Abstract
Purpose
To test whether intrarectal Amifostine limits symptoms of radiation proctitis as measured by the RTOG GI toxicity score and the expanded prostate cancer index composite (EPIC) score.
Methods and Materials
Patients with localized prostate cancer recieved Amifostine as a rectal suspension 30–45 min before daily 3D-conformal radiation treatments (3D-CRT). The first 18 patients received 1gm of Amifostine and the next 12 patients received 2gm. Toxicity was assessed at baseline, during treatment, and at follow-up visits using RTOG grading and the EPIC Quality of Life (QoL) 50 item questionnaire. The “Bowel Function” subset of the bowel domain (EPIC-BF), which targets symptom severity, and “Bowel Bother” subset of the bowel domain (EPIC-BB), which assesses quality of life, were evaluated and compared to the RTOG GI toxicity score.
Results
Median follow-up was 30 months (range 18–36). Overall, the EPIC-BF and EPIC-BB scores both track closely with the RTOG GI toxicity score. Seven weeks after the start of radiation therapy, the incidence of RTOG Grade 2 toxicity was 33% in the 1gm group (6/18) compared with 0% (0/12) in the 2gm group and trended towards statistical significance (p=0.06). A significant difference between Amifostine groups was observed using the EPIC-BF score at 7 weeks (p=0.04). A difference in EPIC-BB score between dose groups was evident at 7 weeks (p=0.07) and was significant at 12 months (p=0.04).
Conclusions
Higher doses of Amifostine produce significant improvements in acute and late bowel QoL (up to one year following therapy) as measured by the EPIC score.
Keywords: Amifostine, Prostate, Radiation-induced Proctitis, EPIC, Quality of Life
Introduction
There are over 200,000 new cases and nearly 30,000 deaths each year from prostate cancer (1). Radiation therapy (RT) is a mainstay of local therapy, and it is known that biochemical disease free survival improves with dose escalation to the prostate (2, 3).
Advances in methods of precise radiation dose delivery, such as 3D-Conformal Radiotherapy (3D-CRT) and Intensity Modulated Radiation Therapy (IMRT), have allowed higher radiation doses (dose escalation) to the prostate while minimizing toxicity by limiting the amount of normal tissue irradiated. Even with precise RT, however, the high dose radiation volume must include the anterior rectal wall to avoid under-dosing the periphery of the prostate. Consequently, if the radiation tolerance of the rectum can be improved, toxicity from therapy at current doses may be diminished and further dose escalation may be possible.
Amifostine is a radioprotector with the potential to improve radiation tolerance of the rectum. Amifostine has been tested in multiple solid tumor systems using several methods of delivery (4–9). Endorectal administration of Amifostine has been assessed in preclinical and pilot clinical trials (10–14).
There are several measures of radiation-induced toxicity. RTOG acute and late gastrointestinal (GI) toxicity is assessed in most current trials and comprises a grading system with scores ranging from 0–5 that are assigned by the physician based on the patient’s symptoms and when the assessment is being made relative to the course of radiation. Newer tools for toxicity assessment have been developed that focus more on the patient’s own assessment of their symptoms, and the change in their overall, and treatment-specific quality of life. We used one such measure, the Expanded Prostate Cancer Index Composite (EPIC) self-assessment questionnaire, in this trial. The EPIC score has been used to measure QoL in the acute and late setting (15–19).
This trial was undertaken to assess the effectiveness and dose-response of daily topical administration of Amifostine in reducing acute and late rectal toxicities in prostate cancer patients treated with 3D-CRT. In a previous publication, we reported acute toxicity profiles using the RTOG grade(20). We noted that use of instruments more sensitive than the RTOG grade may discriminate small but clinically important reductions in proctitis. This analysis was performed to report late toxicity outcomes in patients who received 1 or 2 gm daily endorectal Amifostine during treatment.
Methods
Eligibility and Accrual
All patients underwent history and physical examination as well as routine blood work including CBC, PSA, and alkaline phosphatase. Imaging studies such as bone scan were obtained as warranted. Eligible patients had localized adenocarcinoma of the prostate and were candidates for definitive or post-operative external beam radiotherapy. One patient in each group received post-operative radiation therapy. Use of adjuvant hormonal or experimental PSA vaccine therapy was permitted. All eligible prostate cancer patients evaluated for radiation therapy at the National Cancer Institute were informed about this study. At the time of enrollment, all patients provided written, informed consent for participation in this IRB approved protocol.
Radiation Therapy
All patients underwent computed tomography simulation. Prior to simulation, liquid contrast, of equivalent volume to the amifostine suspension, was instilled endorectally. The prostate, rectum, and bladder were contoured using treatment planning software. The rectum was contoured from the anus (at the level of ischial tuberosities) to a length of 15 cm or until the rectosigmoid flexure could be identified. The clinical target volume (CTV) and the decision to treat the pelvic lymph nodes and seminal vesicles were based on clinical exam findings and whether the risk of involvement was greater than 15% as determined by the Roach equations using Gleason score and pretreatment PSA. 3D-CRT was delivered to all patients. The planning target volume (PTV) was defined as the CTV plus a margin ranging from 0.5 to 1.5 cm at the discretion of the treating physician. The plan was evaluated based on dose-volume-histogram analysis. No more than 25% of the rectal volume was allowed to receive 70 Gy. The prescription dose ranged from 72 to 76 Gy in 2 Gy fractions for most patients. The PTV was covered by at least 97% of the prescribed dose. The 2 post-operative patients received 66 Gy.
Amifostine Application
Amifostine (MedImmune, Inc., Gaithersburg, MD) was reconstituted in saline at a concentration of 50 mg/mL. The first 18 patients enrolled received a 1gm dose (20mL) of Amifostine, while the second cohort was given a 2 gm dose (40mL). Patients were aware of the dose of Amifostine they were receiving, but were not aware of the results of other patients enrolled in the study. Amifostine was administered endorectally at a low pressure using a 60cc syringe 30–45 minutes prior to each daily radiation treatment. The Amifostine preparation used was the same formulation used for intravenous administration and was not specifically formulated for this study. The first 15 patients were alternated between prone and lateral positions every 15 minutes after administration of Amifostine and the remaining 15 patients remained prone for 15 minutes after administration then were allowed to sit upright until radiation treatment. Intrarectal retention of less than 30 minutes was considered inadequate for dosing and documented as such, but this rarely occurred.
On Treatment and Follow-up Evaluations
Patients were evaluated by a physician weekly while on treatment. Upon completion of therapy, follow-up visits occurred at 6 weeks, 3 months, 6 months, then every 6 months until 3 years, and annually thereafter. Formal toxicity measures were assessed and recorded at baseline, at weeks 5 and 7 of therapy, and at each follow-up visit.
Measures of Radiation Related Rectal Injury
At each assessment, an RTOG toxicity score was assigned by the evaluating physician and the EPIC self-assessment questionnaire was completed by the patient. RTOG Acute Radiation Morbidity criteria were used for evaluations conducted during radiation treatment or within the first 90 days following treatment. RTOG Late Radiation Morbidity was used for assessments made greater than 90 days after completion of radiation treatment (See Table 1).
Table 1.
RTOG Toxicity Scoring Criteria
| Acute Toxicity (<120 days) | Late Toxicity (>120 days) | |
|---|---|---|
| Grade 1 | Increased frequency or change in quality of bowel habits not requiring medication
Rectal discomfort not requiring analgesics |
Mild diarrhea or cramping
Bowel movement up to 5 times daily Slight rectal discharge or bleeding |
| Grade 2 | Diarrhea requiring a parasympatholytic drug
Mucous discharge not necessitating sanitary pads Rectal or abdominal pain requiring analgesics |
Moderate diarrhea and colic
Bowel movement >5 times daily Excessive rectal mucus or intermittent bleeding |
| Grade 3 | Diarrhea requiring parenteral support
Severe mucous or blood discharge needing sanitary pads Abdominal distention (flat plate radiograph demonstrates distended bowel loops) |
Obstruction or bleeding requiring surgery |
| Grade 4 | Acute or subacute obstruction, fistula, or perforation
GI bleeding requiring transfusion Abdominal pain or tenesmus requiring tube decompression or bowel diversion |
Necrosis
Perforation Fistula |
The EPIC questionnaire consists of 50 questions divided into four domains (Urinary, Bowel, Sexual, and Hormonal). Within the Bowel Domain, a subset of “Bowel Function” questions target symptom severity while “Bowel Bother” questions assess GI-related quality of life (See Table 2). The questionnaire is scored on a scale of 0–100 with higher scores correlated with higher function and quality of life. For this study, the Bowel Domain was analyzed alongside the RTOG acute and late gastrointestinal morbidity scores. Questionnaires completed by the patients were scored using the SAS software and a scoring macro available from the University of Michigan (http://roadrunner.cancer.med.umich.edu/epic/epicmain.html).
Table 2.
EPIC Bowel Domain Questions
| How often have you had rectal urgency (felt like you had to pass stool, but did nothing) during the past 4 weeks? |
| How often have you had uncontrolled leakage of stool or feces during the past 4 weeks? |
| How often have you had stools (bowel movements) that were loose or liquid (no form, watery, mushy) during the past 4 weeks? |
| How often have you had bloody stools during the past 4 weeks? |
| How often have your bowel movements been painful during the past 4 weeks? |
| How many bowel movements have you had on a typical day during the past 4 weeks? |
| How often have you had cramping pain in your abdomen, pelvis or rectum during the past 4 weeks? |
How big a problem, if any, has each of the following been for you?
|
| Overall, how big a problem have your bowel habits been for you during the past 4 weeks? |
Results of proctoscopic examinations with scoring of mucosal damage were reported using a descriptive scale, described by Wachter et al.(21) Proctoscopic exams were continued until an interim analysis revealed that, in this cohort, this instrument was not useful in the acute setting (15).
Statistical Analysis
Summary statistics, such as sample proportions, means, and median values were used to describe the patient characteristics. A two-sided Fisher’s exact test was used for comparing proportions across groups. A Wilcoxon rank sum test was used to compare medians across groups for continuous variables. All analyses were performed with MATLAB software (The Mathworks, Inc, Natick, MA, USA).
Results
Thirty consecutive patients with localized prostate adenocarcinoma were enrolled on this trial. Median follow-up was 30 months (range 18–36). As shown in Table 1, the 1gm and 2gm groups were similar for most patient, tumor, and treatment characteristics. Median age, however, was 69 and 62 respectively (p=0.01) (Table 3). Amifostine treatment was generally well tolerated. No patient reported any local or systemic toxicity, other than one patient who reported “a metallic taste” following administration of the drug.
Table 3.
Patient Demographics
| 1 gm Group | 2 gm Group | p-value | |
|---|---|---|---|
| Median Age | 69 | 62 | 0.01 |
| Median Follow-up | 19 months | 17 months | NS |
| Clinical Stage T2a or less | 89% (16 of 18) | 83% (10 of 12) | NS |
| Median Gleason Score 6 or less | 22% (4 of 18) | 33% (4 of 12) | NS |
| PSA Less than 10 | 89% (16 of 18) | 83% (10 of 12) | NS |
| Median Radiation Dose (Gy) | 74 | 74 | NS |
| Largest Field Treated | |||
| Prostate + Pelvis | 44% (8 of 18) | 42% (5 of 12) | NS |
| Prostate + Seminal Vesicles | 28% (5 of 18) | 25% (3 of 12) | NS |
| Prostate only | 28% (5 of 18) | 33% (4 of 12) | NS |
| Mean % volume of rectum at 50 Gy | 50 | 45 | NS |
| Mean % volume of rectum at 70 Gy | 17 | 16 | NS |
| Concurrent Hormone Therapy | 56% (10 of 18) | 66% (8 of 12) | NS |
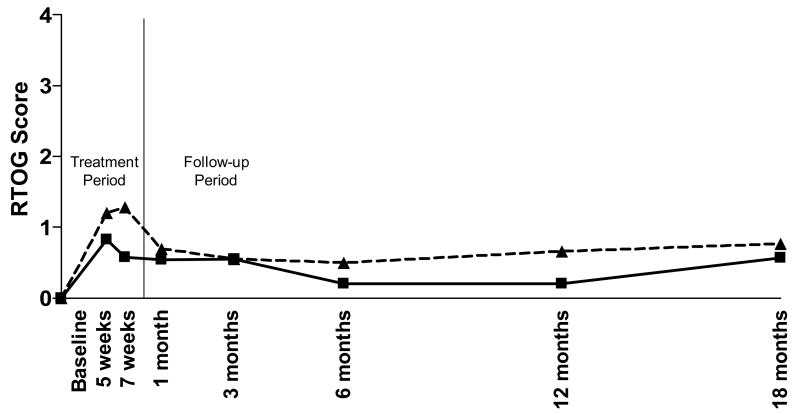
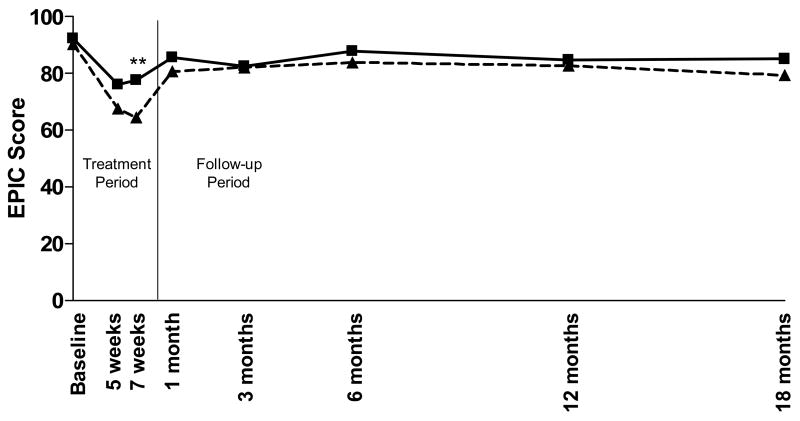
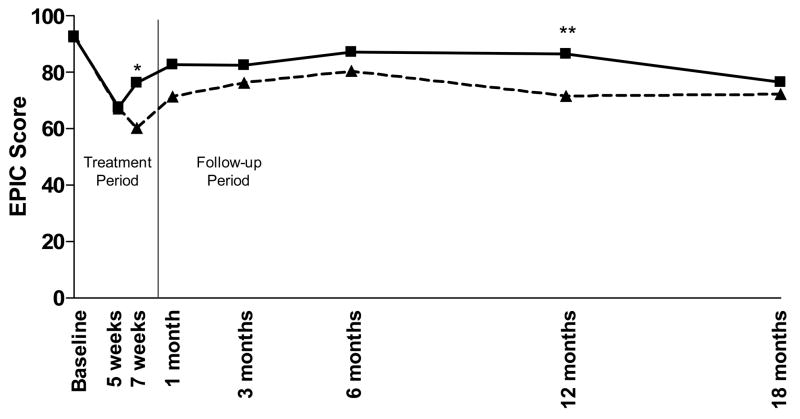
For this analysis, the EPIC Bowel Function (EPIC-BF) composite score, EPIC Bowel Bother (EPIC-BB) composite score, and the acute and late RTOG GI toxicity scores were compared for all enrolled patients. Using both RTOG and EPIC scores, at all assessment points there is a clear trend towards increased rectal protection with the 2 versus 1gm Amifostine. As has been previously reported, 7 weeks after the start of radiation therapy, the incidence of RTOG Grade 2 toxicity was 33% in the 1gm group (6/18) compared with 0% (0/12) in the 2gm group revealing a trend towards increased protection with the 2gm dose (Figure 1) which does not achieve statistical significance (p=0.06).(20) As shown in Figure 2, a significant difference between Amifostine dose groups was observed using the EPIC-BF score at 7 weeks (p=0.04). A difference in EPIC-BB score between dose groups (Figure 3) was also evident at 7 weeks (p=0.07) and was significant at 12 months (p=0.04).
Figure 1. RTOG GI Toxicity Score.
RTOG toxicity was determined at each pre-determined time point for the patients in each group. Mean RTOG scores are presented by dose. Patients who received 1gm Amifostine (---△---) had slightly higher, but not significantly different RTOG scores than the patients who received a 2gm dose (—□—).
Figure 2. EPIC-Bowel Function Composite Score.
EPIC-BF toxicity was determined at each pre-determined time point for the patients in each group. Mean RTOG scores are presented by dose. Patients who received 1gm Amifostine (---△---) had slightly higher, EPIC scores than the patients who received a 2gm dose (—□—). This difference was significant (p=0.04) at the 7-week time point (denoted by **).
Figure 3. EPIC-Bowel Bother Composite Score.
EPIC-BB toxicity was determined at each pre-determined time point for the patients in each group. Mean RTOG scores are presented by dose. Patients who received 1gm Amifostine (---△---) had slightly higher, EPIC scores than the patients who received a 2gm dose (—□—). This difference was nearly significant (p=0.07) at the 7-week time point (denoted by *) and achieved statistical significance (p=0.04) at 12 months (denoted by **).
For each dose group, the mean EPIC scores tend to track each other across time. Further, changes in mean EPIC scores tend to track changes in mean RTOG scores (in a reverse fashion) over time. Further, although a true correlation cannot be calculated since RTOG scores range from 0–4 and EPIC scores range from 1–100, the changes in mean EPIC scores tend to track changes in mean RTOG scores (in a reverse fashion) over time.
Discussion
Our results provide evidence of diminished rectal toxicity with 2 versus 1 gm intrarectal Amifostine. This trend nears significance at the end of therapy (7 weeks) using the RTOG grading system. However, using the EPIC-BF and EPIC-BB scores, at all assessment points there is a clear trend towards improved bowel-related QoL with 2 versus 1 gm intrarectal Amifostine. This trend reached significance (p=0.04) at seven weeks of treatment with the EPIC-BF and at 12 months of follow-up with the EPIC-BB (p=0.04). The improvement in bowel-related QoL with 2 versus 1 gm intrarectal Amifostine appears to be durable at least one year following treatment.
The EPIC score was developed at the University of Michigan as an expansion of the UCLA Prostate Cancer Index and has been validated as a robust instrument that offers a comprehensive assessment of health-related quality of life.(16) EPIC has now been used in several studies to evaluate patient reported health-related QoL outcomes in the management of prostate cancer (17–19).
The findings of this study also suggest that the EPIC score is a sensitive measure to detect changes in both acute and late bowel-related QoL associated with radiation treatment for prostate cancer. The ability to detect even small improvements in radiation-induced side effects is important since it is now clear that treatment of prostate cancer with conventional radiation therapy results in biochemical disease free survival that corresponds predictably to the dose of radiation administered (2, 3). However, increasing radiation dose can produce increased rectal toxicity as well. Acute radiation-induced proctitis can manifest during or after a course of radiation treatment and can include bleeding, rectal pain, mucoid discharge, and fecal urgency. While acute side effects occur within 120 days after completion of treatment, the patient may also experience late radiation-induced proctitis which can be permanent and may include tenesmus, persistent diarrhea, hematochezia, and rectal or anal strictures.
As described more fully in our previous publication (20), several studies have assessed whether the administration of Amifostine prior to radiation therapy confers normal tissue protection. Intravenous Amifostine was shown to decrease the number of patients with RTOG Grade 2 or greater toxicity by approximately 15%, however it is not well tolerated due to hypotension, nausea and rashes (22–25). Subcutaneous administration of Amifostine is better tolerated, but still causes nausea and a fever/rash (7, 23, 26). With regard to rectal mucosal protection, however, subcutaneous administration is inferior to daily topical administration of Amifostine (23).
In a rat model, Ben-Josef et al described preferential accumulation of Amifostine and the active dephosphorylated metabolite in the rectum and not the prostate after intrarectal administration of Amifostine (11). Ben-Josef et al later studied 29 prostate cancer patients treated with Amifostine dose levels ranging from 0.5 to 2.5gm in 40 to 50mL solution, applied before the first 15 radiation treatments. Pharmacokinetic studies were performed on days 1 and 10 of treatment. Neither free parent compound nor free active metabolite was detected in the systemic circulation and, consistent with our findings, there was no systemic toxicity. The authors found that late rectal bleeding developed significantly more often in patients receiving 0.5 to 1gm than in patients receiving 1.5 to 2.5gm Amifostine (50% v 15%; p =0.0325) (12). These results support our finding that 2 versus 1gm of Amifostine has greater efficacy in reducing late radiation proctitis.
Although very few patients experience RTOG Grade 3 or higher toxicity following external beam radiation therapy (27), even mild rectal toxicity can be detrimental to quality of life. Therefore, the efficacy interventions to reduce rectal toxicity must be measured by sensitive quality of life instruments. Traditionally, the RTOG acute and late GI toxicity scores have been assigned by the examining physician. More recently, the EPIC survey has been employed which utilizes a questionnaire completed by the patient allowing for a more comprehensive assessment of the patient’s symptoms and eliminates physician bias. This includes small but important changes in QoL that might not be apparent during brief questioning by a physician or represented by scoring on the broad 0 to 5 RTOG scale.
Perhaps as a result of this ability to discriminate smaller changes, the EPIC-BF and EPIC-BB scores demonstrated significant differences in acute and late GI toxicity in patients treated with 1gm compared to 2gm of Amifostine. Nonetheless, as both the RTOG and EPIC scores are dependent on patient reporting, a high correlation between these measures should be expected and, indeed, was observed in this study.
Conclusion
A higher dose of intrarectal Amifostine, 2gm versus 1gm, produces significant improvements in acute and late bowel QoL as measured by the EPIC score and a trend towards significant improvement in RTOG GI toxicity score. Improvements in QoL persist up to one year following therapy.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Med-Immune contributed the Amifostine for this study, but did not participate in the trial design or in the data analysis.
We would like to thank Edgar Ben-Josef M.D. for input into the study design and for pioneering efforts in this area of research.
Footnotes
Conflict of Interest Notification:
No authors had any conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, et al. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 3.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. Jama. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 4.Sodicoff M, Conger AD, Pratt NE, et al. Radioprotection by WR-2721 against long-term chronic damage to the rat parotid gland. Radiat Res. 1978;76:172–179. [PubMed] [Google Scholar]
- 5.Utley JF, King R, Giansanti JS. Radioprotection of oral cavity structures by WR-2721. Int J Radiat Oncol Biol Phys. 1978;4:643–647. doi: 10.1016/0360-3016(78)90187-6. [DOI] [PubMed] [Google Scholar]
- 6.Stewart FA, Rojas A, Denekamp J. Radioprotection of two mouse tumors by WR-2721 in single and fractionated treatments. Int J Radiat Oncol Biol Phys. 1983;9:507–513. doi: 10.1016/0360-3016(83)90069-x. [DOI] [PubMed] [Google Scholar]
- 7.Koukourakis MI, Kyrias G, Kakolyris S, et al. Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. J Clin Oncol. 2000;18:2226–2233. doi: 10.1200/JCO.2000.18.11.2226. [DOI] [PubMed] [Google Scholar]
- 8.Kligerman MM, Liu T, Liu Y, et al. Interim analysis of a randomized trial of radiation therapy of rectal cancer with/without WR-2721. Int J Radiat Oncol Biol Phys. 1992;22:799–802. doi: 10.1016/0360-3016(92)90527-o. [DOI] [PubMed] [Google Scholar]
- 9.Kuna P. Duration and degree of radioprotection of WR-2721 in mice following its intraperitoneal, intramuscular and subcutaneous administration. Radiobiol Radiother (Berl) 1983;24:357–364. [PubMed] [Google Scholar]
- 10.France HG, Jr, Jirtle RL, Mansbach CM., 2nd Intracolonic WR 2721 protection of the rat colon from acute radiation injury. Gastroenterology. 1986;91:644–650. doi: 10.1016/0016-5085(86)90634-7. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Josef E, Mesina J, Shaw LM, et al. Topical application of WR-2721 achieves high concentrations in the rectal wall. Radiat Res. 1995;143:107–110. [PubMed] [Google Scholar]
- 12.Ben-Josef E, Han S, Tobi M, et al. A pilot study of topical intrarectal application of amifostine for prevention of late radiation rectal injury. Int J Radiat Oncol Biol Phys. 2002;53:1160–1164. doi: 10.1016/s0360-3016(02)02883-3. [DOI] [PubMed] [Google Scholar]
- 13.Delaney JP, Bonsack ME, Felemovicius I. Radioprotection of the rat small intestine with topical WR-2721. Cancer. 1994;74:2379–2384. doi: 10.1002/1097-0142(19941015)74:8<2379::aid-cncr2820740825>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Halberg FE, LaRue SM, Rayner AA, et al. Intraoperative radiotherapy with localized radioprotection: diminished duodenal toxicity with intraluminal WR2721. Int J Radiat Oncol Biol Phys. 1991;21:1241–1246. doi: 10.1016/0360-3016(91)90282-9. [DOI] [PubMed] [Google Scholar]
- 15.Muanza TM, Albert PS, Smith S, et al. Comparing measures of acute bowel toxicity in patients with prostate cancer treated with external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:1316–1321. doi: 10.1016/j.ijrobp.2004.12.083. [DOI] [PubMed] [Google Scholar]
- 16.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 17.Wagner A, Link R, Pavlovich C, et al. Use of a validated quality of life questionnaire to assess sexual function following laparoscopic radical prostatectomy. Int J Impot Res. 2006;18:69–76. doi: 10.1038/sj.ijir.3901376. [DOI] [PubMed] [Google Scholar]
- 18.Merrick GS, Butler WM, Wallner KE, et al. Long-term urinary quality of life after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;56:454–461. doi: 10.1016/s0360-3016(02)04600-x. [DOI] [PubMed] [Google Scholar]
- 19.Kupelian PA, Reddy CA, Klein EA, et al. Short-course intensity-modulated radiotherapy (70 GY at 2. 5 GY per fraction) for localized prostate cancer: preliminary results on late toxicity and quality of life. Int J Radiat Oncol Biol Phys. 2001;51:988–993. doi: 10.1016/s0360-3016(01)01730-8. [DOI] [PubMed] [Google Scholar]
- 20.Singh AK, Menard C, Guion P, et al. Intrarectal amifostine suspension may protect against acute proctitis during radiation therapy for prostate cancer: a pilot study. Int J Radiat Oncol Biol Phys. 2006;65:1008–1013. doi: 10.1016/j.ijrobp.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Wachter S, Gerstner N, Goldner G, et al. Endoscopic scoring of late rectal mucosal damage after conformal radiotherapy for prostatic carcinoma. Radiother Oncol. 2000;54:11–19. doi: 10.1016/s0167-8140(99)00173-5. [DOI] [PubMed] [Google Scholar]
- 22.Athanassiou H, Antonadou D, Coliarakis N, et al. Protective effect of amifostine during fractionated radiotherapy in patients with pelvic carcinomas: results of a randomized trial. Int J Radiat Oncol Biol Phys. 2003;56:1154–1160. doi: 10.1016/s0360-3016(03)00187-1. [DOI] [PubMed] [Google Scholar]
- 23.Kouloulias VE, Kouvaris JR, Pissakas G, et al. Phase II multicenter randomized study of amifostine for prevention of acute radiation rectal toxicity: topical intrarectal versus subcutaneous application. Int J Radiat Oncol Biol Phys. 2005;62:486–493. doi: 10.1016/j.ijrobp.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Liu Y, He S, et al. Use of radiation with or without WR-2721 in advanced rectal cancer. Cancer. 1992;69:2820–2825. doi: 10.1002/1097-0142(19920601)69:11<2820::aid-cncr2820691130>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Schonekas KG, Wagner W, Prott FJ. Amifostine--a radioprotector in locally advanced head and neck tumors. Strahlenther Onkol. 1999;175(Suppl 4):27–29. [PubMed] [Google Scholar]
- 26.Bardet E, Martin L, Calais G, et al. Preliminary data of the GORTEC 2000–02 phase III trial comparing intravenous and subcutaneous administration of amifostine for head and neck tumors treated by external radiotherapy. Semin Oncol. 2002;29:57–60. doi: 10.1053/sonc.2002.37348. [DOI] [PubMed] [Google Scholar]
- 27.Michalski JM, Winter K, Purdy JA, et al. Toxicity after three-dimensional radiotherapy for prostate cancer with RTOG 9406 dose level IV. Int J Radiat Oncol Biol Phys. 2004;58:735–742. doi: 10.1016/S0360-3016(03)01578-5. [DOI] [PubMed] [Google Scholar]