Abstract
Fibulin-5, an extracellular matrix glycoprotein expressed in elastin-rich tissues, regulates vascular cell behaviour and elastic fibre deposition. Recombinant full-length human fibulin-5 supported primary human aortic SMC (smooth-muscle cell) attachment through α5β1 and α4β1 integrins. Cells on fibulin-5 spread poorly and displayed prominent membrane ruffles but no stress fibres or focal adhesions, unlike cells on fibronectin that also binds these integrins. Cell migration and proliferation were significantly lower on fibulin-5 than on fibronectin. Treatment of cells on fibulin-5 with a β1 integrin-activating antibody induced stress fibres, increased attachment, migration and proliferation, and stimulated signalling of epidermal growth factor receptor and platelet-derived growth factor receptors α and β. Fibulin-5 also modulated fibronectin-mediated cell spreading and morphology. We have thus identified the β1 integrins on primary SMCs that fibulin-5 interacts with, and have shown that failure of fibulin-5 to activate these receptors limits cell spreading, migration and proliferation.
Keywords: adhesion, cell migration, cell spreading, fibulin-5, integrin, smooth-muscle cell
Abbreviations: CHO cell, Chinese-hamster ovary cell; DAPI, 4′,6-diamidino-2-phenylindole; DMEM, Dulbecco's modified Eagle's medium; DPBS+, Dulbecco's PBS containing calcium and magnesium; DPBS−, Dulbecco's PBS without calcium and magnesium; ECM, extracellular matrix; EGF, epidermal growth factor; cbEGF, calcium-binding EGF; FBS, fetal bovine serum; HBSS, Hanks balanced salt solution; HEK-293 cell, human embryonic kidney cell; HRP, horseradish peroxidase; mAb, monoclonal antibody; PDGF, platelet-derived growth factor; PDGFRα, PDGF receptor α; RTK, receptor tyrosine kinase; SMC, smooth-muscle cell
INTRODUCTION
Fibulins are widely expressed ECM (extracellular matrix) glycoproteins that are associated with elastic fibres and basement membranes [1,2]. They function as molecular ‘bridges’ that participate in the assembly, organization and stabilization of supramolecular ECM complexes, and play important roles in organogenesis, vasculogenesis and tumorigenesis. Fibulins comprise a family of six glycoproteins that are defined by a common C-terminal FC (fibulin-type) globular domain preceded by a tandem array of cbEGF [calcium-binding EGF (epidermal growth factor)]-like domains (Figure 1A). Fibulin-5, a 55-kDa glycoprotein, is expressed in elastin-rich tissues, such as aorta, skin, lung and uterus, and in adult heart, ovary and colon [3,4]. In the vasculature, it localizes on elastic lamina surfaces adjacent to endothelial cells, and throughout the aortic media [5]. Its expression is down-regulated in adult arteries, but is induced in vascular cells following injury, in atherosclerotic cells and in neointimal cells following balloon angioplasty [3].
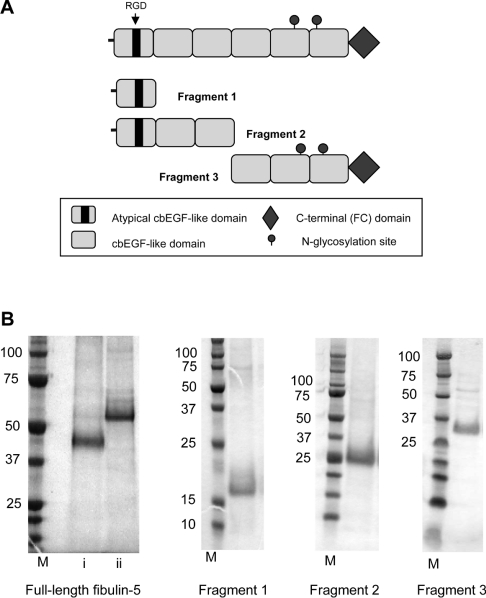
Figure 1. Characterization of recombinant human fibulin-5.
(A) Domain organization of recombinant full-length fibulin-5 and fragments. (B) SDS/PAGE analysis of nickel affinity-purified recombinant full-length fibulin-5, and fragments. Full-length fibulin-5 is shown under (i) non-reducing or (ii) reducing conditions, and fragments 1, 2 and 3 under non-reducing conditions. M indicates molecular-mass markers (sizes in kDa).
The critical contribution of fibulin-5 to elastic tissue integrity is highlighted by serious aberrations caused by mutations in the fibulin-5 gene that result in age-related macular degeneration [6–8] and recessive cutis laxa [9–13]. Fibulin-5-null mice exhibited severe elastic fibre disorganization, resulting in tortuous aorta with loss of compliance, loose skin and emphysematous lung [5,14,15]. Fibulin-5 plays an important role in elastic fibre formation [5,14,16], and interacts with the elastic fibre molecules tropoelastin and fibrillin-1 [5,17–20].
Fibulin-5 supports attachment of HUVECs (human umbilical-vein endothelial cells) in an RGD-dependent manner [4,14]. CHO cells (Chinese-hamster ovary cells) transfected with α9 or β3 integrin subunits adhered to a bacterially expressed N-terminal fibulin-5 RGD-containing fragment through integrins αvβ3, αvβ5 and α9β1 [4,14]. Fibulin-5-null mice have an exaggerated vascular remodelling response, with severe neointima formation and thickened adventitia, while SMCs (smooth-muscle cells) isolated from these mice have enhanced proliferative and migratory responses to PDGF (platelet-derived growth factor)-BB, a potent vascular mitogen and chemoattractant [21]. Skin fibroblasts from wild-type and fibulin-5-null mice showed no significant differences in proliferative or migratory responses to EGF [15]. Fibulin-5 enhances endothelial cell attachment to artificial surfaces under shear stress [22].
We have determined how human fibulin-5, recombinantly expressed by human cells, supports the passive adhesion of SMCs, through integrins α5β1 and α4β1, but fails to stimulate their migration and proliferation. Although fibulin-5 ligates the same integrins as fibronectin, it exerts profoundly different cellular effects.
MATERIALS AND METHODS
Antibodies
Mouse anti-(human fibulin-5) [anti-human DANCE (developing arteries and neural crest EGF-like)] mAb (monoclonal antibody) was obtained from R&D Systems Europe (Abingdon, Oxfordshire, U.K.). Rabbit anti-(human fibronectin) antibody was from Sigma–Aldrich (Poole, Dorset, U.K.). Inhibitory rat anti-(human α5 integrin subunit) mAb (mAb16) and inhibitory rat anti-(human α1 integrin subunit) mAb (mAb13) were gifts from Ken Yamada (National Institute of Dental and Craniofacial Research, Bethesda, MD, U.S.A.). Inhibitory mouse anti-(human integrin α2 subunit) mAb (JA218), non-inhibitory mouse anti-(human integrin β1 subunit) mAb (8E3) [23] and activating mouse anti-(human integrin β1 subunit) mAb (TS2/16) were gifts from Professor Martin Humphries (Wellcome Trust Centre for Cell-Matrix Research, Manchester, U.K.). Inhibitory mouse anti-(human αvβ3 integrin) mAb (LM609) was from Chemicon (Temecula, CA, U.S.A.). Inhibitory mouse anti-(human integrin α4) mAb (HP2/1), mouse anti-(human integrin β1 subunit) mAb (4B4) and mouse anti-(human integrin α9β1) mAb (Y9A2) were from Serotec (Kidlington, Oxford, U.K.). Mouse anti-polyhistidine mAb (AD1.1.10) was from R&D Systems Europe. Mouse anti-(human paxillin) mAb (5H11) and mouse anti-(human α-actinin) mAb (AT6/172) were from Upstate Biotechnology (Milton Keynes, U.K.). Mouse IgG1 (negative control) was from Dako UK (Ely, Cambs., U.K.).
FITC-conjugated rabbit anti-(mouse IgG) secondary antibody, FITC-conjugated goat anti-(rat IgG) secondary antibody, normal mouse IgG and normal rat IgG, used for flow cytometry, were obtained from Jackson ImmunoResearch Laboratories (Avondale, PA, U.S.A.). FITC-conjugated rabbit anti-(mouse IgG) secondary antibody, goat FITC-conjugated anti-(rabbit IgG) secondary antibody, FITC-conjugated goat anti-(mouse IgG) secondary antibody, HRP (horseradish peroxidase)-conjugated rabbit anti(mouse IgG) secondary antibody, HRP-conjugated goat anti-rabbit secondary antibody, HRP-conjugated goat anti-mouse secondary antibody were from Dako UK. Rhodamine-conjugated phalloidin was from Invitrogen (Paisley, Renfrewshire, Scotland, U.K.). Human serum was from Sigma–Aldrich. All mAbs were used as purified IgG.
Fibronectin and fragments
Human plasma fibronectin was obtained from Chemicon. Two recombinant fibronectin peptides, type III repeats 12–15 plus a full-length 120-amino-acid IIICS region (H/120) and type III repeats 12–15 without the IIICS (H/0) [24] were gifts from Professor Martin Humphries.
Cells
Human aortic SMCs from three individuals, SMC medium (Media 231) and SMC supplementation kits (SMGS) were obtained from Cascade Biologics (Mansfield, U.K.). ReagentPack™ Subculture reagent kit [HBSS (Hanks balanced salt solution), TE (trypsin/EDTA) and TNS (trypsin neutralizing solution)] were from Cambrex Bio Science (Wokingham, Berks., U.K.). Only low passage cells (passage 3–6) were used in these studies. HEK-293 cells (human embryonic kidney cells) stably transfected with the EBNA (Epstein–Barr nuclear antigen)-1 gene (designated 293E) were from Invitrogen.
Other materials
Dulbecco's PBS containing calcium and magnesium (DPBS+) and Dulbecco's PBS without calcium and magnesium (DPBS−), DMEM (Dulbecco's modified Eagle's medium) high glucose (4.5 g/l) with Hepes (6.0 g/l) (DMEM/Hepes), and DMEM with L-glutamine (0.6 g/l) (DMEM) were obtained from Cambrex Bio Science. GRGDS and SDGRG synthetic peptides were from Sigma–Aldrich. Microplates (6-, 24- and 96-well) were from Corning (Schiphol-Rijk, The Netherlands). Vectashield® containing DAPI (4′,6-diamidino-2-phenylindole) was from Vector Laboratories (Peterborough, U.K.). PDGF-BB was from R&D Systems Europe. Hi-Trap nickel columns were from GE Healthcare (Chalfont St Giles, Buck, U.K.). All other reagents were from Sigma–Aldrich unless otherwise stated.
Expression of recombinant human fibulin-5
Recombinant human fibulin-5 was expressed using the mammalian episomal expression system pCEP-His and 293E cells, as previously described [18] (Figure 1). Full-length recombinant fibulin-5, an N-terminal fragment (residues 27–69), the N-terminal half (residues 27–206) and the C-terminal half of the molecule (residues 206–448) (Figure 1A) were purified by nickel chromatography, and monitored by SDS/PAGE and size-fractionation chromatography. Full-length fibulin-5 (and fragments) was shown to be monomeric on SDS/PAGE in the presence or absence of 10 mM dithiothreitol (Figure 1B) and by size-fractionation on Superdex 200 HR 10/30 columns, was N-glycosylated as shown by increased electrophoretic mobility after PNGase F (peptide N-glycosidase F) treatment, and bound calcium as judged by an electrophoretic shift following EDTA treatment (results not shown).
ELISAs of protein coating of cell culture plates
Efficient coating of wells with fibulin-5 and fibronectin was confirmed using a published ELISA protocol [25]. Briefly, recombinant fibulin-5, fibulin-5 fragments or fibronectin, diluted to 10–400 nM in DPBS+, were adsorbed on to wells of 96-well microplates overnight at 4 °C. Non-specific binding was blocked with BSA. Bound protein was detected using 100 μl of primary antibody [anti-(human fibulin-5) mAb or anti-(human fibronectin) mAb] at 2 μg/ml in BSA block, followed by washing with DPBS+, addition of 100 μl of secondary antibody [HRP-conjugated goat anti-mouse (1 μg/ml) or goat anti-rabbit (1 μg/ml)], further washing with DPBS+, and then application of ABTS [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] solution and incubation for 5 min at 20 °C. Absorbance was measured at 405 nm.
Cell attachment assays
Cell attachment assays were performed according to well-defined methodologies [26]. Recombinant full-length fibulin-5 or fibulin-5 fragments were diluted to 10–400 nM in DPBS+, and adsorbed on to wells of 96-well microplates overnight at 4 °C. Non-specific binding was blocked with 10 mg/ml heat-denatured BSA for 1 h. BSA was aspirated, and 100 μl of human aortic SMC suspensions (5×105 cells/ml) was added to the wells and incubated for 45 min at 37 °C under 5% (v/v) CO2. To estimate the number of adherent cells, known numbers of cells were added to unblocked wells and fixed with 10 μl of 50% glutaraldehyde. Adherent cells were fixed by the addition of 100 μl of 5% glutaraldehyde for 20 min. Wells were washed three times with 200 μl of distilled water, and cells were stained with 100 μl of 0.1% (w/v) Crystal Violet in 0.2 M Mes buffer (pH 5) for 1 h. Dye taken up by cells was solubilized in 100 μl of 10% (v/v) acetic acid, and absorbance was measured at 570 nm. Absorbance from wells containing the known cell number was used to express results as percentage cell attachment.
Fibronectin supports integrin-mediated cell attachment through interaction of its RGD motif in the tenth type III repeat [25,27,28]. Human plasma fibronectin diluted to 10–400 nM in DPBS+ was used as a positive control.
To examine the effects of exogenous agents (antibodies, EDTA, bivalent cations or synthetic peptides) on attachment, 50 μl of 2× exogenous agent in DPBS− (for cations) or DMEM/Hepes (for antibodies, EDTA and synthetic peptides), followed by 50 μl of 2× cells were added to the wells. As controls for examining the effects of exogenous agents, 50 μl of DPBS− or DMEM/Hepes followed by 50 μl of 2× cells were added to control wells. Results are presented as the means±S.D. for three repeated experiments and were statistically analysed using unpaired Student's t tests (GraphPad Prism 2.0). Results are statistically significant when the P value is <0.05 (*P<0.05, **P<0.001 and ***P<0.0001).
Cell spreading assays
Wells of 96-well microplates were ligand-coated and BSA-blocked as for cell attachment assays. Trypsinized SMC suspensions were adjusted to 2×105 cells/ml with warm DMEM/Hepes gassed with 5–10% CO2. Then 100 μl aliquots of cells were added to wells. As negative controls, cells were added to uncoated BSA-blocked wells. Plates were incubated for 40 min at 37 °C in a 5% CO2 incubator with the lid removed. Cells were immediately fixed by the addition of 10 μl of 37% formaldehyde directly to the well for 20 min. Level of cell spreading was determined by phase-contrast microscopy in three randomly selected fields for each condition. Cells were spread when ‘phase-dark’ with visible nuclei, but unspread when rounded and ‘phase-bright’. Results are presented as the means±S.D. for three repeated experiments and were statistically analysed using unpaired Student's t tests (GraphPad Prism 2.0). Results are statistically significant when the P value is <0.05 (*P<0.05, **P<0.001 and ***P<0.0001).
Flow cytometry
Cell suspensions were prepared by washing confluent cultured cell layers with DPBS− and incubating with 1 ml of 5 mM EDTA in HBSS/10 cm2 flask area at 37 °C for no more than 30 min, diluting with 5 vol. of DMEM/Hepes containing 10% (v/v) FBS (fetal bovine serum), and harvesting by centrifugation at 800 g for 5 min. Cell pellets were resuspended in supplemented DMEM/Hepes gassed with 5% CO2 to a cell density of 1×107 cell/ml. Then 50 μl aliquots of cells were mixed in FACS tubes with 50 μl of the primary antibody diluted to 20 μg/ml in DPBS− containing 0.02% (w/v) sodium azide (final antibody concentration of 10 μg/ml), incubated on ice for 45 min, washed with DPBS− containing 1% FBS and then harvested by centrifugation at 800 g for 5 min. Cell pellets were resuspended in 50 μl of DPBS− containing FITC-conjugated goat anti-mouse or goat anti-rat secondary antibody diluted 1 in 50 in DPBS− containing 10% (v/v) human serum. After incubation on ice for 45 min, the cells were washed twice with 300 μl of DPBS− containing 1% FBS, then once with 300 μl of DPBS−, and harvested by centrifugation at 800 g for 5 min. The cell pellets were resuspended in 400 μl of DPBS−, and 20000 cells were counted using a Cyan flow cytometer (DakoCytomation, Glostrup, Denmark), at a flow rate of less than 200 events/s and a λex of 488 nm (530/40 bandpass filter).
Immunofluorescence analysis of attached cells
Using standard protocols [26], SMCs plated for 3 h on full-length fibulin-5 or fibronectin (each 250 nM), or a combination of full-length fibulin-5 and fibronectin at concentrations indicated, were dual-stained with rhodamine-conjugated phalloidin to stain F-actin (filamentous actin) stress fibres, and either an mAb against the focal-adhesion-associated protein paxillin or an mAb against α-actinin that cross-links stress fibres and displays a periodic distribution.
Briefly, coverslips were coated with 500 μl of recombinant full-length fibulin-5 or fibronectin at 250 nM in DPBS+ overnight at 4 °C, washed using 1 ml/well of 10 mg/ml heat-denatured BSA in DPBS+ and then 500 μl of cell suspensions (2×104 cells/ml) was added to each well. Plates were incubated for 3 h at 37 °C in a 5% CO2 incubator; then cells were fixed by the addition of 45 μl of 37% formaldehyde and then permeabilized in 500 μl of 0.5% (v/v) Triton-X 100 for 4 min. After washing, wells were blocked with 5% (w/v) BSA. Then 500 μl of primary antibody [anti-(human paxillin) mAb, anti-(human α-actinin) mAb or mouse IgG1] diluted to 10 μg/ml in BSA block was added to each well and incubated for 1 h at 20 °C. After washing with DPBS+, 500 μl of secondary antibody [FITC-conjugated rabbit anti-mouse (400 μg/ml) or goat anti-rat (10 μg/ml)] together with rhodamine-conjugated phalloidin (0.1 unit/ml) was added to each well for 30 min at 20 °C, and the wells were washed further with DPBS+. Inverted coverslips were mounted on glass slips using Vectashield® containing DAPI, and viewed using a Leica DM RXA widefield immunofluorescence microscope.
To examine the effects of exogenous agents on SMC cytoskeletal organization on fibulin-5, exogenous agents, including heparin-binding fragments of fibronectin (H0 or H120) [23], PDGF-BB (10 ng/ml) and antibodies (10 μg/ml) were added at the time of plating.
Cell proliferation
Ninety-six-well microplates were coated with full-length fibulin-5 or plasma fibronectin at 250 nM overnight at 4 °C. Following 24 h serum-free incubation, SMCs were trypsinized, plated at 2×103 cells/well and cultured for 1, 2, 3 or 6 days in culture. Cell numbers were quantified by using the CyQuant® cell proliferation assay kit (Invitrogen) according to the manufacturer's protocol. Briefly, frozen samples were thawed at room temperature (20 °C), and 200 μl of the CyQuant® GR dye/cell-lysis buffer was added to each sample well. Samples were mixed briefly and incubated for 2–5 min at 20 °C, protected from light. Sample fluorescence was measured by using a fluorescence microplate reader with filters appropriate for ∼480 nm excitation and ∼520 nm emission maxima. Absolute cell numbers were determined using a standard curve with cell numbers from 500 to 50000 in 200 μl volumes/well. As a negative control, 200 μl of medium was added to a well without cells. All assays were performed in triplicate and were repeated at least twice to confirm observed results. To determine the effect of the β1 integrin subunit-activating antibody (TS2/16) and PDGF-BB on cell proliferation, TS2/16 (10 μg/ml) or PDGF-BB (10 ng/ml) was added to sample wells and refreshed daily. Results are presented as the means±S.D. for three repeated experiments and were statistically analysed using unpaired Student's t tests (GraphPad Prism 2.0). Results are statistically significant when the P value is <0.05 (*P<0.05, **P<0.001 and ***P<0.0001).
Migration assay
For video microscopy analysis of cell migration in real time, wells of a 24-well microplate were coated with either fibulin-5 or fibronectin at 250 nM in DPBS+ overnight at 4 °C, and were blocked with 10 mg/ml heat-denatured BSA for 1 h at 20 °C. Adhered SMCs were trypsinized, neutralized, seeded at confluence (5×104 cells/well) and incubated at 37 °C in a 5% CO2 incubator for up to 6 h, until a confluent monolayer was formed. Cells were serum-starved overnight and washed twice with serum-free DMEM before wounding with a sterile P10 pipette tip. Immunofluorescence analysis confirmed retention of bound ligands [25]. The effect of exogenous agents on cell migration [inhibitory integrin mAbs (anti-α4 (HP2/1), anti-α5 (mAb16) or anti-β1 (mAb13), an activating integrin β1 mAb (TS2/16) or PDGF-BB] was assessed by adding the exogenous agent (mAbs at 10 μg/ml or PDGF-BB at 10 ng/ml) at the time of wounding. Images were acquired every 15 min over a period of 20 h using a Leica AS MDW microscope with an integrated digital camera, and organized into time-lapse movies using ImageJ software. The number of cells that had migrated into the cleared wound was quantified every 60 min.
RTK (receptor tyrosine kinase) phosphorylation assay
Relative levels of tyrosine phosphorylation of RTKs on SMCs seeded for 90 min on fibronectin- and fibulin-5-coated wells (500 nM overnight at 4 °C) were determined by using the Proteome Profiler™ Human Phospho-RTK Array kit (R&D Systems Europe) according to the manufacturer's protocol and as we have previously reported [29]. Adhered cells were lysed in 1 ml of ice-cold NP40 lysis buffer [20 mM Tris/HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P40, 10% (v/v) glycerol, 2.5 mM EDTA, 100 μM Na3VO4, 1% aprotinin, 1% leupeptin and 1 mM PMSF]. Total protein in each lysate was quantified using a BCA (bicinchoninic acid) assay kit (Pierce Biotechnology, Rockford, IL, U.S.A.). Total protein (100 μg) for each lysate (diluted to 1.5 ml with array buffer 1 supplied by the manufacturer) was added to a blocked (1.5 ml of array buffer 1, 1 h at 20 °C) array and incubated on a rocking platform shaker overnight at 4 °C. Each array was washed three times for 10 min with 30 ml of 1 × wash buffer (supplied by the manufacturer). Then 1.5 ml of HRP-conjugated anti-phosphotyrosine detection antibody (1:3000) was added to each array and incubated on a rocking platform shaker for 2 h at 20 °C. Following a further three washes (10 min with 30 ml of 1 × wash buffer), each array was exposed to ECL® (enhanced chemiluminescence) reagents (GE Healthcare) and to an X-ray film. All densitometry data were normalized to positive controls.
RESULTS
SMC adhesion to fibulin-5
SMC adhesion and spreading on fibulin-5
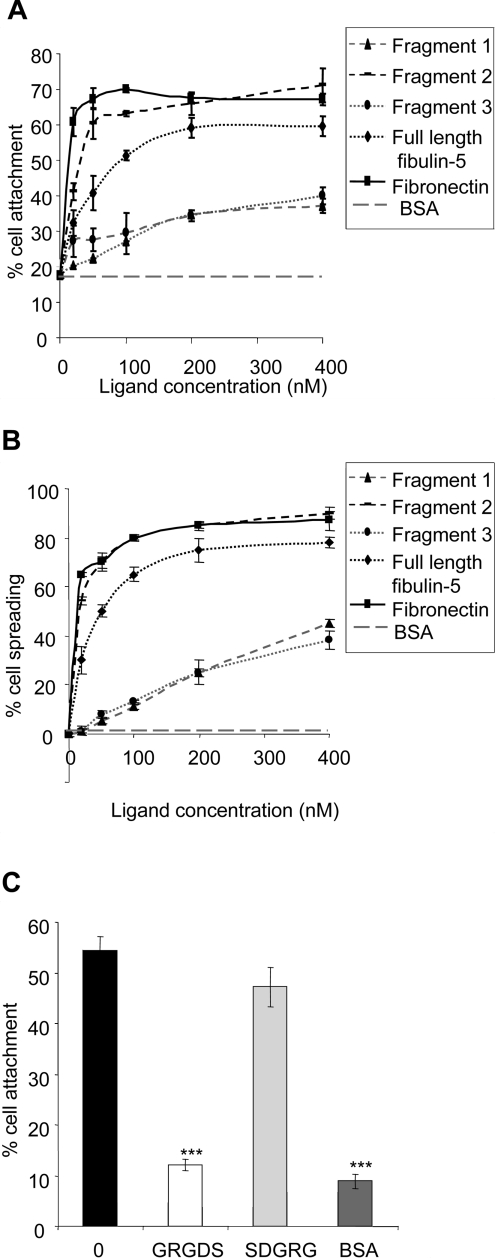
Immobilized full-length human fibulin-5 supported adhesion of SMCs in a concentration-dependent manner. Maximal attachment (58%) was at a fibulin-5 plating concentration of 200 nM (Figure 2A). Immobilized human plasma fibronectin, used as a positive control, supported 70% SMC attachment at 200 nM. BSA-blocked wells, as a negative control, supported 18% SMC attachment. The C-terminal ‘half’ of fibulin-5 (fragment 3) and the RGD-containing N-terminal cbEGF-like domain (fragment 1) (see Figure 1A) supported much lower SMC adhesion (37 and 34% at 400 nM respectively). The N-terminal ‘half’ of fibulin-5 (fragment 2) (see Figure 1A) supported higher attachment than full-length fibulin-5 (63% attachment at 100 nM). A high percentage of SMCs showed spreading on full-length fibulin-5 (72%) and on fragment 2 (85%) at 200 nM, but many fewer cells spread on fragments 1 and 3 (Figure 2B). SMCs on BSA-blocked wells, used as a negative control, did not spread. Full-length fibulin-5 was used in all subsequent experiments.
Figure 2. Attachment and spreading of SMCs on fibulin-5.
Attachment (A) and spreading (B) of SMCs on full-length fibulin-5 (diamonds, black dotted line), fragment 1 (triangles, grey dashed line), fragment 2 (small boxes, black dashed line), fragment 3 (ovals, grey dotted line), and human plasma fibronectin (rectangles, solid black line). Background cell adhesion and spreading on BSA-coated wells is also shown as grey, large dashed lines. Results are means±S.D. for the three experiments. (C) Inhibition of SMC attachment to 250 nM full-length fibulin-5 by synthetic GRGDS peptide (500 μg/ml). Synthetic SDGRG peptide (500 μg/ml) was used as a negative control. Bar 0 indicates cell attachment to 250 nm full-length fibulin-5 in the absence of peptide. BSA indicates background cell attachment to BSA-blocked wells. Pre-incubation of SMCs with the synthetic peptide GRGDS virtually ablated adhesion to full-length fibulin-5 (**P<0.001 compared with untreated SMCs on fibulin-5). Pre-incubation with the control peptide SDGRG showed no inhibition of cell attachment (P=0.059 compared with untreated SMCs on fibulin-5).
SMC attachment to fibulin-5 is through integrins
SMC adhesion to fibulin-5 was bivalent-cation-dependent in a concentration-dependent manner. The bivalent cation chelator EDTA inhibited cell attachment, and Mn2+ or Mg2+ promoted attachment, but Ca2+ failed to stimulate attachment (results not shown). Pre-incubation of SMCs with the synthetic peptide GRGDS virtually ablated adhesion to full-length fibulin-5 (**P<0.001 compared with untreated SMCs on fibulin-5) (Figure 2C). Pre-incubation with the control peptide SDGRG showed no significant inhibition of cell attachment (P=0.059 compared with untreated SMCs on fibulin-5). Thus cell adhesion to fibulin-5 is mediated by integrins.
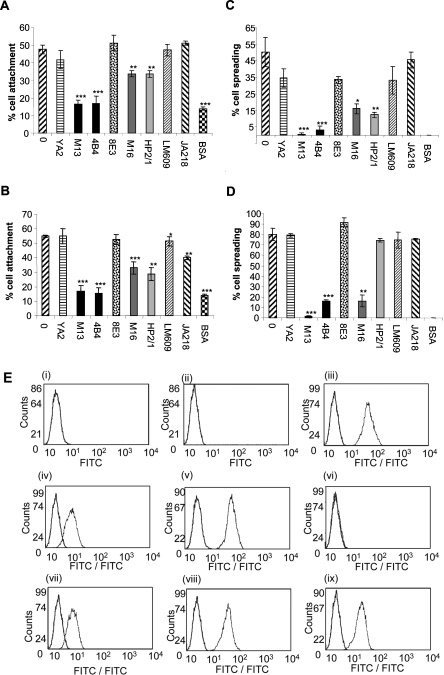
The integrin receptors that mediate SMC adhesion and spreading on fibulin-5 were identified using function-blocking mAbs (Figures 3A and 3C). Human plasma fibronectin, which interacts with α5β1, α4β1 and αvβ3 [30–32], was included as a control (Figures 3B and 3D). SMC adhesion to fibulin-5 was blocked by two function-blocking antibodies to the β1 subunit (mAb13 and 4B4) (***P<0.0001 for both, each compared with untreated SMCs on fibulin-5). Antibodies to α5 (mAb16) and α4 (HP2/1) reduced cell adhesion (**P<0.001 for both, each compared with untreated SMCs on fibulin-5). The LM609 antibody to integrin αvβ3 showed no inhibition (P=0.9282, compared with untreated SMCs on fibulin-5). An antibody to α9β1 (Y9A2) failed to significantly inhibit adhesion (P=0.2730 compared with untreated SMCs on fibulin-5). Antibodies to α2 (JA218) and to a non-inhibitory α1 antibody (8E3) also did not inhibit adhesion significantly (P=0.4021 and 0.4215 respectively, each compared with untreated SMCs on fibulin-5). SMC spreading on fibulin-5 was significantly blocked by antibodies to β1, α5 and α4 integrin subunits (**P<0.001, *P<0.05 and **P<0.001 respectively, each compared with untreated SMCs on fibulin-5). The α9β1 antibody (Y9A2), the αvβ3 antibody (LM609) and the β1 antibody (8E3) all showed small inhibitory effects on cell spreading. The presence of α5β1, α4β1 and α2β1, as well as αvβ3 and αvβ5, was confirmed using flow cytometry, but no expression of integrin α9β1 was detected (Figure 3E). Together, these results show that primary SMCs bind and spread on fibulin-5 through α5β1 and α4β1 integrins.
Figure 3. Integrins that mediate SMC attachment and spreading on fibulin-5.
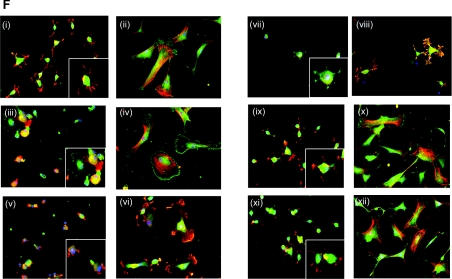
(A–D) Anti-integrin mAb inhibition of SMC attachment (A, B) and spreading (C, D) on full-length fibulin-5 (A, C) or fibronectin (B, D). Y9A2 is an inhibitory α9β1 antibody, mAb13 and 4B4 are both inhibitory anti-β1 antibodies, 8E3 is a non-inhibitory anti-αβ1 antibody, mAb16 is an inhibitory anti-α5 antibody, HP2/1 is an inhibitory α4β1 antibody, LM609 is an inhibitory anti-αVβ3 antibody, and JA218 is an inhibitory anti-α2 antibody. Antibodies were used at 10 μg/ml. BSA indicates background cell attachment to BSA-blocked wells. Results are presented as the means±S.D. for three repeated experiments and were statistically analysed using unpaired Student's t tests (GraphPad Prism 2.0). Results are statistically significant when the P value is <0.05 (*P<0.05, **P<0.001 and ***P<0.0001). SMC adhesion to fibulin-5 was blocked by the β1 function-blocking antibodies, mAb13 and 4B4 (***P<0.0001 for both, each compared with untreated SMCs on fibulin-5). The α5 (mAb16) and α4 (HP2/1) antibodies reduced cell adhesion (**P<0.001 for both, each compared with untreated SMCs on fibulin-5). The LM609 antibody to integrin αvβ3 showed no inhibition (P=0.9282 compared with untreated SMCs on fibulin-5). Antibodies to α9β1 (Y9A2), α2 (JA218) and the non-inhibitory β1 mAb (8E3) all failed to significantly inhibit adhesion (P=0.2730, P=0.4021 and P=0.4215 respectively, each compared with untreated SMCs on fibulin-5). SMC spreading on fibulin-5 was also significantly blocked by antibodies to β1, α5 and α4 integrin subunits (Figure 4C) (***P<0.0001, *P<0.05 and **P<0.001 respectively, each compared with untreated SMCs on fibulin-5). All assays were repeated at least three times, and a representative experiment is shown. (E) FACS detection of SMC cell-surface integrins using the anti-α2 antibody JA218 (iii), anti-α4 antibody HP2/1 (iv), anti-α5 antibody mAb16 (v), anti-α9β1 antibody Y9A2 (vi), anti-αvβ3 antibody LM609 (vii), anti-αvβ5 antibody P1F6 (viii), and anti-β1 antibody 8E3 (ix). Anti-(mouse IgG) antibody (i) and anti-(rat IgG) antibody (ii) were used as negative controls. Antibodies were used at 10 μg/ml. Each integrin detection peak (green) is overlaid with the appropriate IgG negative control (black). (F) Immunofluorescence images of mAb effects on SMC morphology on 250 nM full-length fibulin-5 (i, iii, v, vii, ix and xi) or fibronectin (ii, iv, vi, viii, x and xii) stained for phalloidin (red), and immunostained for the cytoskeletal component, paxillin (green). mAbs to integrin subunits α4 (iii and iv), α5 (v and vi) and β1 (vii and viii), and integrins αvβ3 (ix and x) and αvβ5 (xi and xii) were added (10 μg/ml) at the time of cell seeding. SMCs on full-length fibulin-5 or fibronectin with no antibody (i and ii) were used as negative controls. The anti-β1 mAbs caused cellular rounding and loss of lamellipodia. Images are shown at ×20 (i, iii, v, vii, ix and xi) and ×40 (ii, iv, vi, viii, x and xii; and insets) magnifications for each condition.
To assess the contributions of integrins α5β1 and α4β1 to cell shape on fibulin-5, the effects of β1, α5 and α4 function-blocking mAbs were examined (Figure 3F). The anti-β1 antibody (mAb13) caused loss of cell attachment, with the few remaining cells rounded. The anti-α5 (mAb16) and anti-α4 (HP2/1) antibodies each led to cellular rounding and loss of lamellipodia. We also investigated potential contributions of αvβ3 and αvβ5, since αv has been shown to support CHO cell adhesion to fibulin-5 [4,14]. However, the anti-αvβ3 and anti-αvβ5 inhibitory antibodies had no discernable effect on cytoskeletal organization. These results confirm that α5β1 and α4β1 support SMC spreading on fibulin-5.
Fibronectin activates α5β1, which acts in synergy with syndecan-4 to stimulate robust stress fibres and focal adhesions [33–35]. We found that fibulin-5 does not bind heparin [18], but since fibulin-5 does interact with α5β1, we investigated whether fibronectin fragments (H0 or H120) which activate syndecan-4 on fibronectin [24], could also regulate the SMC cytoskeleton of cells plated on fibulin-5. However, they did not induce stress fibres or focal adhesions (results not shown), as would have occurred if α5β1 had been activated. Thus, when cells are plated on fibulin-5, α5β1 does not synergize with syndecan-4.
Cellular phenotype of SMCs on fibulin-5
Morphology of SMCs on fibulin-5
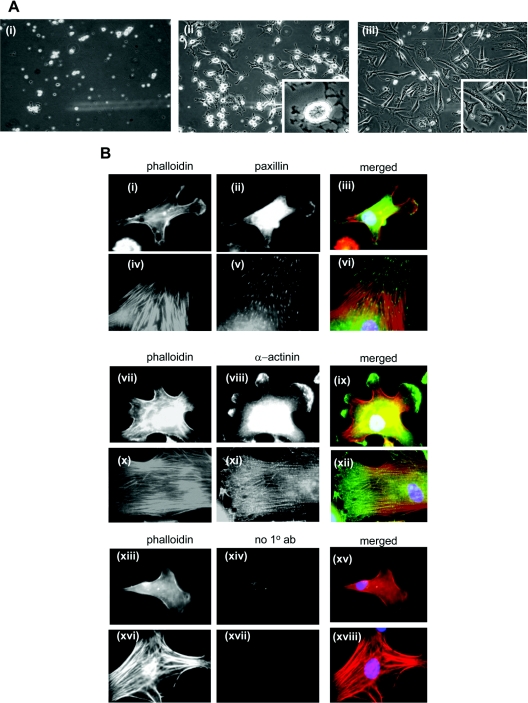
Phase-contrast microscopy revealed profound differences in SMC morphology on fibulin-5, compared with fibronectin (Figure 4A). SMCs on fibronectin were flattened and well spread. However, SMCs on fibulin-5 were smaller and less flattened, with bright centres and numerous membrane ruffles that extended in multiple directions.
Figure 4. SMC spreading on fibulin-5.
(A) Phase-contrast microscopy of SMC spreading on (i) BSA-coated wells, (ii) 250 nM full-length fibulin-5, or (iii) 250 nM human plasma fibronectin. The level of cell spreading was determined by phase-contrast microscopy in three randomly selected fields for each condition. All images are at ×10 magnification, apart from ×20 enlargements in (ii and iii). (B) Immunofluorescence images of SMC spread on full-length fibulin-5 (i–iii, vii–ix and xiii–xv) and fibronectin (iv–vi, x–xii and xvi–xviii). Cells were immunostained for cytoskeletal components paxillin or α-actinin, as indicated, and all were stained with phalloidin for actin stress fibres. Anti-(mouse IgG) primary antibody was a negative control (‘no 1° ab’) (xiv and xvii). Cells plated on BSA-blocked coverslips had not spread by 3 h and did not have an organized actin cytoskeleton (results not shown). All images are at ×40 magnification.
Immunofluorescence microscopy of the SMC cytoskeleton on fibulin-5 showed that there were no stress fibres or focal adhesions. Actin staining was diffuse throughout the cell, and α-actinin localized within the cell and prominently at the periphery in membrane ruffles (Figure 4B). Paxillin immunostaining was mainly diffuse in the cytoplasm, with some localization at membrane ruffles. In contrast, SMCs on fibronectin contained numerous actin stress fibres, with punctate paxillin staining of focal adhesions at tips of actin stress fibres and filamentous α-actinin staining. Cells on BSA-blocked coverslips had not spread by 3 h and had no organized actin cytoskeleton (results not shown). No FITC fluorescence was detectable in mouse IgG negative controls (Figure 2D).
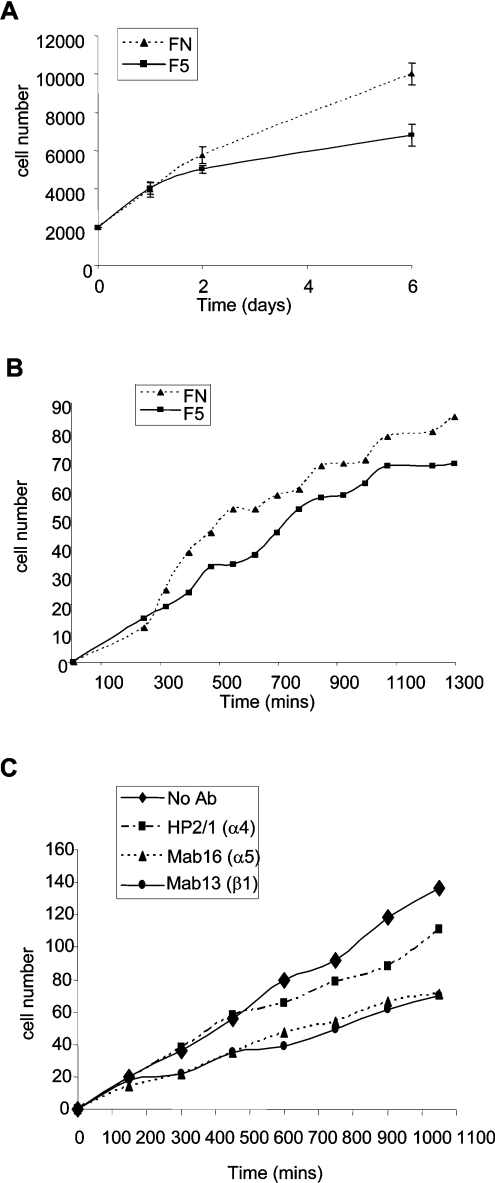
Effects of fibulin-5 on SMC proliferation and migration
To investigate whether SMC adhesion to fibulin-5 influences proliferation, cells (2×103/well) plated on 250 nM fibulin-5 or fibronectin were quantified at 1, 2 and 6 days. SMCs on fibulin-5 proliferated significantly less than cells on fibronectin (Figure 5A). To establish whether SMC adhesion to fibulin-5 influences migration, cells were plated at confluence on 250 nM of fibulin-5 or fibronectin. The confluent monolayer was wounded, and the closure of the wound was monitored by live-cell imaging microscopy for 24 h. SMCs migrated less on fibulin-5 than on fibronectin (Figure 5B). Integrin-neutralizing anti-α5, -β1 and -α4 antibodies all inhibited SMC migration on fibulin-5 (Figure 5C), with the anti-α5 and anti-β1 antibodies most inhibitory, implicating α5β1 in SMC migration on fibulin-5.
Figure 5. SMC proliferation and migration on fibulin-5.
(A) Proliferation and (B) migration of SMCs on 250 nM full-length fibulin-5 (squares, solid lines) or fibronectin (triangles, dotted lines). (C) Inhibition of SMC migration on 250 nM full-length fibulin-5, in the presence of α5, α4 and β1 function-blocking integrin mAbs. No mAb (solid line, diamonds), α4 mAb (dash-dotted line, squares), α5 mAb (dotted line, triangles) and β1 mAb (dotted line, small circles). Results are means±S.D. for three experiments.
Effects of fibulin-5 on fibronectin-mediated cell spreading
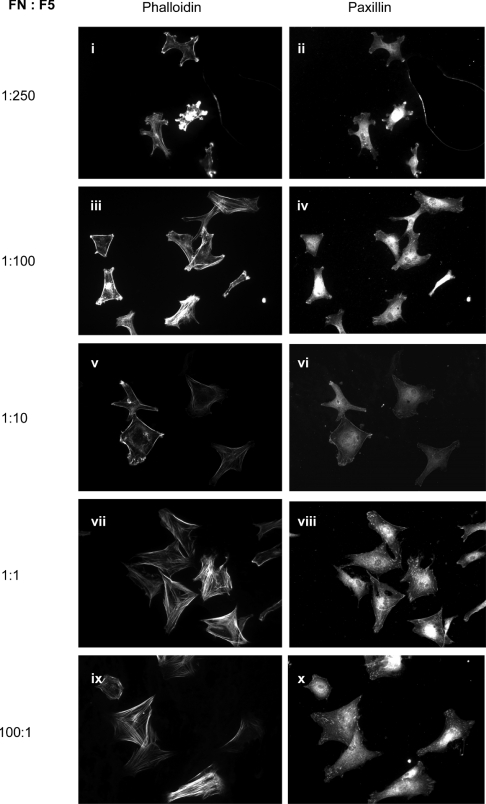
Fibulin-5 and fibronectin both ligate α4β1 and α5β1, so the possibility that fibulin-5 modulates fibronectin-mediated spread cell organization was investigated (Figure 6). At high fibronectin/fibulin-5 levels, cells were well spread with organized actin stress fibres and focal adhesions. At equimolar and lower fibronectin/fibulin-5 ratios, cells were smaller, had reduced or no stress fibres and focal adhesions, and membrane ruffles were increasingly apparent. No FITC fluorescence was detectable in mouse IgG negative controls (results not shown). These results show that, in vascular sites with elevated fibulin-5/fibronectin levels, fibulin-5 will directly inhibit fibronectin-mediated spreading, migration and proliferation.
Figure 6. Effects of fibulin-5 on SMC spreading on fibronectin.
Immunofluorescence analysis of the effects on SMC spreading of co-plating wells at a range of fibronectin/fibulin-5 ratios (FN:F5) as indicated, all shown at ×20 magnification. At high fibronectin/fibulin-5 levels, cells were well spread with organized actin stress fibres and focal adhesions. At equimolar and lower fibronectin/fibulin-5 ratios, cells were smaller, had reduced or no stress fibres and focal adhesions, and membrane ruffles were increasingly apparent. Left-hand-side micrographs, phalloidin staining for actin; right-hand-side micrographs, paxillin immunostaining.
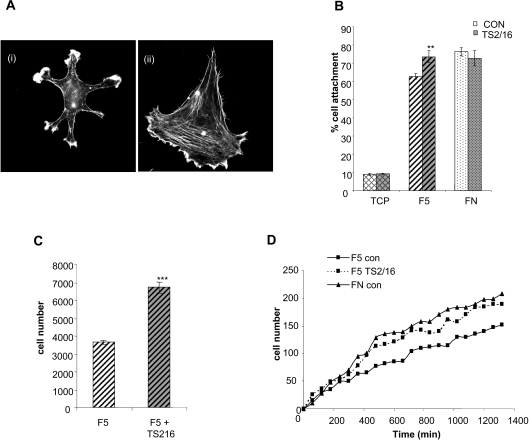
Effects of β1 integrin-activating antibody TS2/16 on SMCs on fibulin-5
Since SMCs bind to fibulin-5 through β1 integrins, but exhibit different adhesive, spreading, proliferative and migratory responses to SMCs on fibronectin, we investigated the effects of the β1 integrin activating mAb TS2/16 (Figure 6). Treatment of SMCs with TS2/16 stimulated spreading and stress fibre formation (Figure 7A). In addition, there was a significant increase in SMC attachment (Figure 7B) (**P<0.001 compared with untreated SMCs on fibulin-5), and in proliferation (Figure 7C) (***P<0.0001 compared with untreated SMCs on fibulin-5). Addition of TS2/16 at the time of wounding also increased cell migration on fibulin-5 (Figure 6D).
Figure 7. Effects of β1-activating antibody TS2/16 on SMC attachment, spreading, migration and proliferation.
(A) Immunofluorescence images of SMCs on 250 nM full-length fibulin-5, before (i) and after (ii) β integrin activation with mAb TS2/16, stained for phalloidin. Images are at×40 magnification. (B) Activation of β1 integrins with mAb TS2/16 induces a small, but significant, increase in SMC attachment to 250 nM full-length fibulin-5 (F5) (**P<0.001 compared with untreated SMCs on fibulin-5). CON, untreated control. Attachment of SMCs to human plasma fibronectin (FN) or to tissue culture plastic (TCP) was not significantly affected by β1 integrin activation. Results shown are the means±S.D. for three experiments and were statistically analysed using unpaired Student's t tests (GraphPad Prism 2.0). *P<0.05, **P<0.001 and ***P<0.0001. (C) Activation of β1 integrin with activating mAb TS2/16 significantly increased SMC proliferation on 250 nM full-length fibulin-5 (F5) (***P<0.0001 compared with untreated SMCs on fibulin-5) following 5 days of culture, in the absence or presence of β1 integrin-activating mAb. Integrin β1 activation of SMCs increased their proliferation on fibulin-5. (D) Time course of SMC migration on 250 nM fibulin-5 (F5 con) or fibronectin (FN con), and the effect of TS2/16 on SMC migration on fibulin-5 (F5 TS2/16).
Growth factor receptor signalling by SMCs on fibulin-5
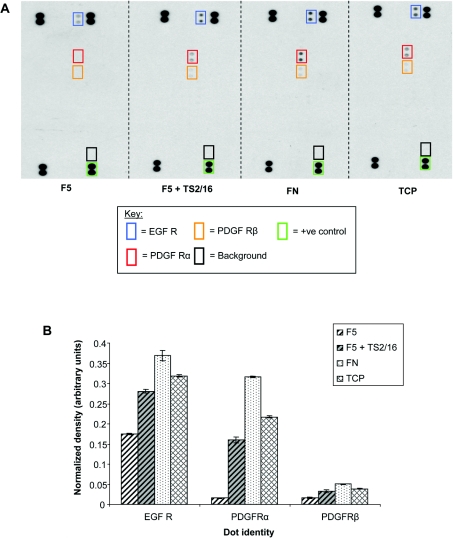
PDGF-BB is a potent vascular mitogen and chemoattractant that stimulates enhanced proliferation and migration in fibulin-5-null murine SMCs [21]. However, skin fibroblasts from the wild-type and fibulin-5-null mice showed no significant differences in proliferative or migratory responses to EGF [15]. PDGFRα (PDGF receptor α) and PDGFRβ, and EGF receptors are RTKs, and integrins can influence their signalling [36,37]. We investigated whether SMCs exhibited altered RTK activity when plated on full-length fibulin-5, compared with fibronectin, using a human phospho-RTK array containing 42 different duplicate RTK phosphorylation antibody spots, which allows relative quantification of RTK phosphorylation (Figure 8). There was undetectable PDGFR signalling and only low EGF signalling activity in SMCs on fibulin-5, compared with cells on fibronectin. However, signalling through both PDGFRs, especially PDGFRα, and through an EGF receptor, were stimulated by TS2/16.
Figure 8. Effects of fibulin-5 on SMC phosphorylation of PDGFRs.
(A) A human phospho-RTK array was used to examine SMC receptor signalling when plated on 500 nM full-length fibulin-5 (F5) in the presence or absence of the β1 integrin-activating mAb TS2/16, or on fibronectin (FN), or on tissue culture plastic (TCP). Cells on fibulin-5 showed no detectable PDGFR signalling, and only traces of EGF receptor (EGF R) signalling. However, cells on fibulin-5 in the presence of TS2/16, or on fibronectin, showed increased signalling, especially by PDGFRα and EGF receptor. (PDGF-AA and PDGF-BB both signal through PDGFRα.) (B) Normalized relative quantification of signalling by PDGFRs and EGF receptor (EGF R) from RTK assays. The mean pixel densities of duplicate RTK spots were normalized against duplicate phosphotyrosine-positive control spots.
DISCUSSION
The importance of fibulin-5 in vascular tissue structure and function has been clearly demonstrated in mouse and disease studies [4,9–14,17]. However, precisely how it interacts with SMCs and influences their phenotype and elastic fibre formation remains unclear. In the present study, we investigated how fibulin-5 interacts with primary human SMCs, and how these interactions regulate their behaviour. These cells adhere to fibulin-5 through integrin receptors α5β1 and α4β1, yet show only modest spreading with a ruffled morphology, and low migration and proliferation due to passive ligation of β1 integrins. Thus, although fibulin-5 ligates the same integrins as fibronectin, it exerts profoundly different cellular effects and can also modulate fibronectin-mediated adhesion and spreading. These results provide a molecular explanation for how fibulin-5 inhibits SMC migration and proliferation in vivo. Moreover, these fibulin-5 interactions with integrins may be important in vascular elastic fibre deposition [16].
Using recombinant human fibulin-5 expressed by human cells to ensure correct folding and glycosylation, we have identified integrins α5β1 and α4β1 as the main receptors supporting primary SMC adhesion and spreading on fibulin-5. A previous study, using a bacterially expressed N-terminal region of fibulin-5 with CHO cells and CHO cell lines stably transfected with α9 and β3, reported that fibulin-5 supported cell attachment through integrins αvβ3, αvβ5 and α9β1 [14]. These differences probably reflect the different cell types used. For example, FACS analysis of our primary SMCs did not detect integrin α9β1.
Integrin α5β1 ligation to fibronectin involves binding to its RGD motif within the central cell-binding domain and to an upstream synergy site (PHSRN) that stabilizes α5β1 in an active conformation [38]. Fibulin-5 has no such synergy sequence. Moreover, the fibulin-5 RGD motif is within an atypical cbEGF-like domain, and its accessibility may be suboptimal for activating β1 integrins. The integrin-activating antibody TS2/16 may exert its effects on fibulin-5 by inducing and stabilizing α5β1 in a high-affinity conformation. Structural studies are now needed to clarify how β1 integrins bind fibulin-5. Comparison of SMC adhesion to full-length fibulin-5 or fragments showed that the N-terminal ‘half’ binds cells more strongly than full-length fibulin-5, but the N-terminal RGD domain (similar to the fragment used in [14]) only supports low cell adhesion. Thus fibulin-5 fragment size and conformation affects cell interactions.
A recent study has shown that integrin ligation to different ECM molecules can result in actively suppressed integrin activation [39]. The failure of fibulin-5 to induce focal adhesions suggests passive adhesion or suppression of β1 integrin activation. Lack of β1 activation was confirmed using the integrin-activating antibody TS2/16, which ‘rescued’ SMC spreading, proliferation, migration and RTK (PDGFRs and EGF receptors) signalling. For cells on fibronectin, engagement of α5β1 and the cell-surface heparan sulfate proteoglycan, syndecan-4, stimulates focal adhesion formation [33,34]. We showed previously that fibulin-5 does not bind heparin [18], and, as expected, the presence of fibronectin fragments that activate syndecan-4 failed to induce focal adhesions on fibulin-5. Integrin α4β1 does not localize to focal adhesions in most cell types, but often promotes lamellipodia formation and cell migration [40]. Ligation of α4β1 by fibulin-5 may therefore contribute to lamellipodia formation in SMCs.
Recent studies have shed light on how fibulin-5 influences cells. Cultured SMCs from fibulin-5-null mice migrated and proliferated normally, but, after treatment with PDGF-BB, showed enhanced proliferation and migration compared with wild-type SMCs [21]. However, skin fibroblasts from the wild-type and fibulin-5-null mice showed no significant differences in proliferative or migratory responses to EGF [15]. Our results show that SMCs on fibulin-5 have undetectable PDGFR signalling, and low EGF receptor signalling compared with cells on fibronectin, owing to lack of β1 activation. The present study thus shows that activated β1 integrin is a critical regulator of PDGFR and EGF receptor signalling. It is also likely that, in vascular sites with elevated fibulin-5/fibronectin levels, fibulin-5 will block fibronectin-mediated spreading, migration and proliferation.
The present study demonstrates how fibulin-5 can regulate SMC behaviour, both directly and by modulating the effects of fibronectin. Fibulin-5 interactions with SMCs may also be critical for ordered elastic fibre formation.
Acknowledgments
This work was conducted within the Wellcome Trust Centre for Cell-Matrix Research, and was supported by the Biotechnology and Biological Sciences Research Council/MRC/Engineering and Physical Sciences Research Council (U.K. Centre for Tissue Engineering) and by EU Contract LSHM-CT-2005-018960. C. M. K. is a Royal Society Wolfson Research Merit Award holder.
References
- 1.Chu M. L., Tsuda T. Fibulins in development and heritable disease. Birth Defects Res. C Embryo Today. 2004;72:25–36. doi: 10.1002/bdrc.20003. [DOI] [PubMed] [Google Scholar]
- 2.Argreaves W. S., Greene L. M., Cooley M. A., Gallagher W. M. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4:1127–1131. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowal R. C., Richardson J. A., Miano J. M., Olson E. N. EVEC, a novel epidermal growth factor-like repeat-containing protein upregulated in embryonic and diseased adult vasculature. Circ. Res. 1999;84:1166–1176. doi: 10.1161/01.res.84.10.1166. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T., Ruiz-Lozano P., Lindner V., Yabe D., Taniwaki M., Furukawa Y., Kobuke K., Tashiro K., Lu Z., Andon N. L., et al. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J. Biol. Chem. 1999;274:22476–22483. doi: 10.1074/jbc.274.32.22476. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa H., Davis E. C., Starcher B. C., Ouchi T., Yanagisawa M., Richardson J. A., Olson E. N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 6.Stone E. M., Braun T. A., Russell S. R., Kuehn M. H., Lotery A. J., Moore P. A., Eastman C. G., Casavant T. L., Sheffield V. C. Missense variations in the fibulin 5 gene and age-related macular degeneration. N. Engl. J. Med. 2004;351:346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- 7.Lotery A. J., Baas D., Ridley C., Jones R. P., Klaver C. C., Stone E., Nakamura T., Luff A., Griffiths H., Wang T., et al. Reduced secretion of fibulin 5 in age-related macular degeneration and cutis laxa. Hum. Mutat. 2006;27:568–574. doi: 10.1002/humu.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullins R. F., Olvera M. A., Clark A. F., Stone E. M. Fibulin-5 distribution in human eyes: relevance to age-related macular degeneration. Exp. Eye Res. 2006;84:378–380. doi: 10.1016/j.exer.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeys B., Van Maldergem L., Mortier G., Coucke P., Gerniers S., Naeyaert J. M., De Paepe A. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum. Mol. Genet. 2002;11:2113–2118. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- 10.de Schepper S., Loeys B., de Paepe A., Lambert J., Naeyaert J. M. Cutis laxa of the autosomal recessive type in a consanguineous family. Eur. J. Dermatol. 2003;13:529–533. [PubMed] [Google Scholar]
- 11.Markova D., Zou Y., Ringpfeil F., Sasaki T., Kostka G., Timpl R., Uitto J., Chu M. L. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am. J. Hum. Genet. 2003;72:998–1004. doi: 10.1086/373940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elahi E., Kalhor R., Banihosseini S. S., Torabi N., Pour-Jafari H., Houshmand M., Amini S. S., Ramezani A., Loeys B. Homozygous missense mutation in fibulin-5 in an Iranian autosomal recessive cutis laxa pedigree and associated haplotype. J. Invest. Dermatol. 2006;126:1506–1509. doi: 10.1038/sj.jid.5700247. [DOI] [PubMed] [Google Scholar]
- 13.Hu Q., Loeys B. L., Coucke P. J., De Paepe A., Mecham R. P., Choi J., Davis E. C., Urban Z. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum. Mol. Genet. 2006;15:3379–3386. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T., Lozano P. R., Ikeda Y., Iwanaga Y., Hinek A., Minamisawa S., Cheng C. F., Kobuke K., Dalton N., Takada Y., et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Q., Choi J., Rouleau L., Leask R. L., Richardson J. A., Davis E. C., Yanagisawa H. Normal wound healing in mice deficient for fibulin-5, an elastin binding protein essential for dermal elastic fiber assembly. J. Invest. Dermatol. 2006;126:2707–2714. doi: 10.1038/sj.jid.5700501. [DOI] [PubMed] [Google Scholar]
- 16.Hirai M., Ohbayashi T., Horiguchi M., Okawa K., Hagiwara A., Chien K. R., Kita T., Nakamura T. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J. Cell Biol. 2007;176:1061–1071. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Q., Davis E. C., Richardson J. A., Starcher B. C., Li T., Gerard R. D., Yanagisawa H. Molecular analysis of fibulin-5 function during de novo synthesis of elastic fibers. Mol. Cell. Biol. 2007;27:1083–1095. doi: 10.1128/MCB.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman L. J., Lomas A., Hodson N., Sherratt M. J., Mellody K. T., Weiss A. S., Shuttleworth A., Kielty C. M. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem. J. 2005;388:1–5. doi: 10.1042/BJ20050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Hallous E., Sasaki T., Hubmacher D., Getie M., Tiedemann K., Brinckmann J., Batge B., Davis E. C., Reinhardt D. P. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adaptor function to tropoelastin. J. Biol. Chem. 2007;282:8935–8946. doi: 10.1074/jbc.M608204200. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi N., Kostka G., Garbe J. H., Keene D. R., Bachinger H. P., Hanisch F. G., Markova D., Tsuda T., Timpl R., Chu M. L., Sasaki T. A comparative analysis of the fibulin protein family: biochemical characterization, binding interactions, and tissue localization. J. Biol. Chem. 2007;282:11805–11816. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- 21.Spencer J. A., Hacker S. L., Davis E. C., Mecham R. P., Knutsen R. H., Li D. Y., Gerard R. D., Richardson J. A., Olson E. N., Yanagisawa H. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2946–2951. doi: 10.1073/pnas.0500058102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preis M., Cohen T., Sarnatzki Y., Ben Yosef Y., Schneiderman J., Gluzman Z., Koren B., Lewis B. S., Shaul Y., Flugelman M. Y. Effects of fibulin-5 on attachment, adhesion, and proliferation of primary human endothelial cells. Biochem. Biophys. Res. Commun. 2006;348:1024–1033. doi: 10.1016/j.bbrc.2006.07.156. [DOI] [PubMed] [Google Scholar]
- 23.Mould A. P., Travis M. A., Barton S. J., Hamilton J. A., Askari J. A., Craig S. E., Macdonald P. R., Kammerer R. A., Buckley P. A., Humphries M. J. Evidence that monoclonal antibodies directed against the integrin β subunit plexin/semaphorin/integrin domain stimulate function by inducing receptor extension. J. Biol. Chem. 2005;280:4238–4246. doi: 10.1074/jbc.M412240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makarem R., Newham P., Askari J. A., Green L. J., Clements J., Edwards M., Humphries M. J., Mould A. P. Competitive binding of vascular cell adhesion molecule-1 and the HepII/IIICS domain of fibronectin to the integrin α4β1. J. Biol. Chem. 1994;269:4005–4011. [PubMed] [Google Scholar]
- 25.Wang R., Clark R. A. F., Mosher D. F., Ren X. D. Fibronectin's central cell-binding domain supports focal adhesion formation and Rho signal transduction. J. Biol. Chem. 2005;280:28803–28810. doi: 10.1074/jbc.M501421200. [DOI] [PubMed] [Google Scholar]
- 26.Bax D. V., Mahalingam Y., Cain S. A., Mellody K., Freeman L. Y., Younger K., Shuttleworth C. A., Humphries M. J., Couchman J. R., Kielty C. M. Cell adhesion to fibrillin-1: identification of an Arg-Gly-Asp-dependent synergy region and a heparin-binding site that regulates focal adhesion formation. J. Cell Sci. 2007;120:1383–1392. doi: 10.1242/jcs.003954. [DOI] [PubMed] [Google Scholar]
- 27.Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc. Natl. Acad. Sci. U.S.A. 1984;81:5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada K. M., Kennedy D. W. Amino acid sequence specificities of an adhesive recognition signal. J. Cell. Biochem. 1985;28:99–104. doi: 10.1002/jcb.240280203. [DOI] [PubMed] [Google Scholar]
- 29.Ball S. G., Shuttleworth C. A., Kielty C. M. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J. Cell Biol. 2007;177:489–500. doi: 10.1083/jcb.200608093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphries J. D., Byron A., Humphries M. J. Integrin ligands at a glance. J. Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mostafavi-Pour Z., Askari J. A., Whittard J. D., Humphries M. J. Identification of a novel heparin-binding site in the alternatively spliced IIICS region of fibronectin: roles of integrins and proteoglycans in cell adhesion to fibronectin splice variants. Matrix Biol. 2001;20:63–73. doi: 10.1016/s0945-053x(00)00131-1. [DOI] [PubMed] [Google Scholar]
- 32.Hsia D. A., Lim S. T., Bernard-Trifilo J. A., Mitra S. K., Tanaka S., den Hertog J., Streblow D. N., Ilic D., Ginsberg M. H., Schlaepfer D. D. Integrin α4β1 promotes focal adhesion kinase-independent cell motility via α4 cytoplasmic domain-specific activation of c-Src. Mol. Cell. Biol. 2005;25:9700–9712. doi: 10.1128/MCB.25.21.9700-9712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods A., Longley R. L., Tumova S., Couchman J. R. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch. Biochem. Biophys. 2000;374:66–72. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- 34.Woods A., Couchman J. R. Syndecan-4 and focal adhesion function. Curr. Opin. Cell Biol. 2001;13:578–583. doi: 10.1016/s0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- 35.Aplin A. E., Howe A., Alahari S. K., Juliano R. L. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol. Rev. 1998;50:197–263. [PubMed] [Google Scholar]
- 36.Baron W., Decker L., Colognato H., ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr. Biol. 2003;13:151–155. doi: 10.1016/s0960-9822(02)01437-9. [DOI] [PubMed] [Google Scholar]
- 37.Hollenbeck S. T., Itoh H., Louie O., Faries P. L., Liu B., Kent K. C. Type I collagen synergistically enhances PDGF-induced smooth muscle cell proliferation through pp60src-dependent crosstalk between the α2β1 integrin and PDGFβ receptor. Biochem. Biophys. Res. Commun. 2004;325:328–337. doi: 10.1016/j.bbrc.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 38.Aota S., Nomizu M., Yamada K. M. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 1994;269:24756–24761. [PubMed] [Google Scholar]
- 39.Orr A. W., Ginsberg M. H., Shattil S. J., Deckmyn H., Schwartz M. A. Matrix-specific suppression of integrin activation in shear stress signaling. Mol. Biol. Cell. 2006;17:4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinco K. A., He W., Yang J. T. α4β1 integrin regulates lamellipodia protrusion via a focal complex/focal adhesion-independent mechanism. Mol. Biol. Cell. 2002;13:3203–3217. doi: 10.1091/mbc.02-05-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]