Abstract
PAR-2 (protease-activated receptor 2) is a GPCR (G-protein-coupled receptor) that can elicit both G-protein-dependent and -independent signals. We have shown previously that PAR-2 simultaneously promotes Gαq/Ca2+-dependent activation and β-arrestin-1-dependent inhibition of class IA PI3K (phosphoinositide 3-kinase), and we sought to characterize further the role of β-arrestins in the regulation of PI3K activity. Whereas the ability of β-arrestin-1 to inhibit p110α (PI3K catalytic subunit α) has been demonstrated, the role of β-arrestin-2 in PI3K regulation and possible differences in the regulation of the two catalytic subunits (p110α and p110β) associated with p85α (PI3K regulatory subunit) have not been examined. In the present study we have demonstrated that: (i) PAR-2 increases p110α- and p110β-associated lipid kinase activities, and both p110α and p110β are inhibited by over-expression of either β-arrestin-1 or -2; (ii) both β-arrestin-1 and -2 directly inhibit the p110α catalytic subunit in vitro, whereas only β-arrestin-2 directly inhibited p110β; (iii) examination of upstream pathways revealed that PAR-2-induced PI3K activity required the small GTPase Cdc (cell-division cycle)42, but not tyrosine phosphorylation of p85; and (iv) β-arrestins inhibit PAR-2-induced Cdc42 activation. Taken together, these results indicated that β-arrestins could inhibit PAR-2-stimulated PI3K activity, both directly and through interference with upstream pathways, and that the two β-arrestins differ in their ability to inhibit the p110α and p110β catalytic subunits. These results are particularly important in light of the growing interest in PAR-2 as a pharmacological target, as commonly used biochemical assays that monitor G-protein coupling would not screen for β-arrestin-dependent signalling events.
Keywords: arrestin, phosphoinositide 3-kinase (PI3K), PI3K regulatory subunit (p85), PI3K regulatory subunit (p110), protease-activated receptor 2 (PAR-2)
Abbreviations: Cdc, cell-division cycle; CRIB, Cdc42/Rac-interacting binding; DKO, double knock-out; 2fAP, 2-furoyl-LIGRL-O-NH2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; GST, glutathione transferase; HA, haemagglutinin; IGF, insulin-like growth factor; IGF-1R, IGF-1 receptor; IPTG, isopropyl β-D-thiogalactoside; IRS, insulin receptor substrate; MEF, mouse embryonic fibroblast; PAK, p21-activated kinase; PAR-2, protease-activated receptor 2; PI3K, phosphoinositide 3-kinase; p85, PI3K regulatory subunit; p110, PI3K catalytic subunit; PKC, protein kinase C; PYK2, proline-rich tyrosine kinase 2; RT, reverse transcriptase; SFK, Src-family kinase; wt, wild-type
INTRODUCTION
PAR-2 (protease-activated receptor 2) is a GPCR (G-protein-coupled receptor) that is capable of eliciting both Gαq-dependent and β-arrestin-dependent signals [1–6]. We have shown previously that PAR-2 simultaneously promotes Gαq/Ca2+-dependent PI3K (phosphoinositide 3-kinase) activation and β-arrestin-1-dependent inhibition of PI3K activity. The Gαq/Ca2+-dependent pathway involves PKC (protein kinase C) and SFK (Src-family kinase) activity, as well as the association of PYK2 (proline-rich tyrosine kinase 2) with p85 (PI3K regulatory subunit). The β-arrestin-dependent pathway involves the direct association of p85/p110 (PI3K catalytic subunit)α with β-arrestin-1, resulting in a decrease in p110α catalytic activity [7]. In a cell line with high endogenous levels of β-arrestins (MDA MB-468, a breast cancer cell line), the inhibitory pathway dominates, resulting in a PAR-2-stimulated decrease in PI3K activity. In contrast, in a cell line with low endogenous levels of β-arrestins (NIH 3T3), an overall PAR-2-induced increase in PI3K activity is observed. These studies highlight the fact that PAR-2 can direct opposing signals in a cell-type-specific fashion, and that the prevalence of certain downstream signalling molecules, such as β-arrestins, can alter the outcome of PAR-2 activation. This inhibitory role for β-arrestins in PI3K activity is in contrast with studies on the receptor tyrosine kinase, IGF (insulin-like growth factor)-1, where β-arrestin was proposed to facilitate PI3K activation in the absence of IRS (insulin receptor substrate)-1 [8]. In those studies, PI3K activity was measured after immunoprecipitation with the p110α catalytic subunit, whereas our previous studies were performed by immunoprecipitation with the p85 subunit [7], which would co-immunoprecipitate both p110α and p110β [8a]. Also, there are two β-arrestin family members, both of which associate with p85 upon PAR-2 activation [7], but it is unknown whether both β-arrestins can inhibit PI3K activity and whether they display specificity for one catalytic subunit. Although they are redundant for some functions, the two β-arrestins have some specific binding partners and downstream effects, raising the possibility that they differentially regulate the PI3K pathway. We therefore investigated which catalytic subunit was preferentially targeted by the PAR-2 pathway and whether both β-arrestins inhibited p110α and p110β catalytic activity. We also examined what other upstream components are involved in PAR-2-induced PI3K signalling and their inhibition by β-arrestins.
MATERIALS AND METHODS
Materials
All chemicals were purchased from Sigma or Fisher Scientific except where otherwise indicated. FLAG-tagged β-arrestin-1 and β-arrestin-2 were gifts from Dr Robert Lefkowitz (Department of Medicine, Duke University Medical Center, Durham, NC, U.S.A.). Lipofectamine™ was bought from Invitrogen. 2fAP (2-furoyl-LIGRL-O-NH2) was synthesized by Genemed (San Fransisco, CA, U.S.A). [γ-32P]ATP was purchased from PerkinElmer. PtdIns was bought from Avanti Polar Lipids and PtdIns(4,5)P2 was purchased from Echelon (Salt Lake City, UT, U.S.A.). Recombinant PI3K (p85α complexed to p110α) was bought from Upstate. GST (glutathione transferase)-tagged β-arrestins were a gift from Dr Kerri Mowen (Department of Chemical Physiology, The Scripps Research Institute, San Diego, CA, U.S.A.). Dominant-negative RhoAGTPases: HA (haemagglutanin)-tagged RhoN19 (DNRHO), GFP-tagged CDC42N17 (DNCDC) (where GFP is green fluorescent protein and Cdc is cell-division cycle) and GFP-tagged RacN17 (DNRAC) were gifts from Dr Martin Schwartz (Department of Microbiology, University of Virginia, Richmond, VA, U.S.A.).
Antibodies
All anti-FLAG antibodies were purchased from Sigma. Rabbit anti-(PI3K p85), anti-p110α and anti-p110β antibodies and Protein A–agarose were bought from Upstate. Rabbit anti-GFP antibody was purchased from Chemicon and the mouse monoclonal anti-HA antibody was bought from Cell Signaling Technology. The IRDye® 800-conjugated mouse anti-phospho-tyrosine (RC20) was from Rockland Immunochemicals (Gilbertsville, PA, U.S.A.). Rabbit anti-β-arrestin-1 and anti-β-arrestin-2 antibodies (A1CT and A2CT) were gifts from Dr Robert Lefkowitz. Mouse anti-β-arrestin-1, anti-RhoA and anti-Cdc42 antibodies were purchased from BD Pharmingen. Rabbit anti-Rac antibody was bought from Cytoskeleton (Denver, CO, U.S.A.). Goat anti-actin was purchased from Santa Cruz Biotechnology. Alexa Fluor® 680-conjugated secondary antibodies were bought from Molecular Probes and IRDye® 800-conjugated secondary antibodies were purchased from Rockland Immunochemicals.
Cell culture and transient transfections
NIH 3T3, MEF (mouse embryonic fibroblast) wt (wild-type), MEF β-arrestin-1, β-arrestin-2-DKO (double knock-out), MEF β-arrestin-1, β-arrestin-2-DKO with β-arrestin-1 and MEF β-arrestin-1, β-arrestin-2-DKO with β-arrestin-2 cell lines were grown in DMEM (Dulbecco's modified Eagle's medium) (Mediatech) supplemented with 10% (v/v) cosmic calf serum (Hyclone) and maintained at 37 °C with in an atmosphere of 5% CO2. NIH 3T3 cells (5×105 cells) were grown on 10 cm tissue-culture dishes overnight to approx. 70% confluency and then were transiently transfected with 10 μg of FLAG-tagged β-arrestin-1, β-arrestin-2, DNRHO, DNCDC or DNRAC using Lipofectamine™ according to the manufacturer's instructions. Cells were harvested for experiments 36–48 h after transfection.
RT (reverse transcriptase)-PCR and electrophoresis of products
Oligonucleotide primers for PCR were synthesized (Sigma Genosys) as follows. Mouse p110α: sense 5′-GTCACCCAAGAAGCAGAAAG-3′ and anti-sense 5′-TCTGCTTGTCGTTGTTTGG-3′ (400 bp: size of amplified fragment); mouse p110β: sense 5′-CAGATGTTATGGAAGCAAG-3′and anti-sense 5′-ATACTCCACTCTCCCACTG-3′ (600 bp: size of amplified fragment); mouse GAPDH (glyceraldehyde-3-phosphate dehydrogenase): sense 5′-GCCCATCACCATCTTCCAG-3′ and anti-sense 5′-ACGCCACAGCTTTCCAGAG-3′ (376 bp: size of amplified fragment). Total cellular RNA was isolated using TRIzol® (Invitrogen) according to the manufacturer's protocol. The concentration of total RNA was determined using spectrophotometric analysis in conjunction with the IBM program (Beckman Coulter), and 2 μg of total RNA was used as template for cDNA first-strand synthesis performed using a first-strand cDNA synthesis kit (Invitrogen) according to manufacturer's instructions. cDNA synthesized from reverse transcription was used as the DNA template for PCR amplifications using Taq polymerase and PCR was performed for 35 cycles. The amplified PCR products were resolved using a 1.5% (w/v) agarose gel containing 1 μg/ml of ethidium bromide, and the DNA bands were visualized by UV illumination. Stored images of the gels were analysed by densitometry using Gel Base/Gel Blot software (Ultraviolet Products, Upland, CA, U.S.A.). GAPDH levels were measured as an internal control.
Recombinant protein expression
GST-tagged β-arrestins, PAK (p21-activated kinase) 3 and Rhotekin were expressed and purified from Escherichia coli cells using glutathione–Sepharose (Pharmacia). Briefly, E. coli BL21DE3 cells were transformed with GST-tagged fusion proteins, grown to exponential phase [where D600 (attenuance) is 0.8 for Rhotekin and PAK3, and D600 is 0.6 for β-arrestin-1 and -2] and induced with IPTG (isopropyl β-D-thiogalactoside). Rhotekin and PAK3 were induced with 0.5 mM IPTG for 2h, and β-arrestin-1 and -2 were induced for 8 h with 1 mM IPTG. Bacterial cells were subsequently lysed by sonication (6 bursts of 10 s at 30% power) in PBS supplemented with 10 μg/ml lysozyme, 10 μg/ml DNase 1 and 1% (v/v) Triton X-100. Cleared lysates were incubated with 300 μl of glutathione–Sepharose for 1 h, washed with 20 volumes of binding buffer supplemented with 1 mM ATP, and the bound protein was eluted using increasing concentrations of free reduced glutathione. Elution fractions containing the fusion protein were dialysed overnight against PBS and stored in 10% (v/v) glycerol at −80 °C until needed. The protein concentration of eluates was determined using the Bradford assay, and the relative purity was determined by SDS/PAGE (10% gels), followed by staining with Coomassie Brilliant Blue R250. Some breakdown of both β-arrestin proteins was observed in each preparation, which was quantified by densitometric analysis of the Coomassie-stained gels. The protein concentrations shown in Figure 3 were calculated on the basis of total purified protein containing 60% full-length β-arrestin-1 and 50% full-length β-arrestin-2.
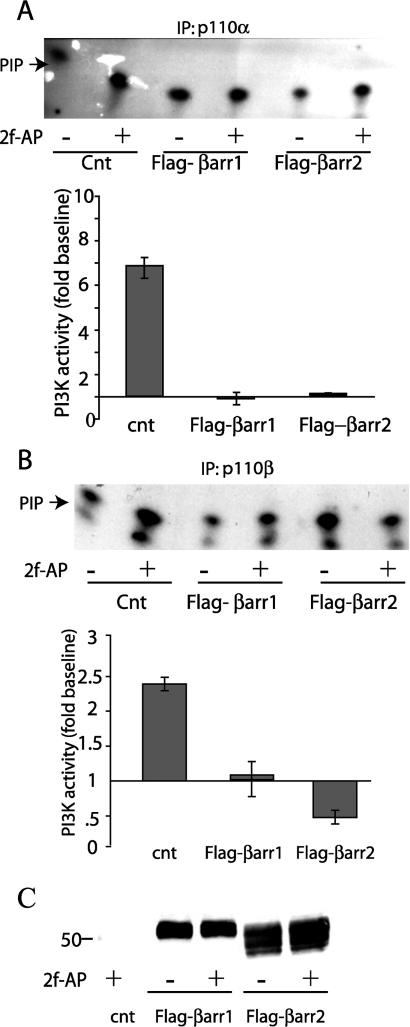
Figure 3. PAR-2 inhibits p110α and p110β activity through both β-arrestin-1 and β-arrestin-2.
NIH 3T3 cells transfected with FLAG-tagged β-arrestin-1 (Flag-βarr1), β-arrestin-2 (Flag-βarr2) or empty vector as a control (Cnt) were treated with 100 nM 2fAP for 0 or 5 min, and PI3K activity was measured from samples immunoprecipitated (IP) using anti-p110α (A) and anti-p110β (B) antibodies as performed in Figure 2. A representative autoradiograph of phospholipids separated by TLC is shown, where PIP is the PtdIns product and a histogram showing PI3K activity in response to 2fAP as fraction baseline. (C) Representative Western blot showing anti-FLAG antibody immunoreactivity in cells transfected with empty vector as a control (cnt) or either FLAG–β-arrestin-1 (Flag-βarr1) or FLAG–β-arrestin-2 (Flag-βarr2).
Immunoblotting
All Western blots were performed by transfer on to PVDF-Fl membranes and blocked in 50 mM Tris/HCl, pH 7.6, 150 mM NaCl and 1% (w/v) fish gelatin. Primary antibodies were added at the following concentrations: anti-(β-arrestin1/2) (A1CT), 1:500; anti-FLAG, 1:1000; anti-RhoA, 1:1000; anti-Rac, 1:250; anti-Cdc42, 1:1000; anti-p110α, 1:1000; anti-p110β, 1:1000; anti-p85, 1:1000, anti-HA-7, 1:750, anti-GFP, 1:1000 and anti-actin, 1:1000. Immunoreactive bands were visualized using the Odyssey infrared imaging system (LI-COR Biosciences) using Alexa Fluor® 680-conjugated and IRDye® 800-conjugated secondary antibodies at a concentration of 1:40000. Integrated band intensities were calculated using the LI-COR Odyssey software.
In vivo and in vitro PI3K assays
NIH 3T3 cells at 80% confluence were transfected with FLAG–β-arrestin-1 or FLAG–β-arrestin-2 and serum-starved for 16 h before stimulation with 100 nM 2fAP or IGF-1 for 0–5 min at 37 °C. The cells were lysed in ice-cold buffer A [20 mM Tris/HCl, pH 7.4, 137 mM NaCl, 1% (v/v) NP40 (Nonidet P40), 1 mM CaCl2, 1 mM MgCl2 and 0.1 mM NaVO4]. Cleared lysates (500 μg of protein) were incubated with 4 μg of either anti-p110α or anti-p110β antibodies at 4 °C for 2 h, followed by incubation with 40 μl of Protein A–agarose for 2 h at 4 °C. The immunoprecipitations were washed using various buffers, with three washes in each of: (i) buffer A, (ii) buffer B (100 mM Tris/HCl, pH 7.4, 5 mM LiCl and 0.1 mM NaVO4) and (iii) TNE buffer (10 mM Tris/HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA and 0.1 mM NaVO4). PI3K reactions were performed in 50 μl of TNE buffer supplemented with 20 μg of PtdIns (approx. 400 μM) or PtdIns(4,5)P2 (approx. 320 μM), 20 mM MgCl2 and 5 μCi of [γ-32P]ATP at 37 °C for 10 min and the reactions were stopped by the addition of 20 μl of 6 M HCl. For in vitro assays, 10 ng (approx. 50 pmol) of recombinant p85α/p110α or p85/p110β were incubated with 20 μg of PtdIns and 5 μCi of [γ-32P]ATP in the presence of 20–40 μl of purified β-arrestin-1 or β-arrestin-2. Purified β-arrestins were prepared from two sources: FLAG–β-arrestins were immunoprecipitated from transfected NIH 3T3 cells and compared with reactions containing anti-FLAG immunoprecipitates from untransfected NIH 3T3 cells as a negative control. Either 20 or 40 μl of anti-FLAG–agarose (containing 25 ng/μl β-arrestin-1 or 2.5 ng/μl β-arrestin-2) was added to the PI3K reactions. The approximate protein concentrations of the purified β-arrestins were determined by SDS/PAGE (10% gels), followed by densitometric analysis of β-arrestin bands compared with serial dilutions of BSA. GST–β-arrestins expressed in E. coli were compared with reactions containing GST alone. Lipids were extracted from the organic layer which was formed after the addition of 160 μl of chloroform/methanol (1:1 ratio), and 32P-labelled phospholipids were resolved by TLC on potassium oxalate-treated silica plates, in chloroform/methanol/water/ammonium hydroxide (60:47:11.3:2 ratio). Migration rates of phospholipid products were compared with those of Molybdenum Blue-stained standards of PtdIns, PtdIns(4)P, PtdIns(4,5)P2 and PtdIns(3,4,5)P3. The density of the radioactive lipids was determined by phosphoimage analysis using a Storm PhosphorImager (Bio-Rad).
Rho GTPase assays
Activation of RhoA GTPases was measured using a CRIB (Cdc42/Rac-interacting binding) domain pull-down assay as described previously [9]. Briefly, GST fusion proteins containing the CRIB domain from PAK3 or Rhotekin were bound to glutathione–Sepharose and incubated for 30 min at 4 °C with lysates from NIH 3T3 cells treated for 5 min with either 100 μM PAR-2-activating peptide or 10% (v/v) serum as a positive control. The beads were washed three times and analysed by SDS/PAGE (12.5% gels), along with total lysates, then transferred on to PVDF membranes and blotted with rabbit anti-CDC42 and mouse anti-Rac-1 antibodies, or with rabbit anti-RhoA antibody.
Data analysis
Percentage changes in PI3K activity were calculated from the phosphoimage band density in PAR-2-treated compared with untreated cells for each experimental group. Band densities were normalized to the total p85 immunoprecipitated as determined by Western blotting. The baseline PI3K levels were those present in the absence of PAR-2 activation and were treated as 100%. Therefore changes to the baseline levels of PI3K activity were not apparent in the results. All experiments were repeated a minimum of three times and results are means±S.E.M. Differences between multiple groups were examined by two-way ANOVA and Tukey t-tests, with P<0.05 considered significant. In this way, both the statistical significance of PAR-2 activation on PI3K activity at each time point, and the difference between paired groups (i.e. PAR-2-activated compared with untreated; PAR-2-activated in the presence of inhibitor compared with the absence of inhibitor) was determined. Apparent IC50 values for in vitro PI3K assays were calculated using Kaleidagraph 4.0 (Synergy Software, Reading, PA, U.S.A.). Data were fitted using a sigmoidal dose–response model with a 95% confidence interval.
RESULTS
PAR-2 can activate both p110α and p110β
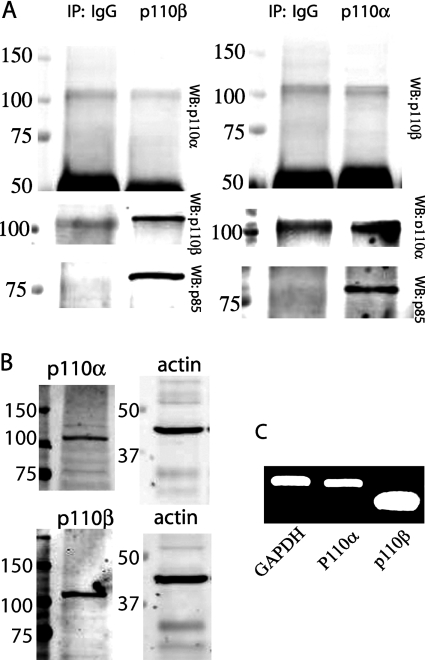
We have shown that PAR-2 promotes β-arrestin-dependent inhibition and Gαq/Ca2+/SFK-dependent stimulation of p85-associated PI3K activity. This was in contrast to previous studies on the IGF-1R (IGF-1 receptor), which suggested that β-arrestin-1 facilitated PI3K activity. Since p85 can associate with both p110α and p110β [8a], we examined the possibility that PAR-2 and IGF-1R can differentially modulate the activity of these two subunits. We first established that both subunits were expressed in NIH 3T3 cells, and that the antibodies used were specific for each catalytic subunit. The specificity of each p110 antibody was confirmed by immunoprecipitation with either anti-p110α or anti-p110β antibodies, followed by Western blotting with antibodies against p110α, p110β and the p85 regulatory subunit (which binds both p110 subunits). There is no visible p110β immunoreactivity in p110α immunoprecipitates and vice versa, but both can co-immunoprecipitate p85 (Figure 1A), confirming their specificity and the existence of separate p85/110α and p85/p110β complexes. To compare the expression of both the catalytic subunits in NIH 3T3 cells, we examined protein levels from whole-cell lysates by Western blotting and mRNA levels by RT-PCR from total cellular RNA (Figures 1B and 1C). In NIH 3T3 cells, the expression of mRNA and protein for both p110α and p110β catalytic subunits was detected.
Figure 1. Expression of p110α and p110β subunits in NIH 3T3 cells.
(A) Specificity of antibodies in NIH 3T3 cell lysates. Lysates were immunoprecipitated (IP) with anti-IgG and anti-p110β antibodies (left-hand side panel) or anti-IgG and anti-p110α antibodies (right-hand side panel), followed by Western blotting with antibodies against p110α, p110β and p85. No p110α was co-immunoprecipitated with anti-p110β antibody and vice versa. (B) Protein expression of p110α and p110β in NIH 3T3 whole-cell lysates. Representative Western blots probed with anti-p110α and anti-actin antibodies (top panel) or p110β and actin (bottom panel). (C) mRNA expression of p110α and p110β in NIH 3T3 cells. cDNA was synthesized from total RNA isolated from NIH 3T3 cells, and used as a template for PCR. A representative agarose gel of PCR products using specific primers directed against p110α, p110β and GAPDH (internal control) is shown.
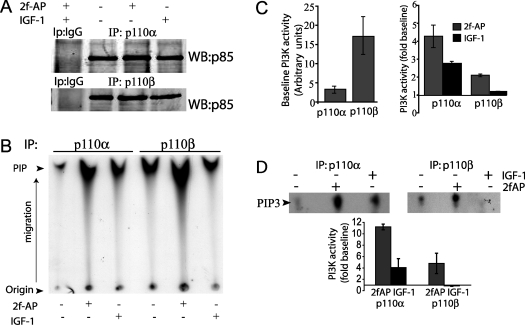
We then compared the activation of either p110α or p110β by PAR-2 and IGF-1 using a standard PI3K assay [10]. NIH 3T3 cells were treated with IGF-1 or 2fAP to activate PAR-2, then the cells were lysed and immunoprecipitated with antibodies against either p110α or p110β. PI3K activity was determined by phosphorylation of purified PtdIns. PAR-2 significantly increased p110α activity 4.2-fold and increased p110β activity 2-fold (Figures 2A–2C). The baseline activity of p110β appeared to be higher than that for p110α (Figure 2C, left-hand side panel), indicating that the induction in response to PAR-2 was lower. In contrast, IGF-1 treatment increased the activity of p110α only 2-fold and had no measurable effect on p110β (Figures 2A–2C). Similar results were observed when the assays were repeated using PtdIns(4,5)P2 as a substrate, confirming that the observed activity was not a result of co-immunoprecipitation of phosphoinositide 4-kinases with any of the PI3K subunits (Figure 2D). These data demonstrate that PAR-2 can activate both of the class IA p110s; thus the p85-associated PI3K activity reported previously [7] is most likely to represent a combination of p110α and p110β catalytic activity.
Figure 2. PAR-2 activates both p110α and p110β subunits of the class IA PI3K kinase.
NIH 3T3 cells were treated with or without 100 nM 2fAP (PAR-2 activating peptide) or 100 nM IGF-1 for 5 min, lysed and immunoprecipitated (IP) with antibodies against p110α or p110β. Immune complexes were incubated with PtdIns in the presence of [γ-32P]ATP and phospholipids were separated by TLC to determine PI3K activity. (A) Western blot with anti-IgG and anti-p85 antibodies to demonstrate equal immunoprecipitation levels. (B) Representative autoradiograph of phospholipids separated by TLC. Migration rates of PtdIns product (PIP) were compared with rates of stained standards, and the migration position is indicated by an arrow. A full TLC plate with both origin and phospholipids is shown. (C) Histogram depicting baseline PI3K activity in p110α and p110β immunoprecipitates as normalized phosphate incorporation into PtdIns (left-hand side panel), and fold increase in PI3K activity after treatment with 2fAP or IGF-1 as a fraction baseline (right-hand side panel). (D) PI3K assay was performed as described above using PtdIns(4,5)P2 as a substrate. Incorporation of phosphate in all PI3K assays was determined by phosphoimage analysis. Fold change was determined by the ratio of band density in treated samples compared with untreated samples. Arbitrary units refer to raw band density, as determined by phosphoimage analysis. All densities were normalized to levels of p85 in p110α or p110β immunoprecipitates.
β-Arrestins inhibit PAR-2-induced p110α and p110β activation
In previous studies, over-expression of β-arrestin-1 inhibited PAR-2-induced PI3K activity associated with p85. However, the ability of β-arrestin-2 to inhibit PI3K was not examined. To determine whether the two β-arrestins differ in their ability to inhibit PAR-2-induced activation of either p110α or p110β, the assay described above was repeated in the presence and absence of over-expressed FLAG–β-arrestin-1 and FLAG–β-arrestin-2. Similar to what was reported previously for p85 immunoprecipitates [7], over-expression of either β-arrestin-1 or β-arrestin-2 eliminated 2fAP-induced p110α and p110β activity (Figure 3). β-Arrestin-2 was a more potent inhibitor of p110β: when β-arrestin-2 was over-expressed, PAR-2 inhibited p110β activity by 50% (Figure 3B), comparable with what was observed previously in MDA MB-468 cells, which express high levels of both β-arrestins [7]. Together with the previous results comparing PI3K signalling in NIH 3T3 and MDA MB-468 cells, these data demonstrate that both β-arrestin-1 and β-arrestin-2 inhibit PAR-2-induced PI3K activity
Inhibition of p110α and p110β catalytic activity by both β-arrestin-1 and β-arrestin-2
To determine whether the β-arrestin-mediated inhibition of PI3K was direct, we investigated the ability of recombinant GST-tagged β-arrestin-1 and β-arrestin-2 to inhibit the activity of recombinant p85/p110α or p85/p110β complexes in vitro (Figures 4A–4D). Expanding on our previous findings that β-arrestin-1 directly binds to and inhibits p85/p110α complexes, we incubated purified GST-tagged β-arrestin-1, GST-tagged β-arrestin-2 and GST alone with either recombinant p85/p110α or p85/p110β complexes and assayed the phosphorylation of PtdIns in vitro (Figures 4A and 4B). β-Arrestin-1 and β-arrestin-2 were equally potent inhibitors of p110α activity, as the apparent IC50 was 8.6 nM for β-arrestin-1 and 6.2 nM for β-arrestin-2, with the Pearson correlation coefficient for sigmodial dose curve fit (R)>0.99, but β-arrestin-2 was a more efficacious inhibitor, providing a maximum inhibition of 94±7% compared with 64±8% for β-arrestin-1. Surprisingly, whereas β-arrestin-2 strongly inhibited p110β activity with an apparent IC50 of 10.6 nM (R>0.99) and maximum inhibition of 97±2%, β-arrestin-1 stimulated p110β activity 2.4±0.2-fold. In contrast, when β-arrestin-1 or β-arrestin-2 was purified from NIH 3T3 cells and added to cell-free kinase reactions, they each inhibited the catalytic activity of both p110α and p110β (Figures 4C and 4D). SDS/PAGE analysis of GST-tagged and FLAG-tagged proteins is displayed in Figures 4(E) and 4(F). These data suggest that β-arrestin-2 may be a more efficacious inhibitor of PI3K activity than β-arrestin-1. Furthermore, p110β may be differentially affected by β-arrestin-1 depending on the presence of post-translational modifications or accessory proteins, and these factors in turn may vary between cell types and the activation state of the cell.
Figure 4. Effect of β-arrestins on the catalytic activity of p110α and p110β in vitro.
(A,B) Increasing concentrations (nM) of GST–β-arrestin-1 (β1GST), GST–β-arrestin-2 (β2GST) or GST alone (negative control) were added to reaction mixtures containing [γ-32P]ATP, purified PtdIns and either recombinant p85/p110α (A) or recombinant p85/p110β (B). Upper panels show representative autoradiographs of phospholipids separated by TLC. Lower panels show semi-log plots of PI3K activity (defined as the fraction of control) as a function of the GST fusion protein concentration (in M). The control activity is that observed in the absence of added GST fusion protein. (C,D) Increasing amounts of anti-FLAG antibody immunoprecipitates from NIH 3T3 cells transfected with FLAG–β-arrestin-1 (β1-Flag), FLAG–β-arrestin-2 (β2-Flag) or empty vector as a control (CT) were added to in vitro recombinant p85/p110α (C) and recombinant p85/p110β (D) and kinase activity was measured as described above. The amount of anti-FLAG–agarose added (20 and 40 μl) corresponded to approx. 500 and 1000 ng of β-arrestin-1 and approx. 50 and 300 ng of β-arrestin-2. Upper panels show representative autoradiographs of phospholipids separated by TLC. Lower panels show plots of PI3K activity as a function of β-arrestin added (in ng). PI3K activity is defined as the fraction of control (activity observed in the presence of an equal volume of anti-FLAG immunoprecipitates from untransfected cells). (E) Representative Coomassie-stained gels of increasing amounts of β-arrestin-2–GST (β-arr-2GST) and GST (left-hand side panel) and β-arrestin-1–GST (β1GST) (right-hand side panel). (F) Representative Coomassie-stained gel of increasing amounts of FLAG–β-arrestin-1 (β1-Flag) and FLAG–β-arrestin-2 (β2-flag).
Mechanism of PAR-2-stimulated PI3K activity
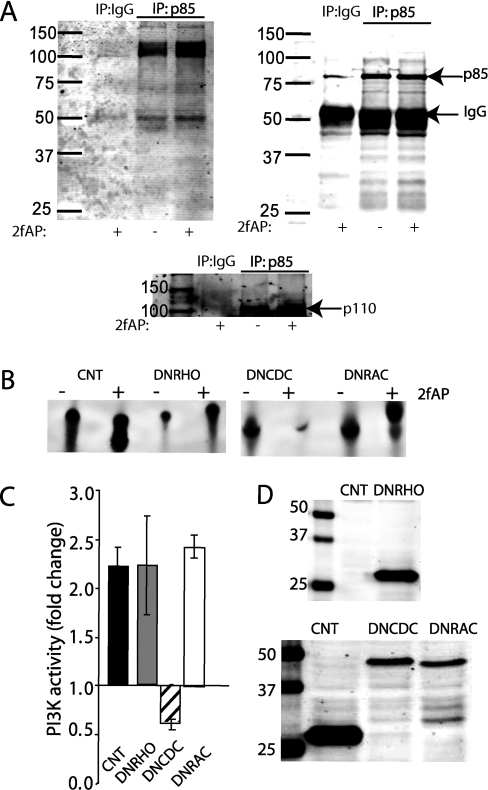
Previous studies have demonstrated that PAR-2 promotes Ca2+-dependent activation of PYK2 and Src, and association of PYK2 with p85. Furthermore, inhibition of SFKs, Ca2+ chelation or Gαq knock-down blocked PAR-2-induced PI3K activity [7,11]. As multiple signalling pathways can converge on PI3K in order to increase its activity, the stimulatory effect of PAR-2 may reflect a contribution from multiple signalling arms. The most common means of PI3K activation by receptor tyrosine kinases such as IGF-1R involves the phosphorylation of tyrosine residues in the SH2 domain of p85 and its association with other phospho-tyrosine proteins. In order to determine whether PAR-2 induced the tyrosine phosphorylation of p85, NIH 3T3 cells were treated with or without 2fAP for 5 min (when maximal PI3K activity is observed), and lysates were immunoprecipitated with anti-p85 antibody, followed by Western blot analysis with anti-phospho-tyrosine antibody (Figure 5A). No significant phosphorylation of p85 was observed. However, two tyrosine phosphorylated bands (at approx. 55 kDa and approx. 100–120 kDa) were associated with p85 after PAR-2 stimulation, consistent with what has been reported for Gαq-coupled chemokine receptors. The 100–120 kDa phospho-tyrosine band is consistent with the migration of p110 (Figure 5A, bottom panel), but could also be consistent with the size of PYK2, which has been demonstrated previously to associate with p85 on PAR-2 activation [7].
Figure 5. PAR-2-stimulated PI3K activity requires Cdc42 but not p85 tyrosine phosphorylation.
(A) NIH 3T3 cells were treated with or without 100 nM 2fAP for 5 min and immunoprecipitated (IP) with anti-IgG or anti-p85 antibodies, followed by Western blotting with anti-phospho-tyrosine (upper left-hand side panel). Blots were then reprobed with anti-p85 antibody (upper right-hand side panel), then stripped and the region corresponding to 100–200 kDa was reprobed with anti-p110 antibody, which recognizes both p110α and p110β (lower panel). (B–D) NIH 3T3 cells were transfected with EGFP (enhanced GFP) N1 control vector (CNT), DNRHO, DNCDC or DNRAC, treated with or without 2fAP, and PI3K activity in anti-p85 antibody immunoprecipitates was determined as described above. (B) Representative autoradiograph of phospholipids separated by TLC. (C) Histogram depicting PAR-2-induced PI3K activity (as fraction of baseline) under each transfected condition. (D) Representative Western blot of GTPase over-expression (upper panel: anti-GFP antibody; lower panel: anti-HA-7 antibody).
RhoA GTPases have also been identified as upstream regulators, as well as downstream effectors, of PI3K [12–14]. To investigate whether RhoA GTPases were involved in PAR-2-stimulated PI3K activation, p85-associated PI3K activity was assayed after treatment of NIH 3T3 cells transfected with dominant negative mutants of RhoA (DNRHO), Cdc42 (DNCDC) and Rac-1 (DNRAC) with 2fAP. Expression of DNCDC, but not DNRHO or DNRAC, abolished PAR-2-stimulated PI3K activation (Figure 5B). p85 was used in these studies, as it co-immunoprecipitates both p110α and p110β. Although p85 has a GTPase-binding domain, we could not detect association of Cdc42 with p85 by co-immunoprecipitation (results not shown). Expression of the dominant-negative GTPases was assessed by Western blotting, and this is shown in Figure 5(D). PAR-2-induced regulation of RhoA, Rac-1 and Cdc42 was confirmed using a GST–CRIB-domain pull-down assay (Rhotekin CRIB domain for RhoA and PAK3 CRIB domain for Cdc42 and Rac-1) [9]. Treatment of NIH 3T3 cells with 2fAP increased the binding of Rac-1 and Cdc42 to PAK3–GST, but did not increase RhoA binding to Rhotekin–GST (Figures 6A and 6B), suggesting that PAR-2 activates both Rac-1 and Cdc42.
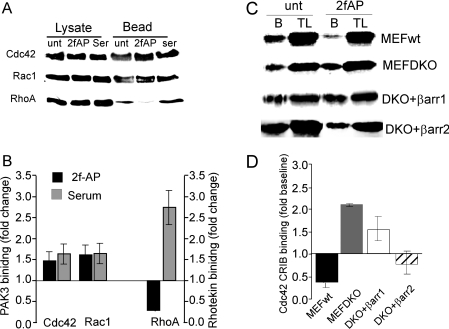
Figure 6. β-arrestin-1 and β-arrestin-2 inhibit PAR-2-stimulated Cdc42 activity.
(A–B) NIH 3T3 cells were treated with or without 2fAP or serum (ser) as a positive control, and lysates were incubated with either PAK3 CRIB–GST or Rhotekin–GST. Total lysates and bound proteins were analysed by SDS/PAGE followed by Western blotting with anti-Cdc42, anti-Rac-1 or anti-RhoA antibodies. (A) Representative Western blots, where the left-hand side lanes contain total lysates and right-hand side lanes contain bound proteins. (B) Histogram depicting PAR-2 and serum-induced changes in CRIB domain binding (as a fraction of baseline). (C–D) MEFwt, MEF β-arrestin-1, β-arrestin-2-DKO, MEF β-arrestin-1, β-arrestin-2-DKO with β-arrestin-1 (DKO+βarr1) and MEF β-arrestin-1, β-arrestin-2-DKO with β-arrestin-2 (DKO+βarr2) cells were treated with 100 nM 2fAP for 0 or 5 min and activation of Cdc42 was determined by association with GST–PAK3 CRIB domain. (C) Representative Western blot of bound proteins (B) and total lysates (TL) blotted with anti-Cdc42 antibody. (D) Histogram depicting PAR-2-stimulated changes in Cdc42/CRIB domain binding (as a fraction of baseline) in each cell line. Unt, untreated.
To examine the role of β-arrestins in this pathway, we compared Cdc42 activation in mouse embryonic fibroblast (MEF) cell lines from wild-type mice (MEFwt), β-arrestin-1/2−/− mice (MEFβarr DKO) or MEFβarr DKO cells transfected with either β-arrestin-1 (DKO+βarr1) or β-arrestin-2 alone (DKO+βarr2). In MEFwt, PAR-2 promoted a decrease in Cdc42 activity, whereas in the absence of both β-arrestins, PAR-2-promoted a 2-fold increase in Cdc42 activation, similar to that observed in NIH 3T3 cells. Transfection of either β-arrestin-1 or β-arrestin-2 inhibited Cdc42 activation (Figures 6C and 6D). These data suggests that the mechanism by which PAR-2 activates PI3K involves input from multiple upstream pathways and differs from that used by the classical receptor-tyrosine kinases.
DISCUSSION
These results are the first to demonstrate the regulation of the individual catalytic subunits of class IA PI3K by PAR-2 and β-arrestins. We have shown that PAR-2 can activate both p110α and p110β in NIH 3T3 cells, and that β-arrestin-1 and β-arrestin-2 can differentially regulate the activity of each subunit.
A model for PAR-2 regulation of the PI3K pathway
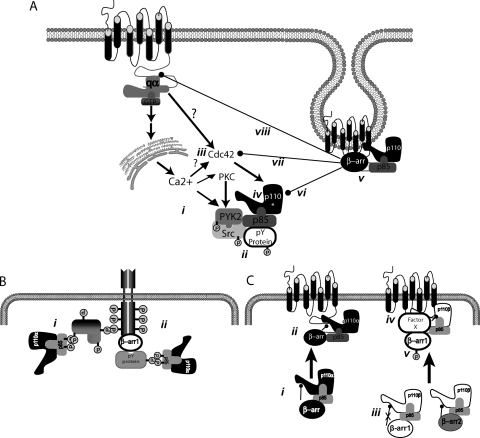
Previous research has demonstrated that PAR-2 sends opposing signals to PI3K by affecting both Gαq/Ca2+-dependent activation and β-arrestin-1-dependent inhibition. PAR-2-dependent PI3K activity was inhibited by broad spectrum SFK and PKC inhibitors, and involved the association of p85 with PYK2 [7]. PAR-2 has also been shown to activate both PYK2 and Src, via a Ca2+-dependent mechanism, and activation of both was enhanced by the inhibition of β-arrestins [1,11]. Together with the findings described here, these data point to a model where PAR-2 regulates PI3K through multiple G-protein-dependent pathways (Figure 7A). We propose that PAR-2-induced PI3K activity involves Ca2+/PKC-dependent activation of tyrosine kinases (PYK2 and Src), incorporation of PI3K into a complex containing PYK2 and possibly other tyrosine phosphorylated proteins, and activation of Cdc42. The identity of the approx. 110–120 kDa phospho-protein that was observed to associate with p85 on PAR-2 activation has not been confirmed. The size is consistent with p110, but other candidates include PYK2 [7] or ACK1, a tyrosine kinase whose activity is increased by association with Cdc42 and clathrin, and decreased by β-arrestin-1 [15]. The ability of β-arrestins to inhibit PAR-2-induced PI3K activity (Figure 7B) probably results from a combination of direct inhibition of the p110 catalytic subunits and their more traditional roles as terminators of G-protein signalling. Both β-arrestin-1 and -2 were shown previously to associate with p85 and with PAR-2 on receptor activation [1,6,7,16,17], and both β-arrestins can inhibit PI3K activity at multiple levels through classic uncoupling of Gαq [1,16,17], through inhibition of Cdc42, and through direct inhibition of the catalytic subunits. Thus there are multiple mechanisms by which PAR-2 can both promote and inhibit PI3K activity. Whether the overall result of PAR-2 activation is stimulation or inhibition of PI3K activity in a given cell depends on the combined effects of each signalling arm, which in turn is partially dependent on the level of β-arrestin expression [7]. β-Arrestins can be viewed as molecular switches, capable of turning off one signal and turning on another.
Figure 7. Model of PI3K regulation by PAR-2.
(A) Gαq compared with β-arrestin-dependent pathways. On activation, PAR-2 promotes Gαq coupling and subsequent mobilization of intracellular Ca2+ and activation of PKC (i). Ca2+ and PKC can promote activation of PYK2 and Src [1,11], association of PYK2 with p85 [7] and possibly phosphorylation of p110 (shown in Figure 5) (ii). PAR-2 also promotes activation of Cdc42 by an unknown mechanism (shown in Figures 5 and 6), perhaps through Gαq/Ca2+-dependent mechanisms or through coupling to a different G-protein (iii). Activation of Cdc42 and Src leads to activation of p110α and p110β (p110*) (iv). β-Arrestin signalling through PAR-2 opposes the Gαq pathway through multiple mechanisms. First, PAR-2 promotes recruitment of both β-arrestins and p85/p110 to the receptor [1,7,17] (v), which can lead to inhibition of p110 catalytic activity (vi). β-Arrestins also inhibit PAR-2-stimulated Cdc42 activation (shown in Figure 6) (vii) and promote receptor uncoupling from Gαq [17] (viii), both of which would inhibit PAR-2-induced PI3K activity. (B) Model of IGF-1-stimulated PI3K activity (for comparison with PAR-2). IGF-1 has been shown to promote tyrosine phosphorylation of p85 SH2 domains, and its recruitment to phospho-tyrosines on IRS-1 or the IRS-1 receptor itself [18,19] (i). β-Arrestins were shown to facilitate IRS-1-induced PI3K activity, possibly through its ability to bind both the IGF-1R and SH3 domain-containing proteins [8,24] (ii). This may indirectly recruit the p85/p110 complex to the receptor. (C) PAR-2 can promote β-arrestin-dependent inhibition of both p110 subunits. Both β-arrestin-1 and β-arrestin-2 can directly inhibit p110α in vitro (i), but can only do so in vivo on PAR-2 activation (ii). Only β-arrestin-2 can directly inhibit p110β in vitro (iii), however, on activation of PAR-2 in vivo β-arrestin-1 becomes inhibitory. This may reflect an association with a third factor (“factor X”) in cells (iv) or post-translational modification of the receptor, e.g. phosphorylation (v).
The β-arrestin paradox: a facilitator or inhibitor of PI3K activity?
In contrast with the findings described in this present paper, previous reports on IGF-1R indicated that β-arrestin-1 facilitated PI3K activity in HEK-293 cells (human embryonic kidney cells) [8]. There are several possibilities which may account for this difference. First, the mechanism of PI3K activation differs between the two receptors. IGF-1 is predicted to utilize two pathways, one involving tyrosine phosphorylation of IRS and p85 and the docking of p85/p110 on to IRS-1 or IGF-1R [18–20], and the other pathway involving β-arrestin-dependent PI3K activation [8] (Figure 7B). Whereas PAR-2-induced PI3K activity is dependent on SFKs and Cdc42, both proteins lie downstream of PI3K in IGF-1 signalling [7,8,21]. Second, no direct interaction between β-arrestin-1 and PI3K has been demonstrated downstream of IGF-1R, whereas PAR-2 promotes association of both β-arrestin-1 and β-arrestin-2 with the PI3K complex [7]. Other work has suggested that β-arrestins can bind IGF-1R and other phospho-tyrosine proteins implicated in PI3K activity, such as p62yes and Src [22–25]. Thus the facilitative effects of β-arrestin-1 on IGF-induced PI3K activity may reflect positive effects on an upstream PI3K regulator. In previous studies, both β-arrestin-1 and β-arrestin-2 appeared to exist in a macromolecular complex with p85/p110 and PAR-2; thus incorporation of β-arrestin-1 into distinct signalling complexes may be able to switch it from an activator to an inhibitor of PI3K [7]. One possibility that should be examined in the future is whether PAR-2 activation can inhibit IGF-1-induced PI3K activity through β-arrestins. Finally, the studies shown here suggest that the two receptors activate different catalytic subunits. Although activation of either IGF-1R or PAR-2 increased p110α activity, only PAR-2 also promoted p110β activity. These data are consistent with other reports in cultured cells and whole animals. For example, insulin receptor and IGF-1R preferentially activate p110α in adipocytes, myotubes and several cancer cell lines [26–28]. Furthermore, transgenic mice expressing kinase-inactive p110α had severe defects in insulin and IGF-1 signalling, whereas specific inhibition of p110β had little effect on IRS-1-associated PI3K activity [29]. We demonstrate that both β-arrestins are capable of inhibiting PI3K activity. In particular, PAR-2-stimulated p110β activity was highly sensitive to inhibition by β-arrestin-2. Thus the difference in β-arrestin requirements in total IGF-1R- and PAR-2-induced PI3K activity may partially reflect the added contribution of β-arrestin-2-dependent inhibition of p110β, induced by PAR-2. This contribution may differ between cell types depending on the relative expression of each subunit.
Differential regulation of p110α and p110β by β-arrestins
We have demonstrated that β-arrestins can directly inhibit the catalytic subunits of PI3K, although the mechanism remains undetermined. Although both β-arrestins inhibit both p110 subunits in vitro, in the absence of PAR-2 activation, they do not inhibit PI3K activity in intact cells, even when constitutively associated with p85 [7]. This suggests that although direct association with PI3K is a key factor in the ability of β-arrestins to inhibit its activity, additional factors contribute to this inhibitory activity. Furthermore, the two β-arrestins are not interchangeable in their regulation of the two catalytic PI3K subunits. β-Arrestin-2 directly inhibits both p110α and p110β, and does so with a higher efficacy, whereas β-arrestin-1 directly inhibits only p110α. The fact that β-arrestin-1 only inhibited p110β when purified from mammalian cells suggests that it may either associate with other proteins in intact cells or undergo a post-translational modification that switches it from a facilitator to an inhibitor of the p110β subunit. One such post-translational modification might be phosphorylation, as β-arrestin-1 is reported to be phosphorylated by MAPK (mitogen-activated protein kinase), which in turn affects its ability to associate with β2-adrenergic receptors [30]. The ability of β-arrestin-1 to act as both a facilitator and inhibitor of p110β activity in vitro may partially explain its positive role in the activation of PI3K downstream of some receptors and in some cell types, whereas it is inhibitory downstream of PAR-2 in NIH 3T3 cells. Future studies are required to address the possibility that PAR-2 promotes association of phosphorylated β-arrestin-1 with p85/p110β and to address whether phosphorylation affects the ability of β-arrestin-1 to activate or inhibit p110β.
The ability of β-arrestins to differentially regulate p110α and p110β has implications beyond explaining their divergent roles in the regulation of PI3K downstream of various receptors. Although the two catalytic subunits are sometimes treated as redundant mediators of PI3K activity, numerous reports suggest distinct and, in some cases, opposing roles in signal transduction. Recent studies on EGFR (epidermal growth factor receptor) have suggested that p110α is the primary mediator of PI3K-induced actin assembly and cell migration in a breast cancer cell line, whereas p110β inhibits these same events [27,28]. Furthermore, several studies suggested that insulin/IGF-1-dependent signalling relies more on p110α than on p110β activity [26,29,31] and that p110β is oncogenic, whereas p110α is not [31a]. Another study found that p110α activity was higher than that of p110β in areas of high phospholipid concentration, whereas in areas of low phospholipid concentration p110β had a higher activity, suggesting that the two subunits might exhibit spatially distinct patterns of activation [32]. PAR-2 promotes β-arrestin-dependent cell migration by a process involving spatial regulation of opposing pathways involved in actin assembly [1,3,18]. Thus regulation of specific p110 catalytic subunits by β-arrestins may represent another mechanism for the spatial control of actin assembly during PAR-2-induced cell migration.
Acknowledgments
This work was supported by the NIH (National Institutes of Health)/NIGMS (National Institute of General Medical Sciences) grant R01GM066151 to K. A. D. We thank Ms Youly Ly and Mr Jonathan Doose for technical assistance, Dr Robert J. Lefkowitz (Duke University Medical Center) for MEFs, β-arrestin constructs and antibodies against β-arrestins; Dr Kerri Mowen (The Scripps Research Institute) for GST–β-arrestin fusion proteins and Dr Martin Schwartz (University of Virginia) for DNCDC, DNRAC, DNRHO, PAK3–GST and Rhotekin–GST.
References
- 1.DeFea K. A., Zalevsky J., Thoma M. S., Dery O., Mullins R. D., Bunnett N. W. β-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoudilova M., Kumar P., Ge L., Wang P., Bokoch G. M., DeFea K. A. β-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J. Biol. Chem. 2007;282:20634–20646. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]
- 3.Ge L., Ly Y., Hollenberg M., DeFea K. A β-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J. Biol. Chem. 2003;278:34418–34426. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- 4.Ge L., Shenoy S. K., Lefkowitz R. J., DeFea K. A. Constitutive protease-activated-receptor-2 mediated migration of MDA MB-231 breast cancer cells requires both β-arrestin-1 and 2. J. Biol. Chem. 2004;279:55419–55424. doi: 10.1074/jbc.M410312200. [DOI] [PubMed] [Google Scholar]
- 5.Jacob C., Yang P. C., Darmoul D., Amadesi S., Saito T., Cottrell G. S., Coelho A. M., Singh P., Grady E. F., Perdue M., Bunnett N. W. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and β-arrestins. J. Biol. Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 6.Stalheim L., Ding Y., Gullapalli A., Paing M. M., Wolfe B. L., Morris D. R., Trejo J. Multiple independent functions of arrestins in the regulation of protease-activated receptor-2 signaling and trafficking. Mol. Pharmacol. 2005;67:78–87. doi: 10.1124/mol.104.006072. [DOI] [PubMed] [Google Scholar]
- 7.Wang P., DeFea K. Protease-activated-receptor-2 simultaneously directs β-arrestin-dependent inhibition and Gaq-dependent activation of PI3K. Biochemistry. 2006;45:9374–9385. doi: 10.1021/bi0602617. [DOI] [PubMed] [Google Scholar]
- 8.Povsic T. J., Kohout T. A., Lefkowitz R. J. β-Arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J. Biol. Chem. 2003;278:51334–51339. doi: 10.1074/jbc.M309968200. [DOI] [PubMed] [Google Scholar]
- 8a.Fruman D. A., Meyers R. E., Cantley L. C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 9.Ren X. D., Schwartz M. A. Determination of GTP loading on Rho. Methods Enzymol. 2000;325:264–272. doi: 10.1016/s0076-6879(00)25448-7. [DOI] [PubMed] [Google Scholar]
- 10.Brachmann S. M., Ueki K., Engelman J. A., Kahn R. C., Cantley L. C. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol. Cell. Biol. 2005;25:1596–1607. doi: 10.1128/MCB.25.5.1596-1607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seatter M. J., Drummond R., Kanke T., Macfarlane S. R., Hollenberg M. D., Plevin R. The role of the C-terminal tail in protease-activated receptor-2-mediated Ca2+ signalling, proline-rich tyrosine kinase-2 activation, and mitogen-activated protein kinase activity. Cell. Signalling. 2004;16:21–29. doi: 10.1016/s0898-6568(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 12.Bokoch G. M., Vlahos C. J., Wang Y., Knaus U. G., Traynor-Kaplan A. E. Rac GTPase interacts specifically with phosphatidylinositol 3-kinase. Biochem. J. 1996;315:775–779. doi: 10.1042/bj3150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genot E. M., Arrieumerlou C., Ku G., Burgering B. M. T., Weiss A., Kramer I. M. The t-cell receptor regulates Akt (protein kinase B) via a pathway involving Rac1 and phosphatidylinositide 3-kinase. Mol. Cell. Biol. 2000;20:5469–5478. doi: 10.1128/mcb.20.15.5469-5478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y., Bagrodia S., Cerione R. A. Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J. Biol. Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]
- 15.Teo M., Tan L., Lim L., Manser E. The tyrosine kinase ACK1 associates with clathrin-coated vesicles through a binding motif shared by arrestin and other adaptors. J. Biol. Chem. 2001;276:18392–18398. doi: 10.1074/jbc.M008795200. [DOI] [PubMed] [Google Scholar]
- 16.Dery O., Thoma M. S., Wong H., Grady E. F., Bunnett N. W. Trafficking of proteinase-activated receptor-2 and β-arrestin-1 tagged with green fluorescent protein. β-Arrestin-dependent endocytosis of a proteinase receptor. J. Biol. Chem. 1999;274:18524–18535. doi: 10.1074/jbc.274.26.18524. [DOI] [PubMed] [Google Scholar]
- 17.Kumar P., Lau C. S., Mathur M., Wang P., DeFea K. A. Differential effects of β-arrestins on the internalization, desensitization and ERK1/2 activation downstream of protease activated receptor-2. Am. J. Physiol. Cell Physiol. 2007;293:C346–C357. doi: 10.1152/ajpcell.00010.2007. [DOI] [PubMed] [Google Scholar]
- 18.Hadari Y. R., Tzahar E., Nadiv O., Rothenberg P., Roberts C. T., Jr, LeRoith D., Yarden Y., Zick Y. Insulin and insulinomimetic agents induce activation of phosphatidylinositol 3′-kinase upon its association with pp185 (IRS-1) in intact rat livers. J. Biol. Chem. 1992;267:17483–17486. [PubMed] [Google Scholar]
- 19.Lavan B. E., Kuhne M. R., Garner C. W., Anderson D., Reedijk M., Pawson T., Lienhard G. E. The association of insulin-elicited phosphotyrosine proteins with src homology 2 domains. J. Biol. Chem. 1992;267:11631–11636. [PubMed] [Google Scholar]
- 20.Yamamoto K., Altschuler D., Wood E., Horlick K., Jacobs S., Lapetina E. G. Association of phosphorylated insulin-like growth factor-I receptor with the SH2 domains of phosphatidylinositol 3-kinase p85. J. Biol. Chem. 1992;267:11337–11343. [PubMed] [Google Scholar]
- 21.Sosa L., Dupraz S., Laurino L., Bollati F., Bisbal M., Caceres A., Pfenninger K. H., Quiroga S. IGF-1 receptor is essential for the establishment of hippocampal neuronal polarity. Nat. Neurosci. 2006;9:993–995. doi: 10.1038/nn1742. [DOI] [PubMed] [Google Scholar]
- 22.Lin F. T., Daaka Y., Lefkowitz R. J. β-Arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J. Biol. Chem. 1998;273:31640–31643. doi: 10.1074/jbc.273.48.31640. [DOI] [PubMed] [Google Scholar]
- 23.Imamura T., Huang J., Dalle S., Ugi S., Usui I., Luttrell L. M., Miller W. E., Lefkowitz R. J., Olefsky J. M. β-Arrestin-mediated recruitment of the Src family kinase Yes mediates endothelin-1-stimulated glucose transport. J. Biol. Chem. 2001;276:43663–43667. doi: 10.1074/jbc.M105364200. [DOI] [PubMed] [Google Scholar]
- 24.Luttrell L. M., Ferguson S. S., Daaka Y., Miller W. E., Maudsley S., Della Rocca G. J., Lin F., Kawakatsu H., Owada K., et al. β-arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 25.Miller W., Lefkowitz R. J. Expanding roles for b-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr. Opin. Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 26.Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., et al. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill K., Welti S., Yu J., Murray J. T., Yip S. C., Condeelis J. S., Segall J. E., Backer J. M. Specific requirement for the p85-p110α phosphatidylinositol 3-kinase during epidermal growth factor-stimulated actin nucleation in breast cancer cells. J. Biol. Chem. 2000;275:3741–3744. doi: 10.1074/jbc.275.6.3741. [DOI] [PubMed] [Google Scholar]
- 28.Yip S. C., El Sibai M., Hill K. M., Wu H., Fu Z., Condeelis J. S., Backer J. M. Over-expression of the p110β but not p110α isoform of PI 3-kinase inhibits motility in breast cancer cells. Cell Motil. Cytoskeleton. 2004;59:180–188. doi: 10.1002/cm.20032. [DOI] [PubMed] [Google Scholar]
- 29.Foukas L. C., Claret M., Pearce W., Okkenhaug K., Meek S., Peskett E., Sancho S., Smith A. J. H., Withers D. J., Vanhaesebroeck B. Critical role for the p110α phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 30.Lin F. T., Miller W. E., Luttrell L. M., Lefkowitz R. J. Feedback regulation of β-arrestin1 function by extracellular signal-regulated kinases. J. Biol. Chem. 1999;274:15971–15974. doi: 10.1074/jbc.274.23.15971. [DOI] [PubMed] [Google Scholar]
- 31.Hooshmand-Rad R., Hajkova L., Klint P., Karlsson R., Vanhaesebroeck B., Claesson-Welsh L., Heldin C. H. The PI 3-kinase isoforms p110α and p110β have differential roles in PDGF- and insulin-mediated signaling. J. Cell Sci. 2000;113:207–214. doi: 10.1242/jcs.113.2.207. [DOI] [PubMed] [Google Scholar]
- 31a.Kang S., Denley A., Vanhaesebroeck B., Vogt P. K. Oncogenic transformation induced by the p110β, -γ, and -δ isoforms of class I phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1289–1294. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beeton C. A., Chance E. M., Foukas L. C., Shepherd P. R. Comparison of the kinetic properties of the lipid- and protein-kinase activities of the p110α and p110β catalytic subunits of class-Ia phosphoinositide 3-kinases. Biochem. J. 2000;350:353–359. [PMC free article] [PubMed] [Google Scholar]