Abstract
Rhizobium etli CFN42 is a symbiotic nitrogen-fixing bacterium of the common bean Phaseolus vulgaris. The symbiotic plasmid p42d of R. etli comprises a gene encoding a putative (strept)avidin-like protein, named rhizavidin. The amino acid sequence identity of rhizavidin in relation to other known avidin-like proteins is 20–30%. The amino acid residues involved in the (strept)avidin–biotin interaction are well conserved in rhizavidin. The structural and functional properties of rhizavidin were carefully studied, and we found that rhizavidin shares characteristics with bradavidin, streptavidin and avidin. However, we found that it is the first naturally occurring dimeric protein in the avidin protein family, in contrast with tetrameric (strept)avidin and bradavidin. Moreover, it possesses a proline residue after a flexible loop (GGSG) in a position close to Trp-110 in avidin, which is an important biotin-binding residue. [3H]Biotin dissociation and ITC (isothermal titration calorimetry) experiments showed dimeric rhizavidin to be a high-affinity biotin-binding protein. Its thermal stability was lower than that of avidin; although similar to streptavidin, it was insensitive to proteinase K. The immunological cross-reactivity of rhizavidin was tested with human serum samples obtained from cancer patients exposed to (strept)avidin. No significant cross-reactivity was observed. The biodistribution of the protein was studied by SPECT (single-photon emission computed tomography) imaging in rats. Similarly to avidin, rhizavidin was observed to accumulate rapidly, mainly in the liver. Evidently, rhizavidin could be used as a complement to (strept)avidin in (strept)avidin–biotin technology.
Keywords: avidin–biotin technology, bacterial avidin, biotin-binding protein, rhizavidin, Rhizobium etli
Abbreviations: DSC, differential scanning calorimetry; DTPA, diethylenetriaminepenta-acetic acid; ESI, electrospray ionization; FT-ICR, Fourier-transform ion cyclotron resonance; lTC, isothermal titration calorimetry; RF, radio-frequency
INTRODUCTION
Avidin is a high-affinity biotin-binding protein found in several bird, reptilian and amphibian species [1–3]. Streptavidins [4,5] and bradavidin [6] are its bacterial analogues. The bradavidin gene was discovered in the completely sequenced genome of Bradyrhizobium japonicum strain USDA110.
Rhizobium etli CFN42 is the nitrogen-fixing symbiont of the common bean Phaseolus vulgaris. R. etli contains six large plasmids including the symbiotic plasmid p42d (∼371 kb), which has been studied intensively [7–9]. Most of the genes involved in nodulation and nitrogen fixation are present in the symbiotic plasmid. In addition, one of the genes in the plasmid encodes a putative new avidin. R. etli is a member of the Rhizobiaceae, whereas B. japonicum belongs to the Bradyrhizobiceae. In addition to these two families, the group of nitrogen-fixing symbiotic bacteria, collectively called rhizobia, also includes the Phyllobacteriaceae.
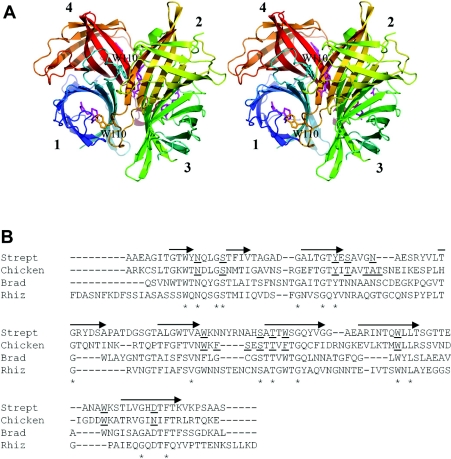
Generally, four identical (strept)avidin subunits form a tetrameric quaternary structure (Figure 1A). Each (strept)avidin monomer has one biotin-binding site located at the open end of the antiparallel β-barrel structure [10–13]. A particular tryptophan residue located at the loop between β-strands 7 and 8 participates in biotin binding on the neighbouring binding site in the tetramer. So far, each characterized avidin-like protein has proved to be tetrameric and has possessed this functionally important tryptophan residue, which is analogous to Trp-110 in avidin and Trp-120 in streptavidin.
Figure 1. Structural characteristics of avidins.
(A) A schematic representation of the tetrameric avidin structure (PDB code 1AVD) in which the eight successive β-strands are indicated by arrows. The subunits are numbered according to Livnah et al. [10]. Trp-110 from the loop between β-strands 7 and 8 is labelled, and its side chain is shown for subunits one and two, in cyan and amber respectively. Biotin molecules in the binding pockets are shown in magenta. (B) The structure-based alignment of avidin and streptavidin was carried out using Swiss-Prot. Multiple sequence alignment was done using ClustalW for all avidins shown in the Figure. On this basis, the bradavidin (Brad) and rhizavidin (Rhiz) sequences were manually inserted and adjusted to the alignment. The eight β-strands indicated by the arrows are positioned according to the structure of chicken avidin, and the biotin-binding residues in chicken avidin and streptavidin (Strept) are underlined [10]. The conserved residues are highlighted by an asterisk. Sequence comparison between rhizavidin and chicken avidin revealed 22.4% sequence identity and 35.0% similarity, whereas the values were 28.1% and 40.4% when compared with streptavidin, and 21.9% and 36.5% when compared with bradavidin.
A wide variety of applications based on the (strept)avidin–biotin interaction have been developed [14,15]. The structure–function relationship behind this protein–ligand pair has been studied extensively, and a wide range of engineered (strept)avidin forms have been described [16]. The structural topology of avidin has been altered (see, for example, [17,18]) with the aim of constructing versatile protein tools displaying properties and possibilities, such as independent binding site modification at the quaternary structure level [19] of applicative value. New avidin-like proteins discovered and characterized in different species provide useful alternatives for applications and expand our understanding of avidin-like proteins in general.
In the present paper, we describe the properties of a new avidin-like protein, named rhizavidin, from R. etli. Unlike all other avidins studied so far, the quaternary structure of rhizavidin was observed to be dimeric instead of tetrameric. In addition, a putative loop located between β-strands 7 and 8 is exceptional in rhizavidin compared with other known avidins. There is no tryptophan residue present in the loop, such as that which in other avidins involved in the functional interplay between the subunits in biotin binding. Thus the GGSGP motif in rhizavidin (Figure 1B) may provide biotin contacts for the same subunit from which it originates, and the biotin-binding mode of rhizavidin is unique among known avidins. In addition to its quaternary structure assembly, its ligand-binding properties, thermal stability, protease sensitivity, immunological cross-reactivity and biodistribution were studied here.
EXPERIMENTAL
Construction of the expression vector
The rhizavidin gene was amplified by PCR using the symbiotic plasmid DNA of R. etli as a template. The first construct was the full-length wild-type form (GenBank® accession number U80928, complement 293047–293586) in which the rare translation initiation codon UUG was converted into AUG for expression in Escherichia coli. The primer used for the 5′-end of the full-length form was 5′-CACCATGATTATTACGAGTTTATATGC-3′. The second construct was N-terminally truncated by removing 24 amino acids from the putative signal peptide in comparison with the wild-type form. The primer for the 5′-end of the truncated form was 5′-CACCATGATCCGTACTAATGCAGTTGC-3′. Also, the N-terminally truncated form possessed a putative signal peptide, 20 residues in length, and both forms were expected to produce identical mature peptide, since the predicted signal cleavage site was identical for both of them (SignalIP program). The primer for the 3′-end of both protein forms was 5′-TTAATCCTTCAAGAGGCTTTTGT-3′. The 5′-ends of the forward primers included CACC, which is necessary for directional TOPO® cloning (Invitrogen). The PCR products were extracted from a 1.5% agarose gel. The constructs were cloned in a pET101/D-vector using the TOPO® cloning protocol. The expression vectors were validated by sequencing with an ABI Prism™ 310 Genetic Analyzer.
Production of rhizavidin
The pET101/D-based expression vectors were transformed into E. coli BL21-(AI) cells (Invitrogen), of which fresh transformants were cultured in Luria–Bertani medium supplemented with 0.1% (w/v) glucose and 10 μg/ml ampicillin (Sigma–Aldrich) at 26 °C, with rotation at 225 rev./min. When the culture density reached a D600 of 0.2–0.3, 0.2% (w/v) L-arabinose was added to induce protein expression. Cultivation was continued for approx. 22 h at 26 °C, after which time the cells were collected by centrifugation at 5000 g for 10 min at 4 °C and resuspended as described previously [20]. The suspension was sonicated twice for 2 min (Vibra cell™). The protein was isolated by affinity chromatography on a 2-iminobiotin column (Affiland) as described by Airenne et al. [21] and subsequently eluted with 0.1 M ethanoic (acetic) acid. Concentrations of the eluted protein fractions were determined using the theoretical molar absorption coefficient of rhizavidin at 280 nm (29160 M−1·cm−1). The fractions were analysed by SDS/PAGE, and gels were stained with Coomassie Brilliant Blue.
Primary structure analysis
The stereo representation of avidin was created using PyMol (DeLano Scientific). The sequence comparisons were made using the Needle program from the European Molecular Biology Open Software Suite (EMBOSS), ClustalW and Swiss-Prot.
Structural analysis
The size of the purified protein was analysed by gel-filtration HPLC in the absence and presence of D-biotin, as described previously [6], using a Superdex 200 10/300 GL column. Formation of intersubunit disulfide bridges was analysed by SDS/PAGE using a sample buffer devoid of the reducing agent 2-mercaptoethanol, and subsequent Coomassie Brilliant Blue staining.
Mass spectrometry
The protein samples were desalted for MS using PD-10 columns. All mass spectrometric experiments were performed on a 4.7-T Bruker APEX IV FT-ICR (Fourier-transform ion cyclotron resonance) mass spectrometer employing ESI (electrospray ionization). The instrument was interfaced to an external Bruker Apollo™ I ion source. The sample solutions were infused directly at a flow rate of 1.5 μl·min−1 (end-plate at −3.8 kV). N2 was used as the drying (69 kPa, 80 °C) and nebulizing gas. ESI-generated ions were passed through a dielectric Pyrex capillary (entrance and exit potentials −4200 and 22–300 V, respectively), accumulated in a RF (radio-frequency)-hexapole for 4.0 s and subsequently transferred to a cylindrical RF-shimmed ICR cell (Infinity Cell) within 2.6 ms. Ions were captured inside the cell by a ‘Sidekick’ trapping technique and were excited to detectable cyclotron radii by an RF-sweep (∼18–90 kHz). A total of 128–256 co-added (512 kword) time-domain transients were fast-Fourier-transformed, followed by magnitude calculation and frequency-to-m/z calibration with respect to the ions of an ES Tuning Mix (Agilent Technologies).
Differential scanning calorimetry
The melting temperatures of rhizavidin, in both the absence and presence of D-biotin, were studied by DSC (differential scanning calorimetry), as described previously [22–24]. The experiments were performed in the absence and presence of biotin in 0.1 M ethanoic acid. The excess of biotin was 2.5 per biotin-binding site. The thermograms (25–130 °C, 0.92 °C/min) were collected with a Nano II high-sensitivity differential scanning calorimeter (Calorimetry Sciences Corp.). Data analysis was carried out using CpCalc 2.1 (Calorimetry Sciences Corp.) and MicroCal Origin 6.0 software.
Heat treatment
The heat stability of rhizavidin was tested by exposing it to high temperatures. Protein samples (0.35 μM in PBS) were heated for 1–30 min at 80, 90 and 100 °C and then chilled. The samples were analysed by ELISA as described previously in detail [25].
Proteinase K assay
Sensitivity to proteinase K was tested in the presence and absence of biotin as described previously [23]. The final concentration of biotin was 61 nM and that of rhizavidin 6.9 nM. The samples were incubated for 0, 15, 30, 60 min and 20 h in the presence of proteinase K (1:25, w/w). After incubation, an equal volume of SDS/PAGE sample buffer was added, and the samples were heated for 5 min at 100 °C and chilled on ice. All of the samples were analysed at the same time by SDS/PAGE (17% gel) and subsequent Coomassie Brilliant Blue staining.
Isothermal titration calorimetry
The thermodynamics of rhizavidin–biotin binding interaction were studied using a MicroCal VP-ITC instrument. Rhizavidin, in ethanoic acid, was dialysed against a sodium phosphate buffer (50 mM NaH2PO4/Na2HPO4 and 100 mM NaCl, pH 7). After dialysis, rhizavidin was diluted to a concentration of 31 μM with the dialysis buffer. D-Biotin (0.4 mM) was diluted with the same buffer. The measurement was conducted at 25 °C using 10 μl titration aliquots [510 rev./min, 30 μcal/s (1 cal=4.184 J)]. In ITC (isothermal titration calorimetry), Gibbs free energy, ΔG=−RTlnKb=ΔH−TΔS, is used to determine the thermodynamic parameters. Binding constant (Kb) and enthalpy change (ΔH) are measured by ITC, temperature (T) and the universal gas constant (R) are known, and therefore change in entropy (ΔS) can be calculated.
Biotin dissociation studies
The dissociation rate constant (kdiss) of rhizavidin was determined by dissociation of D-[8,9-3H]biotin (Amersham Biosciences) at 20, 30, 40 and 50 °C as described previously by Klumb et al. [26]. The labelled biotin was incubated with rhizavidin (50 nM) in sodium phosphate buffer, pH 7, followed by addition of an excess of unlabelled biotin which displaced D-[8,9-3H]biotin. Unbound and displaced biotin was separated by filtration (Microcon YM-30; Millipore). The amount of displaced radiolabelled biotin was detected using a scintillation counter (Wallac 1410). The kdiss was determined from the fraction of biotin bound at each time point from the equation −kofft=ln[(xt−x)/(xt−x0)], in which x is the free biotin at each time point, xt is the total amount of ligand before addition of protein and x0 is the amount of free ligand in the presence of protein before the addition of unlabelled biotin [26].
The kdiss of a fluorescent biotin conjugate (ArcDia BF560™) was measured with a Quanta Master™ spectrofluorimeter (Photon Technology International) with PTI Fluorescence Master System, Felix 32 software as described previously in detail [20]. The fluorescent biotin conjugate was diluted with 50 mM sodium phosphate buffer containing 650 mM NaCl, pH 7, to a concentration of 50 nM, and the initial signal was measured at 560 nm for 100 s. Rhizavidin was added to a final concentration of 50 nM, and the signal of the complex was measured for 300 s. Finally, free biotin was added to a final concentration of 5 μM, and emission of released fluorescent biotin was measured at 575 nm for 1 h. The release percentage was determined from the equation [(xdiss−xini)/(xb−xini)]×100%, in which xdiss is dissociation of fluorescent biotin, xini is the fluorescent signal caused by unbound fluorescent biotin, and xb is the signal caused by the protein fluorescent biotin complex.
Antibody recognition
The cross-reactivity of polyclonal antibodies against (strept)avidin with rhizavidin was tested with cancer patient serum samples. Positive samples were gathered from patients who were exposed to avidin and/or streptavidin and negative serum samples from those exposed to neither of the proteins. All of the samples were obtained from the Division of Nuclear Medicine, European Institute of Oncology, Milan, Italy. Rhizavidin was also exposed to polyclonal rabbit antibodies produced against avidin (University of Oulu, Oulu, Finland) and streptavidin (Weissman Institute, Jerusalem, Israel). Both experiments were conducted by ELISA as described previously in detail [6]. Additionally, recognition of rhizavidin by polyclonal rabbit antibodies against (strept)avidin was studied by Western blot analysis essentially as previously described by Airenne et al. [21].
DTPA (diethylenetriaminepenta-acetic acid) conjugation
Avidin and rhizavidin were labelled with pSCN-Bn-DTPA (Macrocyclics); 2 mg (50 nmol) of avidin or rhizavidin was dissolved in 1 ml of water. An approx. 25-fold molar excess (1 mg/ml of 10 mM carbonate buffer, pH 9.5) was added to both avidin and rhizavidin solutions which were stirred overnight at room temperature (22 °C). After incubation, the conjugates were concentrated with an Amicon ultracentrifugal filter device to 100 μl (Millipore). To remove the remaining free DTPA, 2 ml of PBS was added to the filter devices, and the conjugates were concentrated again. Finally, the concentrates were diluted with 1 ml of PBS and frozen.
Radiolabelling
Two patches of rhizavidin–DTPA (Rz-1 and Rz-2) and one patch of avidin–DTPA (Av-1) conjugates were labelled with [99mTc]pertechnetate. A 150 μl sample (1 mg/ml in water) of DTPA–Rz-1, DTPA–Rz-2 or DTPA–Av-1 was mixed with 50 μl of fresh solution of tin(II) chloride (10 mg/ml in 10 mM HCl) in a nitrogen atmosphere; 200 μl of [99mTc]pertechnetate solution containing 200 MBq was added to each conjugate and incubated at room temperature for 10 min. After incubation radiochemical purity was tested by ITLC (instant thin-layer chromatography) SG (silica gel) (Pall Corporation) in a 13 cm×1.5 cm strip and saline as the mobile phase. The purity was 98.9% for Rz-1, 99.6% for Rz-2 and 100% for Av-1.
SPECT imaging
SPECT studies were conducted using an XSPECT™ system provided by Gamma Medica. Healthy male Wistar rats were anaesthetized with isofluran and the vena femoralis was cannulated. The animals were injected with 99mTc-labelled DTPA–avidin or –rhizavidin in 500 μl of saline. Rat 1 was injected with 117.6 MBq of Rz-1, rat 2 with 70 MBq of Rz-2 and rat 3 with 130.9 MBq of Av-1. After injection, each individual rat was immediately moved to the animal bed of the SPECT equipment, and a nose cone was used to ensure that the animals remained sedated. SPECT acquisition was performed with a high-resolution parallel-hole collimator with a FOV (field of view) of 125 mm×125 mm and a hole diameter of 1.2 mm. In all, 11 planar projections with a duration of 5 min were collected. The energy window for 99mTc was set at 141 keV±10%. Post-reconstruction filtering was performed using the Butterworth method [26a]. After SPECT imaging an X-ray image of the rat skeleton was taken using a CMOS (complementary metal oxide semiconductor) detector with 48 μm pitch and 60 kV voltage.
RESULTS
Production and purification of rhizavidin
The N-terminally truncated form of rhizavidin was produced in E. coli. The yield was, on average, 4 mg/l. As the full-length wild-type form of the protein was not produced efficiently under the same conditions as the truncated form, all of the experiments described in the present paper were performed with the N-terminally truncated rhizavidin. After affinity-chromatography purification, a both pure and homogenous protein was obtained, which was observed subsequently in Coomassie Brilliant Blue-stained SDS/PAGE gels as one band of the expected size.
Gel filtration and intersubunit disulfide bridges
In gel-filtration chromatography (HPLC), only the dimeric form of rhizavidin was observed as a single peak in both the absence and the presence of biotin, at sizes of 29.0 and 31.7 kDa respectively (Table 1). According to non-reducing SDS/PAGE analysis, rhizavidin was not observed to form intersubunit disulfide bridges, although two cysteine residues are present in its sequence. Only a band of the expected size (14 kDa), indicating a monomer, was observed (results not shown).
Table 1. Avidin protein characteristics.
The apparent molecular masses are indicated as determined by gel-filtration HPLC in the absence and presence of biotin (Btn) respectively, followed by calculated pI values and the number of cysteine residues per monomer. The first Tm value (measured by DSC) indicates the temperature at which half of the protein is in a denatured state in the absence of biotin, the second value is that in the presence of biotin. Fluorescent biotin dissociation rate constants and 1 h release percentage values at 50 °C are also indicated.
| Size by gel-filtration HPLC (kDa) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Protein | −Btn | +Btn | pI | Number of cysteine residues | Tm (°C) | Tm with biotin (°C) | Fluorescent biotin kdiss (s−1) | Release 1 h (%) |
| Rhizavidin | 29.0 | 31.7 | 4.0 | 2 | 74.8 | 100.5 | 7.94×10−4 | 46.5 |
| Avidin | 64.0* | 63.1* | 9.5* | 2 | 83.5† | 117.0† | 3.26×10−4 | 76.5 |
| Streptavidin | 51.1* | 53.4* | 6.1* | 0 | 75.0‡ | 112.0‡ | 1.80×10−5 | 21.9 |
| Bradavidin (wild-type) | 50.0* | 57.5* | 6.3* | 2 | – | – | 1.50×10−5* | 4.7* |
Mass spectrometry
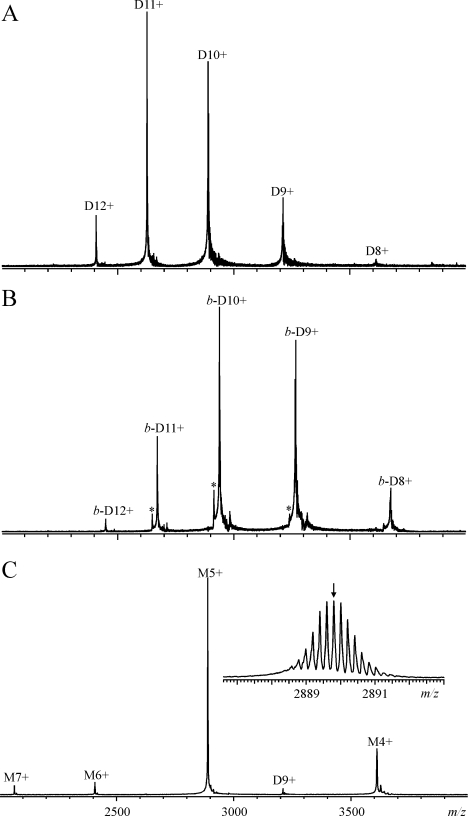
Mass spectrometric experiments demonstrated unequivocally that rhizavidin is indeed a non-covalent dimer. Figure 2 shows the ESI FT-ICR mass spectra of rhizavidin measured in 10 mM ammonium acetate buffer, pH 6.8, in both the absence (Figure 2A) and the presence (Figure 2B) of D-biotin. To avoid dissociation of the weak non-covalent protein complexes in the mass spectrometer, the drying gas temperature was optimized to 80 °C and the capillary exit potential was adjusted to 20–150 V. The peaks in Figure 2(A) represent different charge states of the dimeric protein, with a determined average mass of 28888.5±0.4 Da (theoretical mass of 28888.7 Da). In the presence of D-biotin (Figure 2B), the peaks shifted towards higher m/z, consistent with the binding of two D-biotin molecules, and the mass increased to 29376.1±0.4 Da (theoretical mass of 29376.5 Da). A very small amount of a dimer lacking one D-biotin molecule was observed in the mass spectrum (marked with an asterisk in Figure 2B), this being due to a partial dissociation of the complex during the hexapole accumulation and not related to its solution behaviour. No other protein forms (e.g. a tetramer) were detected. As the charge states appeared in the m/z range 2400–3700, isotopic resolution was not obtained for the dimers, and the masses are therefore reported as average masses. To determine the isotopic mass for the rhizavidin monomer, the capillary exit potential was increased to 300 V to dissociate non-covalent dimers into monomers. The observed charge state distribution in Figure 2(C) represents the monomeric protein, with the most abundant isotopic mass determined as 14443.66±0.02 Da, which agrees well with the theoretical value of 14443.68 Da calculated from the protein sequence with an intact intramolecular disulfide bridge Cys-50–Cys-79.
Figure 2. ESI FT-ICR mass spectra of rhizavidin.
ESI FT-ICR mass spectra of rhizavidin (10 μM) were determined in the absence (A) and presence (B, C) of D-biotin (15 μM), measured in 10 mM ammonium acetate buffer (pH 6.8). The ion source capillary exit potential was adjusted to 150 (A), 22 (B) and 300 (C) V. M refers to the monomeric protein, whereas D and b-D refer to the dimeric proteins, with b designating the binding of two D-biotin molecules. Numbers denote different charge states as [M/D/b-D+zH]z+. The peaks representing a dimer lacking one D-biotin are indicated with asterisks in (B). The inset in (C) shows a magnified view of the isotopically resolved M5+, with a small arrow indicating the peak corresponding to the most abundant isotopic mass.
Thermal stability assays
In ELISA, heat-treated rhizavidin seemed to be more stable than avidin. Avidin lost all of its activity within 15 min at 100 °C, while rhizavidin still retained half. Rhizavidin lost all of its activity within 30 min at 100 °C. In line with this, rhizavidin retained its activity better than avidin at 90 and 80 °C.
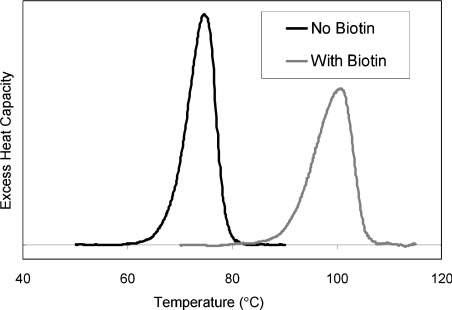
In DSC measurements, the melting temperatures (Tm) of rhizavidin (in 0.1 M ethanoic acid) in the absence and the presence of biotin were 74.8 and 100.5 °C respectively (Table 1), shown as single peaks in the thermogram (Figure 3). The previously measured Tm values for chicken avidin in sodium phosphate buffer, pH 7, were 83.5 and 117.0 °C in the absence and the presence of biotin [27]. In the presence of biotin, the avidin control in ethanoic acid was shown to act highly similarly, with a Tm of 114.2 °C, showing a slight decrease in the Tm value. In the absence of biotin, no transition was observed in 0.1 M ethanoic acid.
Figure 3. Differential scanning calorimetry.
The thermograms of rhizavidin against temperature were measured in the absence and presence of biotin in 0.1 M ethanoic acid. The Tm values (74.8 and 100.5 °C) are obtained at the highest point in the thermograms.
Proteinase K assay
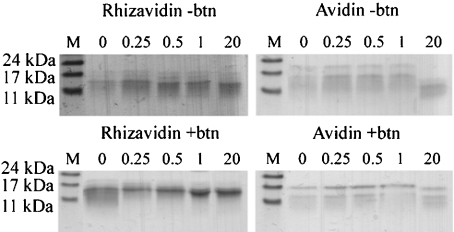
In the presence of biotin, avidin remained intact even under extended incubation with proteinase K, as has been shown previously [28]. Similarly to avidin, rhizavidin evinced high resistance to proteinase K in the presence of biotin (Figure 4). In the absence of biotin, avidin was shown to be almost entirely cleaved under extended incubation, this being observed as the disappearance of the band of 16 kDa in size. Rhizavidin seemed not to be cleaved by proteinase K.
Figure 4. Proteinase K assays for rhizavidin (left) and avidin (right).
The sensitivities of rhizavidin (14 kDa) and avidin (16 kDa) to proteinase K were analysed both in the absence (−btn) and the presence of biotin (+btn). The samples were incubated for 0–20 h followed by SDS/PAGE analysis. The number above each lane indicates the incubation time in h. Molecular mass standards are indicated by M and sizes are given in kDa.
Isothermal titration calorimetry
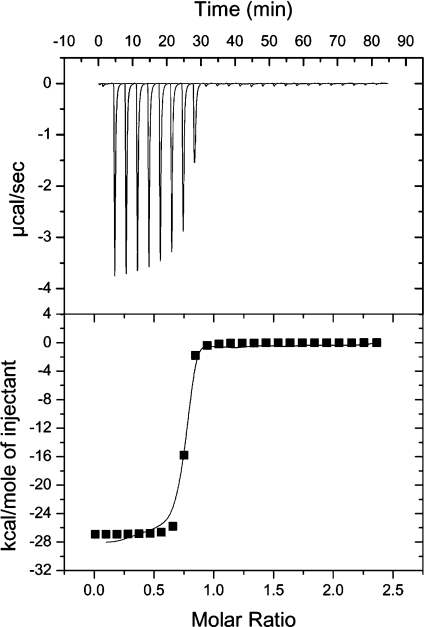
The ITC studies showed rhizavidin to have high affinity toward D-biotin. This experiment showed binding enthalpy (ΔH) to be −26.9 kcal/mol (Figure 5). The corresponding value for avidin was −26.7 kcal/mol. In both cases, entropy was highly negative and the binding constants were higher than is possible to measure by ITC.
Figure 5. Biotin-binding studies of rhizavidin by ITC.
The binding enthalpy (ΔH) of the rhizavidin–biotin interaction was −26.9 kcal/mol, and entropy was highly negative. The sensitivity of the instrument was not adequate to measure the binding constant. However, the saturation of rhizavidin by biotin is clearly observed in the titration curve. Each peak in the upper part and each point in the lower part of the Figure indicates a 10 μl aliquot of 0.4 mM biotin titrated into the rhizavidin solution. The molar ratio (x-axis) indicates the degree of saturation.
Biotin dissociation assays
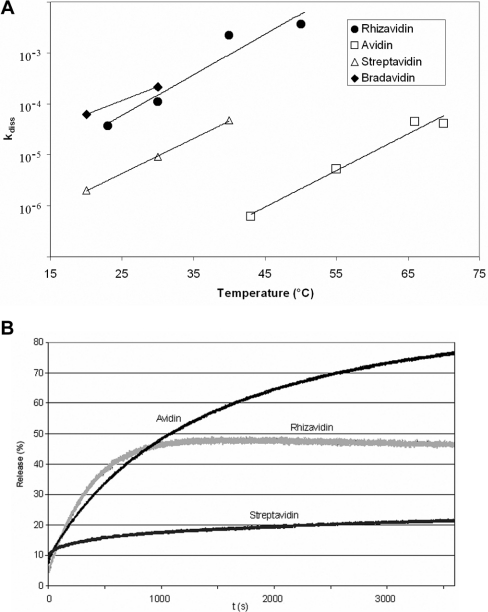
D-[8,9-3H]Biotin was allowed to bind to the protein followed by the addition of an excess amount of unlabelled D-biotin. The replaced D-[8,9-3H]biotin was separated by filtration, and the radioactivity of the solution was used to determine the kdiss of biotin. According to the assay, the kdiss of rhizavidin was the same order of magnitude as that of bradavidin [6], but significantly higher than those of avidin and streptavidin (Figure 6A).
Figure 6. Ligand dissociation analysis.
(A) The kdiss values were determined by displacement of D-[8,9-3H]biotin with free D-biotin. The constants were plotted as functions of temperatures and compared with the other avidin-like proteins. The values for streptavidin are from [26] and those for bradavidin from [6]. The scale of the y-axis is logarithmic. (B) The release of the fluorescent biotin conjugate (ArcDia BF560™) from avidins was determined by displacing it with unlabelled D-biotin. The measurements were conducted as a function of time in the presence of excess D-biotin at 50 °C. The release percentage represents the proportional amount of the fluorescent biotin conjugate which D-biotin had replaced.
The fluorescent biotin conjugate (ArcDia BF560™) was allowed to bind to rhizavidin, which caused quenching of the fluorescence. Upon addition of unlabelled D-biotin, the fluorescent biotin was displaced and the emission signal increased. The kdiss was determined from the initial signal of the unbound fluorescent biotin, the quenched signal of the bound protein complex and the emission signal caused by the released fluorescent biotin. The kdiss of rhizavidin was higher than those of avidin and streptavidin. However, the biotin release percentage of rhizavidin remained at 50% instead of achieving 100% as did that of avidin (Figure 6B). In other words, only half of the ligand was released. The kdiss of rhizavidin still indicated high affinity towards the biotin derivative.
Immunological studies of rhizavidin
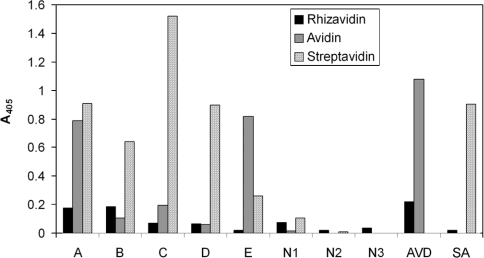
No significant cross-reactivity was observed when rhizavidin was analysed with serum samples from human cancer patients exposed to avidin and/or streptavidin by ELISA. Two out of five positive serum samples, namely samples A and B (Figure 7), evinced a slightly increased response toward rhizavidin. Both signals were close to those of the negative samples. None of the negative samples induced a response with rhizavidin, avidin or streptavidin. A cross-reactivity assay conducted by ELISA clearly showed rhizavidin to be recognized by polyclonal rabbit antibodies against avidin, but only exiguously by those against streptavidin (Figure 7). In contrast with the ELISA, Western blot analysis showed rhizavidin to be recognized by polyclonal rabbit antibodies against streptavidin, but not by those against avidin (results not shown).
Figure 7. Immunological assays.
Serum samples, labelled A–E, were gathered from patients exposed to avidin and/or streptavidin. Negative controls, labelled N1–N3, were from individuals not exposed to either streptavidin or avidin. Cross-reactivity of polyclonal rabbit antibodies against avidin (AVD) and streptavidin (SA) was tested with rhizavidin. Both studies were carried out using ELISA.
Biodistribution in the rat
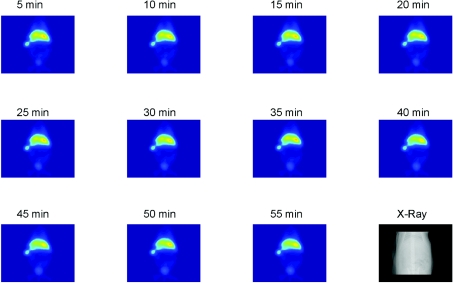
Imaging of the 99mTc-labelled DTPA–avidin or –rhizavidin demonstrated the accumulation of the conjugates in the liver and spleen. Also the bladder can be seen, suggesting clearance of a small part of the tracer. DTPA–avidin or –rhizavidin accumulated immediately after injection into the liver and spleen (Figure 8) and the biodistribution of the molecules did not change during the 55 min measurement period.
Figure 8. Biodistribution SPECT studies.
Serial planar images of a rat injected with 117.6 MBq of [99mTc]DTPA–rhizavidin and a corresponding X-ray image of rat skeleton. The imaging shows that the conjugates accumulated in the liver and spleen. A small part of the tracer is seen in the bladder.
DISCUSSION
In the present study, the structural and functional characteristics of a new avidin-like protein, rhizavidin, were studied. Since the avidin-like protein bradavidin, from B. japonicum, was discovered, new bacterial avidins have offered an interesting field of study, both facilitating the development of avidin–biotin technology and providing more detailed information on avidin-like proteins in general. These studies were successful in illustrating that rhizavidin shares properties with avidin, streptavidin and bradavidin. However, it differs from them radically in being the first naturally occurring dimeric high-affinity biotin-binding protein in the avidin family, which came as a revelation to us.
The rhizavidin gene possesses an extraordinary long signal sequence with an exceptional translation initiation codon, UUG. In addition, it possesses an alternative signal sequence, 24 amino acid residues shorter than the full-length sequence. Both signals are expected to be functional and to produce an identical mature peptide. The biological role of rhizavidin is unknown, but, on the basis of its tight biotin-binding affinity, it could serve as an antimicrobial agent. In our studies, the rare initiation codon UUG was replaced with AUG, because the rare codon was expected to significantly reduce the yield in E. coli production. Here both full-length and truncated signal forms of rhizavidin were produced. The yield of the latter was considerably better and was therefore used in all studies presented in this paper.
Rhizavidin clearly belongs to the avidin protein family and possesses the avidin fingerprint characteristics [6]. Until the three-dimensional structure of rhizavidin is elucidated, only assumptions can be made as to its exact structural properties and relationships to the other members of the calycin superfamily [29]. It seems that rhizavidin differs from most calycins, since, for example, it does not have an obvious last strand arginine or lysine residue equivalent to that in other avidins. The typical calycin structural pattern is thus not present in rhizavidin as such, but it probably forms compensating interactions by some other means.
In contrast with all known avidins studied to date, the loop located between β-strands 7 and 8 in rhizavidin contains a proline residue after a flexible loop stretch, GGSG, in a position close to Trp-110 in avidin and Trp-120 in streptavidin. In avidins, this tryptophan residue encloses the biotin-binding site in the neighbouring subunit, strengthening binding affinity [10,30]. The interaction of Trp-120 in the binding cleft of streptavidin was not assumed to be as tight as that of avidin in the absence of biotin [24,30]. We assume that the rhizavidin dimer is composed of monomers equivalent to those forming the structural subunit pair in (strept)avidin [10]. Therefore only the interface corresponding to the (strept)avidin interface between subunits 1 and 4 might be present in rhizavidin. The biotin-binding mode of rhizavidin is unique, since the functional interplay of (strept)avidin monomers 1 and 2 [10,30] seems to be absent. Instead of forming biotin contacts in the neighbouring binding site, the function of the proline-containing flexible loop between β-strands 7 and 8 (GGSGP) could be to provide novel intramonomeric contacts with biotin. The difference between the biotin-binding affinities of (strept)avidin arises from the divergence of the binding pockets [10]. Particularly, the differences between aromatic residues in the binding pocket and the number of formed hydrogen bonds with biotin are in a key role, as well as differences in certain loop compositions and lengths [10,31]. All known bacterial avidins, including rhizavidin, have aromatic residue analogues with (strept)avidin. The majority of the putative residues which form hydrogen bonds with biotin in rhizavidin are similar to those of streptavidin. Unlike (strept)avidin, the putative loop between β-strands 3 and 4 of rhizavidin contains one cysteine residue which forms an intrasubunit disulfide bridge with the cysteine residue in the loop between β-strands 5 and 6, as indicated by MS.
The DSC studies proved rhizavidin to be less thermostable than avidin [27], in both the presence and the absence of biotin. In this sense it resembles streptavidin, which has Tm values of 75 and 112 °C in the absence and the presence of biotin respectively in a phosphate buffer [24]. When measured by ELISA, rhizavidin appeared to be more thermostable than avidin. However, the different buffers used in ELISA and DSC assays may explain the difference. In ELISA, the samples were diluted with PBS; in DSC, they were diluted in ethanoic acid. In harsh acidic conditions, proteins may be more susceptible to heat-induced denaturation owing to the possible dissociation of monomers or protonation of residues exposed to a solvent and consequent changes in charge interactions. Rhizavidin was seen to be stable under acidic and harsh conditions. Furthermore, the binding mode of avidin and rhizavidin on to an ELISA plate might be dissimilar. Thus, upon binding, they may form dissimilar conformations, which could explain the differences in their ligand-binding ability. In this sense, rhizavidin showed good performance when immobilized to a surface, which makes it a useful substitute for avidin in such applications.
The insensitivity of rhizavidin to proteolytic activity was shown to be similar to that of streptavidin [32]. In avidin, the loop between β-strands 3 and 4 is located within a flexible region having no secondary structure. The region displays high accessibility to solvents and it is therefore most likely to be exposed to proteinase K [28]. The high proteolytic resistance of rhizavidin may arise from a three-dimensional structure of the loop between β-strands 3 and 4 similar to that of streptavidin.
The biotin-binding thermodynamics of rhizavidin as measured by ITC were similar to those of streptavidin [26] and avidin [27]. The sensitivity of ITC is adequate for measuring nanomolar affinities. The affinity of the rhizavidin–biotin interaction exceeds the sensitivity of ITC, therefore only preliminary knowledge of the tight binding affinity was obtained.
The serum samples analysed in immunological studies were gathered from patients subjected to pre-targeting radioimmunotherapy treatment, using both avidin and streptavidin. In the present study, two out of five positive samples caused a higher response to rhizavidin in comparison with that to avidin. In both cases, the responses were small, and therefore counted as insignificant. Overall, rhizavidin clearly induced a smaller response than avidin, but slightly higher than that of bradavidin [6]. Rhizavidin was clearly shown to cause a smaller response to all positive serum samples when set against streptavidin, which is known to be more antigenic than avidin [33,34]. None of the negative serum samples was shown to set up a significant response to the proteins studied.
In cross-reactivity studies using ELISA, rhizavidin was shown to be recognized by polyclonal rabbit antibodies against avidin, in contrast with bradavidin [6], whereas it was hardly recognized by polyclonal rabbit antibodies against streptavidin. In the experiment using Western blotting, rhizavidin was clearly recognized by antibodies against streptavidin, but not by those against avidin. Rhizavidin may possess a few streptavidin-like linear epitopes, which in a denatured state are accessible in Western blotting and are responsible for the recognition by polyclonal rabbit antibodies against streptavidin.
Rapid accumulation of rhizavidin and avidin into the liver was evident in SPECT imaging. Even a low dose of rhizavidin (70 MBq) was clearly detected in the liver 5 min after intravenous administration (results not shown). This is in line with previous findings showing that (strept)avidin has a tendency to target the liver [35,36]. Streptavidin, which resembles rhizavidin more than avidin in respect of its physiological properties (pI, non-glycosylated state), has been observed to accumulate in the kidney in variable magnitudes. Core streptavidin possesses an especially high tendency to accumulate in the kidney [37,38], in contrast with avidin [39]. No kidney accumulation was detected either with rhizavidin or avidin (results not shown), both of which were used in the set-up. This may be due to the inherent low pI of rhizavidin, which has been shown to inhibit accumulation in the kidney [37,38,40,41]. The labelling method used, in which the positive charges on the surface of the molecules are blocked with isothiocyanate and the net charge is changed due to the additional negative charges of DTPA may also explain the results [42]. The localization of streptavidin in the kidney is problematic in pre-targeting set-ups as it may lead to renal toxicity. Hence, rhizavidin provides a potential alternative to streptavidin in therapeutic applications [37,38].
It would thus seem possible that rhizavidin could be used complementarily to (strept)avidin in cancer treatments in that its cross-reactivity with antibodies against (strept)avidin was exiguous, in addition to its favourable biodistribution. Furthermore, it possesses high resistance toward proteolysis, and its affinity towards biotin appears to be high enough for avidin–biotin technology applications.
Acknowledgments
We thank Ulla Kiiskinen for her excellent assistance; Ritva Romppanen for performing the ESI FT-ICR experiments; Dr Victor Gonzales and Dr Guillermo Davila (Universidad Nacional Autonóma de México, Mexico City, Mexico) for providing the symbiotic plasmid of R. etli; the research group of Professor Giovanni Paganelli (Division of Nuclear Medicine, European Institute of Oncology, Milan, Italy) for providing cancer patient serum samples. This work was supported by the Academy of Finland.
References
- 1.Hertz R., Sebrell W. H. Occurrence of avidin in the oviduct and secretions of the genital tract of several species. Science. 1942;96:257. doi: 10.1126/science.96.2489.257. [DOI] [PubMed] [Google Scholar]
- 2.Jones P. D., Briggs M. H. Distribution of avidin. Life Sci. 1962;11:621–623. doi: 10.1016/0024-3205(62)90094-2. [DOI] [PubMed] [Google Scholar]
- 3.Korpela J. K., Kulomaa M. S., Elo H. A., Tuohimaa P. J. Biotin-binding proteins in eggs of oviparous vertebrates. Experientia. 1981;37:1065–1066. doi: 10.1007/BF02085010. [DOI] [PubMed] [Google Scholar]
- 4.Bayer E. A., Kulik T., Adar R., Wilchek M. Close similarity among streptavidin-like, biotin-binding proteins from Streptomyces. Biochim. Biophys. Acta. 1995;1263:60–66. doi: 10.1016/0167-4781(95)00077-t. [DOI] [PubMed] [Google Scholar]
- 5.Chaiet L., Wolf F. J. The properties of streptavidin, a biotin-binding protein produced by streptomycetes. Arch. Biochem. Biophys. 1964;106:1–5. doi: 10.1016/0003-9861(64)90150-x. [DOI] [PubMed] [Google Scholar]
- 6.Nordlund H. R., Hytönen V. P., Laitinen O. H., Kulomaa M. S. Novel avidin-like protein from a root nodule symbiotic bacterium, Bradyrhizobium japonicum. J. Biol. Chem. 2005;280:13250–13255. doi: 10.1074/jbc.M414336200. [DOI] [PubMed] [Google Scholar]
- 7.Girard M. L., Flores M., Brom S., Romero D., Palacios R., Davila G. Structural complexity of the symbiotic plasmid of Rhizobium leguminosarumbv. phaseoli. J. Bacteriol. 1991;173:2411–2419. doi: 10.1128/jb.173.8.2411-2419.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez-Romero M. A., Bustos P., Girard L., Rodriguez O., Cevallos M. A., Davila G. Sequence, localization and characteristics of the replicator region of the symbiotic plasmid of Rhizobium etli. Microbiology. 1997;143:2825–2831. doi: 10.1099/00221287-143-8-2825. [DOI] [PubMed] [Google Scholar]
- 9.Quintero V., Cevallos M. A., Davila G. A site-specific recombinase (RinQ) is required to exert incompatibility towards the symbiotic plasmid of Rhizobium etli. Mol. Microbiol. 2002;46:1023–1032. doi: 10.1046/j.1365-2958.2002.03205.x. [DOI] [PubMed] [Google Scholar]
- 10.Livnah O., Bayer E. A., Wilchek M., Sussman J. L. Three-dimensional structures of avidin and the avidin–biotin complex. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5076–5080. doi: 10.1073/pnas.90.11.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugliese L., Coda A., Malcovati M., Bolognesi M. Three-dimensional structure of the tetragonal crystal form of egg-white avidin in its functional complex with biotin at 2.7 Å resolution. J. Mol. Biol. 1993;231:698–710. doi: 10.1006/jmbi.1993.1321. [DOI] [PubMed] [Google Scholar]
- 12.Weber P. C., Ohlendorf D. H., Wendoloski J. J., Salemme F. R. Structural origins of high-affinity biotin binding to streptavidin. Science. 1989;243:85–88. doi: 10.1126/science.2911722. [DOI] [PubMed] [Google Scholar]
- 13.Hendrickson W. A., Pähler A., Smith J. L., Satow Y., Merritt E. A., Phizackerley R. P. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2190–2194.. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilchek M., Bayer E. Introduction to avidin–biotin technology. Methods Enzymol. 1990;184:5–13. doi: 10.1016/0076-6879(90)84256-g. [DOI] [PubMed] [Google Scholar]
- 15.Wilchek M., Bayer E. A. Foreword and introduction to the book (strept)avidin–biotin system. Biomol. Eng. 1999;16:1–4. doi: 10.1016/s1050-3862(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 16.Laitinen O. H., Hytönen V. P., Nordlund H. R., Kulomaa M. S. Genetically engineered avidins and streptavidins. Cell. Mol. Life Sci. 2006;63:2992–3017. doi: 10.1007/s00018-006-6288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordlund H. R., Laitinen O. H., Hytönen V. P., Uotila S. T., Porkka E., Kulomaa M. S. Construction of a dual chain pseudotetrameric chicken avidin by combining two circularly permuted avidins. J. Biol. Chem. 2004;279:36715–36719. doi: 10.1074/jbc.M403496200. [DOI] [PubMed] [Google Scholar]
- 18.Hytönen V. P., Hörhä J., Airenne T. T., Niskanen E. A., Helttunen K. J., Johnson M. S., Salminen T. A., Kulomaa M. S., Nordlund H. R. Controlling quaternary structure assembly: subunit interface engineering and crystal structure of dual chain avidin. J. Mol. Biol. 2006;359:1352–1363. doi: 10.1016/j.jmb.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 19.Hytönen V. P., Nordlund H. R., Hörhä J., Nyholm T. K., Hyre D. E., Kulomaa T., Porkka E. J., Marttila A. T., Stayton P. S., Laitinen O. H., Kulomaa M. S. Dual-affinity avidin molecules. Proteins. 2005;61:597–607. doi: 10.1002/prot.20604. [DOI] [PubMed] [Google Scholar]
- 20.Hytönen V. P., Laitinen O. H., Airenne T. T., Kidron H., Meltola N. J., Porkka E., Hörhä J., Paldanius T., Määttä J. A., Nordlund H. R., et al. Efficient production of active chicken avidin using a bacterial signal peptide in Escherichia coli. Biochem. J. 2004;384:385–390. doi: 10.1042/BJ20041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Airenne K. J., Oker-Blom C., Marjomäki V. S., Bayer E. A., Wilchek M., Kulomaa M. S. Production of biologically active recombinant avidin in baculovirus-infected insect cells. Protein Expression Purif. 1997;9:100–108. doi: 10.1006/prep.1996.0660. [DOI] [PubMed] [Google Scholar]
- 22.Nordlund H. R., Hytönen V. P., Hörhä J., Määttä J. A., White D. J., Halling K., Porkka E. J., Slotte J. P., Laitinen O. H., Kulomaa M. S. Tetravalent single-chain avidin: from subunits to protein domains via circularly permuted avidins. Biochem. J. 2005;392:485–491. doi: 10.1042/BJ20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hytönen V. P., Laitinen O. H., Grapputo A., Kettunen A., Savolainen J., Kalkkinen N., Marttila A. T., Nordlund H. R., Nyholm T. K., Paganelli G., Kulomaa M. S. Characterization of poultry egg-white avidins and their potential as a tool in pretargeting cancer treatment. Biochem. J. 2003;372:219–225. doi: 10.1042/BJ20021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez M., Bagatolli L. A., Echabe I., Arrondo J. L. R., Argarana C. E., Cantor C. R., Fidelio G. D. Interaction of biotin with streptavidin: thermostability and conformational changes upon binding. J. Biol. Chem. 1997;272:11288–11294. doi: 10.1074/jbc.272.17.11288. [DOI] [PubMed] [Google Scholar]
- 25.Nordlund H. R., Laitinen O. H., Uotila S. T., Nyholm T., Hytönen V. P., Slotte J. P., Kulomaa M. S. Enhancing the thermal stability of avidin: introduction of disulfide bridges between subunit interfaces. J. Biol. Chem. 2003;278:2479–2483. doi: 10.1074/jbc.M210721200. [DOI] [PubMed] [Google Scholar]
- 26.Klumb L. A., Chu V., Stayton P. S. Energetic roles of hydrogen bonds at the ureido oxygen binding pocket in the streptavidin–biotin complex. Biochemistry. 1998;37:7657–7663. doi: 10.1021/bi9803123. [DOI] [PubMed] [Google Scholar]
- 26a.Luo D.-S., King M. A. Combination of Butterworth and variable conductance diffusion approach for filtered back-projection reconstructions. Proc. SPIE Int. Soc. Opt. Eng. 1995;2434:636–647. [Google Scholar]
- 27.Hytönen V. P., Nyholm T. K., Pentikainen O. T., Vaarno J., Porkka E. J., Nordlund H. R., Johnson M. S., Slotte J. P., Laitinen O. H., Kulomaa M. S. Chicken avidin-related protein 4/5 shows superior thermal stability when compared with avidin while retaining high affinity to biotin. J. Biol. Chem. 2004;279:9337–9343. doi: 10.1074/jbc.M310989200. [DOI] [PubMed] [Google Scholar]
- 28.Ellison D., Hinton J., Hubbard S. J., Beynon R. J. Limited proteolysis of native proteins: the interaction between avidin and proteinase K. Protein Sci. 1995;4:1337–1345. doi: 10.1002/pro.5560040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flower D. R., North A. C., Sansom C. E. The lipocalin protein family: structural and sequence overview. Biochim. Biophys. Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 30.Sano T., Cantor C. R. Intersubunit contacts made by tryphtophan 120 with biotin are essential for both strong biotin binding and biotin-induced tighter subunit association of streptavidin. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3180–3184. doi: 10.1073/pnas.92.8.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pazy Y., Kulik T., Bayer E. A., Wilchek M., Livnah O. Ligand exchange between proteins: exchange of biotin and biotin derivatives between avidin and streptavidin. J. Biol. Chem. 2002;277:30892–30900. doi: 10.1074/jbc.M202874200. [DOI] [PubMed] [Google Scholar]
- 32.Wu S. C., Wong S. L. Engineering soluble monomeric streptavidin with reversible biotin binding capability. J. Biol. Chem. 2005;280:23225–23231. doi: 10.1074/jbc.M501733200. [DOI] [PubMed] [Google Scholar]
- 33.Chinol M., Casalini P., Maggiolo M., Canevari S., Omodeo E. S., Caliceti P., Veronese F. M., Cremonesi M., Chiolerio F., Nardone E., et al. Biochemical modifications of avidin improve pharmacokinetics and biodistribution, and reduce immunogenicity. Br. J. Cancer. 1998;78:189–197. doi: 10.1038/bjc.1998.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paganelli G., Chinol M., Maggiolo M., Sidoli A., Corti A., Baroni S., Siccardi A. G. The three-step pretargeting approach reduces the human anti-mouse antibody response in patients submitted to radioimmunoscintigraphy and radioimmunotherapy. Eur. J. Nucl. Med. 1997;24:350–351. doi: 10.1007/BF01728778. [DOI] [PubMed] [Google Scholar]
- 35.Wei R. D., Kou D. H., Hoo S. L. Dissociation of avidin–biotin complex in vivo. Experientia. 1971;27:366–368. doi: 10.1007/BF02137250. [DOI] [PubMed] [Google Scholar]
- 36.Schechter B., Silberman R., Arnon R., Wilchek M. Tissue distribution of avidin and streptavidin injected to mice: effect of avidin carbohydrate, streptavidin truncation and exogenous biotin. Eur. J. Biochem. 1990;189:327–331. doi: 10.1111/j.1432-1033.1990.tb15493.x. [DOI] [PubMed] [Google Scholar]
- 37.Wilbur D. S., Hamlin D. K., Buhler K. R., Pathare P. M., Vessella R. L., Stayton P. S., To R. Streptavidin in antibody pretargeting. 2. Evaluation of methods for decreasing localization of streptavidin to kidney while retaining its tumor binding capacity. Bioconjugate Chem. 1998;9:322–330. doi: 10.1021/bc970182v. [DOI] [PubMed] [Google Scholar]
- 38.Forster G. J., Santos E. B., Smith-Jones P. M., Zanzonico P., Larson S. M. Pretargeted radioimmunotherapy with a single-chain antibody/streptavidin construct and radiolabeled DOTA-biotin: strategies for reduction of the renal dose. J. Nucl. Med. 2006;47:140–149. [PubMed] [Google Scholar]
- 39.Schechter B., Arnon R., Colas C., Burakova T., Wilchek M. Renal accumulation of streptavidin: potential use for targeted therapy to the kidney. Kidney Int. 1995;47:1327–1335. doi: 10.1038/ki.1995.188. [DOI] [PubMed] [Google Scholar]
- 40.Kang Y.-S., Saito Y., Pardridge W. M. Pharmacokinetics of 3H biotin bound to different avidin analogues. J. Drug Targeting. 1995;3:159–165. doi: 10.3109/10611869509059215. [DOI] [PubMed] [Google Scholar]
- 41.Rosebrough S. F., Hartley D. F. Biochemical modification of streptavidin and avidin: in vitro and in vivo analysis. J. Nucl. Med. 1996;37:1380–1384. [PubMed] [Google Scholar]
- 42.Wilbur D. S., Stayton P. S., To R., Buhler K. R., Klumb L. A., Hamlin D. K., Stray J. E., Vessella R. L. Streptavidin in antibody pretargeting: comparison of a recombinant streptavidin with two streptavidin mutant proteins and two commercially available streptavidin proteins. Bioconjugate Chem. 1998;9:100–117. doi: 10.1021/bc970152s. [DOI] [PubMed] [Google Scholar]