Abstract
Objective:
To review and describe randomization techniques used in clinical trials, including simple, block, stratified, and covariate adaptive techniques.
Background:
Clinical trials are required to establish treatment efficacy of many athletic training procedures. In the past, we have relied on evidence of questionable scientific merit to aid the determination of treatment choices. Interest in evidence-based practice is growing rapidly within the athletic training profession, placing greater emphasis on the importance of well-conducted clinical trials. One critical component of clinical trials that strengthens results is random assignment of participants to control and treatment groups. Although randomization appears to be a simple concept, issues of balancing sample sizes and controlling the influence of covariates a priori are important. Various techniques have been developed to account for these issues, including block, stratified randomization, and covariate adaptive techniques.
Advantages:
Athletic training researchers and scholarly clinicians can use the information presented in this article to better conduct and interpret the results of clinical trials. Implementing these techniques will increase the power and validity of findings of athletic medicine clinical trials, which will ultimately improve the quality of care provided.
Keywords: minimization, simple randomization, block randomization, stratified randomization, covariate adaptive randomization
Outcomes research is critical in the evidence-based health care environment because it addresses scientific questions concerning the efficacy of treatments. Clinical trials are considered the “gold standard” for outcomes in biomedical research. In athletic training, calls for more evidence-based medical research, specifically clinical trials, have been issued.1,2
The strength of clinical trials is their superior ability to measure change over time from a treatment. Treatment differences identified from cross-sectional observational designs rather than experimental clinical trials have methodologic weaknesses, including confounding, cohort effects, and selection bias.3 For example, using a nonrandomized trial to examine the effectiveness of prophylactic knee bracing to prevent medial collateral ligament injuries may suffer from confounders and jeopardize the results. One possible confounder is a history of knee injuries. Participants with a history of knee injuries may be more likely to wear braces than those with no such history. Participants with a history of injury are more likely to suffer additional knee injuries, unbalancing the groups and influencing the results of the study.
The primary goal of comparative clinical trials is to provide comparisons of treatments with maximum precision and validity.4 One critical component of clinical trials is random assignment of participants into groups. Randomizing participants helps remove the effect of extraneous variables (eg, age, injury history) and minimizes bias associated with treatment assignment. Randomization is considered by most researchers to be the optimal approach for participant assignment in clinical trials because it strengthens the results and data interpretation.4–9
One potential problem with small clinical trials (n < 100)7 is that conventional simple randomization methods, such as flipping a coin, may result in imbalanced sample size and baseline characteristics (ie, covariates) among treatment and control groups.9,10 This imbalance of baseline characteristics can influence the comparison between treatment and control groups and introduce potential confounding factors. Many procedures have been proposed for random group assignment of participants in clinical trials.11 Simple, block, stratified, and covariate adaptive randomizations are some examples. Each technique has advantages and disadvantages, which must be carefully considered before a method is selected. Our purpose is to introduce the concept and significance of randomization and to review several conventional and relatively new randomization techniques to aid in the design and implementation of valid clinical trials.
What Is Randomization?
Randomization is the process of assigning participants to treatment and control groups, assuming that each participant has an equal chance of being assigned to any group.12 Randomization has evolved into a fundamental aspect of scientific research methodology. Demands have increased for more randomized clinical trials in many areas of biomedical research, such as athletic training.2,13 In fact, in the last 2 decades, internationally recognized major medical journals, such as the Journal of the American Medical Association and the BMJ, have been increasingly interested in publishing studies reporting results from randomized controlled trials.5
Since Fisher14 first introduced the idea of randomization in a 1926 agricultural study, the academic community has deemed randomization an essential tool for unbiased comparisons of treatment groups. Five years after Fisher's introductory paper, the first randomized clinical trial involving tuberculosis was conducted.15 A total of 24 participants were paired (ie, 12 comparable pairs), and by a flip of a coin, each participant within the pair was assigned to either the control or treatment group. By employing randomization, researchers offer each participant an equal chance of being assigned to groups, which makes the groups comparable on the dependent variable by eliminating potential bias. Indeed, randomization of treatments in clinical trials is the only means of avoiding systematic characteristic bias of participants assigned to different treatments. Although randomization may be accomplished with a simple coin toss, more appropriate and better methods are often needed, especially in small clinical trials. These other methods will be discussed in this review.
Why Randomize?
Researchers demand randomization for several reasons. First, participants in various groups should not differ in any systematic way. In a clinical trial, if treatment groups are systematically different, trial results will be biased. Suppose that participants are assigned to control and treatment groups in a study examining the efficacy of a walking intervention. If a greater proportion of older adults is assigned to the treatment group, then the outcome of the walking intervention may be influenced by this imbalance. The effects of the treatment would be indistinguishable from the influence of the imbalance of covariates, thereby requiring the researcher to control for the covariates in the analysis to obtain an unbiased result.16
Second, proper randomization ensures no a priori knowledge of group assignment (ie, allocation concealment). That is, researchers, participants, and others should not know to which group the participant will be assigned. Knowledge of group assignment creates a layer of potential selection bias that may taint the data. Schulz and Grimes17 stated that trials with inadequate or unclear randomization tended to overestimate treatment effects up to 40% compared with those that used proper randomization. The outcome of the trial can be negatively influenced by this inadequate randomization.
Statistical techniques such as analysis of covariance (ANCOVA), multivariate ANCOVA, or both, are often used to adjust for covariate imbalance in the analysis stage of the clinical trial. However, the interpretation of this postadjustment approach is often difficult because imbalance of covariates frequently leads to unanticipated interaction effects, such as unequal slopes among subgroups of covariates.18,19 One of the critical assumptions in ANCOVA is that the slopes of regression lines are the same for each group of covariates (ie, homogeneity of regression slopes). The adjustment needed for each covariate group may vary, which is problematic because ANCOVA uses the average slope across the groups to adjust the outcome variable. Thus, the ideal way of balancing covariates among groups is to apply sound randomization in the design stage of a clinical trial (before the adjustment procedure) instead of after data collection. In such instances, random assignment is necessary and guarantees validity for statistical tests of significance that are used to compare treatments.
How To Randomize?
Many procedures have been proposed for the random assignment of participants to treatment groups in clinical trials. In this article, common randomization techniques, including simple randomization, block randomization, stratified randomization, and covariate adaptive randomization, are reviewed. Each method is described along with its advantages and disadvantages. It is very important to select a method that will produce interpretable, valid results for your study.
Simple Randomization
Randomization based on a single sequence of random assignments is known as simple randomization.10 This technique maintains complete randomness of the assignment of a person to a particular group. The most common and basic method of simple randomization is flipping a coin. For example, with 2 treatment groups (control versus treatment), the side of the coin (ie, heads = control, tails = treatment) determines the assignment of each participant. Other methods include using a shuffled deck of cards (eg, even = control, odd = treatment) or throwing a die (eg, below and equal to 3 = control, over 3 = treatment). A random number table found in a statistics book or computer-generated random numbers can also be used for simple randomization of participants.
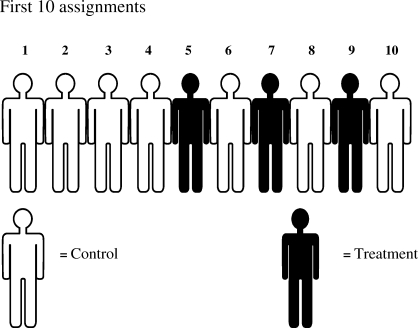
This randomization approach is simple and easy to implement in a clinical trial. In large trials (n > 200), simple randomization can be trusted to generate similar numbers of participants among groups. However, randomization results could be problematic in relatively small sample size clinical trials (n < 100), resulting in an unequal number of participants among groups. For example, using a coin toss with a small sample size (n = 10) may result in an imbalance such that 7 participants are assigned to the control group and 3 to the treatment group (Figure 1).
Figure 1. Imbalance of sample size between treatment arms due to simple randomization (coin toss) in a small trial (n = 10).
Block Randomization
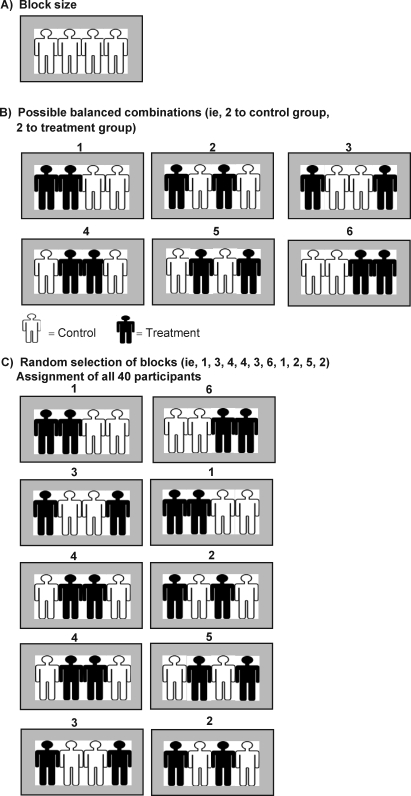
The block randomization method is designed to randomize participants into groups that result in equal sample sizes. This method is used to ensure a balance in sample size across groups over time. Blocks are small and balanced with predetermined group assignments, which keeps the numbers of participants in each group similar at all times. According to Altman and Bland,10 the block size is determined by the researcher and should be a multiple of the number of groups (ie, with 2 treatment groups, block size of either 4 or 6). Blocks are best used in smaller increments as researchers can more easily control balance.7 After block size has been determined, all possible balanced combinations of assignment within the block (ie, equal number for all groups within the block) must be calculated. Blocks are then randomly chosen to determine the participants' assignment into the groups.
For a clinical trial with control and treatment groups involving 40 participants, a randomized block procedure would be as follows: (1) a block size of 4 is chosen, (2) possible balanced combinations with 2 C (control) and 2 T (treatment) subjects are calculated as 6 (TTCC, TCTC, TCCT, CTTC, CTCT, CCTT), and (3) blocks are randomly chosen to determine the assignment of all 40 participants (eg, one random sequence would be [TTCC / TCCT / CTTC / CTTC / TCCT / CCTT / TTCC / TCTC / CTCT / TCTC]). This procedure results in 20 participants in both the control and treatment groups (Figure 2).
Figure 2. Block randomization procedure produces balanced study arms, even with a small sample size.
Although balance in sample size may be achieved with this method, groups may be generated that are rarely comparable in terms of certain covariates.6 For example, one group may have more participants with secondary diseases (eg, diabetes, multiple sclerosis, cancer) that could confound the data and may negatively influence the results of the clinical trial. Pocock and Simon11 stressed the importance of controlling for these covariates because of serious consequences to the interpretation of the results. Such an imbalance could introduce bias in the statistical analysis and reduce the power of the study.4,6,8 Hence, sample size and covariates must be balanced in small clinical trials.
Stratified Randomization
The stratified randomization method addresses the need to control and balance the influence of covariates. This method can be used to achieve balance among groups in terms of participants' baseline characteristics (covariates). Specific covariates must be identified by the researcher who understands the potential influence each covariate has on the dependent variable. Stratified randomization is achieved by generating a separate block for each combination of covariates, and participants are assigned to the appropriate block of covariates. After all participants have been identified and assigned into blocks, simple randomization occurs within each block to assign participants to one of the groups.
The stratified randomization method controls for the possible influence of covariates that would jeopardize the conclusions of the clinical trial. For example, a clinical trial of different rehabilitation techniques after a surgical procedure will have a number of covariates. It is well known that the age of the patient affects the rate of healing. Thus, age could be a confounding variable and influence the outcome of the clinical trial. Stratified randomization can balance the control and treatment groups for age or other identified covariates.
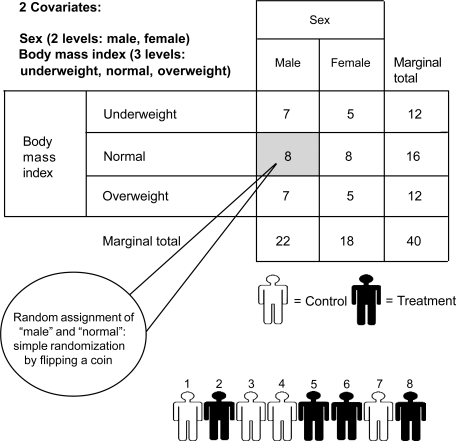
For example, with 2 groups involving 40 participants, the stratified randomization method might be used to control the covariates of sex (2 levels: male, female) and body mass index (3 levels: underweight, normal, overweight) between study arms. With these 2 covariates, possible block combinations total 6 (eg, male, underweight). A simple randomization procedure, such as flipping a coin, is used to assign the participants within each block to one of the treatment groups (Figure 3).
Figure 3. Stratified randomization procedure produces equal-sized study groups that are balanced by covariates.
Although stratified randomization is a relatively simple and useful technique, especially for smaller clinical trials, it becomes complicated to implement if many covariates must be controlled.20 For example, too many block combinations may lead to imbalances in overall treatment allocations because a large number of blocks can generate small participant numbers within the block. Therneau21 purported that a balance in covariates begins to fail when the number of blocks approaches half the sample size. If another 4-level covariate was added to the example, the number of block combinations would increase from 6 to 24 (2 × 3 × 4), for an average of fewer than 2 (40 / 24 = 1.7) participants per block, reducing the usefulness of the procedure to balance the covariates and jeopardizing the validity of the clinical trial. In small studies, it may not be feasible to stratify more than 1 or 2 covariates because the number of blocks can quickly approach the number of participants.10
Stratified randomization has another limitation: it works only when all participants have been identified before group assignment. This method is rarely applicable, however, because clinical trial participants are often enrolled one at a time on a continuous basis. When baseline characteristics of all participants are not available before assignment, using stratified randomization is difficult.7
Covariate Adaptive Randomization
Covariate adaptive randomization has been recommended by many researchers as a valid alternative randomization method for clinical trials.9,22 In covariate adaptive randomization, a new participant is sequentially assigned to a particular treatment group by taking into account the specific covariates and previous assignments of participants.9,12,18,23,24 Covariate adaptive randomization uses the method of minimization by assessing the imbalance of sample size among several covariates. This covariate adaptive approach was first described by Taves.23
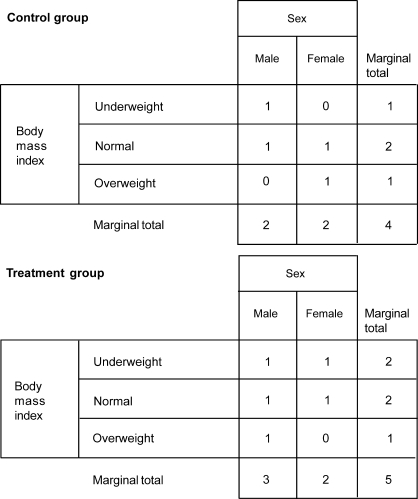
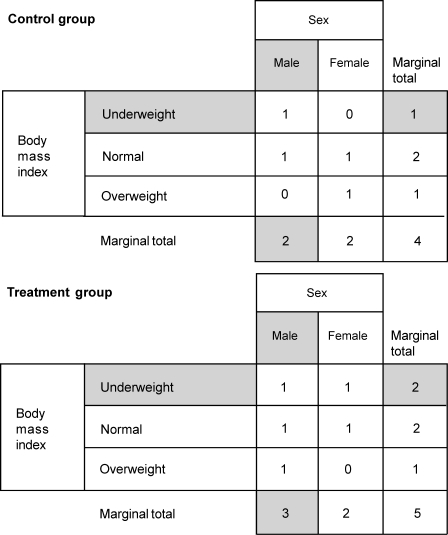
The Taves covariate adaptive randomization method allows for the examination of previous participant group assignments to make a case-by-case decision on group assignment for each individual who enrolls in the study. Consider again the example of 2 groups involving 40 participants, with sex (2 levels: male, female) and body mass index (3 levels: underweight, normal, overweight) as covariates. Assume the first 9 participants have already been randomly assigned to groups by flipping a coin. The 9 participants' group assignments are broken down by covariate level in Figure 4. Now the 10th participant, who is male and underweight, needs to be assigned to a group (ie, control versus treatment). Based on the characteristics of the 10th participant, the Taves method adds marginal totals of the corresponding covariate categories for each group and compares the totals. The participant is assigned to the group with the lower covariate total to minimize imbalance. In this example, the appropriate categories are male and underweight, which results in the total of 3 (2 for male category + 1 for underweight category) for the control group and a total of 5 (3 for male category + 2 for underweight category) for the treatment group. Because the sum of marginal totals is lower for the control group (3 < 5), the 10th participant is assigned to the control group (Figure 5).
Figure 4. Breakdown of the first 9 participants' group assignments by covariates: sex and body mass index.
Figure 5. Taves23 and Pocock and Simon11 covariate adaptive randomization procedures. The 10th participant is male and belongs to the underweight group. Male and underweight categories and marginal totals of initial 9 participants are shaded. Taves method (1974)23: A, Add marginal total in control group = 3 (ie, 2 for male category + 1 for underweight category). Add marginal total in control group = 5 (ie, 3 for male category + 2 for underweight category). B, Assign the 10th participant to the lower marginal total, which is the control group (ie, 3 < 5). Pocock and Simon method (1975)11: A, Marginal total in control group = 3 for male category and 2 for underweight category. B, Male category: 3 − 3 = 0; underweight category: 2 − 2 = 0; sum of the differences = 0 + 0 = 0. C, Marginal total in treatment group = 4 for male category and 3 for underweight category. D, Male category: 2 - 4 = 2; underweight category: 1 - 3 = 2; sum of the differences = 2 + 2 = 4. E, Assign the 10th participant to the control group (ie, 0 < 4).
The Pocock and Simon method11 of covariate adaptive randomization is similar to the method Taves23 described. The difference in this approach is the temporary assignment of participants to both groups. This method uses the absolute difference between groups to determine group assignment. To minimize imbalance, the participant is assigned to the group determined by the lowest sum of the absolute differences among the covariates between the groups. For example, using the previous situation in assigning the 10th participant to a group, the Pocock and Simon method would (1) assign the 10th participant temporarily to the control group, resulting in marginal totals of 3 for male category and 2 for underweight category; (2) calculate the absolute difference between control and treatment group (males: 3 control – 3 treatment = 0; underweight: 2 control – 2 treatment = 0) and sum (0 + 0 = 0); (3) temporarily assign the 10th participant to the treatment group, resulting in marginal totals of 4 for male category and 3 for underweight category; (4) calculate the absolute difference between control and treatment group (males: 2 control – 4 treatment = 2; underweight: 1 control – 3 treatment = 2) and sum (2 + 2 = 4); and (5) assign the 10th participant to the control group because of the lowest sum of absolute differences (0 < 4).
Pocock and Simon11 also suggested using a variance approach. Instead of calculating absolute difference among groups, this approach calculates the variance among treatment groups. Although the variance method performs similarly to the absolute difference method, both approaches suffer from the limitation of handling only categorical covariates.25
Frane18 introduced a covariate adaptive randomization for both continuous and categorical types. Frane used P values to identify imbalance among treatment groups: a smaller P value represents more imbalance among treatment groups.
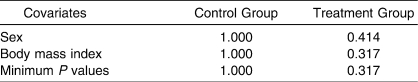
The Frane method for assigning participants to either the control or treatment group would include (1) temporarily assigning the participant to both the control and treatment groups; (2) calculating P values for each of the covariates using a t test and analysis of variance (ANOVA) for continuous variables and goodness-of-fit χ2 test for categorical variables; (3) determining the minimum P value for each control or treatment group, which indicates more imbalance among treatment groups; and (4) assigning the participant to the group with the larger minimum P value (ie, try to avoid more imbalance in groups).
Going back to the previous example of assigning the 10th participant (male and underweight) to a group, the Frane method would result in the assignment to the control group. The steps used to make this decision were calculating P values for each of the covariates using the χ2 goodness-of-fit test represented in the Table. The t tests and ANOVAs were not used because the covariates in this example were categorical. Based on the Table, the lowest minimum P values were 1.0 for the control group and 0.317 for the treatment group. The 10th participant was assigned to the control group because of the higher minimum P value, which indicates better balance in the control group (1.0 > 0.317).
Table.
Probabilities From χ2 Goodness-of-Fit Tests for the Example Shown in Figure 5 (Frane18 Method)
Covariate adaptive randomization produces less imbalance than other conventional randomization methods and can be used successfully to balance important covariates among control and treatment groups.6 Although the balance of covariates among groups using the stratified randomization method begins to fail when the number of blocks approaches half the sample size, covariate adaptive randomization can better handle the problem of increasing numbers of covariates (ie, increased block combinations).9
One concern of these covariate adaptive randomization methods is that treatment assignments sometimes become highly predictable. Investigators using covariate adaptive randomization sometimes come to believe that group assignment for the next participant can be readily predicted, going against the basic concept of randomization.12,26,27 This predictability stems from the ongoing assignment of participants to groups wherein the current allocation of participants may suggest future participant group assignment. In their review, Scott et al9 argued that this predictability is also true of other methods, including stratified randomization, and it should not be overly penalized. Zielhuis et al28 and Frane18 suggested a practical approach to prevent predictability: a small number of participants should be randomly assigned into the groups before the covariate adaptive randomization technique being applied.
The complicated computation process of covariate adaptive randomization increases the administrative burden, thereby limiting its use in practice. A user-friendly computer program for covariate adaptive randomization is available (free of charge) upon request from the authors (M.K., B.G.R., or J.H.P.).29
Conclusions
Our purpose was to introduce randomization, including its concept and significance, and to review several randomization techniques to guide athletic training researchers and practitioners to better design their randomized clinical trials. Many factors can affect the results of clinical research, but randomization is considered the gold standard in most clinical trials. It eliminates selection bias, ensures balance of sample size and baseline characteristics, and is an important step in guaranteeing the validity of statistical tests of significance used to compare treatment groups.
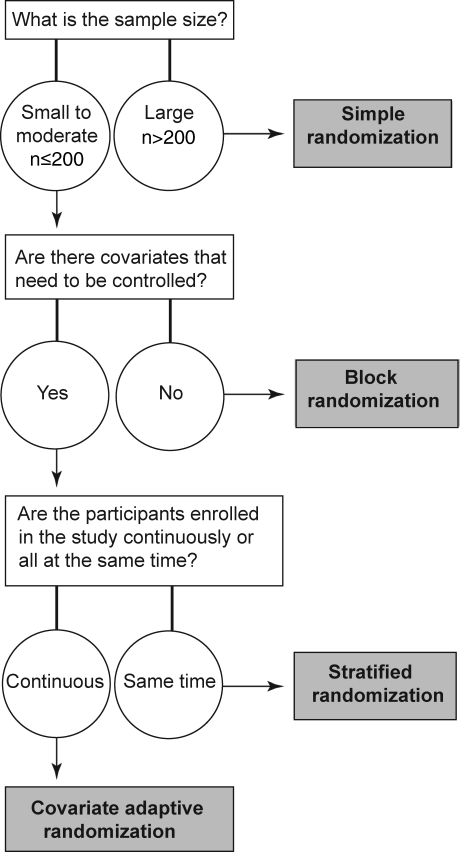
Before choosing a randomization method, several factors need to be considered, including the size of the clinical trial; the need for balance in sample size, covariates, or both; and participant enrollment.16 Figure 6 depicts a flowchart designed to help select an appropriate randomization technique. For example, a power analysis for a clinical trial of different rehabilitation techniques after a surgical procedure indicated a sample size of 80. A well-known covariate for this study is age, which must be balanced among groups. Because of the nature of the study with postsurgical patients, participant recruitment and enrollment will be continuous. Using the flowchart, the appropriate randomization technique is covariate adaptive randomization technique.
Figure 6. Flowchart for selecting appropriate randomization technique. The gray boxes represent appropriate techniques.
Simple randomization works well for a large trial (eg, n > 200) but not for a small trial (n < 100).7 To achieve balance in sample size, block randomization is desirable. To achieve balance in baseline characteristics, stratified randomization is widely used. Covariate adaptive randomization, however, can achieve better balance than other randomization methods and can be successfully used for clinical trials in an effective manner.
Acknowledgments
This study was partially supported by a Faculty Grant (FRCAC) from the College of Graduate Studies, at Middle Tennessee State University, Murfreesboro, TN.
Footnotes
Minsoo Kang, PhD; Brian G. Ragan, PhD, ATC; and Jae-Hyeon Park, PhD, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article.
References
- 1.Ingersoll C.D. It's time for evidence. J Athl Train. 2006;41:7. [PMC free article] [PubMed] [Google Scholar]
- 2. Request for proposals: evidence-based practice and outcomes of care in athletic training. National Athletic Trainers' Association Research & Education Foundation. http://www.natafoundation.org/pdfs/Evidence-BasedPractice20and20Outcomes.pdf. Accessed December 24, 2006. [Google Scholar]
- 3.Singer J.D, Willett J.B. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. pp. 3–15. [Google Scholar]
- 4.McEntegart D.J. The pursuit of balance using stratified and dynamic randomization techniques: an overview. Drug Inf J. 2003;37(3):293–308. [Google Scholar]
- 5.Altman D.G, Bland J.M. Statistics notes: treatment allocation in controlled trials. Why randomise? BMJ. 1999;318(7192):1209. doi: 10.1136/bmj.318.7192.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedden S.L, Woolson R.F, Malcolm R.J. Randomization in substance abuse clinical trials. Subst Abuse Treat Prev Policy. 2006;1(1):6. doi: 10.1186/1747-597X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lachin J.M, Matts J.P, Wei L.J. Randomization in clinical trials: conclusions and recommendations. Control Clin Trials. 9;(4):365–374. doi: 10.1016/0197-2456(88)90049-9. 1988. [DOI] [PubMed] [Google Scholar]
- 8.Schulz K.F, Grimes D.A. Generation of allocation sequences in randomised trials: chance, not choice. Lancet. 2002;359(9305):515–519. doi: 10.1016/S0140-6736(02)07683-3. [DOI] [PubMed] [Google Scholar]
- 9.Scott N.W, McPherson G.C, Ramsay C.R, Campbell M.K. The method of minimization for allocation to clinical trials: a review. Control Clin Trials. 2002;23(6):662–674. doi: 10.1016/s0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 10.Altman D.G, Bland J.M. How to randomise. BMJ. 1999;319(7211):703–704. doi: 10.1136/bmj.319.7211.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pocock S.J, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 12.Fleiss J.L, Levin B, Paik M.C. Statistical Methods for Rates and Proportions. 3rd ed. Hoboken, NJ: John Wiley & Sons; 2003. How to randomize. pp. 86–94. [Google Scholar]
- 13.Simon S.D. Is the randomized clinical trial the gold standard of research? J Androl. 2001;22(6):938–943. doi: 10.1002/j.1939-4640.2001.tb03433.x. [DOI] [PubMed] [Google Scholar]
- 14.Fisher R.A. The arrangement of field experiments. J Ministry Ag. 1926;33:503–513. [Google Scholar]
- 15.Amberson J.B, McMahon B.T, Pinner M.A. Clinical trial of sanocrysin in pulmonary tuberculosis. Am Rev Tuberc. 1931;24:401–435. [Google Scholar]
- 16.Kalish L.A, Begg C.B. Treatment allocation methods in clinical trials: a review. Stat Med. 1985;4(2):129–144. doi: 10.1002/sim.4780040204. [DOI] [PubMed] [Google Scholar]
- 17.Schulz K.F, Grimes D.A. Allocation concealment in randomised trials: defending against deciphering. Lancet. 2002;359(9306):614–618. doi: 10.1016/S0140-6736(02)07750-4. [DOI] [PubMed] [Google Scholar]
- 18.Frane J.W. A method of biased coin randomization, its implementation, and its validation. Drug Inf J. 1998;32(2):423–432. [Google Scholar]
- 19.Lomax R.G. Statistical Concepts: A Second Course for Education and the Behavioral Sciences. Mahwah, NJ: Lawrence Erlbaum Assoc; 2001. pp. 186–199. [Google Scholar]
- 20.Weir C.J, Lees K.R. Comparison of stratification and adaptive methods for treatment allocation in an acute stroke clinical trial. Stat Med. 2003;22(5):705–726. doi: 10.1002/sim.1366. [DOI] [PubMed] [Google Scholar]
- 21.Therneau T.M. How many stratification factors are “too many” to use in a randomization plan? Control Clin Trial. 1993;14(2):98–108. doi: 10.1016/0197-2456(93)90013-4. [DOI] [PubMed] [Google Scholar]
- 22.Kang M. Clinical trials 101: randomization in clinical trials. Paper presented at: Annual Meeting of the American Alliance for Health, Physical Education, Recreation and Dance; April 13, 2005; Chicago, IL. [Google Scholar]
- 23.Taves D.R. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15(5):443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 24.Treasure T, MacRae K.D. Minimization: the platinum standard for trials? Randomisation doesn't guarantee similarity of groups; minimization does. BMJ. 1998;317(7155):362–363. doi: 10.1136/bmj.317.7155.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begg C.B, Iglewicz B. A treatment allocation procedure for sequential clinical trials. Biometrics. 1980;36(1):81–90. [PubMed] [Google Scholar]
- 26.Blair E. Gold is not always good enough: the shortcomings of randomization when evaluating interventions in small heterogeneous samples. J Clin Epidemiol. 2004;57(12):1219–1222. doi: 10.1016/j.jclinepi.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58(3):403–417. [Google Scholar]
- 28.Zielhuis G.A, Straatman H, van't Hof-Grootenboer A.E, van Lier H.J, Rach G.H, van den Broek P. The choice of a balanced allocation method for a clinical trial in otitis media with effusion. Stat Med. 1990;9(2):237–246. doi: 10.1002/sim.4780090306. [DOI] [PubMed] [Google Scholar]
- 29.A Computerized Covariate Adaptive Randomization Program [Computer program] Murfreesboro, TN: Kang M, Park JH; Version 1.0. [Google Scholar]