Abstract
Background and purpose:
We investigated the ability of celecoxib, a selective cyclooxygenase-2 (COX-2) inhibitor, to modulate expression of ICAM-1 and VCAM-1 in the colon cancer cell line HT29.
Experimental approach:
We analysed the effect of celecoxib on ICAM-1 and VCAM-1 protein and mRNA expression in HT29 cells. Experiments were performed in the presence of mitogen-activated protein kinases (MAPK) inhibitors to evaluate the involvement of these kinases in this phenomenon. We evaluated adhesion of HT29 cells to FCS-coated plastic wells in the presence of celecoxib or MAPK inhibitors. Furthermore, we studied the effect of celecoxib on apoptosis.
Key results:
Celecoxib down-regulated ICAM-1 and VCAM-1 expression in HT29 cells in a time- and dose-dependent way. Celecoxib reduced activation of p38 and p55 c-Jun terminal NH2 kinase (JNK) MAPKs, but did not affect p46 JNK or p42/44 MAPK phosphorylation. Pretreatment with SB202190 or SP600125, specific inhibitors of p38 and JNK MAPKs, respectively, reduced ICAM-1 and VCAM-1 expression in HT29 cells dose-dependently. Adhesion of HT29 cells to FCS-coated plastic wells was inhibited dose-dependently by celecoxib, and also by SB202190 and SP600125. Celecoxib showed a pro-apoptotic effect, inducing Bax and BID but down-regulating Bcl-2.
Conclusions and implications:
Our findings show that celecoxib caused down-regulation of ICAM-1 and VCAM-1, affecting the adhesive properties of HT29 cells in a COX-2 independent way, inhibiting p38 and p55 MAPKs and activating a pro-apoptotic pathway.
Keywords: celecoxib, HT29 cells, ICAM-1, VCAM-1, MAPKs, apoptosis
Introduction
Cyclooxygenase-2 (COX-2) is an enzyme that regulates prostaglandin synthesis; it is overexpressed at sites of inflammation and in several epithelial cancers, such as colon and breast cancer (Eberhart et al., 1994; Hwang et al., 1998). COX-2 is involved in resistance to apoptosis (Tsujii and DuBois, 1995), in angiogenesis (Tsujii et al., 1998) and in tumour-cell invasiveness (Tsujii et al., 1997; Kakiuchi et al., 2002), suggesting that it may play a role in tumour initiation and promotion.
Epidemiological studies have demonstrated a correlation between the use of non-steroidal anti-inflammatory drugs and a reduced development and growth of malignancies (Giovannucci et al., 1995). Non-steroidal anti-inflammatory drugs are chemopreventive against colorectal cancer, in part through their inhibitory effects on COX-2. The selective COX-2 inhibitors developed in recent years have in some cases been found to be effective in the prevention of colorectal cancer (Arber et al., 2006).
Celecoxib is a selective COX-2 inhibitor (IC50=0.04 μM; Penning et al., 1997) with a chemical structure characterised by a diarylheterocycle moiety and a sulphonamide group. It was approved in 1999 by the FDA, and in 2001 by the EMEA, for use as an adjunct to surgery and for further endoscopic surveillance, with the aim of reducing the number of adenomatous intestinal polyps in familial adenomatous polyposis (FDA, 1999; EMEA, 2001). Treatment with celecoxib had been shown to reduce the number of polyps, both in patients and in an animal model of familial adenomatous polyposis (Giovannucci et al., 1995; Oshima et al., 1996). The mechanisms of the cancer chemopreventive action of celecoxib and other COX inhibitors are complex and not clearly understood (Grosch et al., 2006). Inhibition of COX-2 certainly contributes to the chemopreventive effects of these drugs, but it is also clear that they can work even in cells with minimal COX-2 expression and that many of the cell-growth-inhibitory responses they induce are COX-2 independent (Smith et al., 2000; Gately and Li, 2004).
A less-studied aspect is the ability of COX-2 inhibitors to interfere with cellular adhesion machinery. Cell adhesion is a pivotal mechanism for the growth and spread of metastases, and specific adhesion molecules are required by tumour cells to localise in different tissues. Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are members of the immunoglobulin superfamily of proteins and are crucially involved not only in trafficking of leucocytes through endothelial and epithelial barriers, but also in metastasis formation and in the immune surveillance of tumours (Neeson et al., 2003; Yamada et al., 2006). Human epithelial cells are normally devoid of ICAM-1 and VCAM-1 expressions, but induction of such expressions has been reported in response to pro-inflammatory cytokines such as IFNγ, IL-1β and IL-6 (Kaiserlian et al., 1991; Parkos et al., 1996). Ligands for ICAM-1 and VCAM-1 are, respectively, LFA-1 (αLβ2 complex) and VLA-4 (α4β1 complex), which are expressed on blood cells and microvascular endothelial cells (Steward et al., 1995; ten Kate et al., 2004). The interaction between ICAM-1 and VCAM-1 and their specific ligands expressed on microvascular endothelial cells or on peripheral blood cells could be involved in adhesion mechanisms that facilitate adhesion of cancer cells to specific microvascular endothelium, such as in the lung, and subsequently in the formation of metastases.
HT29 cells are a colon cancer cell line widely utilised as experimental models in colon cancer research. It is known that HT29 cells normally express not only COX-2, but also ICAM-1 and VCAM-1, and so this cell line may be a useful model to study drug modulation of adhesion molecules. The present study addressed the question of whether celecoxib affects ICAM-1 and VCAM-1 expressions in the HT29 cell line. The time- and dose-dependence of ICAM-1 and VCAM-1 expressions on HT29 cells evoked by celecoxib were investigated. The involvement of mitogen-activated protein kinases (MAPKs) in adhesion molecule expression on HT29 cells was evaluated using selective inhibitors of these kinases. The effects of celecoxib on apoptosis in HT29 cells were also studied, evaluating its involvement in the expression of some of the Bcl-2 family proteins such as Bcl-2, Bax and BID.
Methods
Cell culture
Cells of the human carcinoma cell line HT29 were grown in culture dishes as a monolayer in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS) (vol/vol), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin and maintained at 37 °C in 5% CO2-humidified atmosphere. Cells were subcultured following enzymatic digestion using trypsin/EDTA solution.
Cell incubation
The incubation medium was composed of RPMI 1640 supplemented with 2% heat-inactivated FCS (vol/vol), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. In time-course experiments, HT29 cells (3 × 105 cells per well) were treated with suitable stimuli from time 0 to 4 or 8 h and the cells were processed at 30 min and then every hour for mRNA or protein extraction, respectively. In dose–response experiments, HT29 cells were treated with 0.001–10 μM celecoxib or rofecoxib for 4 h. Cells were pre-incubated for 30 min with the irreversible inhibitor SB202190 for p38 MAPK and washed twice or pre-incubated with the reversible p46/55 inhibitor SP600125 and without washing incubated with celecoxib or rofecoxib in time course (10 μM ) or dose–response (0.001–10 μM) experiments. In refilling experiments, HT29 cells were treated with 10 μM celecoxib and every 2 h they were washed twice. Celecoxib was then re-added 2 hourly up to 8 h. Following the appropriate treatments, cells were lysed for western blot analysis.
Protein extraction and western blot
HT29 cells were lysed with ice-cold lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 5 mM EDTA, 1 μl ml−1 protease inhibitors, 0.1 mM ZnCl2 and 1 mM phenylmethylsulphonyl fluoride) or sucrose buffer (220 mM mannitol, 70 mM sucrose, 50 mM HEPES-KOH (pH 7.2), 10 mM KCl, 5 mM EGTA, 2 mM MgCl2 and 1 μl ml−1 protease inhibitor mixture) to obtain, respectively, total or subcellular fractionation. Protein concentrations were determined using a BCA protein assay following the manufacturer's directions. HT29 cell lysate samples containing 50 μg of protein underwent SDS-PAGE using an 8 or 15% gel. Proteins were transferred to a polyvinyldenedifluoride membrane and then incubated with SuperBlock blocking buffer. Membranes were probed with primary antibodies diluted in PBS containing 0.1% Tween-20 (PBS-T) raised against ICAM-1 or VCAM-1 (both 1:200, 2 h room temperature); phospho-p38, phospho-p42/44 or phospho-p46/55 (all three 1:2000, overnight at 4 °C); Bax, BID or Bcl-2 (all three 1:200, 1 h room temperature). The appropriate secondary antibodies used were diluted 1:10 000 in PBS-T and incubated for 30 min at room temperature. To confirm the homogeneity of cytosolic or mitochondrial proteins loaded, the membranes were stripped and incubated, respectively, with β-actin (1:5000) or COX 4 (cytochrome c oxidase subunit IV; 1:1000) monoclonal antibodies and subsequently with secondary antibodies for 30 min at room temperature. The membranes were covered with Western Lightning Chemiluminescence Reagent Plus and then exposed to Hyperfilm ECL film. Protein bands were quantified using the Gel Pro.Analyser 4.5, 2000 software.
RNA extraction and reverse transcription
Total RNA was isolated from HT29 cells with the NucleoSpin RNA II kit, following the manufacturer's directions as described in the instructions included in the kit. About 1 μg of total RNA was reverse-transcribed into cDNA in a total volume of 20 μl using the RevertAid H Minus M-MuLV Reverse Strand cDNA Synthesis kit using 0.5 μg of oligo(dT)18 primers.
About 3–5 μl of reverse transcription-PCR reactions was subjected to 35 cycles of PCR for amplification of VCAM-1, ICAM-1 or β-actin. PCR was performed in a 50 μl reaction volume containing 1 μM primers, 200 μM of each dNTPs and 1.25 U of Taq DNA polymerase. After denaturing at 94 °C for 5 min, cDNA was subjected to 35 cycles of PCR amplification, performed using a Tpersonal 48 Whatman Biometra thermal cycler. PCR conditions were 95 °C for 30 s, 60 °C for 30 s and 72 °C for 45 s for VCAM-1 amplification; 94 °C for 30 s, 62 °C for 30 s and 72 °C for 2 min for ICAM-1 amplification; and 95 °C for 45 s, 60 °C for 45 s and 72 °C for 90 s for β-actin amplification, with a final extension of 70 °C for 10 min. Positive- and negative-strand PCR primers used were as follows: VCAM-1—forward primer, 5′-TCCGTCTCATTGACTTGCAG-3′; reverse primer, 5′-TTCCAGGGACTTCC TGTCTG-3′ (399 bp fragment); ICAM-1—forward primer, 5′-GCAAGCTCCCAGTGAAATGCAAAC-3′; reverse primer, 5′-TGTCTACTGACCCCAACCCTTGATG-3′ (498 bp fragment); β-actin—forward primer, 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′; reverse primer, 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′ (660 bp fragment). The PCR products were separated by gel electrophoresis, stained with ethidium bromide and visualised and photographed (Digital camera Canon Power Shot G6) under UV transillumination (Vilber Lourmat). Amplicon size was verified by comparison with a DNA mass ladder.
Fluorescent labelling of HT29 cells
Commercial fluorescent cell linker kit PKH67 was used for membrane labelling of HT29 cells, following the manufacturer's directions as described in the kit. The staining efficiency was monitored by fluorescent microscopy.
Adhesion assay
HT29 cells, labelled as described above, were plated at 7 × 104 cells per well in a final volume of 0.25 ml buffered salt solution (138 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 1 mM MgCl2, 1 mM CaCl2, pH 7.4). Celecoxib or rofecoxib were incubated with HT29 cells for 4 h at 37 °C in 5% CO2 in 24-well plates. Some experiments were performed pretreating HT29 cells with SB202190 or SP600125 at 0.001–10 μM or anti-ICAM-1 or anti-VCAM-1 monoclonal antibodies (mAbs) at 5 μM for 30 min. After incubation, non-adherent HT29 cells were removed by washing three times with 1 ml buffered salt solution.
The centre of each well was analysed by fluorescence image analysis (Dianzani et al., 2003). Adherent cells were counted using Image Pro Plus Software for micro-imaging. Single experimental points were assayed in quadruplicate, and the standard error of the four replicates was below 10% in all cases. Data are presented as percentage adhesion versus the control value, control adhesion being measured on untreated HT29 cells. Control adhesion was 65±6 cells per microscope field (n=15).
Statistical analysis
Data are expressed as means±s.e.m. Statistical analysis was performed with GraphPad Prism 3.0 software. One-way ANOVA was performed, and Dunnett's multiple comparison was used to determine significant differences between means. P⩽0.05 was considered significant.
Materials
Mouse mAb against β-actin, protease inhibitors cocktail, green fluorescent cell linker kit PKH67 and heparin were from Sigma (Chemical Co., St Louis, MO). RPMI 1640 and FCS were from GIBCO BRL (Grand Island, NY). Celecoxib and rofecoxib were from Sequoia Research Products Ltd. (Pangbourne, UK). The MAPK inhibitors SB202190, PD98059 and SP600125 were from Calbiochem (San Diego, CA). The BCA Protein Assay and SuperBlock blocking buffer were from Pierce Biotechnology Inc. (Rockford, IL); Polyvinyldenedifluoride was from Millipore (Bedford, MA). Rabbit polyclonal antibodies against human ICAM-1, Bax, BID, Bcl-2, goat polyclonal antibodies against VCAM-1, anti-mouse, anti-goat and anti-rabbit Ig horseradish peroxidase-linked whole antibodies were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibodies against the phosphorylated forms of p38, p42/44 and p46/55 MAPKs were from Cell-Signalling (Beverly, MA). The COX 4 anti-mouse mAb was from Abcam plc (Cambridge, UK). Hyperfilm ECL was from Amersham Biosciences Corp. (Piscataway, NJ) and Western Lightning Chemiluminescence Reagent Plus was from PerkinElmer Life Science (Cetus, Norwalk, CT). Gel Pro.Analyser 4.5, 2000 was from Media Cybernetics Inc. (Leiden, The Netherlands). Image Pro Plus Software for micro-imaging was from Media Cybernetics (version 5.0). GraphPad Prism 3.0 software was from GraphPad software (San Diego, CA). All the other reagents utilised were from Sigma. NucleoSpin RNA II was from Macherey-Nagel (Düren, Germany). RevertAid H Minus M-MuLV Reverse Strand cDNA Synthesis kit and Taq DNA Polymerase were from Fermentas (Harrington Court, Burlington, Ontario). All primers were synthesised and purified by MGW-Biotech (Ebersberg, Germany).
Results
Effect of celecoxib on ICAM-1 and VCAM-1 expressions on HT29 cells
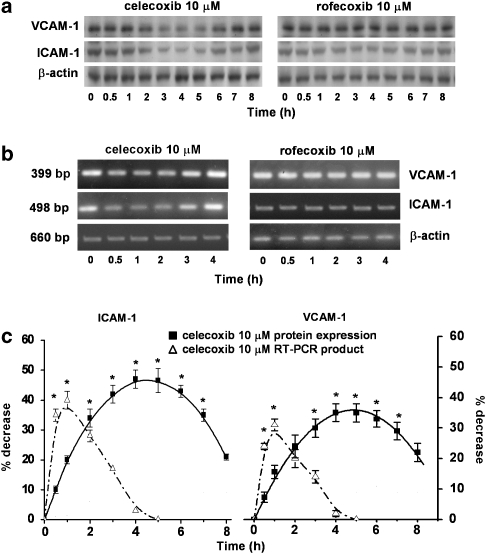
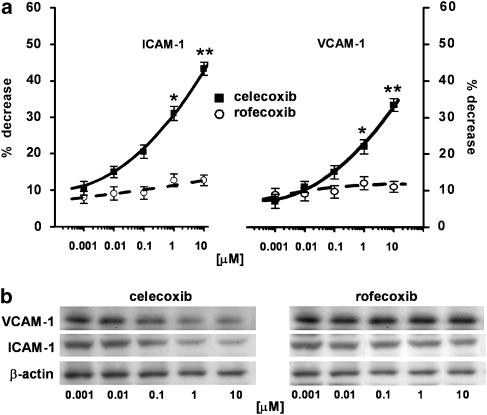
HT29 cells normally express ICAM-1 and VCAM-1. We had already shown that stimulation of HT29 cells with 10 ng ml−1 TNF-α for 4 h did not significantly modify VCAM-1 and ICAM-1 expressions. Thus, the following experiments were performed without exposing the cells to this cytokine. Time-course experiments (0–8 h) demonstrated that the inhibitory effect of 10 μM celecoxib on ICAM-1 and VCAM-1 expressions was maximal after 4 h incubation and this was maintained up to 6 h (Figure 1a). Densitometric analysis of the autoradiograms showed that celecoxib induced maximum decreases of approximately 45 and 35%, respectively, of ICAM-1 and VCAM-1 expressions, versus celecoxib-untreated cells, with a loss of effect after 6 h (n=3; Figure 1c). In the same experimental conditions, rofecoxib, another selective COX-2 inhibitor with a different chemical structure, did not have any significant effect on ICAM-1 or VCAM-1 expressions (Figure 1a). When the time-dependence of celecoxib's effect on ICAM-1 and VCAM-1 was studied by evaluating mRNA expression, this effect was similarly lost at longer time periods of incubation (Figures 1b and c). However, inhibition of the expression of mRNA showed its maximum much earlier, betweeen 30 min and 2 h incubation (n=3; Figures 1b and c). As shown in Figure 2, dose–response experiments performed in the range 0.001–10 μM gave maximum inhibition of adhesion molecules at 10 μM celecoxib with a higher inhibition (∼50%) of ICAM-1. Over this concentration range, rofecoxib did not affect ICAM-1 or VCAM-1 expressions (n=4; Figures 2a and b). As the maximum inhibition of ICAM-1 and VCAM-1 expressions was achieved at 10 μM and after 4 h incubation with celecoxib, we chose these two experimental conditions for all following experiments.
Figure 1.
Time course of celecoxib and rofecoxib on ICAM-1 and VCAM-1 expressions. (a) HT29 cells were incubated with celecoxib or rofecoxib (10 μM) from time 0 to 8 h. At the time indicated, cells were processed for western blot analysis as described in the Methods section. The immunoblot shown is representative of three separate experiments. (b) Agarose gel showing products of RT-PCR assay for ICAM-1 and VCAM-1 cDNA from HT29 cells. Cells were incubated with celecoxib or rofecoxib (10 μM) from time 0 to 4 h. At the time indicated, cells were processed for mRNA extraction. Single transcripts corresponding to the sizes predicted for ICAM-1 (498 bp) and VCAM-1 (399 bp) were detected. β-actin was used as a housekeeping gene. Data are representative of three separate experiments. (c) Densitometric analysis of western blot and RT-PCR products. Values of protein expression, normalised to β-actin, are expressed as percentage decrease versus control, mean±s.e.m., n=3. Values of RT-PCR products were obtained from HT29 cells incubated with celecoxib (10 μM) from time 0 to 4 h. Data were analysed by one-way ANOVA and the Dunnett's test (*P<0.05 versus control). RT-PCR, reverse transcription-PCR.
Figure 2.
Dose–response relationships of celecoxib and rofecoxib on ICAM-1 and VCAM-1 expression. (a) HT29 cells were incubated with celecoxib or rofecoxib 0.001–10 μM for 4 h and then subjected to western blot analysis. Data, normalised to β-actin, are expressed as percentage decrease versus control, mean±s.e.m., n=4. Data were analysed by one-way ANOVA and the Dunnett's test (*P<0.05; **P<0.01 versus control). (b) The immunoblot shown is representative of four separate experiments.
Effect of celecoxib on MAPK activation
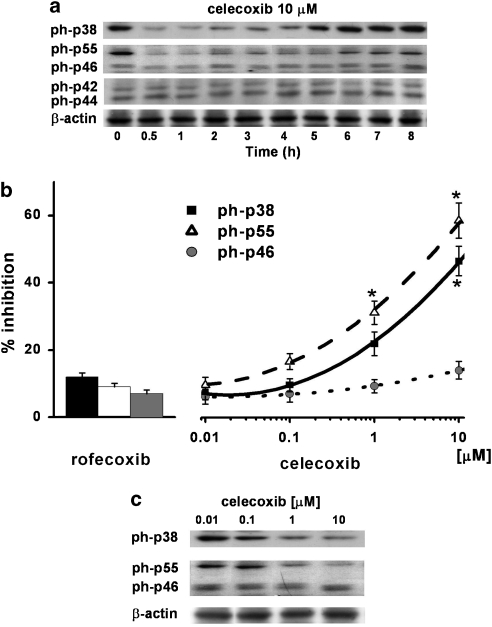
Based on the reported ability of celecoxib to inhibit different MAPKs (Chun et al., 2004; Steffel et al., 2005) and the structural analogy between celecoxib and the p38 MAPK inhibitor SB202190 (see Discussion), we evaluated the effect of celecoxib on p38, c-Jun terminal NH2 kinase (JNK) p46/55 and p42/44 activation in HT29 cells. Time-course experiments (0–8 h) demonstrated that the inhibitory effect of 10 μM celecoxib on the activation of p38 and p55 MAPKs started after 30 min of stimulation and was maintained up to 4 h, returning to initial levels after 6–8 h (n=3). Neither p42/44 nor p46 MAPK was affected by celecoxib treatment (Figure 3a). Treatment of HT29 cells with celecoxib (0.001–10 μM) for 4 h resulted in the inhibition of the phosphorylation of p38 and p55 MAPKs, with a maximum of about 50 and 60%, respectively, at 10 μM (n=3; Figures 3b and c). In the same experimental conditions, rofecoxib showed no effect on the activation of any of the MAPKs evaluated.
Figure 3.
Effect of celecoxib on MAPKs. (a) HT29 cells were incubated with celecoxib (10 μM) from time 0 to 8 h. At the time indicated, cells were processed for western blot analysis as described in the Methods section. The immunoblot shown is representative of three separate experiments. ph indicates the phosphorylated form of each MAPK analysed. (b) Densitometric analysis of ph-p38, ph-p55 and ph-p46 MAPK activation. HT29 cells were incubated with celecoxib (0.01–10 μM) or rofecoxib (10 μM) for 4 h and then processed for western blot analysis as described in the Methods section. Data, normalised to β-actin, are expressed as percentage inhibition versus control, mean±s.e.m., n=3. Data were analysed by one-way ANOVA and the Dunnett's test (*P<0.05 versus control). (c) The immunoblot shown is representative of three separate experiments.
Effect of MAPK inhibitors on ICAM-1 and VCAM-1 expressions
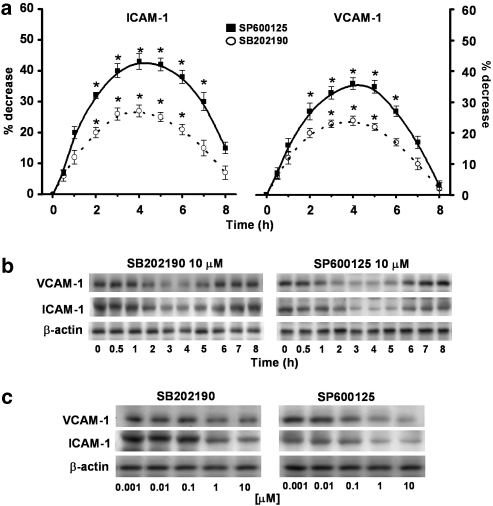
In order to assess whether the inhibitory effect of celecoxib on ICAM-1 and VCAM-1 expression may be related to drug effects on the reported MAPKs, well-known experimental inhibitors of these enzymes were compared. Time-course experiments (0–8 h) demonstrated that SB202190 or SP600125 (10 μM) down-regulated ICAM-1 and VCAM-1 expressions, with the maximal inhibition achieved after 4 h incubation, maintained up to 6 h and fading thereafter (n=3; Figures 4a and b). The extent of the inhibition that could be obtained with SP600125 (maximum at ∼40%) was higher than that measured with SB202190 (maximum at ∼25%). Treatment of HT29 cells, either with SB202190 or SP600125, decreased ICAM-1 and VCAM-1 expressions, respectively, in a dose-dependent way (0.001–10 μM; n=4; Figure 4c).
Figure 4.
Effect of MAPK inhibitors on ICAM-1 and VCAM-1 expressions. (a) HT29 cells were pretreated with SB202190 or SP600125 (10 μM) for 30 min and then incubated from time 0 to 8 h. At the time indicated, cells were processed for western blot analysis as described in the Methods section and analysed by densitometry. Data, normalised to β-actin, are expressed as percentage inhibition versus control, mean±s.e.m., n=3. Data were analysed by one-way ANOVA and the Dunnett's test (*P<0.05; **P<0.01 versus control). (b) The immunoblot shown is representative of three separate experiments. (c) HT29 cells were pretreated with SB202190 (0.001–10 μM) for 30 min and then plated for 4 h or directly incubated with SP600125 (0.001–10 μM) for 4 h and 30 min. Cells were processed for western blot analysis as described in the Methods section. The immunoblot shown is representative of four separate experiments.
Effect of celecoxib and MAPK inhibitors on HT29 cell adhesion to FCS-coated plastic wells
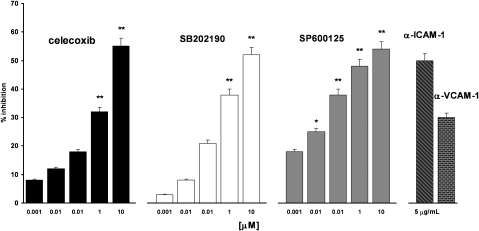
ICAM-1 and VCAM-1 are involved in the adhesion mechanisms that underlie interactions between cancer cells or between cancer cells with other cell types or the extracellular matrix. We previously showed that celecoxib inhibits the expression of these adhesion molecules on HT29 cells, and thus to evaluate the functional significance of the observation, we assessed the adhesion of HT29 cells to FCS-coated plastic wells. As shown in Figure 5, celecoxib inhibited HT29 cell adhesion to FCS-coated plastic wells in a dose-dependent way and in the same concentration range evaluated previously (n=5). The maximum inhibition (∼55%), achieved even here at 10 μM, was comparable with those measured in assays of the effects of celecoxib on ICAM-1 and VCAM-1 expressions. In the same experimental conditions, rofecoxib inhibited HT29 cells adhesion to plastic only at 10 μM and to a lower extent than celecoxib (∼30%) (data not shown). The effect of celecoxib in the adhesion test was matched by those of the MAPK inhibitors SB202190 and SP600125. Both drugs, in the same concentration range evaluated previously (Figure 5), inhibited HT29 cell adhesion to FCS-coated plastic wells. The maximum inhibition (∼50%) was quite close for both drugs, but SP600125 was significantly active even at 0.01 μM. To evaluate the mechanism of HT29 cell adhesion to FCS-coated plastic wells, experiments were performed by pre-incubating HT29 cells with mAbs blocking each of the two adhesion molecules. Adhesion of HT29 cells to FCS-coated plastic wells was inhibited by 50% (ICAM-1 mAb) and 35% (VCAM-1 mAb), thus demonstrating a direct involvement of the two adhesion molecules in the adhesion of HT29 cells to FCS-coated plastic wells (Figure 5).
Figure 5.
Effect of celecoxib, SB202190, SP600125 and α-ICAM-1 and α-VCAM-1 mAbs on HT29 cell adhesion to FCS-coated plastic wells. HT29 cells were plated with celecoxib or pretreated for 30 min with SB202190 and plated for 4 h at 37 °C for 4 h or incubated for 4 h and 30 min with SP600125. All compounds were tested at 0.001–10 μM. HT29 cells were treated with mAbs to α-ICAM-1 or α-VCAM-1 (5 μg ml−1) for 30 min and then plated on FCS-coated plastic wells for 4 h. Data are expressed as percentage inhibition versus control, as mean±s.e.m., n=5. Control adhesion was 65±6 cells per microscope field (mean±s.e.m., n=15). Data were analysed by one-way ANOVA and the Dunnett's test (*P<0.05, **P<0.01 versus control).
Effect of celecoxib on apoptosis
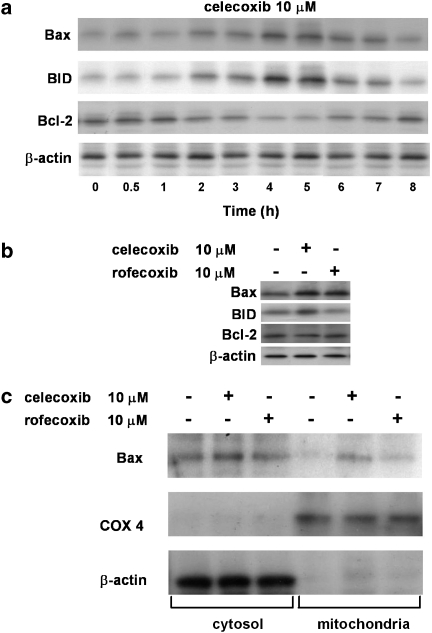
Celecoxib has been reported to affect cancer cells in a number of ways. The antineoplastic properties of celecoxib have been attributed, at least in part, to its ability to directly inhibit COX-2 by binding to its active site (Hood et al., 2003). We evaluated PGE2 synthesis in HT29 cells as a measure of COX-2 activity, by dose–response experiments performed at the same time and concentrations as those used to evaluate the effect on adhesion molecule expression. Treatment of HT29 cells with celecoxib inhibited PGE2 production in a dose-dependent way. COX-2 is basally expressed at a high level in HT29 cells that also possess COX-1. At the highest dose tested (10 μM celecoxib), PGE2 production was inhibited by about 30% (data not shown). This extent of inhibition is similar to those measured in the other assays reported here. To ascertain whether the drug could also exert other effects in experimental conditions suitable for inhibiting adhesion molecule expression, the ability of celecoxib to affect apoptosis in HT29 cells was evaluated. In time-course experiments, treatment of HT29 cells with 10 μM celecoxib up to 8 h induced Bax and BID expressions with a maximum at about 4 h, whereas the drug inhibited Bcl-2 expression with a maximal inhibition at the same time (n=3; Figure 6a). In the same experimental conditions, rofecoxib induced a significantly lesser increase in Bax expression and did not significantly modify BID and Bcl-2 expressions (n=4; Figures 6b and c). As Bax overexpression is correlated with translocation to mitochondria, we have also demonstrated that 10 μM celecoxib, with 4 h incubation, induced Bax translocation (n=3; Figure 6c). These data indicate that celecoxib activates a proapoptotic response in HT29 cells, involving both the intrinsic and the extrinsic pathways.
Figure 6.
Effect of celecoxib and rofecoxib on expressions of Bax, BID and Bcl-2. (a) HT29 cells were incubated with celecoxib (10 μM) from time 0 to 8 h. At the time indicated, cells were processed for western blot analysis as described in the Methods section. The immunoblot shown is representative of three separate experiments. (b) HT29 cells were treated with celecoxib or rofecoxib (10 μM) for 4 h and processed for western blot analysis as described in the Methods section. The immunoblot shown is representative of four separate experiments. (c) Immunoblot representing translocation of Bax to mitochondria in HT29 cells treated with celecoxib or rofecoxib (10 μM) for 4 h. COX 4 and β-actin are mitochondrial and cytosolic protein markers, respectively. The immunoblot shown is representative of three separate experiments.
Effect of continuous celecoxib exposure on ICAM-1 and VCAM-1 expressions on HT29 cells
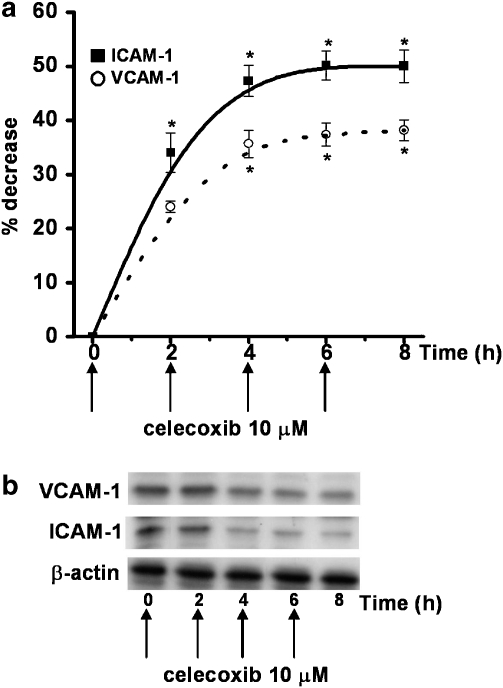
To evaluate the duration of the effect of celecoxib on ICAM-1 and VCAM-1 expressions in HT29 cells, we replenished celecoxib in the culture medium every 2 h up to 8 h. Under these experimental conditions, the ability of celecoxib to decrease ICAM-1 and VCAM-1 expressions was maintained over the whole time (8 h), until it reached a plateau, demonstrating the drug's ability to maintain the inhibition of expression of the two adhesion molecules (n=3; Figures 7a and b).
Figure 7.
Effect of continuing treatment with celecoxib (replenishment) on ICAM-1 and VCAM-1 expressions. (a) HT29 cells were incubated with celecoxib (10 μM) and cells were washed twice every 2 h and fresh celecoxib added, up to 8 h (arrows). Every 2 h, cells were processed for western blot analysis as described in the Methods section and analysed by densitometry. Data, normalised to β-actin, are expressed as percentage inhibition versus control, mean±s.e.m., n=3. Data were analysed by one-way ANOVA and the Dunnett's test (*P<0.05 versus control). (b) The immunoblot shown is representative of three separate experiments.
Discussion and conclusion
Adhesion molecules play a key role in tumour development, as they mediate both interactions between cancer cells and interactions between cancer and other cells (e.g. endothelial cells). Specific adhesion molecules are involved in processes that allow cancer cell migration, tissue invasion and metastasis formation (Fidler, 2002). Colon cancer metastases are frequently localised in the lung, and this could be related to the ability of colon cancer cells to adhere to microvascular endothelial cells of the lung that express the ligands for ICAM-1 and VCAM-1, present on tumour cells (ten Kate et al., 2004). Microvascular endothelial cells of the lung normally express LFA-1 and VLA-4, ligands for ICAM-1 and VCAM-1, respectively, which are not present on macrovascular endothelial cells (ten Kate et al., 2004). Celecoxib, the only anti-inflammatory drug approved by the FDA (1999) for adjuvant therapy in patients with familial adenomatous polyposis, was recently proposed to be useful in reducing the occurrence of colorectal sporadic adenomatous polyps within 3 years after polypectomy (Arber et al., 2006). Celecoxib has never been studied as a modulator of these adhesion molecules. Using HT29 cells as an experimental model, we have demonstrated that celecoxib down-regulated ICAM-1 and VCAM-1, decreasing their expression by about 40% with no comparable effect with rofecoxib. To our knowledge, this is the first demonstration of the effect of celecoxib on the expression of these adhesion molecules in HT29 cells. The demonstration that continuous exposure to the drug was able to maintain down-regulation of ICAM-1 and VCAM-1 is an important finding, also from a therapeutic point of view. The chemopreventive effect displayed by celecoxib may be linked to its ability to maintain the down-regulation of adhesion molecules, preventing the attachment of tumour cells either among themselves or to other cells.
At the highest concentrations tested here, celecoxib cannot be regarded as a selective inhibitor of COX-2 (Fitzgerald and Patrono, 2001). Both COX isoforms are present in HT29 cells (Goel et al., 2001). While evaluating the possible mechanism(s) of the action of celecoxib, we focused our attention on data showing the drug's ability to interfere with the activity of some MAPKs (Chun et al., 2004; Steffel et al., 2005). Chun et al. (2004) have shown that celecoxib inhibits both the catalytic activity and the phosphorylation of p38 MAPK in an experimentally induced carcinogenesis model. This data can be explained by the common chemical structure shared by p38 MAPK inhibitors, such as SB203580, and celecoxib (Muller, 2003). Moreover, Steffel et al. (2005) demonstrated that, in human aortic endothelial cells, 10 μM celecoxib decreased TNFα-induced tissue-factor expression by 50% by inhibiting JNK phosphorylation. Based on these observations, we tried to confirm the inhibitory effects of celecoxib on HT29 cells, in order to ascertain a possible relationship between inhibition of MAPKs and a decrease in ICAM-1 and VCAM-1 expressions. In HT29 cells, celecoxib inhibited p38 and activation of p55 JNK MAPKs in a time- and dose-dependent way, without affecting p42/44 and p46 JNK. It appears that celecoxib inhibition of MAPKs occurs at the same time and concentration as those needed to reduce ICAM-1 and VCAM-1 expressions. These results are in keeping with a possible relationship between inhibition of MAPKs and a decrease in ICAM-1 and VCAM-1 expressions, both affected by micromolar concentrations of celecoxib. Moreover, the refilling experiments showed that celecoxib could induce a long-lasting inhibition of p38 and p55 JNK MAPKs, suggesting a prolonged inhibitory effect of the drug also on MAPKs (data not shown).
To confirm the role of MAPKs in the expression of ICAM-1 and VCAM-1, we performed experiments with selective inhibitors of MAPKs previously used to evaluate celecoxib's effect. We utilised the p38 inhibitor SB202190, which like SB203580 has a chemical structure shared also by celecoxib, and JNK inhibitor SP600125. SB202190 and SP600125 inhibited ICAM-1 and VCAM-1 expressions in a time- and dose-dependent way, and also in this case we obtained a curve with a shape that significantly overlapped with all the previously described dose–response curves for celecoxib. PD98059 was also tested as a negative comparator, confirming that p42/44 is not involved in celecoxib-mediated inhibition of adhesion molecule expression (data not shown). Evidence for the functional meaning of these results is provided by adhesion data. Celecoxib inhibited HT29 cell adhesion to FCS-coated plastic wells in a dose-dependent way (0.001–10 μM) and at 4 h incubation. Similar results were obtained with MAPK inhibitors SB202190 and SP600125, but not with PD98059 (data not shown). We demonstrated the direct involvement of ICAM-1 and VCAM-1 in mediating HT29 cell adhesion to FCS-coated plastic wells by blocking ICAM-1 and VCAM-1 with mAbs. The extent of celecoxib's inhibition of HT29 cell adhesion was of the same order as the inhibition recorded with mAbs to the adhesion molecules.
Various experimental studies have shown a positive correlation between the expression of COX-2 and inhibition of apoptosis. In HT29 cells, we found that two pro-apoptotic proteins Bax and BID were induced by treatment with celecoxib, whereas the expression of the anti-apoptotic protein Bcl-2 decreased after treatment with celecoxib in a time-dependent way with a maximum effect, in both cases, at 4 h. In our experimental model, Bax was translocated to mitochondria, demonstrating that both intrinsic and extrinsic apoptosis pathways are activated. In the same experimental conditions, rofecoxib increased Bax, without affecting BID and Bcl-2 expression. Refilling experiments showed, once again, that continuous exposure to celecoxib induced a lasting effect on Bax and BID up-regulation and Bcl-2 down-regulation, demonstrating the drug's ability to produce a prolonged effect (data not shown). Increased expression of Bax and BID has been reported in another colon cancer cell line, COLO 205, after stimulation with nimesulide, another COX-2 inhibitor that, in this cell line, induced a pro-apototic pathway (Godlewski et al., 2002). In other cell lines and in other experimental conditions (Hsu et al., 2000), celecoxib did not affect Bcl-2 expression, underlining a cell- and dose-dependence of this effect. The different behaviour between celecoxib and rofecoxib is in agreement with data reported by Zhu et al. (2002), suggesting a key role for the sulphonamide moiety in the pro-apoptotic activity of celecoxib (Zhu et al., 2002). Our results provide the first demonstration of the involvement of Bcl-2 family proteins in celecoxib-mediated apoptosis in HT29 cells.
It is very important to stress that the celecoxib concentrations required to achieve an apoptotic effect in cell culture experiments are well in excess (5–20 fold higher) of human plasma concentrations achieved after administration of one 400 mg dose per day (Marx, 2001; Maier et al., 2005). This discrepancy may be attributed to the time required to obtain tumour regression in vivo (weeks or months), whereas an anti-proliferative effect in cell cultures is achieved after hours or days (Maier et al., 2005).
Celecoxib-mediated down-regulation of ICAM-1 and VCAM-1 on HT29 cells could explain new aspects of the chemopreventive effects of this drug on colon cancer. Celecoxib may reduce the ability of tumour cells to invade local issues and to spread to distant sites, not only through reduced secretion of matrix metalloproteinases (Cha et al., 2004; Peluffo et al., 2004), but also by inhibiting the adhesiveness of colon cancer cells, thus reducing the interactions between different cell types. In conclusion, this study contributes to our knowledge about the mechanism of celecoxib's action in an in vitro experimental model. This demonstrates a role for celecoxib, not only in apoptosis, but also in the modulation of the expression of adhesion molecules in colon cancer cells, reducing cell adhesiveness, inhibiting MAPKs activity and thus suggesting a role for the drug in reducing the formation of metastases.
Acknowledgments
This research was supported by Ministry of Education, Universities and Research (MIUR, 60% and PRIN 2004 Projects).
Abbreviations
- COX-2
cyclooxygenase-2
- FCS
foetal calf serum
- ICAM-1
intercellular adhesion molecule-1
- JNK
c-Jun terminal NH2 kinase
- mAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- VCAM-1
vascular cell adhesion molecule-1
Conflict of interest
The authors state no conflict of interest.
References
- Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- Cha HS, Ahn KS, Jeon CH, Kim J, Koh EM. Inhibitory effect of cyclo-oxygenase-2 inhibitor on the production of matrix metalloproteinases in rheumatoid fibroblast-like synoviocytes. Rheumatol Int. 2004;24:207–211. doi: 10.1007/s00296-003-0359-3. [DOI] [PubMed] [Google Scholar]
- Chun KS, Kim SH, Song YS, Surh YJ. Celecoxib inhibits phorbol ester-induced expression of COX-2 and activation of AP-1 and p38 MAP kinase in mouse skin. Carcinogenesis. 2004;25:713–722. doi: 10.1093/carcin/bgh076. [DOI] [PubMed] [Google Scholar]
- Dianzani C, Collino M, Lombardi G, Garbarino G, Fantozzi R. Substance P increases neutrophil adhesion to human umbilical vein endothelial cells. Br J Pharmacol. 2003;139:1103–1110. doi: 10.1038/sj.bjp.0705344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- EMEA 18th Meeting of the committee for Orphan Medicinal Products, 22 November, 2001 2001. EMEA/CPMP/1747/04
- FDA The FDA medical products reporting programs 1999. Summary of safety-related drug labeling changes approved by FDA December 1999, posted: 4 February, 2000
- Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GA, Patrono C. The COXIBS, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy. Semin Oncol. 2004;31:2–11. doi: 10.1053/j.seminoncol.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, et al. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- Godlewski MM, Gajkowska B, Lamparska-Przybysz M, Motyl T. Colocalization of BAX with BID and VDAC-1 in nimesulide-induced apoptosis of human colon adenocarcinoma COLO 205 cells. Anticancer Drugs. 2002;13:1017–1029. doi: 10.1097/00001813-200211000-00006. [DOI] [PubMed] [Google Scholar]
- Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer lett. 2001;172:111–118. doi: 10.1016/s0304-3835(01)00655-3. [DOI] [PubMed] [Google Scholar]
- Grosch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98:736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- Hood WF, Gierse JK, Isakson PC, Kiefer JR, Kurumbail RG, Seibert K, et al. Characterization of celecoxib and valdecoxib binding to cyclooxygenase. Mol Pharmacol. 2003;63:870–877. doi: 10.1124/mol.63.4.870. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- Kaiserlian D, Rigal D, Abello J, Revillard JP. Expression, function and regulation of the intercellular adhesion molecule-1 (ICAM-1) on human intestinal epithelial cell lines. Eur J Immunol. 1991;21:2415–2421. doi: 10.1002/eji.1830211018. [DOI] [PubMed] [Google Scholar]
- Kakiuchi Y, Tsuji S, Tsujii M, Murata H, Kawai N, Yasumaru M, et al. Cyclooxygenase-2 activity altered the cell-surface carbohydrate antigens on colon cancer cells and enhanced liver metastasis. Cancer Res. 2002;62:1567–1572. [PubMed] [Google Scholar]
- Maier TJ, Janssen A, Schmidt R, Geisslinger G, Grosch S. Targeting the beta-catenin/APC pathway: a novel mechanism to explain the cyclooxygenase-2-independent anticarcinogenic effects of celecoxib in human colon carcinoma cells. FASEB J. 2005;19:1353–1355. doi: 10.1096/fj.04-3274fje. [DOI] [PubMed] [Google Scholar]
- Marx J. Cancer research. Anti-inflammatories inhibit cancer growth—but how. Science. 2001;291:581–582. doi: 10.1126/science.291.5504.581. [DOI] [PubMed] [Google Scholar]
- Muller G. Medicinal chemistry of target family-directed masterkeys. Drug Discov Today. 2003;8:681–691. doi: 10.1016/s1359-6446(03)02781-8. [DOI] [PubMed] [Google Scholar]
- Neeson PJ, Thurlow PJ, Jamieson GP, Bradley C. Lymphocyte-facilitated tumour cell adhesion to endothelial cells: the role of high affinity leucocyte integrins. Pathology. 2003;35:50–55. [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Parkos CA, Colgan SP, Diamond MS, Nusrat A, Liang TW, Springer TA, et al. Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: consequences for CD11b/CD18-mediated interactions with neutrophils. Mol Med. 1996;2:489–505. [PMC free article] [PubMed] [Google Scholar]
- Peluffo GD, Stillitani I, Rodriguez VA, Diament MJ, Klein SM. Reduction of tumor progression and paraneoplastic syndrome development in murine lung adenocarcinoma by nonsteroidal antiinflammatory drugs. Int J Cancer. 2004;110:825–830. doi: 10.1002/ijc.20226. [DOI] [PubMed] [Google Scholar]
- Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- Smith ML, Hawcroft G, Hull MA. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer. 2000;36:664–674. doi: 10.1016/s0959-8049(99)00333-0. [DOI] [PubMed] [Google Scholar]
- Steffel J, Hermann M, Greutert H, Gay S, Luscher TF, Ruschitzka F, et al. Celecoxib decreases endothelial tissue factor expression through inhibition of c-Jun terminal NH2 kinase phosphorylation. Circulation. 2005;111:1685–1689. doi: 10.1161/01.CIR.0000160358.63804.C9. [DOI] [PubMed] [Google Scholar]
- Steward M, Thiel M, Hogg N. Leukocyte integrins. Curr Opin Cell Biol. 1995;7:690–696. doi: 10.1016/0955-0674(95)80111-1. [DOI] [PubMed] [Google Scholar]
- ten Kate M, Hofland LJ, Van Grevenstein WM, Van Koetsveld PV, Jeekel J, Van Eijck CH. Influence of proinflammatory cytokines on the adhesion of human colon carcinoma cells to lung microvascular endothelium. Int J Cancer. 2004;112:943–950. doi: 10.1002/ijc.20506. [DOI] [PubMed] [Google Scholar]
- Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- Yamada M, Yanaba K, Hasegawa M, Matsushita Y, Horikawa M, Komura K, et al. Regulation of local and metastatic host-mediated anti-tumour mechanisms by L-selectin and intercellular adhesion molecule-1. Clin Exp Immunol. 2006;143:216–227. doi: 10.1111/j.1365-2249.2005.02989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Song X, Lin HP, Young DC, Yan S, Marquez VE, et al. Using cyclooxygenase-2 inhibitors as molecular platforms to develop a new class of apoptosis-inducing agents. J Natl Cancer Inst. 2002;94:1745–1757. doi: 10.1093/jnci/94.23.1745. [DOI] [PubMed] [Google Scholar]