Abstract
The nature of the mechanisms underlying the age-related decline in glutathione (GSH) synthetic capacity is at present unclear. Steady-state kinetic parameters of mouse liver GCL (glutamate–cysteine ligase), the rate-limiting enzyme in GSH synthesis, and levels of hepatic GSH synthesis precursors from the trans-sulfuration pathway, such as homocysteine, cystathionine and cysteine, were compared between young and old C57BL/6 mice (6- and 24-month-old respectively). There were no agerelated differences in GCL Vmax, but the apparent Km for its substrates, cysteine and glutamate, was higher in the old mice compared with the young mice (∼800 compared with ∼300 μM, and ∼710 compared with 450 μM, P<0.05 for cysteine and glutamate in young and old mice respectively). Amounts of cysteine, cystathionine and Cys-Gly increased with age by 91, 24 and 28% respectively. Glutathione (GSH) levels remained unchanged with age, whereas GSSG content showed an 84% increase, suggesting a significant pro-oxidizing shift in the 2GSH/GSSG ratio. The amount of the toxic trans-sulfuration/glutathione biosynthetic pathway intermediate, homocysteine, was 154% higher (P<0.005) in the liver of old mice compared with young mice. The conversion of homocysteine into cystathionine, a rate-limiting step in trans-sulfuration catalysed by cystathionine β-synthase, was comparatively less efficient in the old mice, as indicated by cystathionine/homocysteine ratios. Incubation of tissue homogenates with physiological concentrations of homocysteine caused an up to 4.4-fold increase in the apparent Km of GCL for its glutamate substrate, but had no effect on Vmax. The results suggest that perturbation of the catalytic efficiency of GCL and accumulation of homocysteine from the trans-sulfuration pathway may adversely affect de novo GSH synthesis during aging.
Keywords: aging, glutamate–cysteine ligase, glutathione, homocysteine, oxidative stress, redox state
Abbreviations: BSO, L-buthionine-(SR)-sulfoximine; CBS, cystathionine β-synthase; CYSlim, limiting L-cysteine substrate concentration; DTT, dithiothreitol; GC, γ-glutamylcysteine; GCL, glutamate–cysteine ligase; GCLholo, GCL holoenzyme; GCLc, GCL catalytic subunit; GCLm, GCL modulatory subunit; GLUlim, limiting L-glutamate substrate concentration; GS, glutathione synthase
INTRODUCTION
The tripeptide, glutathione or γ-glutamylcysteinylglycine (GSH), is often present in millimolar concentrations in mammalian tissues [1]. It is a versatile reductant, serving multiple biological functions, including acting as a quencher of free radicals, a co-substrate in the enzymatic reduction of peroxides, a conjugant of drugs to enhance their water solubility, in cellular transport of amino acids, and thiolation/dethiolation of proteins, among others [2]. Depletion of GSH increases susceptibility of tissues to oxidative damage [1]. GSH is synthesized by the consecutive actions of two enzymes, GCL (glutamate–cysteine ligase; EC 6.3.2.2), which catalyses the rate-limiting step in the de novo synthesis of GSH, followed by GS (glutathione synthase; EC 6.3.2.3), which couples glycine with GC (γ-glutamylcysteine) [3,4]. Under normal physiological conditions, GC concentrations are extremely low (nanomolar levels; [5]), suggesting that the GCL-catalysed reaction is rate limiting in GSH biosynthesis [6].
The eukaryotic GCLholo (GCL holoenzyme) is a heterodimer consisting of a ∼31 kDa modulatory subunit (GCLm) and a ∼73 kDa catalytic subunit (GCLc) [7]. The activity of the GCLc subunit is substantially increased by interaction with GCLm [8,9]. Mechanisms by which the GCLm subunit regulates the catalytic activity of GCLc include disulfide-dependent covalent interactions between the subunits, which leads to a decrease in the Km for glutamate and a reduction in the feedback inhibition of the holoenzyme by GSH [7]. The GCLholo heterodimer is credited with almost all of the GC synthetic activity in vivo [8,9]. The Kcat/Km of GCL, an indicator of the catalytic efficiency of the enzyme, is much higher for GCLholo compared with the free GCLc subunit [9]. At physiologically relevant substrate and inhibitor (GSH) concentrations, GCLc activity may be as low as 2% of GCLholo [9].
A ubiquitous age-related alteration, observed in a variety of phylogenetically divergent species, is a pro-oxidizing shift in the redox state of tissues, as reflected by the decrease in the 2GSH/GSSG ratio [10,11]. Furthermore, aged organisms have a diminished ability to maintain relatively high GSH levels and 2GSH/GSSG ratios in response to oxidant challenges [12]. The nature of the mechanisms underlying the deterioration of GSH homoeostasis is at present unclear. Although an aging-related loss in GCL activity has been reported under substrate saturating conditions [12,13], it is unknown whether comparable changes in GCL activity also occur at physiologically relevant substrate concentrations. Indeed, currently available data can be alternatively interpreted to suggest either a substantial decrease or no significant age-related change in GCL activity [12–14].
Plasma GSH and cysteine levels are largely determined by hepatic efflux [15] via GSH transporters [16], suggesting that liver is essential in ‘systemic’ GSH and cysteine distribution and thus plays a critical role in inter-organ GSH and redox homoeostasis [17]. Liver has one of the highest organ contents of GSH, in addition to being one of the few organs that possesses a trans-sulfuration pathway (Scheme 1; [17]). Thus it seems reasonable to hypothesize that hepatic de novo GSH synthesis and trans-sulfuration pathways may have key roles in age-related loss of GSH homoeostasis. In this context, the present study reports age-dependent variations in (i) kinetics of hepatic GCL activity and (ii) amounts of the intermediates that represent ‘metabolic commitment junctions’ regulating de novo glutathione synthetic capacity in mouse liver.
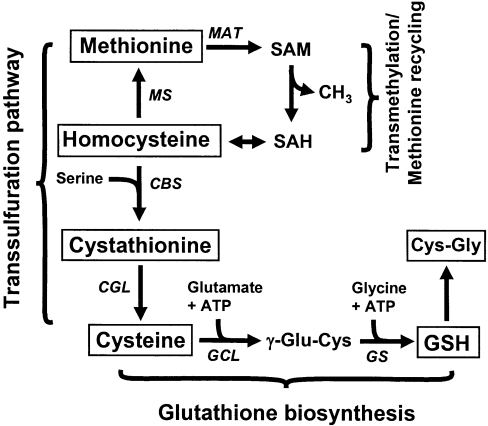
Scheme 1. Schematic representation of the hepatic trans-sulfuration and glutathione biosynthetic pathways.
L-γ-Glutamylcysteinylglycine, or glutathione, is composed of glutamate, cysteine and glycine residues. In the trans-sulfuration pathway, methionine acts as the sulfur source for cysteine synthesis via a number of intermediates. Initially, methionine undergoes adenylation, catalysed by methionine adenosyltransferase (MAT) to form S-adenosylmethionine (SAM), which, following demethylation, generates S-adenosylhomocysteine (SAH). Homocysteine (Hcy) is generated by the hydrolysis of SAH to adenosine and homocysteine. Next, in a committed reaction catalysed by cystathionine β-synthase (CBS), homocysteine condenses with serine to form cystathionine, which in turn is rapidly converted into cysteine by the activity of cystathionine γ-lyase (CGL). GCL joins glutamate and cysteine in an ATP-dependent reaction, to form GC, which upon addition of glycine, forms GSH. Cys-Gly is formed by the breakdown of GSH. The metabolites that were measured in the present study are boxed. Italicized abbreviations represent enzyme activities. MS, methionine synthase; GS, glutathione synthase.
EXPERIMENTAL
Chemicals and reagents
The HPLC calibration standards (L-cysteine, DL-cystathionine, L-homocysteine, GSH, GSSG, L-methionine, Cys-Gly and GC) were obtained from Sigma Chemical Company (St. Louis, MO, U.S.A.). Orthophosphoric acid was purchased from EMD Science (Gibbstown, NJ, U.S.A.). All chemicals were either of analytical grade or of the highest purity commercially available.
Animals
Male C57BL/6 mice (6 and 24 months of age) were obtained from the National Institute on Aging, NIH (National Institutes of Health, Bethesda, MD, U.S.A.) and were fed ad libitum on Teklad Global 16% Protein Rodent Diet (Harlan Teklad, Indianapolis, IN, U.S.A.).
Tissue preparation for enzyme assays
Mice were killed at approx. 09:00–10:00 h by cervical dislocation, and livers were removed and placed in ice-cold antioxidant buffer (50 mM potassium phosphate buffer, pH 7.4, containing 2 mM EDTA and 0.1 mM butylated hydroxytoluene). All solutions were sparged with oxygen-free nitrogen gas for a minimum of 10 min, and livers were homogenized in Kontes glass homogenizers (Kontes, Vineland, NJ, U.S.A.), by using 10 vol. of extraction buffer (320 mM sucrose, 1 mM PMSF, 1 mM ϵ-amino-n-hexanoic acid and 10 mM Tris/HCl, pH 7.4), supplemented with Complete® protease inhibitor cocktail tablets (Roche, Indianapolis, IN, U.S.A.), at a concentration of 1 tablet per 10 ml of buffer [18]. Homogenates were centrifuged at 3000 g for 10 min, and resulting supernatants were centrifuged at 14000 g (for 15 min at 4 °C) through Nanosep 10K ω centrifugal filters (Pall, Ann Arbor, MI, U.S.A.).
Measurement of enzymatic activity
Supernatants resulting from 3000 g centrifugation of tissue homogenates were passed through 0.45 μm PTFE Acrodisc® syringe filters (Gelman Laboratory, Ann Arbor, MI, U.S.A.) directly into Pall centrifugal devices. Following centrifugation at 14000 g for 15 min, the protein samples were washed with 100 μl of wash buffer (200 mM sucrose, 1 mM PMSF, 1 mM ϵ-amino-n-hexanoic acid and 10 mM Tris/HCl, pH 7.4) and subsequently made up to a known volume with wash buffer. Aliquots of the preparation were immediately used for GCL assays, as described below. Following GCL assays, samples were either directly injected on to the HPLC column or stored at −80 °C for <24 h before analysis.
HPLC-based GCL enzyme assay and kinetic analysis
The GCL assay mixture consisted of 5–20 μl aliquots of protein sample (∼30–50 μg), 100 mM Tris/HCl, 20 mM MgCl2 (pH 8.2), 10 mM ATP, 5 mM L-cysteine, 50 mM L-glutamate, 1 mM EDTA and 100 μM acivicin in a total assay volume of 250 μl. Assays were carried out for 10 min at 25 °C. In preliminary experiments, reaction linearity with various substrate concentrations, time and protein content was established. A specific inhibitor of GCL, BSO [L-buthionine-(SR)-sulfoximine], was used to determine the specificity of the assay. Briefly, liver extracts were incubated at room temperature (25 °C) for 10 min with up to 1 mM BSO and 10 mM ATP in incubation buffer (0.1 M Tris/HCl and 20 mM MgCl2, pH 8.2) prior to the measurement of GCL activity. Preliminary experiments with BSO and the mouse liver homogenate indicated that the Ki was <100 μM, in close agreement with rat GCL [19]. For kinetic studies, L-glutamate was varied between 0 and 25 mM and L-cysteine between 0 and 5 mM. Apparent Km and Vmax were determined by using Hanes–Woolf plots, which represent substrate concentration divided by the reaction velocity ([S]/Vo) compared with substrate concentration ([S]). GCL reactions were terminated with an equivalent volume of 15 mM orthophosphoric acid. Precipitated proteins were removed by centrifugation at 14000 g for 10 min at 4 °C; the supernatant was refiltered through 0.45 μm PTFE Acrodisc® syringe filters and injected on to the HPLC either immediately or within 24 h. GC was detected by an HPLC-based procedure, as described recently in [20]. Compounds were resolved by a reverse-phase C18 Luna column (particle size 5 μm; 250 mm×4.6 mm; Phenomenex, Torrance, CA, U.S.A.) by using isocratic elution with 15 mM orthophosphoric acid (pH 2.0) as the mobile phase at a flow rate of 1.0 ml·min−1, and detected with a model 5011 CoulArray electrochemical detector (ESA, Chelmsford, MA, U.S.A.) set at a potential of +600 mV.
HPLC-based measurement of free aminothiols
Aminothiols (L-cysteine, DL-cystathionine, Cys-Gly, L-methionine, L-homocysteine, GSH and GSSG) were resolved and quantified according to the procedure described in [20]. Separation was achieved by using a C18 Luna column (5 μm; 150 mm×4.6 mm), obtained from Phenomenex, and isocratic elution with a mobile phase composition of 50 mM monobasic sodium phosphate, 1 mM 1-octanesulfonic acid and 1% (v/v) acetonitrile (pH 2.7), delivered via a Waters 515 HPLC pump at a flow rate of 1.0 ml·min−1. Compounds were detected with a model 5011 CoulArray electrochemical detector (ESA), by using potentials of +600 for L-cysteine, GSH and Cys-Gly and +850 mV for methionine and GSSG. Each sample was injected twice and the average of the peak areas was used for quantification. Linear calibration curves were constructed by using four to five concentrations of each compound. In all cases, concentrations of calibration curves for all tested aminothiols were linear (R2≥0.995; results not shown).
Incubation of homogenates with L-homocysteine
Mouse liver homogenates (∼1.0 mg·ml−1) were incubated with 100–500 μM L-homocysteine at 37 °C with gentle agitation [21]. Aliquots were washed free of unbound L-homocysteine as described above, and the resulting mixtures were used in GCL assays with both saturating (Vmax) and limiting L-cysteine (CYSlim; containing 1.5 mM cysteine) or L-glutamate (GLUlim; containing 5 mM glutamate) substrate concentrations. Kinetic parameters were determined in experiments with appropriate dilution series of L-cysteine and L-glutamate substrates, as described above and in the Figure legends.
Western blot analysis of protein samples
The subunits GCLc and GCLm and the holoenzyme (GCLholo) were resolved by PAGE and then subjected to Western blot analysis by using previously published methods [8,9]. Total protein mixtures (10–20 μg) were resolved by SDS/10% PAGE and electrotransferred to Immobilon-P® PVDF membranes (Millipore, Bedford, MA, U.S.A.). GCLc was detected by incubation of blots with anti-GCLc primary antibodies (Lab Vision, Freemont, CA, U.S.A.). GCLc and GCLm subunits were also detected by using a different set of polyclonal antibodies kindly provided by Dr Timothy P. Dalton (University of Cincinnati). The primary antibodies were diluted in TBS-T (1:10000 dilution in 20 mM Tris/HCl, pH 7.6, 8 g·l−1 NaCl and 0.1% Tween 20) containing 5% (w/v) non-fat dry milk, and incubated overnight at 4 °C. The blots were washed four times, 10 min each, with TBS-T before addition of horseradish peroxidase-conjugated anti-rabbit IgG or anti-chicken IgY secondary antibodies (at 1:50000 dilution), for GCLc and GCLm respectively. Multiple rounds of preliminary Western blot analyses were conducted by using variable concentrations of liver proteins separated on polyacrylamide gels, to generate standard curves. Antigen and antibody concentrations used in the analyses were within the linear (R2=0.97–0.99) region of autoradiograph band intensity. For reducing conditions, loading buffer was adjusted to contain 50 mM DTT (dithiothreitol) and heated at 95 °C for 5 min before PAGE. For native separations, proteins were not treated with DTT or heat prior to loading and were resolved on 7.5% (w/v) polyacrylamide gels. Bands were visualized with an Amersham ECL® Plus Western blotting detection kit (Piscataway, NJ, U.S.A.). Relative band intensities on autoradiographs were digitally quantified by using NIH ImageJ software (http://rsb.info.nih.gov/ij/).
Sequence alignments and phylogenetic analysis
Published cDNA or amino acid sequences for GCLc subunits were obtained by using the public sequence databases and analysis services at NCBI (National Center for Biotechnology Information). The mouse and Trypanosoma deduced amino acid sequences for GCLc were used for BLAST searches (see http://www.ncbi.nlm.nih.gov/BLAST). Construction of phylogenetic trees from deduced GCLc amino acid sequences was performed by using TreeTop, Phylogenetic tree prediction software (see http://www.genebee.msu.su/genebee.html). Conserved cysteine residues were aligned by using the above software in addition to manual inspection and visual verification of primary structures.
Protein determinations
The protein content of the extracts was determined by using the sodium BCA (bicinchoninic acid) protein assay (Pierce, Rockford, IL, U.S.A.), according to the manufacturer's instructions.
Statistical analysis
Results are presented as means±S.E.M. Experiments typically included six to thirteen replicates per treatment unless indicated otherwise. Treatments were compared statistically by using Student's t tests on Microsoft Excel® 2002 software. P values of <0.05 were considered statistically significant.
RESULTS
Aging-associated decline in the utilization of L-cysteine and L-glutamate substrates by mouse hepatic GCL
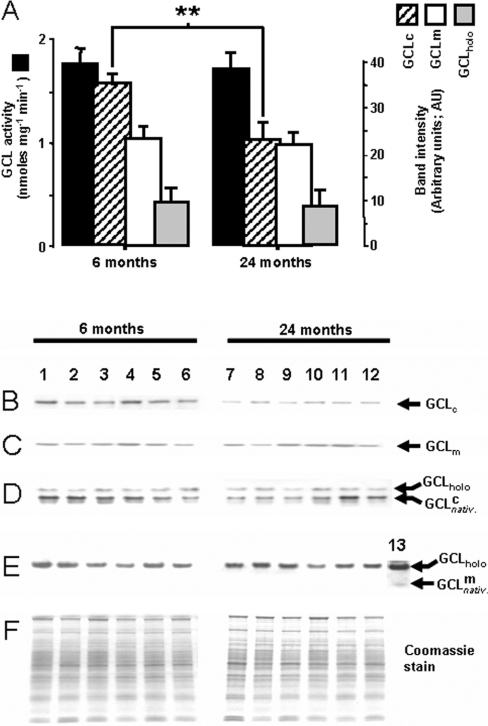
GSH is synthesized de novo by the consecutive activities of two enzymes, namely GCL, which catalyses the first and the rate-limiting step, and GS, which links glycine to GC to form GSH. The ability to synthesize GSH de novo declines in aged organisms, particularly under conditions that promote increased fluxes of reactive oxygen species [22]. To understand the basis of this phenomenon, the maximal activity (Vmax) of GCL and the apparent Km for its two substrates, cysteine and glutamate, were compared in the liver homogenates of 6- and 24-month-old mice. The Vmax, determined under saturating substrate conditions (10 mM ATP, 5 mM L-cysteine and 50 mM L-glutamate), was similar in the young and the old mice (1.8 and 1.7 nmol·min−1·mg−1 respectively; Figure 1). The apparent Km of GCL for its limiting substrate, cysteine [KmCYS (app)], was ∼300±15 μM in the 6-month-old and ∼800±72 μM in the 24-month-old mice (P<0.05). The KmGLU (app) of GCL was ∼450±36 μM in the young and ∼710±85 μM (P<0.05) in the old mouse liver. Thus there was a significant loss in catalytic efficiency of GCL for both of these substrates.
Figure 1. Maximal catalytic activity (Vmax) of hepatic GCL (upper panel), and Western blot analysis of GCL holoenzyme, catalytic and regulatory subunits (lower panel) in young (6-month-old) and old (24-month-old) C57BL/6 mice.
Upper panel (A): maximal velocity determinations of GCL activity assay (dark bars) were made in liver homogenates under substrate saturating conditions (10 mM ATP, 5 mM L-cysteine, 50 mM L-glutamate and 100 μM acivicin, pH 8.2). Lower panel: liver homogenates from 6-month-old (lanes 1–6) and 24-month-old (lanes 7–12) mice resolved by: (B) SDS/PAGE separation followed by Western blot analysis with anti-GCLc antibodies; (C) SDS/PAGE separation, followed by Western blot analysis with anti-GCLm antibodies; (D) native PAGE separation followed by Western blot analysis using GCLc antibodies; (E) native PAGE followed by Western blot analysis with anti-GCLm antibodies; (F) Coomassie Blue-stained gels. Lane 13 in (E) is a representative liver homogenate, loaded on to the gels at ∼10-fold protein concentration; the sample illustrates the barely detectable free GCLm subunit in native separations. Autoradiographs were digitally quantified by using NIH ImageJ software (http://rsb.info.nih.gov/ij/). Results are means±S.E.M. (n=13); **P<0.00006 compared with mice at 6 months of age. AU, arbitrary units.
Aging-related changes in protein levels of GCL subunits in mouse liver
To determine whether the observed decrease in the catalytic efficiency of GCL may be due to corresponding losses in the amounts of GCLc and GCLm subunits, proteins in liver homogenates were separated by SDS/PAGE and quantified by Western blot analysis (Figures 1A–1F; hatched bars and open bars respectively). The immunoreactive bands, corresponding to the deduced Mr of the mouse GCLc and GCLm subunits, were ∼73 and ∼31 kDa in mass respectively. The amount of the catalytic GCLc subunit protein was ∼35% lower (P<0.05) in the old compared with the young mice (Figures 1A and 1B). In contrast, there was no significant loss in the amount of GCLm protein with age (Figures 1A and 1C). To determine whether there were any age-related alterations in the association between the two GCL subunits, native PAGE was used to resolve GCL heterodimer, GCLc and GCLm species (Figures 1D and 1E), thereby allowing quantification of any age-related changes in subunit association. Virtually all of the available GCLm subunit protein was found to be complexed with GCLc in both the young and old mice (Figure 1E). However, densitometric measurements showed no age-related difference in the amount of GCLholo protein, which constitutes the primary enzymatic unit for catalysis under in vivo conditions [9].
Effect of age on the intermediates of the hepatic trans-sulfuration pathway
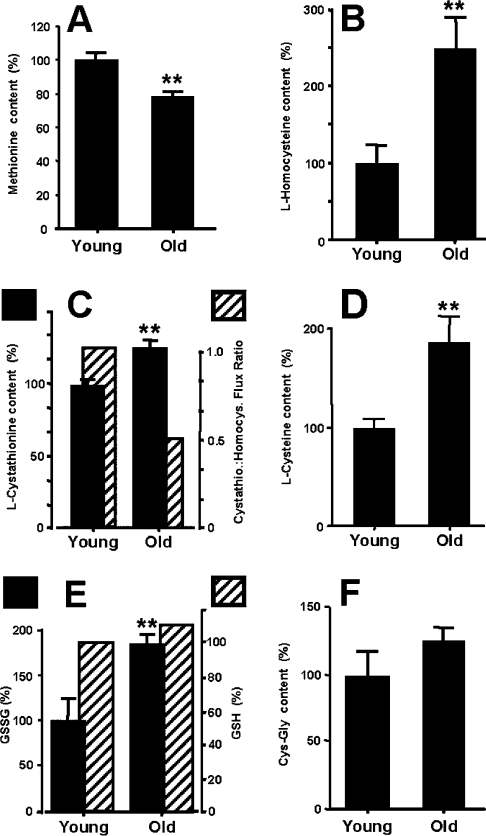
The function of the trans-sulfuration pathway (methionine→homocysteine→cystathionine→cysteine; Scheme 1) is to transfer sulfur from methionine to cysteine, the rate-limiting substrate for GCL activity. Age-related alterations in the trans-sulfuration pathway were assessed on the basis of the amounts of the intermediates in the pathway [2]. Compared with the young, the old mice showed a 22% decrease in methionine concentration (Figure 2; P<0.05), whereas there was a 91% increase in cysteine, 154% increase in homocysteine and a 24% increase in cystathionine (Figure 2; P<0.005). Thus metabolic flux coefficients [ratios of L-cystathionine/L-homocysteine (nmol/mg of protein) of young and old animals; Figures 2B and 2C] suggested that the conversion of homocysteine into cystathionine, carried out by the regulatory enzyme CBS (cystathionine β-synthase; Scheme 1), was relatively less efficient in the older animals compared with the young.
Figure 2. Comparison of aminothiol levels in the livers of 6- and 24-month-old mice.
The closed bars indicate levels (in percentages), of the specified aminothiol: (A) L-methionine; (B) L-homocysteine; (C) L-cystathionine and L-cystathionine/L-homocysteine ratio (closed and hatched bars respectively); (D) L-cysteine; (E) GSSG and glutathione (GSH) (closed and hatched bars respectively); and (F) Cys-Gly content in liver homogenates. Absolute amounts of aminothiols, in nmol/mg of protein from liver homogenates of young mice, were as follows: GSH, 41±3.9; GSSG, 2.2±0.4; Cys-Gly, 1.1±0.19; methionine, 1.8±0.1; cysteine, 2.5±0.22; cystathionine, 0.05±0.01; homocysteine, 0.3±0.07. Results are means±S.E.M. (n=6); **P<0.05 compared with mice at 6 months of age.
The amount of the dipeptide Cys-Gly, which is both a breakdown product and a precursor of GSH, was 28% (P<0.03) higher in the livers of the old animals compared with the young (Figure 2F). Although the steady-state amounts of GSH were similar in the young and the old mice (∼41 and 45 nmol/mg of protein respectively), the GSSG content was 84% higher in the old, thereby resulting in a decrease in the 2GSH/GSSG ratio (Figure 2E).
Sequence analysis of mouse GCL substrate-binding determinants
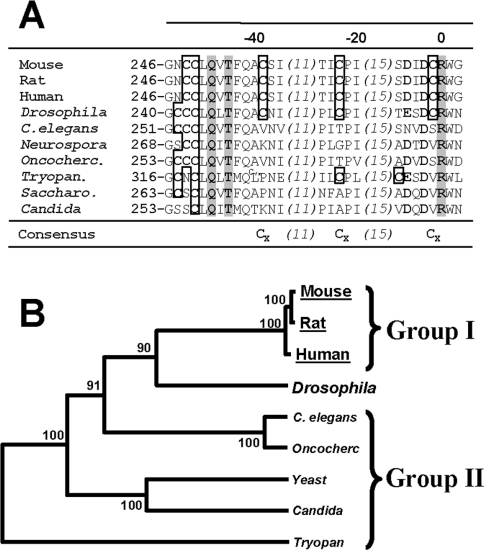
GCL activity has been shown to be modulated by changes in the ambient redox state [17]. It was therefore postulated that the observed age-related changes in the affinity of GCL for its substrates might emanate from shifts in the redox state occurring during aging, as indicated by the observed decrease in the 2GSH/GSSG ratio (Figure 2E). To identify the potential substrate-binding sites of GCL, its deduced amino acid sequence was compared with that of glutamine synthase, a structural homologue [23,24]. By homology, Arg296 and Arg427 in mouse GCLc appeared to be the key sequences for glutamate binding (Figure 3A; also discussed in [24]). Based on results from studies in site-directed mutagenesis [24], it was reasoned that cysteine residues located in the vicinity of Arg296 could play a role in the modulation of GCL activity in some species (designated as ‘group I’; Figure 3B; [24,25]). It was thus hypothesized that homocysteine, a nucleophile that was observed here to accumulate with age, and which reaches concentrations of up to 500 μM under pathological conditions, such as hyperhomocysteinaemia [26], may be responsible for the decrease in the affinity of GCL for glutamate in the aged mice. To test this idea, liver homogenates were incubated with 0–500 μM homocysteine, followed by GCL assays using different amounts of cysteine and glutamate. A 3 h pre-incubation of liver homogenates with 500 μM homocysteine had no effect on GCLc or GCLm protein abundance (Figure 4A), but it resulted in a 56% decrease (P<0.002) in GCL activity, using the GLUlim buffer (containing 5 mM glutamate). No differences in GCL activity were obtained with CYSlim (containing 1.5 mM cysteine) or Vmax (saturating substrates: 50 mM glutamate and 5 mM cysteine) buffers. Additional kinetic studies using physiologically relevant concentrations of glutamate (1–25 mM) indicated a 4.4-fold decrease in the GCL catalytic efficiency following pre-incubation of the homogenate with homocysteine (Figure 4B; compare open circle controls with closed diamonds, homocysteine treated).
Figure 3. Local multiple sequence alignment (A) and phylogenetic analysis (B) of different species.
(A) The sequences were analysed and aligned as described previously [18]. Conserved cysteine residues near the L-glutamate-binding site are shown within the boxes in the aligned sequences. Boldface residues represent glutamate-binding determinants, which are conserved between species. Numbers in the top row of the sequence alignment arbitrarily set Arg296 of the mouse GCLc, which is essential in glutamate binding, as the ‘origin’ in the alignment. Numbers to the left of each row of the alignment are provided as an estimate of the distance of each glutamate-binding domain from the beginning of the cloned GCLc subunit sequence. The numbers in parentheses indicate the number of amino acid residues omitted from the sequence in an effort to facilitate clarity. Shaded amino acid residues are strictly conserved amino acids critical in glutamate binding. (B) Bootstrap values are shown on the nodes of the phylogenetic tree as an indicator of significance for the local alignments. The abbreviations used for the various species are: mouse, Mus musculus; rat, Rattus norvegicus; human, Homo sapiens; Drosophila, Drosophila melanogaster; C. elegans, Caenorhabditis elegans; Neurospora, Neurospora crassa; Oncocherc., Onchocerca volvulus; Tryopan., Trypanosoma brucei; Saccharo., Saccharomyces cerevisiae; Candida, Candida albicans.
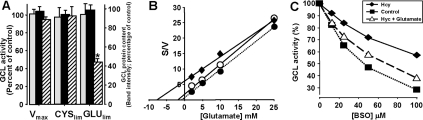
Figure 4. Effect of L-homocysteine pre-incubation on the kinetic characteristics of GCL activity.
(A) The liver homogenates were pre-incubated with 100 μM homocysteine, and aliquots were sampled at 0 and 3 h (results at 6 h are discussed in the main body of the text) for GCL assays with saturating (Vmax) and limiting (GLUlim and CYSlim) substrate concentrations. Western blot analysis was performed on each sample to quantify GCL protein following the homocysteine pre-incubations; grey bars, GCLc, black bars, GCLm protein content; hatched bars, GCL activity. Results are means±S.E.M. (n=6–13). *P<0.05. (B) L-BSO inhibitability of GCL activity; following pre-incubations with 500 μM homocysteine, the kinetics of L-glutamate utilization by GCL were determined by using Hanes–Woolf analysis; ●, activity prior to incubation; ○, activity following pre-incubation without homocysteine; ◆, activity after 3 h pre-incubation with 500 μM homocysteine. (C) BSO inhibition of hepatic GCL activity following homocysteine pre-incubations. Liver homogenates were pre-incubated with homocysteine (◆), homocysteine + glutamate (△) and with buffer only (■) as the control. The experiment was repeated three times with essentially identical results.
The above experiments were conducted after a 3 h pre-incubation with homocysteine. The effects of longer exposure were also determined because homocysteine binds to proteins in a biphasic manner; the initial phase of rapid binding is followed by progressive denaturation [21]. Compared with controls, a 6 h pre-incubation of liver homogenates with 100 μM homocysteine resulted in decreases in GCL activity respectively of 72.5% (P<0.00003), 87.1% (P<0.000007) and 96.1% (P<0.00003) with Vmax, CYSlim and GLUlim buffers, suggesting that GCL was largely inactivated. There were no significant changes in subunit association detectable in native gels (results not shown).
Using another approach to determine whether GCL kinetics could be affected by possible binding with homocysteine, liver homogenates that had been pre-incubated with 100 μM homocysteine for 3 h were subsequently incubated with 0–100 μM BSO, a well-characterized competitive inhibitor of GCL activity. Glutamate and BSO have been demonstrated to bind to GCL at nearly identical sites and undergo a similar ‘mechanism-based phosphorylation’ of the bound metabolite [27]. Thus homogenates were incubated in the presence or absence of homocysteine and/or glutamate, and GCL activity was determined in the presence of 0–100 μM BSO (Ki for BSO was determined to be <100 μM; results not shown). Homocysteine treatment caused a significant decrease in the effectiveness of BSO inhibition of GCL (Figure 4C, closed diamond symbols). The inhibition was greatest in the absence of homocysteine (Figure 4C, closed square symbols). Notably, incubation with glutamate in addition to homocysteine was able to partially ‘rescue’ the effectiveness of inhibition by BSO. Together, these experiments show that homocysteine can cause alterations in the apparent Km and inhibition kinetics of GCL.
DISCUSSION
The main findings of this study were that: (i) there was an age-related decrease in the affinity of hepatic GCL towards cysteine and glutamate, as reflected by Km values; (ii) concentrations of homocysteine, GSSG, as well as other oxidative-stress-related metabolites, such as Cys-Gly, increased in the livers of old mice; (iii) the protein amount of the GCLc subunit decreased with age; and (iv) homocysteine was shown to affect GCL activity in vitro, implying that the age-related increase in the level of homocysteine could have a detrimental effect on de novo GSH synthesis.
GSH is a highly versatile biological antioxidant, which has both the ability to quench radicals directly as well as to donate electrons in enzyme-mediated reductions of peroxides. It has been consistently noted that the level of oxidative stress in various tissues increases during aging, as reflected by the increase in the rates of mitochondrial production of superoxide anion radical and H2O2 as well as the steady-state amounts of oxidatively damaged macromolecules [28]. Liver plays a critical role in the maintenance of redox homoeostasis in the body, as it is the primary site of synthesis, storage, and the supply of GSH to other organs [17]. A decrease in GSH synthetic ability in the liver during aging, when basal levels of oxidative stress are elevated, could be particularly detrimental to the tissues dependent on liver-derived GSH.
Although there is general agreement that steady-state levels of GSH tend to decline in skeletal muscle and brain during aging, a similar trend does not seem to exist in other tissues, with different studies reporting no change, elevation or a decline with age (see [29] and references therein). Nevertheless, in this study GSH levels in mouse liver were found to remain unaltered during aging. As discussed in detail elsewhere [30], discrepancies in results of various studies on age-related changes in GSH levels can arise from a variety of sources, including the procedures used for extraction and measurement of GSH, the age of the sampled animals, and species/strain-specific differences. In contrast to the discrepancies in the age-related GSH levels, there is fairly broad consensus that the amount of GSSG increases and the ratio of 2GSH/GSSG decreases during aging, indicating a pro-oxidizing shift in the redox state [30]. The amount of GSH consumed in the liver for the purposes of detoxification of xenobiotics, conjugation of nucleophiles and mixed disulfide formation is small [17], whereas the GSH efflux into the plasma and by ‘metabolic turnover’, which is estimated to be ∼20% of the total GSH pool per hour, is relatively much larger and must be adequately balanced by the de novo synthesis of GSH. Thus the capacity of the liver for GSH synthesis has a broad impact on the redox status of a variety of tissues.
Under normal physiological conditions, the rate of GSH synthesis is determined by (i) the catalytic efficiency of GCL, (ii) the availability of GSH precursors and (iii) levels of GCLholo protein. In the present study, the maximal activity of GCL and the levels of GCLholo protein were not found to be affected by aging, whereas KmCYS (app) and KmGLU (app) increased. The age-related increase in KmCYS (app), ∼300 and ∼800 μM in the young mice compared with old mice, is particularly relevant because cysteine is normally the limiting substrate under physiological conditions [31]. The issue whether such an increase in KmCYS (app) could be due to changes in the relative abundance of GCL subunits or their interaction to form GCLholo has been debated extensively in the literature [7–9]. In summary, there is now near consensus that the kinetics of cysteine utilization by GCL [i.e. KmCYS (app)] are independent of GCL subunit interactions or GCLholo formation. Because of the observed decrease in the affinity of GCL for cysteine, it was of interest to determine whether an adequate supply of this GSH precursor was maintained during aging. Existing reports on age-related changes in cysteine levels are discrepant, showing an increase, decrease or no change [32,33]. In the present study, the level of cysteine in the liver was found to be higher in the old compared with the young, which may be reflective of an adaptive response to the corresponding increase in KmCYS (app). The extent to which such a compensatory response is sufficient to overcome the increased KmCYS (app) cannot be determined on the basis of the present study. Nevertheless, it can be argued that although no age-related decrease in hepatic GSH was noted in this study, the decline in the affinity of GCL for its substrates, cysteine and glutamate, would adversely affect the capacity of the old mice for de novo GSH synthesis. For instance, aged organisms could become particularly vulnerable to rapid surges in oxidative stress.
Trans-sulfuration is the major metabolic pathway for the conversion of methionine into cysteine via homocysteine. The elevation in the concentration of homocysteine observed in old mice suggests that there is an increase in metabolic flux towards trans-sulfuration in the liver during aging. Increased levels of homocysteine, however, can lead to exacerbation of oxidative stress by a variety of mechanisms [31]. Because of the high intrinsic pKa of its -SH group (∼10), homocysteine can target -SH-containing side chains, often displacing other less reactive thiol adducts [34]. In humans, there appears to be no definite quantitative threshold in the relationship between elevated homocysteine and resulting increases in observed pathology [26]; the incidence of ischaemic heart disease and stroke are elevated by even modest rises in the levels of homocysteine [35].
Results of the present study are the first to suggest that the relatively high levels of homocysteine that accumulate during aging can interfere with de novo GSH synthesis. Although the precise nature of the underlying mechanism is at present unclear, our results demonstrate that physiological concentrations of free homocysteine can inhibit the efficiency of cysteine utilization by GCL in a competitive manner. Homocysteine has been shown to bind GCLc at the active site in vitro [25] as well as in vivo [35], forming γ-glutamylhomocysteine [36], which is then rapidly degraded enzymatically. An additional mechanism by which an age-related increase in free homocysteine can inhibit the catalytic efficiency of GCL in a competitive manner is described in [35]. It is possible that an aging-related increase in the competition between cysteine and homocysteine for the cysteine binding site of GCL might lead to a decrease in GC synthesis.
Apart from the Cys553 residue in the human GCLc, which has been proposed to be involved in intersubunit disulfide bond formation [24], none of the other cysteine residues in GCL have been unambiguously assigned any functional role. However, kinetic evidence gathered in the present and other studies (Figures 4A and 4B; [23]), using cystamine- and BSO-dependent inactivation of GCL (Figure 4C; [19]) and sequence alignments (Figures 3A and 3B), suggest that organisms lacking cysteine residues near Arg296 (Figures 3A and 3B; i.e. ‘group II’ species) usually exhibit reduced susceptibility to BSO and/or cystamine inhibition (e.g. see [23,24] and references therein). It is thus plausible that cysteine residues near the substrate-binding site (Figure 3A) could bestow a regulatory function, as a ‘redox sensor’, to the GCL enzyme. Based on these results and conservation of cysteine residues around the glutamate-binding site (Figure 4A), we propose that GCL activity in mice (and possibly other ‘group I species’; Figure 4B) could be modulated in a sequence-specific and redox-sensitive manner.
In conclusion, hepatic GCL, the major enzymatic rate determinant in de novo GSH biosynthesis, shows an age-related kinetic deterioration in the utilization of its substrates, cysteine and glutamate, as indicated by an increase in KmCYS (app) and KmGLU (app). Levels of the toxic metabolite homocysteine, a precursor of cysteine, increase during aging. In vitro, kinetic evidence suggests that the interaction between homocysteine and GCL can lead to an increase in KmCYS (app) and KmGLU (app). Furthermore, homocysteine accumulation during aging can cause a plethora of other deleterious alterations.
Acknowledgments
This study was supported by the grant RO1AG13563 from the National Institute on Aging National Institute of Aging. D. T. thanks Dr Timothy P. Dalton for kindly sharing anti-GCLm and -GCLc antibodies used in the study.
References
- 1.Meister A., Anderson M. E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 2.Wu G., Fang Y. Z., Yang S., Lupton J. R., Turner N. D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 3.Meister A. Glutathione metabolism. Methods Enzymol. 1995;251:3–7. doi: 10.1016/0076-6879(95)51106-7. [DOI] [PubMed] [Google Scholar]
- 4.Cotgreave I. A., Gerdes R. G. Recent trends in glutathione biochemistry: glutathione–protein interactions: a molecular link between oxidative stress and cell proliferation? Biochem. Biophys. Res. Commun. 1998;242:1–9. doi: 10.1006/bbrc.1997.7812. [DOI] [PubMed] [Google Scholar]
- 5.Ristoff E., Hebert C., Njalsson R., Norgren S., Rooyackers O., Larsson A. Glutathione synthetase deficiency: is γ-glutamylcysteine accumulation a way to cope with oxidative stress in cells with insufficient levels of glutathione? J. Inherit. Metab. Dis. 2002;25:577–584. doi: 10.1023/a:1022095324407. [DOI] [PubMed] [Google Scholar]
- 6.Orr W. C., Radyuk S. N., Prabhudesai L., Toroser D., Benes J. J., Luchak J. M., Mockett R. J., Rebrin I., Hubbard J. G., Sohal R. S. Overexpression of glutamate–cysteine ligase extends life span in Drosophila melanogaster. J. Biol. Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- 7.Huang C. S., Anderson M. E., Meister A. Amino acid sequence and function of the light subunit of rat kidney γ-glutamylcysteine synthetase. J. Biol. Chem. 1993;268:20578–20583. [PubMed] [Google Scholar]
- 8.Lee J. I., Kang J., Stipanuk M. H. Differential regulation of glutamate–cysteine ligase subunit expression and increased holoenzyme formation in response to cysteine deprivation. Biochem. J. 2006;393:181–190. doi: 10.1042/BJ20051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Shertzer H. G., Schneider S. N., Nebert D. W., Dalton T. P. Glutamate–cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J. Biol. Chem. 2005;280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
- 10.Asensi M., Sastre J., Pallardo F. V., Lloret A., Lehner M., Garcia-de-la Asuncion J., Vina J. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol. 1999;299:267–276. doi: 10.1016/s0076-6879(99)99026-2. [DOI] [PubMed] [Google Scholar]
- 11.Hernanz A., Fernandez-Vivancos E., Montiel C., Vazquez J. J., Arnalich F. Changes in the intracellular homocysteine and glutathione content associated with aging. Life Sci. 2000;67:1317–1324. doi: 10.1016/s0024-3205(00)00722-0. [DOI] [PubMed] [Google Scholar]
- 12.Suh J. H., Wang H., Liu R. M., Liu J., Hagen T. M. (R)-α-lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: evidence for increased cysteine requirement for GSH synthesis. Arch. Biochem. Biophys. 2004;423:126–135. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu R., Choi J. Age-associated decline in γ-glutamylcysteine synthetase gene expression in rats. Free Radical Biol. Med. 2000;28:566–574. doi: 10.1016/s0891-5849(99)00269-5. [DOI] [PubMed] [Google Scholar]
- 14.Mosoni L., Breuille D., Buffiere C., Obled C., Mirand P. P. Age-related changes in glutathione availability and skeletal muscle carbonyl content in healthy rats. Exp. Gerontol. 2004;39:203–210. doi: 10.1016/j.exger.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Ookhtens M., Kaplowitz N. Role of the liver in interorgan homeostasis of glutathione and cyst(e)ine. Semin. Liver Dis. 1998;18:313–329. doi: 10.1055/s-2007-1007167. [DOI] [PubMed] [Google Scholar]
- 16.Lu S. C., Sun W. M., Yi J., Ookhtens M., Sze G., Kaplowitz N. Role of two recently cloned rat liver GSH transporters in the ubiquitous transport of GSH in mammalian cells. J. Clin. Invest. 1996;97:1488–1496. doi: 10.1172/JCI118571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu S. C. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 18.Toroser D., Yarian C. S., Orr W. C., Sohal R. S. Mechanisms of γ-glutamylcysteine ligase regulation. Biochim. Biophys. Acta. 2006;1760:233–244. doi: 10.1016/j.bbagen.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J. Biol. Chem. 1982;257:13704–13712. [PubMed] [Google Scholar]
- 20.Toroser D., Sohal R. S. Kinetic characteristics of native γ-glutamylcysteine ligase in the aging housefly, Musca domestica L. Biochem. Biophys. Res. Commun. 2005;326:586–593. doi: 10.1016/j.bbrc.2004.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Togawa T., Sengupta S., Chen H., Robinson K., Nonevski I., Majors A. K., Jacobsen D. W. Mechanisms for the formation of protein-bound homocysteine in human plasma. Biochem. Biophys. Res. Commun. 2000;277:668–674. doi: 10.1006/bbrc.2000.3723. [DOI] [PubMed] [Google Scholar]
- 22.Liu H., Wang H., Shenvi S., Hagen T. M., Liu R. M. Glutathione metabolism during aging and in Alzheimer disease. Ann. N.Y. Acad. Sci. 2004;1019:346–349. doi: 10.1196/annals.1297.059. [DOI] [PubMed] [Google Scholar]
- 23.Abbott J. J., Ford J. L., Phillips M. A. Substrate binding determinants of Trypanosoma brucei γ-glutamylcysteine synthetase. Biochemistry. 2002;41:2741–2750. doi: 10.1021/bi0159128. [DOI] [PubMed] [Google Scholar]
- 24.Tu Z., Anders M. W. Identification of an important cysteine residue in human glutamate–cysteine ligase catalytic subunit by site-directed mutagenesis. Biochem. J. 1998;336:675–680. doi: 10.1042/bj3360675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orlowski M., Meister A. Partial reactions catalyzed by γ-glutamylcysteine synthetase and evidence for an activated glutamate intermediate. J. Biol. Chem. 1971;246:7095–7105. [PubMed] [Google Scholar]
- 26.Aguilar B., Rojas J. C., Collados M. T. Metabolism of homocysteine and its relationship with cardiovascular disease. J. Thromb. Thrombolysis. 2004;18:75–87. doi: 10.1007/s11239-004-0204-x. [DOI] [PubMed] [Google Scholar]
- 27.Kelly B. S., Antholine W. E., Griffith O. W. Escherichia coli γ-glutamylcysteine synthetase: two active site metal ions affect substrate and inhibitor binding. J. Biol. Chem. 2002;277:50–58. doi: 10.1074/jbc.M107961200. [DOI] [PubMed] [Google Scholar]
- 28.Sohal R. S., Mockett R. J., Orr W. C. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radical Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 29.Voss P., Siems W. Clinical oxidation parameters of aging. Free Radical Res. 2006;40:1339–1349. doi: 10.1080/10715760600953859. [DOI] [PubMed] [Google Scholar]
- 30.Rebrin I., Bayne A. C., Mockett R. J., Orr W. C., Sohal R. S. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem. J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stipanuk M. H. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 32.Rebrin I., Kamzalov S., Sohal R. S. Effects of age and caloric restriction on glutathione redox state in mice. Free Radical Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Droge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos. Trans. R. Soc. London Ser. B. 2005;360:2355–2372. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobsen D. W., Catanescu O., Dibello P. M., Barbato J. C. Molecular targeting by homocysteine: a mechanism for vascular pathogenesis. Clin. Chem. Lab. Med. 2005;43:1076–1083. doi: 10.1515/CCLM.2005.188. [DOI] [PubMed] [Google Scholar]
- 35.Stokke O., Marstein S., Jellum E., Lie S. O. Accumulation of pyroglutamic acid (5-oxoproline) in homocystinuria. Scand. J. Clin. Lab. Invest. 1982;42:361–369. [PubMed] [Google Scholar]
- 36.Schwahn B., Kameda G., Wessalowski R., Mayatepek E. Severe hyperhomocysteinaemia and 5-oxoprolinuria secondary to antiproliferative and antimicrobial drug treatment. J. Inherit. Metab. Dis. 2005;28:99–102. doi: 10.1007/s10545-005-5084-5. [DOI] [PubMed] [Google Scholar]