Abstract
Polyamines are naturally occurring intracellular polycations that are essential for viability and growth of eukaryotes. Dysregulation of polyamine metabolism is a hallmark of cancer and the carcinogenic process, and consequently development of polyamine analogues has emerged as a viable strategy for therapeutic intervention. Previously, we showed that the naturally occurring polyamines spermidine and spermine were quite effective at inducing the oligomerization of nucleosomal arrays in vitro, suggesting that polyamines may play a key role in regulating higher order chromatin structures in vivo. Here, we analyse the ability of a number of synthetic polyamine analogues to potentiate formation of higher order chromatin structures in vitro. We find that a class of long-chain polyamines called oligoamines are potent inducers of nucleosomal array oligomerization in vitro and that these same polyamine analogues rapidly block yeast cell growth.
Keywords: chromatin, nucleosomal array, oligoamine, polyamine analogue, spermine, yeast
Abbreviations: ODC, ornithine decarboxylase
INTRODUCTION
The polyamines, putrescine, spermidine and spermine, are naturally occurring intracellular polycations that are essential for viability and growth of eukaryotes [1–3]. Polyamines are synthesized by an exquisitely regulated biosynthetic pathway, and regulation of their catabolism and transport is likewise finely tuned [1,4,5]. Dysregulation of these pathways is the hallmark of cancer and the carcinogenic process. Elevations of polyamine levels and of the polyamine biosynthetic enzyme ODC (ornithine decarboxylase) are observed in neoplastic tissues and are induced by the application of tumour promoters [4]. ODC transcription is a target for the proto-oncogene c-myc [6,7], and activation of c-jun and c-fos pathways involves polyamines [8]. Overexpression of ODC has been shown to transform cells in culture, and high levels of ODC production in transgenic mice cause increased susceptibility to tumour formation [4]. Thus the polyamine pathway has been identified as a target for therapeutic intervention for cancer, as well as for other hyperproliferative diseases.
While pharmacological inhibition of polyamine biosynthetic enzymes has found some clinical utility, inherent problems related to the availability of polyamines in food and from normal bacterial flora in the gastrointestinal tract have necessitated the evolution of new approaches to the creation of potentially useful compounds. The development of a variety of polyamine analogues that gain entry into cells through active polyamine transport, that may modulate normal polyamine biosynthesis through natural feedback mechanisms, that may up-regulate catabolism and, importantly, that may compete with natural polyamines for intracellular binding sites, but with altered function, is an emerging approach that is now being tested clinically [9–14].
While the biochemistry and molecular biology of the polyamines are being elucidated rapidly, knowledge of their specific molecular functions is more embryonic. Emerging functions include association with nucleic acids [15], maintenance and modulation of structure and function of chromatin [16,17], regulation of specific gene expression, ion channel regulation [18], maintenance of membrane stability [19], essential incorporation into active eIF5A (eukaryotic translation initiation factor 5A) [20] and free radical scavenging. Of particular interest are the effects of polyamines on chromatin structure. Previously we found that depletion of cellular polyamines bypassed the transcriptional requirements for the yeast histone acetyltransferase Gcn5p [17]. Furthermore, we found that polyamines facilitated nucleosomal array condensation in vitro and that this activity was antagonized by histone hyperacetylation [17]. These genetic and biochemical data indicated that cellular polyamines function in opposition to chromatin-modifying enzymes to facilitate formation of chromatin higher order structures, leading to transcriptional repression. Likewise, Gilmour and co-workers [21–23] have found that increased levels of polyamines in mammalian cells also lead to changes in chromatin structure and alterations in the activities of histone-modifying activities. Thus it seems likely that chromatin is a key cellular target for polyamines and that dysregulation of this chromatin function may play a key role in development or progression of cancers.
Given the therapeutic promise of several polyamine analogues, we have investigated the potency of a variety of polyamine analogues in facilitating condensation of chromatin in vitro. All polyamine analogues tested retained the ability to induce oligomerization of nucleosomal arrays in vitro, and our results lead to a classification of polyamine analogues into the following two groups: (i) group I analogues are similar in potency to the natural polyamine, spermine, in chromatin condensation assays, and (ii) group II analogues are 3–4-fold more potent than spermine, and thus induce chromatin condensation at low micromolar concentrations. These novel group II polyamine analogues are composed mostly of oligoamines [24], and each member of this class is a potent inhibitor of yeast cell growth. Previous studies have indicated that members of the group II class of polyamine analogues show promise as therapeutics for treatment of breast cancer and other cancers [25], and our results suggest that chromatin structure may play a role as a key molecular target.
EXPERIMENTAL
Polyamine analogues
The polyamine analogues were obtained from Cellgate and each was resuspended in sterile water and stored at −20 °C. These compounds are highly stable in aqueous solutions even at elevated temperature.
Nucleosomal array reconstitution
Array DNA template was isolated by digestion of plasmid pCL7c with NotI, HindIII and HhaI (New England Biolabs) followed by FPLC purification on Sephacryl-500 (Amersham Biosciences) essentially as described in [26,27]. Chicken erythrocyte histone octamers were purified from chicken whole blood (Pel-Freez Biologicals) as described previously [28]. Arrays were reconstituted on to the 208-11S DNA template in a Slide-a-Lyzer dialysis cassette (Pierce) by using the salt dialysis protocol of Hansen et al. [29]. Octamer concentrations were determined by measuring A230 (absorbance) [30].
Array oligomerization assay
Arrays (50 nM) were incubated with polyamines or polyamine analogues in TE buffer (10 mM Tris, pH 7.4, and 0.1 mM EDTA). Samples were incubated for 15 min at room temperature (24 °C), and then samples were centrifuged for 10 min in a microfuge. A260 of the resulting supernatant was then determined and compared with the initial reading prior to polyamine addition to yield the percentage array remaining in supernatant.
Analysis of polyamine levels within yeast cells
Yeast cultures (50 ml) were grown at 30 °C in rich YPD medium [1% (w/v) yeast extract, 2% (w/v) bactopeptone and 2% (w/v) glucose] to an A600 of 1.0. Polyamine analogues were added to 100 μM final concentration and cultures were grown for an additional 2 h at 30 °C. An identical cell culture was grown in the absence of polyamine analogue. Cells were harvested by centrifugation (4000 g for 5 min at 4 °C) and washed three times with distilled water. Cell pellets (0.25 g) were resuspended in ice-cold 5% HClO4 and a cell pellet volume equivalent of zirconium beads (Biospec Products) was added. Cells were broken by vigorous vortex-mixing. Extracts were clarified by centrifugation (14000 g for 10 min at 4 °C), and extracts were stored at −20 °C. Polyamine extracts were subjected to dansylation reactions and analysed by HPLC. Since different preparations vary due to different efficiencies of cell breakage, polyamine analogue levels were normalized to spermine, since spermine levels are relatively constant within cells.
RESULTS AND DISCUSSION
In order to investigate the potential for various polyamine analogues to induce chromatin condensation, model nucleosomal arrays were reconstituted with purified histones. Nucleosomal arrays were assembled by salt dialysis using purified chicken erythrocyte histone octamers and a DNA template composed of 11 head to tail repeats of a 208 bp 5S rRNA gene from Lytechinus variegatus (208-12S template). Each 5S repeat can rotationally and translationally position a nucleosome after in vitro salt dialysis reconstitution, yielding a positioned array of nucleosomes. These model nucleosomal arrays undergo complex hierarchical structural changes in vitro in the presence of bivalent cations [31]. Low concentrations of Mg2+ ions (<2 mM) induce intramolecular compaction of individual nucleosomal arrays through association of neighbouring nucleosomes (‘folding’), while progressively higher concentrations of Mg2+ (>2 mM Mg2+) or low concentrations of polyamines (∼200 μM) can induce nucleosomal arrays to reversibly oligomerize. The intramolecular folding of model arrays at low Mg2+ concentrations is believed to mimic the formation of 30 nm-like chromatin fibres, while intermolecular oligomerization generates relatively defined, soluble structures that sediment in the thousands of S, and are believed to mimic the fibre–fibre interactions that stabilize higher order chromosomal domains such as chromonema fibres [16].
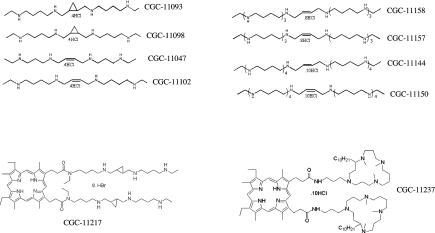
Figure 1 illustrates the polyamine analogues used in the present study. Several of the analogues are conformationally restricted spermine-like compounds (CGC-11047, CGC-11093, CGC-11098 and CGC-11102) [32], several analogues are longer chain oligoamines (CGC-11157, CGC-11158, CGC-11144 and CGC-11150), while CGC-11217 is mesoporphyrin IX covalently bound to two molecules of CGC-11093, and CGC-11237 is mesoporphyrin IX covalently bound to two molecules of a cyclic polyamine. All of these compounds are active against a variety of human tumour cells both in vitro and in vivo [9,12,24,25,32]. CGC-11047 and CGC-11093 are in human clinical trials.
Figure 1. Schematic representation of polyamine analogues.
Polyamine analogues induce chromatin condensation
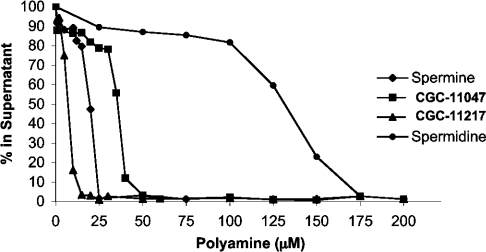
To investigate the ability of polyamine analogues to induce oligomerization of nucleosomal arrays, increasing concentrations of each polyamine analogue were incubated with nucleosomal arrays (50 nM) for 10 min at room temperature, and oligomerized arrays were pelleted by centrifugation. The amount of array remaining in the supernatant was then quantified and plotted as a function of analogue concentration. Similar to our previous study [17], spermidine was quite effective at oligomerizing nucleosomal arrays, as a concentration of 140 μM was sufficient to oligomerize 50% of the arrays (Figure 2 and Table 1). Spermine was approx. 10-fold more potent than spermidine, as 50% oligomerization occurred at 18 μM. When each of the analogues was analysed in parallel, two groups emerged from the data (Figure 2 and Table 1). Group I analogues were similar in effectiveness to spermine, with 50% oligomerization occurring between 16 and 60 μM concentrations (CGC-11047, CGC-11093, CGC-11098, CGC-11102 and CGC-11237). On the other hand, group II analogues were clearly more effective than spermine, with oligomerization requiring only 4–7 μM of each compound (CGC-11157, CGC-11158, CGC-11144, CGC-11150 and CGC-11217). Interestingly, with only one exception, CGC-11217, all of the group II polyamine analogues are oligoamines, indicating that potent nucleosomal array oligomerization is a common feature of this class of compounds.
Figure 2. Representative nucleosomal array oligomerization profiles for four different polyamine analogues.
Nucleosomal arrays (50 nM) were incubated with increasing concentrations of spermine, spermidine or the polyamine analogues CGC-11047 or CGC-11217 for 15 min at room temperature. Samples were centrifuged for 10 min and the concentration of nucleosomal array remaining in the supernatant was determined by measuring A260.
Table 1. Summary of chromatin condensation by polyamine analogues.
Values are means of at least three independent experiments. All analogues were assayed in parallel.
| Compound | Concentration inducing 50% chromatin condensation |
|---|---|
| Controls | |
| Spermidine | 140 μM |
| Spermine | 18 μM |
| Group I analogues | |
| CGC-11047 | 36 μM |
| CGC-11102 | 20 μM |
| CGC-11093 | 16 μM |
| CGC-11098 | 18 μM |
| CGC-11237 | 60 μM |
| Group II analgoues | |
| CGC-11157 | 5 μM |
| CGC-11158 | 5 μM |
| CGC-11150 | 5 μM |
| CGC-11144 | 4 μM |
| CGC-11217 | 7 μM |
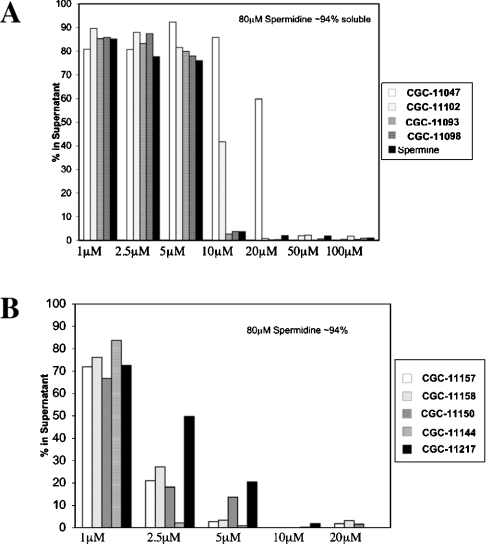
In a therapeutic setting, polyamine analogues must compete with cellular polyamine pools for interactions with cellular targets such as chromatin. One possibility is that chromatin, which is already bound by cellular polyamines, will be insensitive to the effects of polyamine analogues added subsequently. To test this idea, nucleosomal arrays were pre-incubated with 80 μM spermidine, a concentration in which ∼95% of the arrays are soluble, and then increasing concentrations of the polyamine analogues were added and array oligomerization was assayed by centrifugation. As shown in Figure 3, prebinding spermidine to nucleosomal arrays did not inhibit the ability of the polyamine analogues to mediate array oligomerization. For instance, analogue CGC-11102 which induces 50% oligomerization at ∼20 μM by itself, only requires ∼10 μM when the arrays were prebound by low concentrations of spermidine. Likewise, each of the group II polyamine analogues (e.g. CGC-11144) were potent in the presence of spermidine, with greater than 50% oligomerization occurring at 2.5 μM. Thus the results suggest that endogenous polyamines do not grossly antagonize polyamine analogue-mediated nucleosomal array oligomerization.
Figure 3. Polyamine analogues synergize with spermidine for chromatin condensation.
Nucleosomal arrays (50 nM) were incubated with 80 μM spermidine for 5 min at room temperature. Group I (A) or group II (B) polyamine analogues were then added at varying concentrations and the incubation was continued for 15 min. Nucleosomal array oligomerization was then assayed as in the legend of Figure 2.
Group II polyamine analogues inhibit yeast cell growth
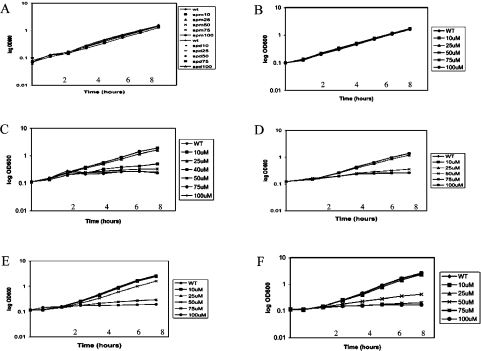
The potent chromatin condensation activity of the group II polyamine analogues may have a detrimental effect on cell growth and/or nuclear function. As an initial investigation, increasing concentrations of each polyamine analogue were added to cultures of yeast cells and growth rates were monitored over time. A representative set of results for a subset of the polyamine analogues is shown in Figure 4. As was the case for the chromatin condensation assays, these yeast cell studies were consistent with the classification of the polyamine analogues into the same two groups. The group I polyamine analogues were identical with spermine and spermidine, as no effects were observed on yeast cell growth at the concentrations used (10–100 μM). In contrast, the group II polyamine analogues, which were more potent in chromatin condensation, dramatically inhibited yeast cell growth within the first cell cycle following addition of the polyamine analogue. For instance, the group II analogue, CGC-11144, blocked yeast cell growth at a concentration of 40 μM, while addition of 100 μM of any of the group I analogues had no detrimental effect. Previous studies have shown that polyamine synthesis is required for yeast cell growth and that depletion of cellular polyamines leads to cell cycle arrest in the G1-phase of the cell cycle [33,34]. Likewise, FACS analysis indicates that yeast cells treated with the group II analogue, CGC-11144, rapidly accumulate in G1 (results not shown). Thus the ability of polyamine analogues to inhibit yeast cell growth correlates quite well with their ability to induce chromatin condensation.
Figure 4. Group II polyamine analogues inhibit yeast cell growth.
Yeast cells (CY1019) were grown at 30 °C in rich YPD medium until mid-exponential phase and then diluted to an A600 of 0.1 prior to addition of spermine (A), spermidine (A), CGC-11047 (B), CGC-11144 (C), CGC-11150 (D), CGC-11157 (E) or CGC-11158 (F) at the indicated concentrations. Cultures were grown at 30 °C with shaking and A600 was monitored at the indicated times. Results shown are representative of at least two independent experiments. spm, spermine; spd, spermidine.
One possibility is that the toxicity of group II polyamines may be due to enhanced uptake or stability within yeast cells compared with group I polyamines. To test this idea, yeast cultures were treated for 2 h with 100 μM CGC-11144 or CGC-11093, cells were harvested, and 5% HClO4 extracts were analysed for polyamine content by HPLC analysis. Results from two independent extracts indicate that both of the polyamine analogues accumulate to similar levels within yeast cells. The class I analogue CGC-11093 was found at 183±9 nmol/g of yeast cells (normalized to endogenous spermine levels), while the potent class II analogue CGC-11144 was found at 150±22 nmol/g of yeast cells (normalized to endogenous spermine). In both cases polyamine analogues were ∼10% the level of cellular spermidine. Thus these results indicate that the increased toxicity of the class II polyamine analogues is not due to greatly enhanced uptake, but that this phenotype may be due to their effects on chromatin structure.
As select polyamine analogues are being advanced in preclinical and clinical studies for cancer and other hyperproliferative diseases, such as macular degeneration, further understanding of the mechanisms of action of these compounds is essential. Recent results showing that intracellular polyamine levels can modulate the activity of HDAC (histone deacetylase) inhibitors [21] and that the histone lysine-specific demethylase LSD1 (lysine-specific demethylase 1) shares significant characteristics with spermine oxidase [35] further tie the polyamines to the regulation of chromatin structure and function. Of particular interest is the fact that the presence of the natural polyamine spermidine does not interfere with the oligomerization activity of the polyamine analogues studied. The unexpected outcome of these experiments lends further credence to the potential intracellular utility of these compounds. Finally, just as the oligoamines' particular abilities to oligomerize arrays in the present study correlate with their cell-killing ability against yeast, a similar correlation between oligoamines and their ability to aggregate DNA has been shown for human prostate cells in culture [24]. The critical roles that polyamines play in all cells suggest the potential therapeutic utility of their analogues in a wide spectrum of diseases.
Acknowledgments
This work was supported by a grant to C. L. P. from the National Institutes of Health (GM54096). We thank John Mitchell (N. Illinois University) for performing HPLC analysis of yeast cell extracts. L. M. C. was supported by a postdoctoral NRSA (National Research Service Award) award from the NIH (National Institutes of Health; F32 GM066452).
References
- 1.Cohen S. S. Oxford, U.K.: Oxford University Press; 1998. A Guide to the Polyamines. [Google Scholar]
- 2.Marton L. J., Pegg A. E. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharmacol. Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 3.Gerner E. W., Meyskens F. L., Jr Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 4.Pegg A. E. Regulation of ornithine decarboxylase. J. Biol. Chem. 2006;281:14529–14532. doi: 10.1074/jbc.R500031200. [DOI] [PubMed] [Google Scholar]
- 5.Coffino P. Regulation of cellular polyamines by antizyme. Nat. Rev. Mol. Cell Biol. 2001;2:188–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson J. A., Keller U. B., Baudino T. A., Yang C., Norton S., Old J. A., Nilsson L. M., Neale G., Kramer D. L., Porter C. W., Cleveland J. L. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell. 2005;7:433–444. doi: 10.1016/j.ccr.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson J. A., Maclean K. H., Keller U. B., Pendeville H., Baudino T. A., Cleveland J. L. Mnt loss triggers Myc transcription targets, proliferation, apoptosis, and transformation. Mol. Cell. Biol. 2004;24:1560–1569. doi: 10.1128/MCB.24.4.1560-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J. Y., McCormack S. A., Viar M. J., Wang H., Tzen C. Y., Scott R. E., Johnson L. R. Decreased expression of protooncogenes c-fos, c-myc, and c-jun following polyamine depletion in IEC-6 cells. Am. J. Physiol. 1993;265:G331–G338. doi: 10.1152/ajpgi.1993.265.2.G331. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y., Hager E. R., Phillips D. L., Dunn V. R., Hacker A., Frydman B., Kink J. A., Valasinas A. L., Reddy V. K., Marton L. J., et al. A novel polyamine analog inhibits growth and induces apoptosis in human breast cancer cells. Clin. Cancer Res. 2003;9:2769–2777. [PMC free article] [PubMed] [Google Scholar]
- 10.Wilding G., King D., Tutsch K., Pomplun M., Feierabend C., Alberti D., Arzoomanian R. Phase I trial of the polyamine analog N1,N14-diethylhomospermine (DEHSPM) in patients with advanced solid tumors. Invest. New Drugs. 2004;22:131–138. doi: 10.1023/B:DRUG.0000011789.79368.ae. [DOI] [PubMed] [Google Scholar]
- 11.Wolff A. C., Armstrong D. K., Fetting J. H., Carducci M. K., Riley C. D., Bender J. F., Casero R. A., Jr, Davidson N. E. A phase II study of the polyamine analog N1,N11-diethylnorspermine (DENSpm) daily for five days every 21 days in patients with previously treated metastatic breast cancer. Clin. Cancer Res. 2003;9:5922–5928. [PubMed] [Google Scholar]
- 12.Frydman B., Porter C. W., Maxuitenko Y., Sarkar A., Bhattacharya S., Valasinas A., Reddy V. K., Kisiel N., Marton L. J., Basu H. S. A novel polyamine analog (SL-11093) inhibits growth of human prostate tumor xenografts in nude mice. Cancer Chemother. Pharmacol. 2003;51:488–492. doi: 10.1007/s00280-003-0598-8. [DOI] [PubMed] [Google Scholar]
- 13.Faaland C. A., Thomas T. J., Balabhadrapathruni S., Langer T., Mian S., Shirahata A., Gallo M. A., Thomas T. Molecular correlates of the action of bis(ethyl)polyamines in breast cancer cell growth inhibition and apoptosis. Biochem. Cell Biol. 2000;78:415–426. [PubMed] [Google Scholar]
- 14.Schipper R. G., Deli G., Deloyer P., Lange W. P., Schalken J. A., Verhofstad A. A. Antitumor activity of the polyamine analog N1, N11-diethylnorspermine against human prostate carcinoma cells. Prostate. 2000;44:313–321. doi: 10.1002/1097-0045(20000901)44:4<313::aid-pros8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Ouameur A. A., Tajmir-Riahi H. A. Structural analysis of DNA interactions with biogenic polyamines and cobalt(III)hexamine studied by Fourier transform infrared and capillary electrophoresis. J. Biol. Chem. 2004;279:42041–42054. doi: 10.1074/jbc.M406053200. [DOI] [PubMed] [Google Scholar]
- 16.Belmont A. S., Bruce K. Visualization of G1 chromosomes: a folded, twisted, supercoiled chromonema model of interphase chromatid structure. J. Cell Biol. 1994;127:287–302. doi: 10.1083/jcb.127.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollard K. J., Samuels M. L., Crowley K. A., Hansen J. C., Peterson C. L. Functional interaction between GCN5 and polyamines: a new role for core histone acetylation. EMBO J. 1999;18:5622–5633. doi: 10.1093/emboj/18.20.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurata H. T., Marton L. J., Nichols C. G. The polyamine binding site in inward rectifier K+ channels. J. Gen. Physiol. 2006;127:467–480. doi: 10.1085/jgp.200509467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheliaskova A., Naydenova S., Petrov A. G. Interaction of phospholipid bilayers with polyamines of different length. Eur. Biophys. J. 2000;29:153–157. doi: 10.1007/s002490050261. [DOI] [PubMed] [Google Scholar]
- 20.Park M. H. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A) J. Biochem. (Tokyo) 2006;139:161–169. doi: 10.1093/jb/mvj034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs C. A., Gilmour S. K. High levels of intracellular polyamines promote histone acetyltransferase activity resulting in chromatin hyperacetylation. J. Cell. Biochem. 2000;77:345–360. [PubMed] [Google Scholar]
- 22.Hobbs C. A., Paul B. A., Gilmour S. K. Deregulation of polyamine biosynthesis alters intrinsic histone acetyltransferase and deacetylase activities in murine skin and tumors. Cancer Res. 2002;62:67–74. [PubMed] [Google Scholar]
- 23.Hobbs C. A., Paul B. A., Gilmour S. K. Elevated levels of polyamines alter chromatin in murine skin and tumors without global changes in nucleosome acetylation. Exp. Cell Res. 2003;290:427–436. doi: 10.1016/s0014-4827(03)00352-5. [DOI] [PubMed] [Google Scholar]
- 24.Valasinas A., Reddy V. K., Blokhin A. V., Basu H. S., Bhattacharya S., Sarkar A., Marton L. J., Frydman B. Long-chain polyamines (oligoamines) exhibit strong cytotoxicities against human prostate cancer cells. Bioorg. Med. Chem. 2003;11:4121–4131. doi: 10.1016/s0968-0896(03)00453-x. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y., Keen J. C., Pledgie A., Marton L. J., Zhu T., Sukumar S., Park B. H., Blair B., Brenner K., Casero R. A., Jr, Davidson N. E. Polyamine analogues down-regulate estrogen receptor α expression in human breast cancer cells. J. Biol. Chem. 2006;281:19055–19063. doi: 10.1074/jbc.M600910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logie C., Peterson C. L. Catalytic activity of the yeast SWI/SNF complex on reconstituted nucleosome arrays. EMBO J. 1997;16:6772–6782. doi: 10.1093/emboj/16.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logie C., Peterson C. L. Purification and biochemical properties of yeast SWI/SNF complex. Methods Enzymol. 1999;304:726–741. doi: 10.1016/s0076-6879(99)04044-6. [DOI] [PubMed] [Google Scholar]
- 28.Hansen J. C., Ausio J., Stanik V. H., van Holde K. E. Homogeneous reconstituted oligonucleosomes, evidence for salt-dependent folding in the absence of histone H1. Biochemistry. 1989;28:9129–9136. doi: 10.1021/bi00449a026. [DOI] [PubMed] [Google Scholar]
- 29.Hansen J. C., van Holde K. E., Lohr D. The mechanism of nucleosome assembly onto oligomers of the sea urchin 5 S DNA positioning sequence. J. Biol. Chem. 1991;266:4276–4282. [PubMed] [Google Scholar]
- 30.Stein A. DNA folding by histones: the kinetics of chromatin core particle reassembly and the interaction of nucleosomes with histones. J. Mol. Biol. 1979;130:103–134. doi: 10.1016/0022-2836(79)90421-2. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher T. M., Hansen J. C. The nucleosomal array: structure/function relationships. Crit. Rev. Eukaryotic Gene Expr. 1996;6:149–188. doi: 10.1615/critreveukargeneexpr.v6.i2-3.40. [DOI] [PubMed] [Google Scholar]
- 32.Valasinas A., Sarkar A., Reddy V. K., Marton L. J., Basu H. S., Frydman B. Conformationally restricted analogues of 1N,14N-bisethylhomospermine (BE-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cells. J. Med. Chem. 2001;44:390–403. doi: 10.1021/jm000309t. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz B., Hittelman A., Daneshvar L., Basu H. S., Marton L. J., Feuerstein B. G. A new model for disruption of the ornithine decarboxylase gene, SPE1, in Saccharomyces cerevisiae exhibits growth arrest and genetic instability at the MAT locus. Biochem. J. 1995;312:83–90. doi: 10.1042/bj3120083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balasundaram D., Tabor C. W., Tabor H. Spermidine or spermine is essential for the aerobic growth of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 1991;88:5872–5876. doi: 10.1073/pnas.88.13.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Y., Lan F., Matson C., Mulligan P., Whetstine J. R., Cole P. A., Casero R. A., Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]