Aldrich and administered as an i.v. infusion of 1 mg kg−1 in 60 min or as an p.o. dose of 2 mg kg−1. Diclofenac was dissolved in 0.9% NaCl for i.v. and i.p administration and in 1% methylcellulose for p.o. administration.
Experimental protocols
All experiments were started between 0830 and 0930 hours to exclude the influence of potential circadian variation in EHC. An infusion pump (Bioanalytical Systems Inc., Indiana, USA) was used for i.v. administration of diclofenac and rofecoxib. Per os administration was performed by oral gavage (5 ml kg−1). Serial arterial blood samples were taken at pre-defined time points and the total volume of blood samples was kept to 2.0 ml with volume replacement during each experiment. For study A, blood samples were heparinized and immediately centrifuged at 3200 g for 10 min for plasma collection and stored at −20 °C until analysis. For study B, blood samples were collected into tubes containing K2EDTA. Each sample was subsequently diluted with an equal volume of purified water and stored at –80 °C until analysis.
Bioanalysis of diclofenac and its 4-hydroxy metabolite
For study A, concentrations of diclofenac were determined by HPLC with ultraviolet detection, as described by Giagoudakis and Markantonis (1998) with slight modifications. The concentrations of 4-hydroxy diclofenac were not determined in this study. Briefly, samples were prepared by adding 50 μl internal standard solution (5 μg ml−1 flurbiprofen) and 50 μl 0.1 M phosphate buffer to 50 μl plasma, followed by acidification with 500 μl of 2.5 M o-phosphoric acid solution and liquid–liquid extraction with 5 ml chloromethane. The aqueous supernatant was removed by suction. After freezing, the organic layer was transferred into clean tubes and evaporated to dryness. The residue was re-dissolved in 100 μl mobile phase and injected into the HPLC system. The HPLC system used for the diclofenac assay consisted of Waters 501 solvent pump (Millipore-Waters, Milford, MA, USA), a Waters 717plus autosampler (Millipore-Waters), a Superflow 757 Kratus UV absorbance detector (Spark Holland BV, Emmen, The Netherlands) and a Chromatopac C-R3A reporting integrator (Shimadzu, Kyoto, Japan). Determination of diclofenac was performed using a reversed-phase stainless steel pre-conditioned Microsphere C18 3 μm cartridge column (100 × 4.6 mm id) (Chrompack, Bergen op Zoom, The Netherlands) equipped with a guard column (20 × 2 mm id) (Upchurch Scientific, Oak Harbor, WA, USA). The mobile phase consisted of 0.1 M phosphate buffer pH 6/acetonitrile (74:26 v/v) at ambient temperature. The mobile phase buffer was filtered through a 0.45 μm nylon filter (Gelman Scientific), mixed with acetonitrile and degassed with helium. The detector wavelength and the flow rate were 278 nm and 1 ml min−1, respectively. The run time was of 20 min with the diclofenac peak at approximately 15 min and the flurbiprofen (IS; Sigma-Aldrich, Poole, Dorset, UK) peak at approximately 10 min. The signal showed linearity over the range of 50–50 000 ng ml−1 and the lower limit of quantification was 50 ng ml−1. Bias and precision of the rat plasma calibration curves were assessed by quality control samples at nominal concentrations of 800 and 20 000 ng ml−1. Results were acceptable if variation in the measurements remained within 20% of the nominal concentration. The within- and between-day coefficients of variation of the assay were less than 10 and 20%, respectively.
For study B, diclofenac plasma samples (50 μl) were extracted using protein precipitation with acetonitrile/10 mM ammonium acetate (80:20) containing an internal standard (250 μl, 0.2 μg ml−1). An aliquot of the supernatant was analysed by reverse-phase HPLC/MS/MS using a heat-assisted electrospray interface in positive ion mode. Nominal multiple reaction monitoring (MRM) transitions for diclofenac, its 4-hydroxy metabolite and internal standard (lumiracoxib) were 298 to 216 and 294 to 248, respectively. Samples (2 μl) were injected using a CTC Analytics HTS Pal autosampler (Presearch, Hitchin, UK) onto a Hypersil Aquastar 3.0 mm × 30 mm, 3 μm column (Thermo, Runcorn, Cheshire, UK) operated at 40 °C and at an eluent flow rate of 1 ml min−1. Analytes were eluted using a high-pressure linear gradient programme, by means of an HP1100 binary HPLC system (Agilent, Stockport, Cheshire, UK), using 1 mM ammonium acetate containing 0.1% (v/v) as solvent A and acetonitrile as solvent B. The gradient was held at 5% solvent B for 2 min, before increasing to 80% at 1.2 min, remaining at 80% until 1.6 min before returning to the starting conditions. The cycle time was 2.5 min per sample. An Aquastar column (30 mm × 3.0 mm, 3 μm id; Thermo) was used for the chromatographic separation coupled to an API4000 tandem mass spectrometer (Applied Biosystems, Toronto, ON, Canada). Samples were assayed in the range 5–5000 ng ml−1 and the lower limit of quantification was 2 ng ml−1. Bias and precision of the rat plasma calibration curves were assessed with quality control samples (n=6) at the following concentrations 5, 20, 2000 and 5000 ng ml−1 and results were accepted based on these quality controls being within 20% of the nominal expected concentration. The within- and between-day coefficients of variation of the assay were less than 8 and 10%, respectively.
Bioanalysis of rofecoxib
Rofecoxib plasma samples (50 μl) from studies A and B were extracted using protein precipitation with acetonitrile/10 mM ammonium acetate (80:20) containing an internal standard (250 μl, 0.2 μg ml−1). An aliquot of the supernatant was analysed by reverse-phase HPLC/MS/MS using a heat-assisted electrospray interface in negative ion mode. Nominal MRM transitions for rofecoxib and internal standard (celecoxib) were 313 to 284 and 380 to 316, respectively. Samples (5 μl) were injected using a CTC Analytics HTS Pal autosampler (Presearch) onto a Supelco Discovery Cyano 4.6 mm × 50 mm, 5-μm column (Sigma-Aldrich, UK) operated at 40 °C and at an eluent flow rate of 1 ml min−1. Analytes were eluted isocratically by means of an HP1100 binary HPLC system (Agilent), using 10 mM ammonium acetate containing 0.1% (v/v) as solvent A (50%) and acetonitrile as solvent B (50%). The cycle time was 2.2 min per sample. The eluent was injected into an API4000 tandem mass spectrometer (Applied Biosystems). Rat plasma samples were assayed in the range 5–5000 ng ml−1 and the lower limit of quantification was 5 ng ml−1. Bias and precision of the rat plasma calibration curves were assessed with quality control samples at the following concentrations, 5, 20, 2000 and 5000 μg ml−1 and results were accepted based on these quality controls being within 20% of the nominal expected concentration. Samples showing plasma concentrations above the upper limit of quantification were re-assayed by appropriate dilution in plasma to within the range of the calibration curve. The within- and between-day coefficients of variation of the assay were less than 10 and 10%, respectively.
PK data analysis
The PK of diclofenac and rofecoxib was analysed using nonlinear mixed effects modelling as implemented in NONMEM version V, level 1.1 (Beal and Sheiner, 1999). This approach allows the estimation of intra-individual as well as inter-individual variability in model parameters. All fitting procedures were performed on a computer (AMD-Athlon XP-M 3000+) running under Windows XP with the Fortran compiler Compaq Visual Fortran version 6.1. An in-house available S-Plus 6.0 (Insightful Corp., Seattle, WA, USA) interface to NONMEM version V was used for data processing, data management and graphical data display. The first-order conditional estimation method (FOCE) with interaction was used for fitting the data. Model building details will be given for diclofenac first and complemented subsequently by specific requirements for rofecoxib. During model building, we have attempted to move from a fully empirical approach to a more physiologically meaningful parameterization of EHC.
Structural model building
The PK analysis was performed using the ADVAN6 and ADVAN5 routine in NONMEM. Model selection was based on the likelihood ratio test, parameter point estimates and their respective confidence intervals, parameter correlations and goodness-of-fit plots. For the likelihood ratio test, the significance level was set at P=0.01, which corresponds to a decrease of 6.6 points, after the inclusion of one parameter, in the minimum value of the objective function under the assumption that the difference in minimum value of the objective function between two nested models is χ2 distributed. The following goodness-of-fit plots were subjected to visual inspection to detect systematic deviations in model fits: individual observed vs population or individual predicted values and weighted residuals vs time or population predicted values. Diagnostic evaluation of the final model included visual and posterior predictive checks (Yano et al., 2001).
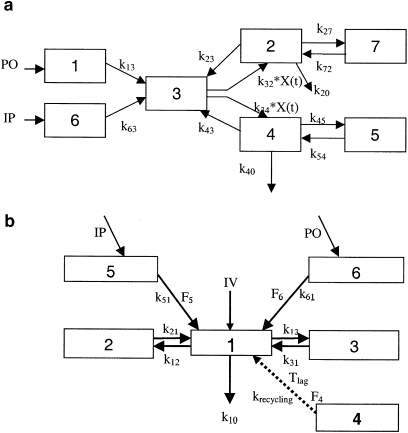
Diclofenac concentration–time profiles were analysed using an oscillatory EHC model, which was first proposed by Wajima et al. (2002); Figure 1a. In this model, it is assumed that extrahepatic and first-pass metabolism occurs following i.p. and p.o. administration. The periodic transfer rate of the liver-bile flow to central compartment of parent and metabolite is a nonlinear function and described by the following equation:
where frequency is the periodicity of recycling (which represented a cycle of enterohepatic circulation), which is constrained to be a divisor of 24 h.
Figure 1.
Pharmacokinetic (PK) models accounting for enterohepatic recycling. (a) PK model for diclofenac and its metabolite 4-hydroxydiclofenac. CMT1 represents the administration site for p.o. dosing, CMT6 represents the administration site for i.p. dosing, CMT2 represents the central compartment for the parent, CMT7 describes the disposition of the parent, CMT4 represents the central compartment for the metabolite, CMT5 describes the disposition of the metabolite and CMT3 represents the enterohepatic recirculation (EHC) compartment. The periodic transfer rate of EHC to central compartment is a nonlinear function (k32*X(t)and k34*X(t)). See PK data analysis for further details. (b) Mixture model with a conversion compartment for rofecoxib. CMT1–CMT3 depict the central and peripheral compartments, respectively. CMT5 and CMT6 represent the depot compartments following i.p. and p.o. administration of rofecoxib. EHC is described by CMT4, Tlag is the lag time associated with the start of EHC and krecycling the re-absorption rate constant. F4–F6 are estimates of the bioavailability for compartments 4–6, respectively. Dashed arrow represents administration of a fictitious dose into the EHC compartment.
The central compartment for the parent (compartment 2) and metabolite (compartment 4) is linked directly to the EHC compartment (compartment 3), through which the metabolite is also cleared from the systemic circulation. Subsequent uptake of either compound into the central compartment is described by the periodic transfer rate. It was assumed that all the drug cleared into the EHC compartment remains bioavailable (F3). The systemic disposition of diclofenac and 4-hydroxydiclofenac is described by separate compartments (compartments 5 and 7, respectively). Drug clearance was tested for parent and metabolite, and elimination was determined on central compartment of parent and metabolite. Complementary metabolite formation, which is not associated with EHC was characterized by a separate elimination rate constant (k20). At the start of the model building process, the volume of distribution of the central compartment of the metabolite (CMT4) was fixed at 1. The differential equations specifying the model are presented in the appendix.
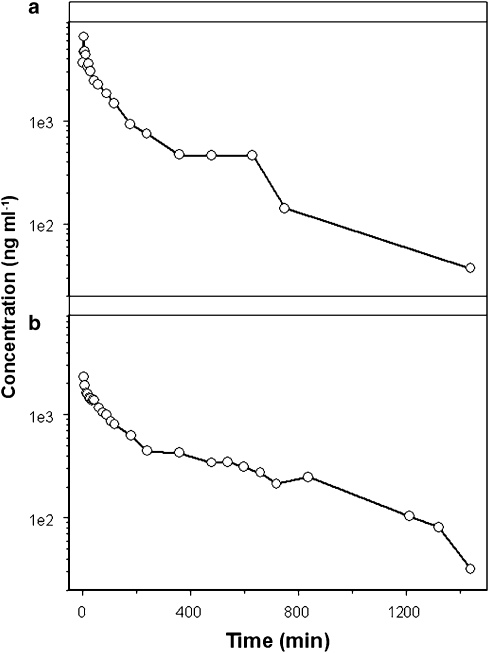
In contrast to diclofenac, the PK of rofecoxib was highly variable and no concurrent metabolite data were available for an integrated PK analysis of parent drug and metabolite. The use of a model with a steady oscillatory behaviour was therefore not sufficient to describe in a semiphysiological manner the impact of EHC on systemic exposure, leading to parameter identifiability problems and model mis-specifications. Hence, a slightly more complex model structure was proposed to describe EHC after administration of rofecoxib. Rofecoxib data were evaluated using one-, two- and three-compartment models. These models were extended by a chain of compartments describing the transit of the drug from the central compartment to the gut and back. This chain constitutes a recycling loop with continuous transit and appeared sufficient for characterizing EHC in animals without a gall bladder, such as rats. Despite the numerous attempts to account for and explain inter-individual variability, parameter estimates for the recycling loops could not be calculated. Model building was subsequently accomplished by adding a ‘conversion' compartment for the introduction of a delay, which allowed the estimation of a single first-order recirculation rate (krecycling), reducing the number of parameters needed in the model (Figure 1b). As metabolite concentrations were not available for this analysis, the appearance of parent drug derived from EHC into the central compartment was based on zero-order rate from a fictitious dose into compartment 4. Furthermore, a mixture model was used to discriminate different populations in the data. Some rats displayed a clear secondary peak in their concentration–time profile, whereas others displayed a plateau phase (Figure 2). The algorithm identified two sub-populations with respect to the duration of the recycling process (D4). Each individual was classified into a sub-population of the mixture according to an empirical Bayesian computation, conditional on the individual's data and on the final estimates of the population parameters.
Figure 2.
A mixture model approach, which assumes the existence of sub-populations, was used to account for distinct patterns in the kinetic disposition of rofecoxib (10 mg kg−1). The upper panel (a) shows secondary peaks in the concentration vs time profile after i.v. administration, whereas the lower panel (b) illustrates the plateau phase in the concentration vs time profile observed after i.p. administration.
To corroborate the model estimates obtained for EHC and assess its contribution to the decrease in the total clearance of diclofenac and rofecoxib, published data from Tabata et al. (1995) and Baillie et al. (2001) in bile duct-cannulated animals were reanalysed using the PK models proposed above with rate constants associated with the recycling compartment set to zero. This is equivalent to reducing the PK model to a standard two-compartment model for diclofenac and a three-compartment model for rofecoxib. The differential equations specifying the model are presented in the appendix.
Stochastic model building
The stochastic part of the model was aimed at describing inter-individual variability in PK under the assumption of log-normal distribution of all population parameters. Therefore, an exponential model was used to account for inter-individual variability:
where θ is the population estimate for parameter P, Pi is the individual estimate and ηi is the normally distributed between-subject random variable with mean zero and variance ω2.
A model with a proportional error component was required to describe residual error in the plasma drug concentration:
where Cobs,ij is the jth observed concentration in the ith individual, Cpred,ij is the predicted concentration and ɛij is the normally distributed residual random variable with mean zero and variance σ2. The residual error term contains the remaining random variability that cannot be explained by fixed effects and refers to measurement and experimental error, such as errors in recording sampling times, and structural model mis-specification.
Influence of EHC on PD
Simulations were performed to explore the relevance of EHC on PD using the software package Berkeley Madonna 8.0 (Macey and Oster, University of California at Berkeley, USA). The inhibition of PGE2 was selected as a marker of the PD response for diclofenac, its main metabolite 4-hydroxydiclofenac and rofecoxib. Different scenarios were evaluated under the assumption that the concentration–effect relationship and protein binding are not altered by EHC. The selection of the various scenarios was based on the experimental conditions typically observed in pre-clinical models of inflammatory pain with the tested drugs.
The competitive interaction between diclofenac and 4-hydroxydiclofenac was modelled by the equation originally proposed by Holford and Sheiner (1981),
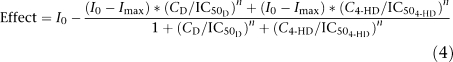 |
where I0 represents the baseline production of PGE2, which is assumed to be 100%, Imax the maximal response, which was set to 0.1% and n represents the Hill factor, with a value of 1, CD is the concentration of diclofenac and C4−HD is the concentration of 4-hydroxydiclofenac. In view of in vitro data of diclofenac and 4-hydroxydiclofenac in the whole blood assay for COX inhibition, IC50 values of 190 and 1600 ng ml−1, respectively, have been found for PGE2 inhibition (Menasse et al., 1978; Jett et al., 1999). Menasse et al. (1978) also suggest that the difference in potency between parent drug and metabolite is approximately 10-fold. Therefore, this ratio was used for the purpose of the simulations, that is, IC50 values of 190 and 1900 ng ml−1 for diclofenac and 4-hydroxydiclofenac, respectively. Data from bile duct-cannulated animals were obtained from a publication by Tabata et al. (1995).
As the metabolite of rofecoxib does not have pharmacological activity, the inhibition of PGE2 was described by an inhibitory Imax model:
where I0 represents the baseline production of PGE2, which is assumed to be 100%, Imax the maximal response to diclofenac and rofecoxib, which was set to 0.1% and n is the Hill factor, with a value of 1. In view of in vitro data of rofecoxib in the whole blood assay, an IC50 value of 100 ng ml−1 was used for PGE2 inhibition (Chan et al., 1999; Warner et al., 1999; Esser et al., 2005). PK data from bile duct-cannulated animals were obtained from publication by Baillie et al. (2001).
Three different doses of 1, 5 and 10 mg kg−1 were selected to investigate the relevance of increased exposure of diclofenac (parent drug and metabolite) and rofecoxib on PGE2 inhibition. An assessment of different ranges of variability and a formal sensitivity analysis were not deemed necessary for the purposes of this investigation.
Results
Pharmacokinetics
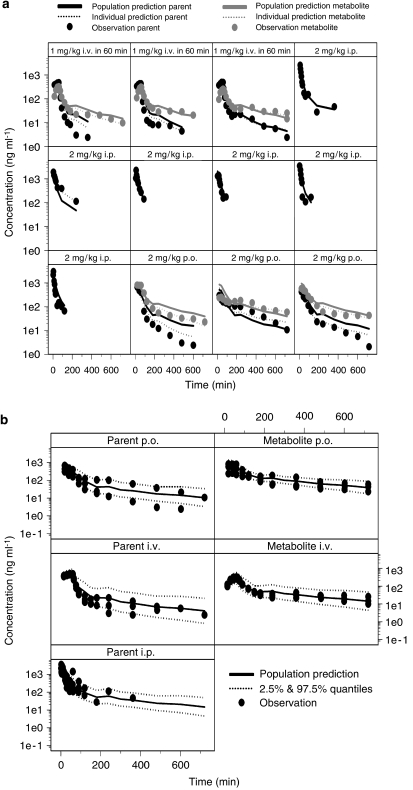
Plasma concentrations of diclofenac were analysed together with its main metabolite 4-hydroxydiclofenac to allow integrated modelling of parent drug and metabolite. A summary of the population parameter estimates is shown in Table 1. Individual measured plasma concentrations of diclofenac and 4-hydroxydiclofenac are shown in Figure 3, along with predictions of the individual and population concentration–time profiles. The concentrations of 4-hydroxydiclofenac were very high compared with the parent compound after p.o. and i.v. administration and showed no time delay associated with its appearance or re-absorption. The structural model parameters were estimated accurately with acceptable coefficients of variation (Table 1), except for k27, which shows high uncertainty (>100%). Inter-individual variability was found on diclofenac elimination as represented by k20, distribution rate constants k43 and k45 for 4-hydroxydiclofenac. Addition of inter-individual variability on k20 resulted in a reduction of the objective function of 52 (P<0.001). Bioavailability after i.p. administration was not significantly different from 100% and therefore was removed from the model. An attempt was made to estimate all parameters separately but the model failed to converge due to overparameterization. Therefore, model parameters were reduced by assuming that k20 equals k40 and k13 equals k32 equals k34. F3 was fixed to 1.
Table 1.
Population pharmacokinetic parameters and inter- and intra-individual variability of diclofenac after i.p., p.o. and i.v. administration based on model 1 (Figure 1a)
|
Parameters |
Estimate |
IIV % |
|---|---|---|
| Fixed effects | Random effects | |
| k20* (min−1) | 0.728 (60) | 39 (50) |
| V2 (ml) | 8.5 (74) | |
| k32** (min−1) | 0.048 (23) | |
| k23 (min−1) | 0.857 (60) | |
| Frequency (min) | 330 (1) | |
| F1 (%) p.o. | 72.1 (25) | |
| k43 (min−1) | 5.12 (38) | 15 (99) |
| k45 (min−1) | 3.23 (41) | 47 (67) |
| k54 (min−1) | 0.0061 (30) | |
| k27 (min−1) | 0.184 (183) | |
| k72 (min−1) | 0.0139 (57) | |
| Residual error | ||
| Proportional parent (%) | 39 (38) | |
| Proportional metabolite (%) | 20 (45) | |
Values in parentheses are relative standard errors (in percent) of the estimates. IIV % is inter-individual variability in percent.
*k20 equals k40.
**k32 equals k13 equals k34. F3 and k63 were fixed at 1 and 1000 min−1, respectively.
Figure 3.
(a) Concentration vs time profile of diclofenac and its metabolite 4-hydroxy diclofenac after a 60-min i.v. infusion of 1 and 2 mg kg−1 dose of diclofenac administered either orally or intraperitoneally. Dose was administered at time zero. The lines represent the population and individual predictions of parent and metabolite according to the oscillatory model described in Figure 1a. (b) Visual predictive check of the model for diclofenac (parent) and its metabolite. The measured plasma concentrations are shown along with lines representing the median population predictions and 2.5 and 97.5% quantiles according to the oscillatory model described in Figure 1a.
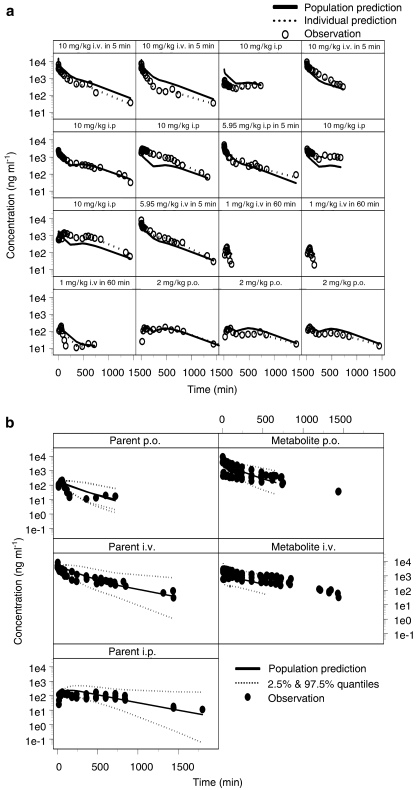
The PK profiles of rofecoxib showed large inter-individual variability. In total, 11 out of 16 rats displayed a second peak in their plasma profiles, which was independent of dose and route of administration, whereas the other five rats showed a plateau phase. Measured plasma concentrations of rofecoxib are shown in Figure 4, along with predictions of the individual and population concentration–time profiles, which were obtained by analysis of the data based on model 2 (Figure 1b). A summary of the population parameter estimates is shown in Table 2. Fixed and random effects were estimated accurately, as indicated by the diagnostic procedures and by the estimates of variability. The duration of the enterohepatic recycling process for rats with a second peak (sub-population 1) was 76 and 336 min for rats with a plateau (sub-population 2). A large proportion of animals were assigned to sub-population 1 (P(1)=0.875; Table 2). The estimate of 61% for inter-individual variability in the rate constant for enterohepatic recycling, krecycling, reveals large variations in this process despite the absence of gall bladder and periodic bile excretion pattern.
Figure 4.
(a) Concentration vs time profile of rofecoxib after a 60-min i.v. infusion of 0.5, 10 and 5.95 mg kg−1 and a 5-min i.v. infusion of 10 mg kg−1 dose of rofecoxib administered intraperitoneally and 2 mg kg−1 orally. Dose was administered at time zero. The measured plasma concentrations are shown with lines representing the population and individual predictions according to the recycling model described in Figure 1b. (b) Visual predictive check of the model for rofecoxib. The measured plasma concentrations are shown with lines representing the median population predictions and 2.5 and 97.5% quantiles according to the recycling model described in Figure 1b.
Table 2.
Population pharmacokinetic parameters and inter- and intra-individual variability of rofecoxib based on model 2 (Figure 1b)
|
Parameters |
Estimate |
IIV % |
|---|---|---|
| Fixed effects | Random effects | |
| CL (ml min−1) | 4.00 (17) | 49 (31) |
| V1 (ml) | 137 (28) | — |
| k12 (min−1) | 0.173 (79) | — |
| K21 (min−1) | 0.0699 (45) | — |
| K13* (min−1) | 0.345 (17) | — |
| krecycling (min−1) | 0.0026 (19) | 61 (56) |
| k5 (min−1) | 10 (−) | — |
| k6 (min−1) | 0.0138 (22) | — |
| F4 (%) | 30.0 (14) | |
| F5 (%) | 33.0 (37) | 77 (62) |
| F6 (%) | 15.6 (15) | — |
| Tlag (min) | 260 (−) | — |
| D4 (min) | 76.4a (28)/336b (4) | — |
| P(1)c | 0.87 (15) | — |
| Residual error | ||
| Exponential (%) | 27 (21) | — |
Values in parentheses are relative standard errors (in percent) of the estimates. IIV % is inter-individual variability in percent.
*k13 was set to be equal to k31.
Duration for sub-population 1.
Duration for sub-population 2.
Proportion of individuals assigned to sub-population 1.
In addition to parameterizing the recirculation process into an integrated PK model, data from bile duct-cannulated rats were used to substantiate the findings and estimate the contribution of EHC to changes in total clearance. Population estimates for clearance in these animals were 12 ml min−1 for diclofenac and 4.6 ml min−1 for rofecoxib. Using this information, the total transfer rate (that is, clearance to the recycling compartment and central clearance) in rats with EHC was calculated. The estimated parameter values were 6.2 ml min−1 for diclofenac and 4 ml min−1 for rofecoxib. For diclofenac, clearance to the recycling compartment was found to be as fast as the clearance from the central compartment, that is, the rate constants k23 is 0.857 min−1 vs k20 is 0.728 min−1. For rofecoxib, it was not possible to estimate the rate transfer to the recycling compartment due to the large variability in the PK data. In view of the values for clearance, the absolute increase in drug exposure due to EHC has been calculated. The relevance of EHC to overall systemic exposure was assessed by estimating the ratio between CLbile duct cannulation/CLEHC, which was 1.95 and 1.15 for diclofenac and rofecoxib, respectively. For diclofenac, EHC represents an increase of 95% in exposure (CLbile duct cannulation/CLEHC), whereas for rofecoxib this process corresponds to approximately 15% increase in total exposure, under the assumption of first-order PK at the investigated dose range. These figures may vary considerably in the presence of nonlinear PK.
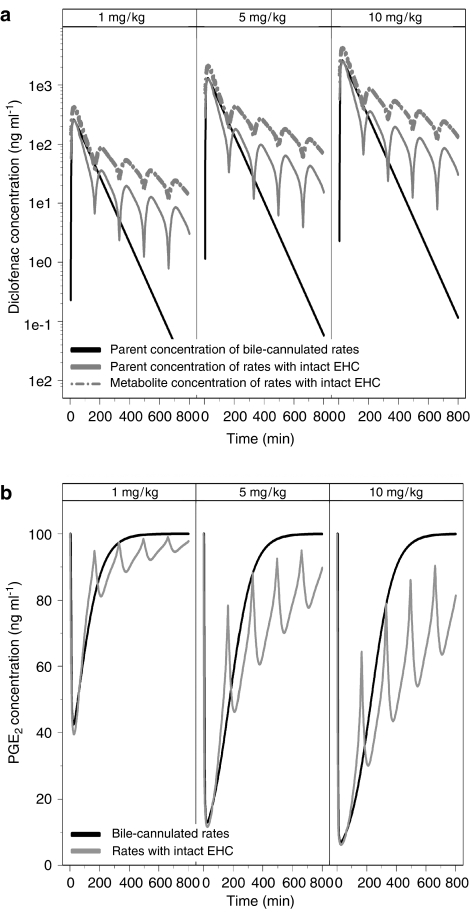
The final step in this investigation consisted of assessing the consequences of EHC for the anti-inflammatory activity by simulations. The assessment of the effect of EHC on PGE2 inhibition was based on total plasma concentration. The use of free concentration was deemed not necessary. Model-predicted population parameter estimates were used to simulate concentrations of diclofenac and rofecoxib over a period of 12 and 24 h, respectively. In Figure 5, the simulated PK profiles of diclofenac and 4-hydroxydiclofenac are displayed for rats with intact or cannulated bile ducts. These results show that the maximum inhibition of PGE2 in animals with cannulated bile duct is comparable to the values achieved in rats with intact EHC. However, the duration of inhibition is considerably shorter. At a dose of 10 mg kg−1, inhibition due to EHC is prolonged for more than 16 h. In contrast, maximum inhibition of PGE2 following a dose of 1 mg kg−1 diclofenac is decreased in rats with intact EHC as compared with bile duct-cannulated animals. This difference can be assigned to the interaction between parent compound and metabolite, which is 10-fold less potent than the parent compound. At higher doses, the contribution of 4-hydroxydiclofenac results in prolonged inhibition of PGE2.
Figure 5.
(a) Simulated concentration vs time profile of diclofenac and its 4-hydroxy metabolite after p.o. administration. (b) Simulated PGE2 inhibition vs time profile based on the competitive interaction model proposed by Holford and Sheiner (1981) (Equation (4), see text for details). The lines represent diclofenac pharmacokinetics (PK) in rats with cannulated bile duct and the PK of diclofenac and its 4-hydroxy metabolite in rats with intact enterohepatic recirculation.
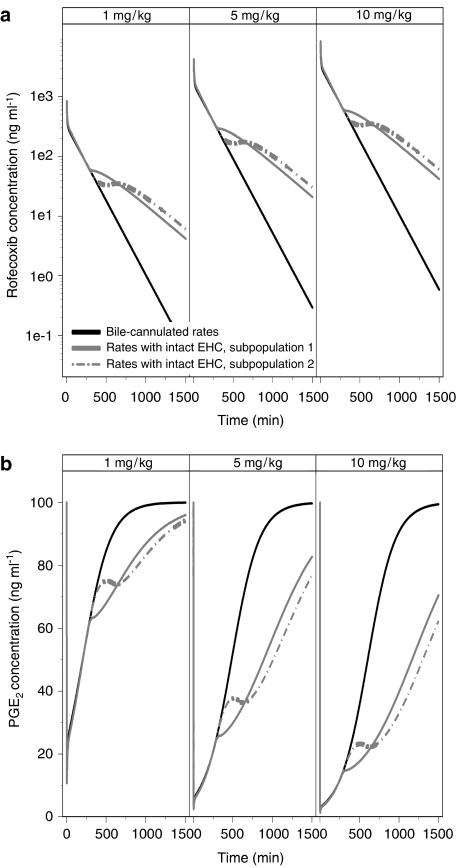
In Figure 6, the simulated concentration–time course of rofecoxib is displayed for both sub-populations in conjunction with the PK profiles after cannulation of the bile duct (Baillie et al., 2001). Due to EHC, rofecoxib concentrations in plasma are almost 100-fold higher at 24 h post-dose, as compared with bile duct-cannulated animals. This difference results in an increase in the duration of drug effect on PGE2 by more than 24 h. From Figure 6, it is also clear that EHC does not have a significant effect on the maximum inhibition of PGE2.
Figure 6.
(a) Simulated concentration vs time profile of rofecoxib after i.v. administration. (b) Simulated PGE2 inhibition vs time profile based on the inhibitory Imax model (Equation (5), see text for details). The lines represent rats with cannulated bile duct and the sub-populations 1 and 2 of rats with intact EHC.
Discussion and conclusions
In the current study, we have assessed different parameterizations of the EHC process in rats in an integrated PK model for two compounds showing distinct PK, metabolic and PD properties. Previously, different modelling approaches have been developed to describe EHC in humans, in which PK compartments have been modified to incorporate additional input into the intestine from EHC. Often these models are based on cyclic biliary emptying even though more complex models with irregular intervals may better describe true physiological variation. Yet, little attention has been given to the description of this PK phenomenon in animals and its relevance for extrapolation and scaling of PK and PD data across species, particularly when the gall bladder is absent.
Semiparametric approaches, such as the CLS model have been proposed to analyse noisy data with large inter-individual variability and interpolate PK data (Park et al., 1997; Bressolle and Gomeni, 1998). This empirical method allows estimation of model-independent PK parameters such as Tmax, Cmax and area under the concentration time curve, without any assumption on the structural form of the kinetic model. Thus, parameter estimates derived from CLS are only influenced by the data and not by any prior assumption (Bressolle and Gomeni, 1998). However, this approach is only suitable for interpolation and requires a balanced sampling design, which limits its use in drug development. Moreover, dose and route of administration have to be analysed independently, which reduces its statistical power. We have tested a series of CLS models with varying breakpoints in NONMEM (data not shown), but not all data sets resulted in a successful minimization. While one should try to increase sampling frequency in time intervals where concentrations are changing the most, as in the case of the recirculation process, CLS models seem to perform better in the analysis of noisy sparse data (Fattinger and Verotta, 1995). Such a restriction adds up to the difficulties in finding a physiological interpretation for model parameters.
On the other hand, a mechanistic approach to PK modelling that incorporates EHC by measuring portal and systemic blood concentration differences has been developed for several drugs, including diclofenac in rats (Tabata et al., 1995, 1996; Moriwaki et al., 2003). These models provide a scientific basis for the characterization of the extent and rate of local drug absorption and disposition, including EHC. However, the use of such models is limited in pre-clinical drug development or standard PK–PD studies, as portal blood sampling is required. The parametric, semiphysiological approach presented in the current investigation to assess EHC was developed taking into account the practical limitations and methodological issues discussed above. Our approach also illustrates the implication of nonlinear mixed effects modelling for accurate characterization of PK processes in pre-clinical species, in that it enables concurrent analysis of data from different experimental settings.
Previous publications have shown that both diclofenac and rofecoxib are subject to EHC (Peris-Ribera et al., 1991; Fukuyama et al., 1994; Halpin et al., 2000; Baillie et al., 2001). Our results show that the model proposed by Wajima et al. (2002) with a periodic transfer rate can describe the patterns of EHC of diclofenac in rats. The approach was used initially to describe single-subject profile in humans (Wajima et al., 2002). In addition, we have extended the model to analyse diclofenac and its metabolite, 4-hydroxy diclofenac concurrently. A separate disposition compartment was required to account for the contribution of 4-hydroxy diclofenac after p.o. and i.v. dosing.
Despite the fairly variable PK of diclofenac, model parameters were estimated accurately, as indicated by the coefficients of variation (Table 1). Inter-individual variability could be determined for k20, k43 and k45. The latter parameter clearly reflects the varying pattern in the timing and magnitude of the secondary peaks that occur over time. The frequency parameter was estimated at 330 min after i.v., p.o. and i.p. administration, indicating that the recirculation process for diclofenac is not route dependent. The relative bioavailability after p.o. dosing was estimated at 72%, which is comparable to non-compartmental results by Peris-Ribera et al. (1991) who reported 79%. We acknowledge that the proposed model for diclofenac and its metabolite may appear physiologically unrealistic, in that all the drug in the EHC compartment can re-enter the central compartment. A model with separate compartments for bile, liver and gastrointestinal tract and estimation of the bioavailable fraction from these compartments would provide a more physiological description of the recycling process. However, such an attempt has resulted in model overparameterization. An integrated analysis, including experimental data from bile duct-cannulated rats, is required to further describe metabolite formation and recycling rate.
In contrast to diclofenac, rofecoxib data were not suitable for analysis by a compartmental model, including an EHC compartment that transfers drug periodically into the central compartment. Due to the large variability in a relatively small data set, minimization was unsuccessful for all tested models based on periodic transfer rates. Therefore, we have simplified the model by the addition of a so-called conversion compartment with first-order rate and a lag time to account for EHC. A similar PK model was developed to describe EHC of ezetimibe in humans (Ezzet et al., 2001). Model parameters were estimated accurately with acceptable values for the coefficients of variation (Table 2). Additional PK data are required to obtain more precise estimates of re-absorption process associated with EHC. Despite the limitations in the current data set, the use of nonlinear mixed effects modelling enabled identification of inter-individual variability in the recycling process, as indicated by krecycling, the re-absorption rate constant. Furthermore, implementation of a mixture model allowed the identification of two distinct sub-populations in the concentration vs time profiles of rofecoxib. The duration of the re-absorption was estimated to be 76 and 336 min in sub-populations 1 and 2, respectively. Even though rats had limited access to food during the experiments, the identification of two sub-populations could be associated physiologically with food intake and differences in bile flow rate. The re-absorption rate parameter, krecycling, was also tested as a mixture parameter, but no significant improvement was observed. The fraction of rofecoxib that was re-absorbed was estimated at 30%.
From a modelling perspective, our results illustrate that the concepts of periodic transfer rate and a conversion compartment with first-order re-absorption offer a feasible alternative to characterizing EHC in animals. However, this exercise would be incomplete without further consideration of two questions relevant to pre-clinical drug development, namely what is the consequence of EHC to PK and PD and how to translate the findings in animals to humans?
We have attempted to evaluate the aforementioned questions by looking at the change in clearance in the presence and absence of EHC and subsequently simulating drug response for a biomarker common to humans and rats, namely PGE2 inhibition.
The consequences of EHC to systemic exposure were assessed by estimating the ratio between CLbile duct cannulation/CLEHC, which was 1.95 and 1.15 for diclofenac and rofecoxib, respectively. For diclofenac, the increase in exposure due to EHC is significant and could have direct implications for the PD response. For rofecoxib, it may be claimed that due to EHC the dose of 10 mg kg−1 given intravenously becomes an effective dose of 11.5 mg kg−1. However, this finding must be interpreted carefully, as for rofecoxib we have not been able to estimate the clearance to the central compartment. An extended model that includes this rate constant would be helpful to estimate the exact rofecoxib amount reaching the central compartment over time.
Our simulations to evaluate the impact of EHC on PD and, potentially, its meaning for the scaling of data from animals to humans, reveal that EHC can prolong the inhibition of PGE2. However, EHC does not seem to affect the maximum PGE2 inhibition. From the simulations with diclofenac, total PGE2 inhibition in bile duct-cannulated rats was slightly higher than in rats with intact EHC, but such effect was short-lasting. This effect is reduced if one takes into account the contribution of the metabolite, which is 10-fold less potent than the parent compound.
Whereas drugs such as diclofenac and rofecoxib do exhibit significant EHC in humans, this PK phenomenon has often been identified at phase I clinical studies or even later in clinical development. Thus far, we are not aware of any research attempt aimed at describing EHC in a quantitative manner in animals. We have shown that modelling of EHC in pre-clinical species enables early prediction of the influence of EHC to PK and PD. Prolonged exposure to drug as a consequence of EHC may have serious implications, in particular when drug safety is a concern, as is the case for many non-steroidal anti-inflammatory drugs.
In summary, we have shown that empirical and semiphysiological PK models can be used in conjunction with nonlinear mixed-effect modelling to characterize data with large inter-individual variability. The approach allows concomitant analysis of data from different experimental sources, with fewer restrictions to frequency or intervals for sample collection.
Abbreviations
- CLS
constrained longitudinal spline
- COX
cyclo-oxygenase
- EHC
enterohepatic recirculation
- PD
pharmacodynamics
- PG
prostaglandins
- PK
pharmacokinetics
Appendix
Equations used for the pharmacokinetic modelling of diclofenac and rofecoxib
For diclofenac and its metabolite 4-hydroxydiclofenac:
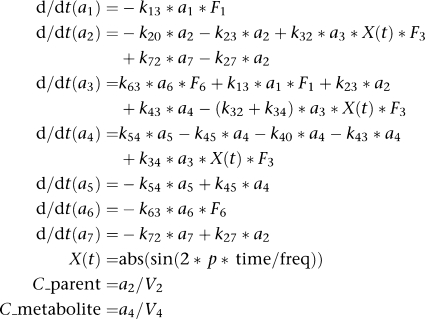 |
where V4 and F3 were fixed to 1, ki,j are transfer rates, F1 is the bioavailable fraction of rofecoxib after p.o. administration, F6 is the bioavailable fraction of rofecoxib after i.p. administration, F3 is the bioavailable fraction of drug from enterohepatic recirculation (EHC), V2 is the volume of the central compartment of diclofenac and V4 is the volume of the central compartment of 4-hydroxydiclofenac, freq is the frequency time of recycling. X(t) is the periodic transfer rate of parent and metabolite from EHC to the central compartment.
For rofecoxib:
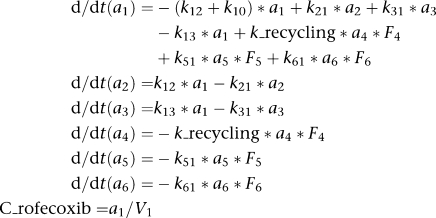 |
where ki,j are transfer rates, F4 is the bioavailable fraction of rofecoxib that undergoes recycling, F5 is the bioavailable fraction of rofecoxib after i.p. administration, F6 is the bioavailable fraction of rofecoxib after p.o. administration, V1 is the volume of the central plasma compartment of rofecoxib. The delay in EHC was described by a lag time for compartment 4.
Conflict of interest
The authors state no conflict of interest.
References
- Alvarez-Bujidos L, Ortiz AI, Molina-Martinez IT, Cubria C, Ordonez D. Pharmacokinetics of intravenous luxabendazole in rabbits: influence of the enterohepatic circulation. Biopharm Drug Dispos. 1998;19:341–347. doi: 10.1002/(sici)1099-081x(199807)19:5<341::aid-bdd110>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Baillie TA, Halpin RA, Matuszewski BK, Geer LA, Chavez-Eng CM, Dean D, et al. Mechanistic studies on the reversible metabolism of rofecoxib to 5-hydroxyrofecoxib in the rat: evidence for transient ring opening of a substituted 2-furanone derivative using stable isotope-labeling techniques. Drug Metab Dispos. 2001;29:1614–1628. [PubMed] [Google Scholar]
- Beal SL, Sheiner LB. NONMEM User's Guide. NONMEM Project Group, University of California at San Fransisco: San Fransisco, CA; 1999. [Google Scholar]
- Bressolle F, Gomeni R. Predictive performance of a semiparametric method to estimate population pharmacokinetic parameters using NONMEM. J Pharmacokinet Biopharm. 1998;26:349–361. doi: 10.1023/a:1023289527297. [DOI] [PubMed] [Google Scholar]
- Chan CC, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, et al. Rofecoxib [Vioxx, MK-0966; 4-(4′-methylsulfonylphenyl)-3-phenyl-2-(5H)-furanone]: a potent and orally active cyclooxygenase-2 inhibitor. Pharmacological and biochemical profiles. J Pharmacol Exp Ther. 1999;290:551–560. [PubMed] [Google Scholar]
- Eeckhoudt SL, Evrard PA, Verbeeck RK. Biliary excretion and enterohepatic cycling of R- and S-flurbiprofen in the rat. Drug Metab Dispos. 1997;25:428–430. [PubMed] [Google Scholar]
- Esser R, Berry C, Du Z, Dawson J, Fox A, Fujimoto RA, et al. Preclinical pharmacology of lumiracoxib: a novel selective inhibitor of cyclooxygenase-2. Br J Pharmacol. 2005;144:538–550. doi: 10.1038/sj.bjp.0706078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzet F, Krishna G, Wexler DB, Statkevich P, Kosoglou T, Batra VK. A population pharmacokinetic model that describes multiple peaks due to enterohepatic recirculation of ezetimibe. Clin Ther. 2001;23:871–885. doi: 10.1016/s0149-2918(01)80075-8. [DOI] [PubMed] [Google Scholar]
- Fattinger KE, Verotta D. A nonparametric subject-specific population method for deconvolution: I. Description, internal validation, and real data examples. J Pharmacokinet Biopharm. 1995;23:581–610. doi: 10.1007/BF02353463. [DOI] [PubMed] [Google Scholar]
- Fukuyama T, Yamaoka K, Ohata Y, Nakagawa T. A new analysis method for disposition kinetics of enterohepatic circulation of diclofenac in rats. Drug Metab Dispos. 1994;22:479–485. [PubMed] [Google Scholar]
- Giagoudakis G, Markantonis SL. An alternative high-performance liquid chromatographic method for the determination of diclofenac and flurbiprofen in plasma. J Pharm Biomed Anal. 1998;17:897–901. doi: 10.1016/s0731-7085(97)00258-6. [DOI] [PubMed] [Google Scholar]
- Halpin RA, Geer LA, Zhang KE, Marks TM, Dean DC, Jones AN, et al. The absorption, distribution, metabolism and excretion of rofecoxib, a potent and selective cyclooxygenase-2 inhibitor, in rats and dogs. Drug Metab Dispos. 2000;28:1244–1254. [PubMed] [Google Scholar]
- Holford NH, Sheiner LB. Pharmacokinetic and pharmacodynamic modeling in vivo. Crit Rev Bioeng. 1981;5:273–322. [PubMed] [Google Scholar]
- Jett MF, Ramesha CS, Brown CD, Chiu S, Emmett C, Voronin T, et al. Characterization of the analgesic and anti-inflammatory activities of ketorolac and its enantiomers in the rat. J Pharmacol Exp Ther. 1999;288:1288–1297. [PubMed] [Google Scholar]
- Menasse R, Hedwall PR, Kraetz J, Pericin C, Riesterer L, Sallman A, et al. Pharmacological properties of diclofenac sodium and its metabolites. Scand J Rheumatol. 1978;22 Suppl:5–16. doi: 10.3109/03009747809097211. [DOI] [PubMed] [Google Scholar]
- Moriwaki T, Yasui H, Yamamoto A. A recirculatory model with enterohepatic circulation by measuring portal and systemic blood concentration difference. J Pharmacokinet Pharmacodyn. 2003;30:119–144. doi: 10.1023/a:1024415730100. [DOI] [PubMed] [Google Scholar]
- Ogiso T, Kitagawa T, Iwaki M, Tanino T. Pharmacokinetic analysis of enterohepatic circulation of etodolac and effect of hepatic and renal injury on the pharmacokinetics. Biol Pharm Bull. 1997;20:405–410. doi: 10.1248/bpb.20.405. [DOI] [PubMed] [Google Scholar]
- Park K, Verotta D, Blaschke TF, Sheiner LB. A semiparametric method for describing noisy population pharmacokinetic data. J Pharmacokinet Biopharm. 1997;25:615–642. doi: 10.1023/a:1025769431364. [DOI] [PubMed] [Google Scholar]
- Peris-Ribera JE, Torres-Molina F, Garcia-Carbonell MC, Aristorena JC, Pla-Delfina JM. Pharmacokinetics and bioavailability of diclofenac in the rat. J Pharmacokinet Biopharm. 1991;19:647–665. doi: 10.1007/BF01080872. [DOI] [PubMed] [Google Scholar]
- Ploeger BA, Meulenbelt J, DeJongh J. Physiologically based pharmacokinetic modeling of glycyrrhizic acid, a compound subject to presystemic metabolism and enterohepatic cycling. Toxicol Appl Pharmacol. 2000;162:177–188. doi: 10.1006/taap.1999.8843. [DOI] [PubMed] [Google Scholar]
- Priymenko N, Garnier F, Ferre JP, Delatour P, Toutain PL. Enantioselectivity of the enterohepatic recycling of carprofen in the dog. Drug Metab Dispos. 1998;26:170–176. [PubMed] [Google Scholar]
- Reuter BK, Davies NM, Wallace JL. Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology. 1997;112:109–117. doi: 10.1016/s0016-5085(97)70225-7. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Magnusson BM, Burczynski FJ, Weiss M. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet. 2002;41:751–790. doi: 10.2165/00003088-200241100-00005. [DOI] [PubMed] [Google Scholar]
- Schaiquevich P, Niselman A, Rubio M. Comparison of two compartmental models for describing ranitidine's plasmatic profiles. Pharmacol Res. 2002;45:399–405. doi: 10.1006/phrs.2002.0954. [DOI] [PubMed] [Google Scholar]
- Seitz S, Boelsterli UA. Diclofenac acyl glucuronide, a major biliary metabolite, is directly involved in small intestinal injury in rats. Gastroenterology. 1998;115:1476–1482. doi: 10.1016/s0016-5085(98)70026-5. [DOI] [PubMed] [Google Scholar]
- Tabata K, Yamaoka K, Fukuyama T, Nakagawa T. Evaluation of intestinal absorption into the portal system in enterohepatic circulation by measuring the difference in portal-venous blood concentrations of diclofenac. Pharm Res. 1995;12:880–883. doi: 10.1023/a:1016217221977. [DOI] [PubMed] [Google Scholar]
- Tabata K, Yamaoka K, Fukuyama T, Nakagawa T. Local absorption kinetics into the portal system using the portal-venous concentration difference after an oral dose of diclofenac in the awakening rat. Accelerative effect of bile on intestinal absorption of diclofenac. Drug Metab Dispos. 1996;24:216–220. [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wajima T, Yano Y, Oguma T. A pharmacokinetic model for analysis of drug disposition profiles undergoing enterohepatic circulation. J Pharm Pharmacol. 2002;54:929–934. doi: 10.1211/002235702760089045. [DOI] [PubMed] [Google Scholar]
- Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn. 2001;28:171–192. doi: 10.1023/a:1011555016423. [DOI] [PubMed] [Google Scholar]