Abstract
Regulatory elements that lie outside the basal promoter of a gene may be revealed by local changes in chromatin structure and histone modifications. The promoter of the CFTR (cystic fibrosis transmembrane conductance regulator) gene is not responsible for its complex pattern of expression. To identify important regulatory elements for CFTR we have previously mapped DHS (DNase I-hypersensitive sites) across 400 kb spanning the locus. Of particular interest were two DHS that flank the CFTR gene, upstream at −20.9 kb with respect to the translational start site, and downstream at +15.6 kb. In the present study we show that these two DHS possess enhancer-blocking activity and bind proteins that are characteristic of known insulator elements. The DHS core at −20.9 kb binds CTCF (CCCTC-binding factor) both in vitro and in vivo; however, the +15.6 kb core appears to bind other factors. Histone-modification analysis across the CFTR locus highlights structural differences between the −20.9 kb and +15.6 kb DHS, further suggesting that these two insulator elements may operate by distinct mechanisms. We propose that these two DHS mark the boundaries of the CFTR gene functional unit and establish a chromatin domain within which the complex profile of CFTR expression is maintained.
Keywords: CCCTC-binding factor (CTCF), chromatin structure, cystic fibrosis transmembrane conductance regulator (CFTR), DNase I-hypersensitive site, histone modification, insulator
Abbreviations: ASZ1, ankyrin repeat, SAM and basic leucine zipper; ATF1, activating transcription factor 1; CFTR, cystic fibrosis transmembrane conductance regulator; ChIP, chromatin immunoprecipitation; CTCF, CCCTC-binding factor; CTTNBP2, cortactin-binding protein 2; DHS, DNase I- hypersensitive site(s); DMR, differentially methylated region; DTT, dithiothreitol; EMSA, electrophoretic mobility-shift assay; FCS, fetal calf serum; GD, genital duct; GR, glucocorticoid receptor; HNF1, hepatocyte nuclear factor 1; HRE, hormone-response element; IVT, in vitro translated; Oct-1, octamer-binding protein 1; PR, protected region; RXR, retinoid X receptor; SP1, specificity protein 1; TR, thyroid receptor; VDR, vitamin D receptor; YAC, yeast artificial chromosome
INTRODUCTION
Actively transcribed genes are generally associated with increased levels of histone acetylation and are found within open, accessible regions of euchromatin. In contrast, inactive genes demonstrate basal levels of histone acetylation, show increased DNA methylation and are usually located within condensed, inaccessible regions of heterochromatin. This heterogeneous composition enables genes and their cis-acting regulatory sequences to exist within discrete chromosomal domains. The mechanisms that demarcate the transition between active and inactive chromatin states are of interest and may involve insulator elements that maintain distinct expression domains. When positioned between an enhancer and promoter, insulators block their interaction (enhancer-blocking activity). These elements may also protect against positional effects due to the chromosomal environment. The CTCF (CCCTC-binding factor) protein plays a crucial role in maintaining enhancer-blocking activity and CTCF-binding sites have been characterized in many vertebrate insulators [1,2]. However, the precise mechanism by which insulators function remains to be elucidated. A looping mechanism, with CTCF sites being tethered to either the nucleolus [3] or the nuclear matrix [4] has been suggested. Alternatively, CTCF may form part of a protein complex directly involved in epigenetic remodelling and histone modification [5].
The CFTR (cystic fibrosis transmembrane conductance regulator) gene, which when mutated causes cystic fibrosis, is located at chromosome 7q31.2. It is flanked by two genes, ASZ1 (ankyrin repeat, SAM and basic leucine zipper) on the 5′ side and CTTNBP2 (cortactin-binding protein 2) on the 3′ side, which have very different expression profiles. CFTR is expressed primarily in specialized epithelial cells, whereas ASZ1 is transcribed exclusively in the testis and ovary [6], and CTTNBP2 is highly expressed in the brain, kidney and pancreas, with lower levels of expression in other tissues [7]. We have tested the hypothesis that CFTR is flanked by chromatin insulators, to prevent interference between the regulatory elements of these neighbouring loci, and to maintain the independent expression domains.
We previously screened 400 kb encompassing the CFTR locus for DHS (DNase I-hypersensitive sites), which are often associated with regulatory elements. In addition to the DHS that were observed in introns 1, 2, 3, 10, 16, 17a, 18, 20 and 21, which have been characterized further [8–11], a number of DHS were also identified 5′ and 3′ to the CFTR gene [12,13]. Two of these DHS, one located 5′ to CFTR at −20.9 kb with respect to the translational start site [13] and the second 3′ to CFTR at +15.6 kb [12], are candidates for insulator activity. The −20.9 kb DHS was present in all cells analysed, but the +15.6 kb DHS was restricted to epithelial cells, showing a moderate correlation with CFTR expression. Despite the identification of a number of transcription factors binding to the −20.9 kb and +15.6 kb DHS in vitro [12,14,15], the precise role of these regions remains to be elucidated.
In the present study we tested the hypothesis that either, or both, of the −20.9 kb and +15.6 kb DHS may function as insulator elements. We demonstrate, by evaluating histone modifications across the CFTR locus, that these two sites may be functioning by distinct mechanisms. Next, using a well-established assay for insulator activity, we determine that both DHS possess enhancer-blocking activity comparable with that of known insulators. CTCF is shown to bind to the −20.9 kb DHS in vitro [measured by EMSA (electrophoretic mobility-shift assay)] and in vivo [measured by ChIP (chromatin immunoprecipitation)]. CTCF does not bind to the +15.6 kb DHS, although we provide in vitro evidence for the interaction of other factors that may be involved in insulator activity.
EXPERIMENTAL
Cell culture
The K562 erythroleukaemia cell line [16] and the 37566 lymphoblastoid cell lines were cultured in RPMI 1640 medium supplemented with 10% (v/v) FCS (fetal calf serum). The Caco2 [17] and Calu3 [18] cell lines were cultured in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) FCS. Primary human fetal male GD (genital duct) cells [19] were cultured in CMRL1066 medium containing 15% (v/v) FCS, 1 μg/ml hydrocortisone, 0.2 units/ml insulin and 10−10 M cholera toxin. Human fetal pancreas-derived fibroblasts were cultured in CMRL1066 medium containing 10% (v/v) FCS.
Plasmid resources
The plasmids pNI and pNI-FII were a gift from Dr Gary Felsenfeld (Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, U.S.A.) [20]. The pNI plasmid contains a neomycin-resistance cassette (neor) driven by the human γ-globin promoter and stimulated by the mouse HS2 enhancer of the β-globin LCR (locus control region). In the pNI-FII plasmid, a 42 bp region from the chicken β-globin 5′ insulator is located between the HS2 enhancer and γ-globin promoter. This 42 bp fragment corresponds to footprint two of the chicken β-globin 5′ insulator, shown to possess enhancer-blocking activity that is dependent upon CTCF binding.
The plasmids pSG5-VDR (where VDR is vitamin D receptor) and pSG5-RXRα (where RXRα is retinoid X receptor α) were gifts from Dr C. Carlberg (Department of Biochemistry, University of Kuopio, Kuopio, Finland) [21], and the plasmid pCTCF was kindly donated by Dr J. Lee (Department of Molecular Biology, Howard Hughes Medical Institute, Boston, MA, U.S.A.) [22].
Protein resources
Caco2 nuclear extract was prepared using standard methods [23]. IVT (in vitro translated) proteins were created by in vitro transcription and translation of pCTCF, pSG5-RXRα or pSG5-VDR [using the TNT-Kit (Promega) and following the manufacturer's instructions].
ChIP
ChIP was carried out following the Upstate protocol with minor modifications. Briefly, 5×107 cells were cross-linked with either 0.37% formaldehyde for histone-modification ChIP, or 1% formaldehyde for CTCF ChIP, for 10 min in each case. Cross-linking was stopped by the addition of glycine to 0.125 M. Cells were washed in ice-cold PBS containing complete protease inhibitors (Roche) and lysed in 1 ml of 1% SDS, 10 mM EDTA, 50 mM Tris/HCl (pH 8.1) plus protease inhibitors. Sonication was carried out to produce fragments of 500 bp or under.
Immunoprecipitations were performed overnight at 4 °C with 10 μl of the antibody of interest in 200 μl of chromatin, and complexes were collected for 1 h with Protein A-agarose. Immunoprecipitations were washed, DNA was eluted and crosslinks were reversed according to the Upstate protocol. Samples were then treated with RNase (10 μg/ml) and proteinase K (40 μg/ml) before phenol/chloroform extraction [phenol/chloroform/isopentanol (25:24:1)] and ethanol precipitation. Immunoprecipitated samples were resuspended in 150 μl of 0.5×TE [Tris EDTA (1×TE is 10 mM Tris and 1 mM EDTA, pH 8.0)] and input DNA was resuspended in 200 μl of 0.5×TE.
The antibodies used for ChIP were anti-(acetyl-histone H3), anti-(acetyl-histone H4) and anti-(dimethyl-histone H3) (Lys4) and anti-CTCF (all from Upstate). All immunoprecipitations were carried out in duplicate or triplicate, using different chromatin preparations. Immunoprecipitated and 1/10-diluted input DNA were used as templates for real-time quantitative PCR using the ABIprism 7700 sequence detection system (Applied Biosystems). Primer and probe sets corresponding to regions of interest of the CFTR locus were designed using primer express 1.0 software and obtained from Eurogentec. Reactions were carried out following the Applied Biosystems Taqman Gold PCR protocol and were performed in triplicate. Results were analysed using ABI sequence detector software and following the standard curve method.
Constructs and enhancer-blocking assay
Fragments within the −20.9 kb and +15.6 kb DHS regions were generated by PCR from human genomic DNA, as described previously [12,14] and were then transferred as BssHII fragments into pNI at the AscI site, located between the HS2 enhancer and γ-globin promoter. Site-directed mutagenesis was performed using QuikChange® II XL (Stratagene).
Enhancer-blocking assays were performed as previously described [20,24]. G418-resistant colonies were counted after 2–3 weeks of selection, and data were subjected to statistical analysis by one-way ANOVA followed by Dunnett's multiple-comparison test.
EMSAs
Complementary single-stranded oligonucleotides were annealed and labelled with [α-32P]dCTP by fill-in reactions with Klenow DNA polymerase, prior to purification on microspin G-25 columns (Amersham Biosciences). DNA-binding reactions were typically performed in 20 mM Hepes (pH 7.9), 100 mM KCl, 1 mM EDTA, 1 mM DTT (dithiothreitol), 0.1 mM ZnSO4 and 12% (v/v) glycerol, using standard methods [8]. For antibody supershifts, nuclear extract and anti-CTCF (Upstate), anti-Sp1 (specificity protein 1; SC-420x; Santa Cruz Biotechnology), anti-Oct-1 (octamer-binding protein 1; SC-232x; Santa Cruz Biotechnology) anti-RXRα (SC-553x; Santa Cruz Biotechnology), anti-HNF1 (hepatocyte nuclear factor 1; SC-8936x; Santa Cruz Biotechnology), anti-ATF1 (activating transcription factor 1; SC-243x; Santa Cruz Biotechnology) or anti-GR (glucocorticoid receptor; SC-8992x; Santa Cruz Biotechnology) antibody were incubated on ice for 2 h, then the labelled DNA probe was added as above. Binding reactions with IVT protein were performed in 20 mM Hepes (pH 7.9), 50 mM KCl, 15 mM MgCl2, 3 mM DTT, 0.3 mg/ml BSA and 5% (v/v) glycerol, with 5 μl of IVT protein. DNA–protein complexes were resolved on 4.5% (29:1 acrylamide/bisacrylamide) gels in 0.5×TBE (1×TBE is 89 mM Tris, 89 mM boric acid and 2 mM EDTA) at 300 V for 1.5 h at 4 °C. Dried gels were exposed to PhosphorImager screens.
RESULTS
We have shown previously the presence of DHS at −20.9 kb relative to the CFTR translational start point and at +15.6 kb from the 3′ end of the gene. Although a number of protected regions were identified by in vitro DNase I footprinting reactions at both DHS regions, we were unable to determine the function of these elements [12,14]. In the present study we test the hypothesis that the −20.9 kb and +15.6 kb DHS function as insulator elements for the CFTR locus.
Chromatin structure at the −20.9 kb and +15.6 kb DHS regions
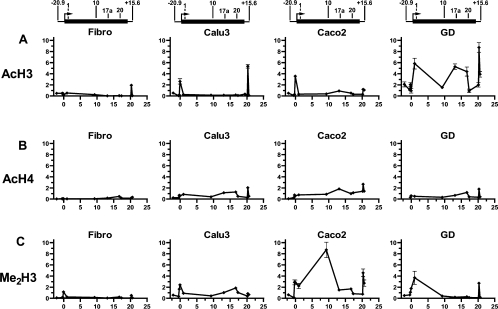
Since the functional boundaries of a locus, including insulators, are often demarcated by changes in chromatin modifications we evaluated histone modifications across the CFTR locus (Figure 1). ChIP was carried out using antibodies to known histone modifications that are indicative of an open chromatin structure. These were anti-(acetyl-histone H3) (Figure 1A), anti-(acetyl-histone H4) (Figure 1B) and anti-(dimethyl-histone H3) (Lys 4) (Figure 1C). Twelve Taqman primer and probe sets were designed across the CFTR locus in regions that correspond to known DHS and the promoter regions (see Supplementary Table 1 at http://www.BiochemJ.org/bj/408/bj4080267add.htm). The B13 probe/primer set was used as a negative control region of the CFTR locus with no known DHS. Histone modifications were evaluated in Caco2 colon carcinoma cells, Calu3 lung adenocarcinoma cells and primary fetal male GD cells, all of which express high levels of the CFTR gene. Fibroblasts provided a control which do not express CFTR. Expression of CFTR in these cells was verified by RT (reverse transcriptase)–PCR at the time of harvest for ChIP (results not shown).
Figure 1. Investigation of chromatin structure across the CFTR locus by ChIP.
Immunoprecipitation with antibodies specific for (A) acetylated histone H3 (AcH3), (B) acetylated histone H4 (AcH4) and (C) dimethylated histone H3 (Lys4; Me2H3) from fibroblast (Fibro), Calu3, Caco2 and primary human fetal male GD cell chromatin. Each point represents the abundance of a site measured by a specific Taqman probe shown as the fold-enrichment over input DNA. The lines joining the points on each graph are included for clarity but do not represent measured values of histone modification. The x-axis denotes the base numbers (×104) within the CFTR locus (1 is the translation start site). Above each set of graphs is a scale diagram to show the CFTR gene (thick black line) with arrows above the line denoting the location of each DHS evaluated by ChIP. Locations of DHS are as follows (where 1 is the CFTR translational start point): −20.9 kb DHS, (−20964); DHS1, intron 1 at 185 +10 kb (9952); DHS10ab, first two DHS in intron 10 at 1716 +13.2/13.7 kb (92706); DHS17a, intron 17a at 3271 +0.7 kb (130878); DHS20, intron 20 at 4005 +4 kb (166556); and +15.6 kb DHS, (202828).
For all panels in Figure 1, the x-axis shows the location of the Taqman probe within the CFTR locus with 1 being the translational start site of the gene. Results are shown as enrichment in chromatin-immunoprecipitated sample over input DNA and are normalized to the 18S rRNA gene.
ChIP with an antibody specific for acetylated histone H3 (Figure 1A) showed peaks of the modification at the CFTR promoter in both Calu3 and Caco2 cells. In Calu3 cells, a second peak of acetylated histone H3 was demonstrated at the +15.6 kb DHS, whereas Caco2 cells exhibited minor peaks at the DHS in intron 17a (DHS17a) and the +15.6 kb DHS. The primary human GD cells showed much higher levels of acetylated H3 across the whole locus with peaks at the first DHS in intron 1 (DHS1), at DHS17a, at the DHS in intron 20 (DHS20) and at the +15.6 kb DHS. In contrast, the whole of the CFTR locus showed low levels of acetylated H3 in fibroblasts, with the exception of a small peak at the +15.6 kb DHS.
ChIP with an antibody specific for acetylated histone H4 (Figure 1B) showed a moderate level of this modification across the whole CFTR locus in Calu3, Caco2 and GD cells, with a prominent peak at the +15.6 kb DHS in all three cell types. Additional peaks of acetylated histone H4 were observed at DHS17a and DHS20 in Calu3 cells, DHS17a in Caco2 cells and DHS20 in GD cells. Again, fibroblasts showed low acetylation across the whole locus, except for a slight increase at DHS20.
Anti-(dimethyl-histone H3) (Lys4) ChIP (Figure 1C) in Caco2 and Calu3 cells showed methylation across the locus, with both cell types exhibiting peaks over the promoter region. Caco2 cells, which had generally higher levels of methylation, had further peaks at the first two DHS in intron 10 (DHS10ab) and the +15.6 kb DHS, whereas Calu3 cells showed a peak at DHS20. The primary GD cells had moderate levels of methylation at the 5′ end of the locus, which were not maintained along the rest of the locus, and a peak at the +15.6 kb DHS. Fibroblasts had low methylation across the locus except for a slight increase at the promoter.
The +15.6 kb DHS region was enriched following ChIP for acetylated histone H3, acetylated histone H4 and dimethylated histone H3 (Lys4), with generally much larger peaks of each of these modifications in CFTR-expressing cells compared with fibroblasts. These peaks of histone modification were no longer evident at +17.8 kb. These results for the +15.6 kb DHS are consistent with histone modifications at other insulators [25]. In contrast, the −20.9 kb DHS did not show increased levels of acetylated histone H3 or H4, or dimethylated histone H3 (Lys4), and furthermore, no acetylation of histone H3 was seen at −25 kb (results not shown). Since not all insulators are enriched for euchromatin-specific modifications [25], this does not rule out an insulator function for the −20.9 kb DHS. However, it does suggest different mechanisms for the −20.9 kb and +15.6 kb DHS elements.
Enhancer-blocking activity of the −20.9 kb and +15.6 kb DHS
One property of insulators is their ability to block enhancers in a directional manner. To determine whether fragments from either the −20.9 kb or +15.6 kb DHS of CFTR possessed such activity, we used a previously described assay [20,24]. Briefly, this system uses the pNI plasmid, in which the chicken β-globin enhancer is positioned upstream of a neor gene, which is driven by the human γ-globin promoter. Sequences to be assayed for insulator activity are placed at an AscI site located between the enhancer and promoter. Transfection of these constructs into K562 human erythroleukaemia cells yields G418-resistant colonies, with a frequency dependent on the level of enhancer-promoter communication (and hence enhancer-blocking activity of the intervening fragment).
For functional analysis, the −20.9 kb and +15.6 kb DHS regions were previously divided into three overlapping fragments of approx. 200 and 300 bp respectively [12,14]. For the −20.9 kb DHS these fragments were named AB, CD and EF, and the +15.6 kb DHS fragments were named PQ, RS and TU (Table 1, and Supplementary Figure 1 at http://www.BiochemJ.org/bj/408/bj4080267add.htm).
Table 1. Genomic locations of −20.9 kb and +15.6 kb DHS fragments.
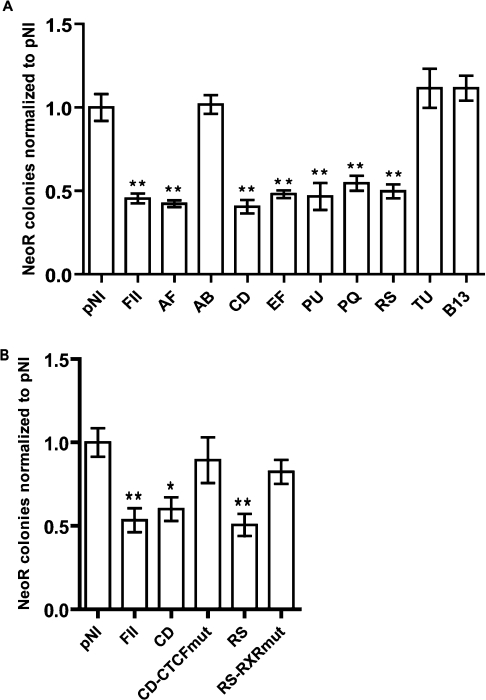
The number of G418-resistant colonies obtained with the empty pNI plasmid was normalized to 1, and the number of colonies for all other constructs was expressed relative to this (Figure 2A). FII, a 42 bp core from the known 5′HS4 chicken β-globin insulator [1,24], significantly reduced the number of colonies (2–3-fold reduction; P<0.01) when placed between the enhancer and promoter, as reported previously [26]. In contrast, B13, a 549 bp fragment from intron 21 of CFTR with no predicted CTCF-binding sites, had no significant effect upon the number of colonies (P>0.05). The 560 bp AF fragment spanning the −20.9 kb DHS showed equivalent enhancer-blocking activity to FII (P<0.01), as did the 775 bp PU fragment encompassing the +15.6 kb DHS region (P<0.01). Following evaluation of the smaller fragments within AF, enhancer-blocking activity was shown to reside in fragments CD and EF (P<0.01), with AB demonstrating no insulator activity (P>0.05). Similarly, following evaluation of the smaller fragments within PU, enhancer-blocking activity was shown to reside in fragments PQ and RS (P<0.01), with TU demonstrating no insulator activity (P>0.05).
Figure 2. Enhancer-blocking activity of −20.9 kb and +15.6 kb DHS regions.
(A) pNI is the empty pNI plasmid and FII contains a known insulator from the chicken β-globin locus. AF and PU are the entire −20.9 kb and +15.6 kb DHS. AB, CD and EF, PQ, RS and TU are overlapping fragments from within AF and PU respectively. B13 is a 549 bp fragment from intron 21 of CFTR with no DHS or predicted CTCF-binding sites. (B) CD-CTCFmut was formed by mutating 4 bp (bold and underlined) of the CD CTCF-binding core from CCAGCAGAGGGCAG to CCAGCAGAGTCATG. RS-RXRmut was formed by mutating 2 bp (bold and underlined) of middle HRE half-site in RS from AAAGGGCATT to AAAGCACATT. For all experiments, the number of neor colonies obtained for empty pNI was given a value of 1, and the number of colonies obtained with all other constructs was expressed relative to this. Error bars represent S.E.M. for triplicate experiments carried out on at least two separate occasions. *P<0.05 and **P<0.01.
Search for CTCF-binding sites
As both the −20.9 kb and +15.6 kb DHS showed insulator activity, we inspected these regions for CTCF-binding sites in silico. CTCF is able to interact with DNA using different combinations of its 11 zinc fingers, and as a result CTCF-binding sites are extremely variable and difficult to predict. However, a consensus sequence obtained from the chicken β-globin and IGF2/H19 loci has been used with some success [22,27]. This 14 bp consensus was used to search 560 bp covering the −20.9 kb DHS region, and 780 bp covering the +15.6 kb DHS region (Table 2). Within the −20.9 kb DHS region, the greatest similarity was at AC003045 23778–23791, within fragment CD, with eight out of 14 bp identical with the consensus sequence. A second site at AC003045 23854–23867, within fragment EF, showed eight out of 14 bp similarity. At the +15.6 kb DHS region. The best matches to the CTCF consensus were at AC000061 72973–73986 (in fragment PQ), 73107–73120 (overlapping region of fragments PQ and RS) and 73219–73232 (in fragment RS), with ten out of 14, nine out of 14 and nine out of 14 matches respectively (Table 2).
Table 2. Predicted CTCF-binding sites within the −20.9 kb and +15.6 kb DHS.
 |
CTCF binds to the −20.9 kb DHS in vitro
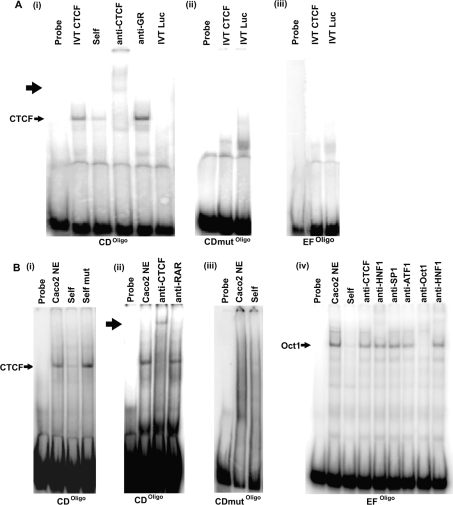
Oligonucleotides were synthesized to evaluate the two potential CTCF-binding sites in the −20.9 kb region. As CTCF often interacts with extended regions of DNA, producing a large footprint of up to 50 bp [28], oligonucleotides were designed to be at least 70 bp in length, with the 14 bp core-binding consensus positioned centrally. Oligonucleotides were named according to the original overlapping fragment in which they are located. For example, for the site at AC003045 23748–23819, located in fragment CD, the corresponding oligonucleotide was named CDoligo (Table 2). EMSAs were performed to investigate DNA–protein interactions in vitro. CDoligo formed a complex with IVT CTCF, which was supershifted with an anti-CTCF antibody, but not with an isotype control (IgG) antibody against the GR [Figure 3A(i)]. When the 14 bp core of the CDoligo CTCF site was mutated (CDmutoligo; see Table 2), no interaction with IVT CTCF was observed [Figure 3A(ii)]. EFoligo did not bind IVT CTCF [Figure 3A(iii)].
Figure 3. DNA–protein interactions at the −20.9 kb DHS core.
(A) EMSA using IVT CTCF and the following probes: (i) CDoligo. The complex formed with IVT CTCF is indicated, and supershift obtained with anti-CTCF is marked with a bold arrow. (ii) CDmutoligo; (iii) EFoligo. In all cases competition was 100× molar excess. For all probes, binding reactions with IVT luciferase (Luc) provided negative controls. Results are representative of three separate experiments. (B) EMSAs using Caco2 cell nuclear extracts. (i) CDoligo-binding assay competed with self and CDmutoligo (self- mutated); (ii) CDoligo-binding assay supershifted with an anti-CTCF antibody. Supershift is indicated by bold arrow; (iii) CDmutoligo-binding assay competed with self; (iv) EFoligo-binding assay, competed with self. Lanes 5–9 show the EFoligo-binding assay with various antibodies. An apparent Oct-1 complex is indicated. In all cases competition was with 100× molar excess. Results are representative for three separate experiments.
CDoligo also formed a complex with nuclear extract from Caco2 cells [Figure 3B(i)]. This complex was of the same mobility as the complex formed with IVT CTCF. The complex was competed by an excess of unlabelled self, but not by CDmutoligo (self-mutated). The CDoligo complex with nuclear extract was also supershifted by an anti-CTCF antibody [Figure 3B(ii)]. When the CDmutoligo was incubated with Caco2 cell nuclear extract it was unable to form any complexes [Figure 3B(iii)]. These results demonstrate a strong in vitro interaction between CDoligo and CTCF. By mutating 4 bp of the CTCF-binding core from within the CD fragment (CD-CTCFmut), enhancer-blocking activity was abolished (Figure 2B), indicating that CTCF is responsible for the enhancer-blocking activity of CD.
EFoligo formed a complex with Caco2 cell nuclear extract that was of a lower mobility than that formed with CDoligo [Figure 3B(iv)]. The EFoligo complex was not competed by FII (results not shown), nor did it supershift with the anti-CTCF antibody, indicating further that the protein binding EFoligo is probably not CTCF. In an attempt to determine the identity of the protein(s) responsible for this complex, we conducted a search using Alibaba2 [29] for possible transcription-factor-binding sites within EFoligo. Among several predicted sites were binding motifs for Sp1 and Oct-1. Both of these proteins have been implicated in insulator activity [30]. When used in EMSA reactions, an antibody specific for Oct-1 competed the complex formed between EFoligo and Caco2 cell nuclear extract. An antibody specific for Sp1 had no effect on the complex. Thus Oct-1 is a candidate for the complex generated by Caco2 nuclear extracts with EFoligo and may have a role in its enhancer-blocking activity.
In vitro binding of RXR–VDR heterodimers at the +15.6 kb DHS
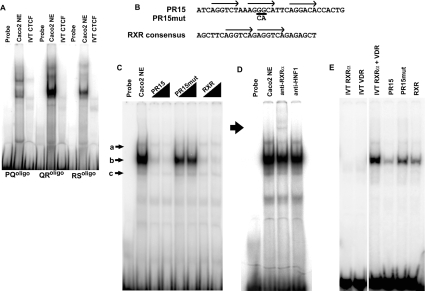
Oligonucleotides (70-mers) encompassing predicted CTCF-binding sites (e.g. PQoligo) were generated for EMSA experiments to evaluate proteins binding to the +15.6 kb DHS in vitro. The predicted site at AC000061 73079–73147, located on the overlapping region between fragments PQ and RS, was denoted QRoligo (Table 2). Mutant versions of each oligonucleotide were also generated. In contrast with the demonstrated in vitro interaction of CTCF with sequences in the −20.9 kb DHS region no interactions were observed between IVT CTCF and any of the three potential binding sites within the +15.6 kb DHS (Figure 4A). Although each of the three probes from this region did form complexes with Caco2 cell nuclear extracts there was no evidence of competition with FII, nor did mutating the core of the putative CTCF site for each probe abolish these complexes (results not shown).
Figure 4. DNA–protein interactions at the +15.6 kb DHS.
(A) EMSA assaying for CTCF-binding at PQoligo, QRoligo and RSoligo probes, using both Caco2 cell nuclear extract and IVT CTCF. (B) Sequence of PR15, PR15mut and RXR consensus probes. Arrows represent HRE half-sites. Bases underlined are those that are mutated in PR15mut, as indicated. Note that for ease of highlighting the HRE half-sites, the PR15 antisense strand is shown (AC000061 73261–73230). (C) EMSA using PR15 probe with Caco2 cell nuclear extract. Three complexes, termed a, b and c, are indicated. Competition reactions were carried out with increasing amounts of PR15, PR15mut and RXR consensus. (D) PR15-binding assay with Caco2 cell nuclear extract, supershifted with an anti-RXRα antibody. Supershift is indicated with a bold arrow. (E) PR15 EMSA with IVT RXRα and VDR. Reactions were carried out with IVT RXRα alone, IVT VDR alone and both IVT RXRα and VDR. Subsequent competition reactions were carried out on the IVT RXRα–VDR complex, as indicated.
The lack of demonstrated CTCF binding in the +15.6 kb DHS region implicated the involvement of other proteins at this site. A search for potential transcription-factor-binding sites was carried out using Alibaba2 and MatInspector [31]. Within the +15.6 kb DHS, a number of potential binding sites for nuclear-receptor-type proteins were present. These proteins form homo- or hetero-dimers that bind at HREs (hormone-response elements), usually consisting of two half-sites with the consensus sequence AGGTCA [32,33]. The orientation of these half-sites (either direct repeats or inverted palindromes), together with the distance with which they are separated, determines which nuclear hormone receptors are able to bind [34–36]. Within fragment RS of the +15.6 kb DHS there are three potential HRE half-sites (AC000061 73236–73258), each with a five out of six homology with the consensus sequence. They are in a direct repeat arrangement, separated by 2 and 3 bp respectively, and furthermore they are located within a protected region (PR15) identified by DNase I footprinting [12]. A 35 bp oligonucleotide probe (PR15) was synthesized that encompassed these three potential HRE half-sites (Figure 4B). EMSA experiments using nuclear extract from Caco2 cells demonstrated the presence of three distinct complexes a, b and c (marked with arrows in Figure 4C), with complex b being the most abundant. All three complexes were diminished by self-competition, but PR15mut, in which the two central bases of the middle half-site were mutated, had little effect. Within nuclear receptor heterodimers, the most common binding partner is the RXR [37,38]. Hence a consensus oligonucleotide sequence for RXR homodimers [39] was synthesized and utilized in EMSA competition experiments (Figure 4B). Complex b was effectively abolished by competition with the RXR consensus sequence, suggesting binding of RXR to PR15 (Figure 4C). A supershift was obtained using an antibody specific for RXRα, confirming that RXRα formed part of the complex at PR15 (Figure 4D).
To determine further the identity of proteins binding at PR15, EMSA experiments were carried out using IVT proteins. Although there are no precise rules as to which nuclear receptors bind at a particular HRE arrangement, half-sites in a direct repeat separated by 3 bp have a tendency to be bound by heterodimers formed between RXR and the VDR [36]. Furthermore, inspection of the PR15 sequence by MatInspector predicted an RXR–VDR interaction at this site. When used individually in EMSA reactions, IVT RXRα and VDR formed very weak complexes with PR15, with a similar mobility to that of complex c seen with nuclear extract (Figure 4E). However, when added to EMSA reactions together, two further complexes were generated: a very intense complex with the same mobility as that of b, together with a weaker complex with similar mobility to that of a. Mutation of the middle PR15 HRE half-site within the RS fragment (RS-RXRmut), abolished enhancer-blocking activity (Figure 2B), suggesting that a nuclear-receptor complex bound at PR15 confers CTCF-independent enhancer-blocking activity to RS.
In vivo analysis of CTCF binding
Since CTCF shows a strong in vitro interaction with CDoligo, in vivo binding at this site was investigated by ChIP using an antibody specific for CTCF, followed by quantitative PCR. Chromatin from three cell lines was evaluated: Caco2 and Calu3 colon and lung adenocarcinoma cells respectively, both of which express abundant CFTR, and 37566, a lymphoblastoid cell line which does not express CFTR.
The anti-CTCF ChIP revealed a 2–3-fold enrichment of the −20.9 kb DHS CD sequence in comparison with a control sequence from intron 17 of CFTR (the intron 17a DHS region at 3271 +0.7kb), not predicted in silico to bind CTCF (Figure 5). This enrichment of CD was detected in all cell lines, regardless of CFTR expression. In contrast, a probe designed to detect the QR-predicted CTCF-binding site from the +15.6 kb DHS was not enriched by anti-CTCF ChIP. Enrichment of the CTCF-binding site of the IGF2/H19 imprinted region (H19) is shown as a positive control for CTCF immunoprecipitation. H19 was enriched 18- and 6-fold in Calu3 and 37566 cells respectively. Lack of enrichment in Caco2 cells can be explained by colorectal cancers often showing biallelic methylation of the IGF2/H19 DMR (differentially methylated region), thus preventing CTCF binding to its usual site on the maternal allele [40].
Figure 5. ChIP using an antibody specific for CTCF.
Chromatin from the three cell types was immunoprecipitated with an anti-CTCF antibody. DNA was prepared and subjected to Taqman real-time PCR analysis using probes specific for the −20.9 kb and +15.6 kb DHS. Enrichment of these products in anti-CTCF immunoprecipitated samples are shown relative to a no antibody control and are normalized to a probe specific for the CFTR intron 17a region, which contains no predicted CTCF-binding sites. CTCF binding at the H19 DMR is shown as a positive control. Error bars shown are S.E.M. PCRs were performed in triplicate and immunoprecipitations were repeated three times.
DISCUSSION
Although the CFTR gene shows tight control of its temporal and spatial expression, the underlying mechanisms responsible for controlling this are poorly understood. Elements within the CFTR basal promoter are not able to confer these complex expression patterns, indicating that key elements must lie elsewhere within the gene or in adjacent chromatin. In this context it may be relevant that ASZ1 and CTTNBP2, which flank the CFTR locus, are located within the functional range of enhancers and other promiscuous regulatory elements, yet exhibit distinct tissue-specific expression from CFTR [6,7]. We have shown that two DHS, at −20.9 kb from the CFTR translational start site and at +15.6 kb from the 3′ end of the gene, show functional properties of insulator elements. We propose that these two regions are responsible for establishing an independent expression domain at the CFTR locus. Using a standard assay for insulator activity [20,24], the −20.9 kb DHS was shown to have enhancer-blocking activity comparable with that of the chicken β-globin HS4 insulator [1,24]. The insulator activity exists within two fragments of approx. 200 bp, termed CD and EF. In the case of CD, but not EF, this activity was attributed to binding of CTCF, a multivalent protein that confers enhancer-blocking activity at the majority of vertebrate insulators [1]. An in vitro interaction was demonstrated between EF and Oct-1, a protein that has also been implicated in insulator function [30]. A high level of conservation of the −20.9 kb DHS CTCF and Oct-1-binding sites, across a range of mammalian species (see Supplementary Figure 2 at http://www.BiochemJ.org/bj/408/bj4080267add.htm), provides additional support for their important regulatory function.
Previous evidence for an important role for the −20.9 kb DHS in CFTR expression was provided by an experiment in which the DHS region was deleted from a 310 kb YAC (yeast artificial chromosome) containing the CFTR gene. Lack of the −20.9 kb site and 5′ sequences caused a 60% decrease in transcription from the YAC transgene in Caco2 human colon carcinoma cells [14]. Furthermore, 19 kb of genomic DNA 5′ to the CFTR gene failed to drive reporter-gene expression in transgenic mice [41]. Absence of an enhancer-blocking insulator within the −20.9 kb DHS might remove protection against positional effects, and hence result in silencing of the CFTR transgene in this experiment.
The +15.6 kb DHS shows some correlation with CFTR expression in cell lines and, in transgenic mice carrying the 310 kb human CFTR YAC, tissue-specific appearance of the DHS correlated with CFTR expression [12]. In the present study we demonstrate that the +15.6 kb DHS also possesses enhancer-blocking activity that resides in two fragments of approx. 300 bp, named PQ and RS. Although partial matches to the CTCF consensus were identified in both fragments, none of these bound CTCF in vitro or in vivo. Although we currently have no information on the proteins responsible for the enhancer-blocking activity of the PQ fragment, candidate proteins have been identified that may contribute to the properties of the RS fragment. We demonstrate that proteins bound to a previously identified DNase I footprint (PR15) [12] confer enhancer-blocking activity to the RS fragment, and we provide evidence for an in vitro interaction between PR15 and an RXRα–VDR heterodimer complex. RXRα and VDR are members of the nuclear receptor superfamily, a large family of ligand-activated transcription factors that share several features with CTCF. Specific DNA binding is mediated by two zinc-finger motifs, which, like CTCF, induce DNA bending [42]. Once bound to DNA, nuclear receptors recruit cofactors, such as the histone deacetylase Sin3A, which is also recruited by CTCF [43]. Nuclear-receptor-type proteins and CTCF have also been shown to functionally cooperate. For example, upstream of both the chicken lysozyme and human c-myc genes, CTCF-binding sites are found in close proximity to TREs (thyroid-response elements), able to bind TR (thyroid receptor)–RXR heterodimers [44,45]. In both of these cases, enhancer-blocking by the CTCF–TR composite element is abrogated when TR is bound by thyroid hormone [44]. Interestingly, some nuclear receptors, including RXR, can also interact with Oct-1 [46].
The nuclear-receptor superfamily has many members and, due to heterodimerization between them, a very large number of potential complexes may be formed. In our analysis of PR15, we focussed upon RXRα–VDR heterodimers, since there is a tendency for the half-sites involved in their binding to be separated by 3 bp [36], as is the case for two of the half-sites within PR15. The ligands for RXRα and VDR are 9-cis retinoic acid and vitamin D3 respectively. With the exception of experiments on oestrogen [47,48], hormonal regulation of CFTR expression has not been well-studied and although there are reports of CFTR regulation by retinoic acid [49], there is no evidence for the involvement of vitamin D. Future experiments will determine the physiological importance of RXRα–VDR binding to the +15.6 kb DHS.
Comparison of specific histone modifications across the CFTR locus with the observed location of enhancer-blocking elements was of interest. As expected, a peak of acetylated histone H3, acetylated histone H4 and dimethylated histone H3 was observed at the CFTR promoter region only in cell types that express CFTR. The +15.6 kb DHS region also showed a prominent peak in CFTR-expressing cells. In contrast there was no enrichment of euchromatin-specific modifications at the −20.9 kb DHS or immediately 5′ to it (−25 kb). Such a divergence between the histone modifications seen at 5′- and 3′-flanking insulator elements is also observed at the chicken β-globin locus. The chicken β-globin 5′HS4 insulator has elevated levels of histone H3 acetylation, in contrast with the 3′HS insulator [25]. Interestingly, the 5′HS4 insulator, but not the 3′HS, can protect against positional effect variegation [50]. Thus recruitment of a high density of euchromatin-specific histone modifications may enable insulators to protect against positional effects [51] and this would be predicted to be a property of the CFTR +15.6 kb DHS.
An emerging theme from current insulator studies is that a variety of regulatory elements may exhibit the properties of ‘enhancer-blocking’ or ‘barrier’ insulators, and that there may even be overlap between the mechanism of action of insulators and other regulatory elements [52]. In the present study we have shown that 5′ to CFTR, within the −20.9 kb DHS, there is a classical CTCF-dependent enhancer-blocking insulator. However, both within the −20.9 kb DHS and 3′ to CFTR in the +15.6 kb DHS, other elements are present that possess CTCF-independent enhancer-blocking activity. We have identified proteins that are potentially responsible for these activities, some of which have been implicated previously at insulator elements for other genetic loci.
Our current model for regulation of expression of the CFTR gene involves multiple regulatory elements interacting in chromatin in vivo, probably utilizing different mechanisms in individual cell types that exhibit specific sets of DHS within the gene. We propose that the −20.9 kb and +15.6 kb DHS help to create a specific chromatin environment that allows such interactions to occur. The characterization of boundary elements flanking the CFTR locus may be of direct practical relevance in the design of vectors for effective gene therapy of cystic fibrosis. One of the problems encountered in gene therapy protocols is the relatively rapid loss of expression from the CFTR cDNA once it is introduced into mammalian cells [53]. Incorporation of the −20.9 kb and +15.6 kb DHS regions into the CFTR expression vectors might enable the establishment of an open chromatin domain around the transgene, following integration into the host cell, thus promoting its prolonged expression.
Online data
Acknowledgments
This work was supported in part by the Cystic Fibrosis Trust, U.K. N.B. holds a Medical Research Council studentship. We are grateful to Dr Adam West and Dr Gary Felsenfeld (Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, U.S.A.) for providing the pNI and pNI-FII plasmids and for helpful discussions. We also wish to acknowledge the Computational Biology Research Group, Medical Sciences Division, Oxford University, Oxford, U.K. for use of their services in this project.
References
- 1.Bell A. C., West A. G., Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387–396. doi: 10.1016/s0092-8674(00)81967-4. [DOI] [PubMed] [Google Scholar]
- 2.Hark A. T., Schoenherr C. J., Katz D. J., Ingram R. S., Levorse J. M., Tilghman S. M. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486–489. doi: 10.1038/35013106. [DOI] [PubMed] [Google Scholar]
- 3.Yusufzai T. M., Felsenfeld G. The 5′-HS4 chicken β-globin insulator is a CTCF-dependent nuclear matrix-associated element. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8620–8624. doi: 10.1073/pnas.0402938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn K. L., Zhao H., Davie J. R. The insulator binding protein CTCF associates with the nuclear matrix. Exp. Cell Res. 2003;288:218–223. doi: 10.1016/s0014-4827(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara K., Oshimura M., Nakao M. CTCF-dependent chromatin insulator is linked to epigenetic remodeling. Mol. Cell. 2006;23:733–742. doi: 10.1016/j.molcel.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Yan W., Rajkovic A., Viveiros M. M., Burns K. H., Eppig J. J., Matzuk M. M. Identification of Gasz, an evolutionarily conserved gene expressed exclusively in germ cells and encoding a protein with four ankyrin repeats, a sterile-α motif, and a basic leucine zipper. Mol. Endocrinol. 2002;16:1168–1184. doi: 10.1210/mend.16.6.0864. [DOI] [PubMed] [Google Scholar]
- 7.Cheung J., Petek E., Nakabayashi K., Tsui L. C., Vincent J. B., Scherer S. W. Identification of the human cortactin-binding protein-2 gene from the autism candidate region at 7q31. Genomics. 2001;78:7–11. doi: 10.1006/geno.2001.6651. [DOI] [PubMed] [Google Scholar]
- 8.Mouchel N., Henstra S. A., McCarthy V. A., Williams S. H., Phylactides M., Harris A. HNF1α is involved in regulation of expression of the CFTR gene. Biochem. J. 2004;378:909–918. doi: 10.1042/BJ20031157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phylactides M., Rowntree R., Nuthall H., Ussery D., Wheeler A., Harris A. Evaluation of potential regulatory elements identified as DNase I hypersensitive sites in the CFTR gene. Eur. J. Biochem. 2002;269:553–559. doi: 10.1046/j.0014-2956.2001.02679.x. [DOI] [PubMed] [Google Scholar]
- 10.Rowntree R., Vassaux G., McDowell T. L., Howe S., McGuigan A., Phylactides M., Huxley C., Harris A. An element in intron 1 of the CFTR gene augments intestinal expression in vivo. Hum. Mol. Genet. 2001;11:1455–1464. doi: 10.1093/hmg/10.14.1455. [DOI] [PubMed] [Google Scholar]
- 11.Smith D. J., Nuthall H. N., Majetti M. E., Harris A. Multiple potential intragenic regulatory elements in the CFTR gene. Genomics. 2000;64:90–96. doi: 10.1006/geno.1999.6086. [DOI] [PubMed] [Google Scholar]
- 12.Nuthall H. N., Moulin D. S., Huxley C., Harris A. Analysis of DNase I hypersensitive sites at the 3′ end of the cystic fibrosis transmembrane conductance regulator gene. Biochem. J. 1999;341:601–611. [PMC free article] [PubMed] [Google Scholar]
- 13.Smith A. N., Wardle C. J., Harris A. Characterization of DNASE I hypersensitive sites in the 120 kb 5′ to the CFTR gene. Biochem. Biophys. Res. Commun. 1995;211:274–281. doi: 10.1006/bbrc.1995.1807. [DOI] [PubMed] [Google Scholar]
- 14.Nuthall H. N., Vassaux G., Huxley C., Harris A. Analysis of a DNase I hypersensitive site located −20.9 kb upstream of the CFTR gene. Eur. J. Biochem. 1999;266:431–443. doi: 10.1046/j.1432-1327.1999.00872.x. [DOI] [PubMed] [Google Scholar]
- 15.Rowntree R., Harris A. DNA polymorphisms in potential regulatory elements of the CFTR gene alter transcription factor binding. Hum. Genet. 2002;111:66–74. doi: 10.1007/s00439-002-0737-z. [DOI] [PubMed] [Google Scholar]
- 16.Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- 17.Fogh J., Wright W. C., Loveless J. D. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J. Natl. Cancer Inst. 1977;58:209–214. doi: 10.1093/jnci/58.2.209. [DOI] [PubMed] [Google Scholar]
- 18.Fogh J., Trempe G. New York: Plenum Press; 1975. Human Tumor Cells In Vitro; pp. 115–159. [Google Scholar]
- 19.Harris A., Coleman L. Ductal epithelial cells cultured from human foetal epididymis and vas deferens: relevance to sterility in cystic fibrosis. J. Cell Sci. 1989;92:687–690. doi: 10.1242/jcs.92.4.687. [DOI] [PubMed] [Google Scholar]
- 20.Chung J. H., Whiteley M., Felsenfeld G. A 5′ element of the chicken β-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- 21.Carlberg C., Bendik I., Wyss A., Meier E., Sturzenbecker L. J., Grippo J. F., Hunziker W. Two nuclear signalling pathways for vitamin D. Nature. 1993;361:657–660. doi: 10.1038/361657a0. [DOI] [PubMed] [Google Scholar]
- 22.Chao W., Huynh K. D., Spencer R. J., Davidow L. S., Lee J. T. CTCF, a candidate trans-acting factor for X-inactivation choice. Science. 2002;295:345–347. doi: 10.1126/science.1065982. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber E., Matthias P., Muller M. M., Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung J. H., Bell A. C., Felsenfeld G. Characterization of the chicken β-globin insulator. Proc. Natl. Acad. Sci. U.S.A. 1997;94:575–580. doi: 10.1073/pnas.94.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litt M. D., Simpson M., Recillas-Targa F., Prioleau M. N., Felsenfeld G. Transitions in histone acetylation reveal boundaries of three separately regulated neighboring loci. EMBO J. 2001;20:2224–2235. doi: 10.1093/emboj/20.9.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippova G. N., Thienes C. P., Penn B. H., Cho D. H., Hu Y. J., Moore J. M., Klesert T. R., Lobanenkov V. V., Tapscott S. J. CTCF-binding sites flank CTG/CAG repeats and form a methylation-sensitive insulator at the DM1 locus. Nat. Genet. 2001;28:335–343. doi: 10.1038/ng570. [DOI] [PubMed] [Google Scholar]
- 27.Magdinier F., Yusufzai T. M., Felsenfeld G. Both CTCF-dependent and -independent insulators are found between the mouse T cell receptor α and Dad1 genes. J. Biol. Chem. 2004;279:25381–25389. doi: 10.1074/jbc.M403121200. [DOI] [PubMed] [Google Scholar]
- 28.Ohlsson R., Renkawitz R., Lobanenkov V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 2001;17:520–527. doi: 10.1016/s0168-9525(01)02366-6. [DOI] [PubMed] [Google Scholar]
- 29.Grabe N. AliBaba2: context specific identification of transcription factor binding sites. In Silico Biol. 2002;2:S1–S15. [PubMed] [Google Scholar]
- 30.Acuto S., Di Marzo R., Calzolari R., Baiamonte E., Maggio A., Spinelli G. Functional characterization of the sea urchin sns chromatin insulator in erythroid cells. Blood Cells Mol. Dis. 2005;35:339–344. doi: 10.1016/j.bcmd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Cartharius K., Frech K., Grote K., Klocke B., Haltmeier M., Klingenhoff A., Frisch M., Bayerlein M., Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- 32.Brent G. A., Harney J. W., Chen Y., Warne R. L., Moore D. D., Larsen P. R. Mutations of the rat growth hormone promoter which increase and decrease response to thyroid hormone define a consensus thyroid hormone response element. Mol. Endocrinol. 1989;3:1996–2004. doi: 10.1210/mend-3-12-1996. [DOI] [PubMed] [Google Scholar]
- 33.Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988;336:262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- 34.Forman B. M., Casanova J., Raaka B. M., Ghysdael J., Samuels H. H. Half-site spacing and orientation determines whether thyroid hormone and retinoic acid receptors and related factors bind to DNA response elements as monomers, homodimers, or heterodimers. Mol. Endocrinol. 1992;6:429–442. doi: 10.1210/mend.6.3.1316541. [DOI] [PubMed] [Google Scholar]
- 35.Naar A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991;65:1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- 36.Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992;355:441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- 39.Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor–COUP-TF interactions modulate retinoic acid signaling. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa H., Chadwick R. B., Peltomaki P., Plass C., Nakamura Y., de La Chapelle A. Loss of imprinting of the insulin-like growth factor II gene occurs by biallelic methylation in a core region of H19-associated CTCF-binding sites in colorectal cancer. Proc. Natl. Acad. Sci. U.S.A. 2001;98:591–596. doi: 10.1073/pnas.011528698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griesenbach U., Suen T.-C., Chamberlain J., Olek K., L-C. T. Study of the human CFTR promoter in transgenic mice. Pediatr. Pulmonol. 1994;Supp 10:119. [Google Scholar]
- 42.Arnold R., Burcin M., Kaiser B., Muller M., Renkawitz R. DNA bending by the silencer protein NeP1 is modulated by TR and RXR. Nucleic Acids Res. 1996;24:2640–2647. doi: 10.1093/nar/24.14.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke L. J., Zhang R., Lutz M., Renkawitz R. The thyroid hormone receptor and the insulator protein CTCF: two different factors with overlapping functions. J. Steroid Biochem. Mol. Biol. 2002;83:49–57. doi: 10.1016/s0960-0760(02)00256-x. [DOI] [PubMed] [Google Scholar]
- 44.Lutz M., Burke L. J., LeFevre P., Myers F. A., Thorne A. W., Crane-Robinson C., Bonifer C., Filippova G. N., Lobanenkov V., Renkawitz R. Thyroid hormone-regulated enhancer blocking: cooperation of CTCF and thyroid hormone receptor. EMBO J. 2003;22:1579–1587. doi: 10.1093/emboj/cdg147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-Juste G., Garcia-Silva S., Aranda A. An element in the region responsible for premature termination of transcription mediates repression of c-myc gene expression by thyroid hormone in neuroblastoma cells. J. Biol. Chem. 2000;275:1307–1314. doi: 10.1074/jbc.275.2.1307. [DOI] [PubMed] [Google Scholar]
- 46.Kakizawa T., Miyamoto T., Ichikawa K., Kaneko A., Suzuki S., Hara M., Nagasawa T., Takeda T., Mori J., Kumagai M., Hashizume K. Functional interaction between Oct-1 and retinoid X receptor. J. Biol. Chem. 1999;274:19103–19108. doi: 10.1074/jbc.274.27.19103. [DOI] [PubMed] [Google Scholar]
- 47.Rochwerger L., Dho S., Parker L., Foskett J. K., Buchwald M. Estrogen-dependent expression of the cystic fibrosis transmembrane regulator gene in a novel uterine epithelial cell line. J. Cell Sci. 1994;107:2439–2448. doi: 10.1242/jcs.107.9.2439. [DOI] [PubMed] [Google Scholar]
- 48.Trezise A. E., Linder C. C., Grieger D., Thompson E. W., Meunier H., Griswold M. D., Buchwald M. CFTR expression is regulated during both the cycle of the seminiferous epithelium and the oestrous cycle of rodents. Nat. Genet. 1993;3:157–164. doi: 10.1038/ng0293-157. [DOI] [PubMed] [Google Scholar]
- 49.Chazaud C., Dolle P., Rossant J., Mollard R. Retinoic acid signaling regulates murine bronchial tubule formation. Mech. Dev. 2003;120:691–700. doi: 10.1016/s0925-4773(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 50.Saitoh N., Bell A. C., Recillas-Targa F., West A. G., Simpson M., Pikaart M., Felsenfeld G. Structural and functional conservation at the boundaries of the chicken β-globin domain. EMBO J. 2000;19:2315–2322. doi: 10.1093/emboj/19.10.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pikaart M., Recillas-Targa F., Felsenfeld G. Loss of transcriptional activity of a transgene is accompanied by DNA methylation and histone deacetylation and is prevented by insulators. Genes Dev. 1998;12:2852–2862. doi: 10.1101/gad.12.18.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaszner M., Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat. Rev. Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- 53.Rosenecker J., Huth S., Rudolph C. Gene therapy for cystic fibrosis lung disease: current status and future perspectives. Curr. Opin. Mol. Ther. 2006;8:439–445. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.