Abstract
Studies on the reinstatement of drug-seeking after withdrawal from chronic drug self-administration have varied in terms of the procedures by which animals are initially trained to self-administer the drug. The current study directly compared whether prior operant training for food pellet reinforcement and/or maintained dietary restriction significantly altered the reinstatement of extinguished cocaine-seeking in the presence of cocaine-paired cues, a priming injection of cocaine (10 mg/kg; i.p.), and the pharmacological stressor, yohimbine (1.25 or 2.5 mg/kg, i.p.). Male Long-Evans rats were divided into four groups as follows: a) trained to lever press for food pellets prior to cocaine self-administration and then maintained on a restricted diet, b) trained to lever press for food pellets prior to cocaine self-administration and then placed on an ad libitum diet, c) untrained and maintained on a restricted diet, or d) untrained and placed on ad libitum feeding. All rats readily self-administered cocaine (0.2 mg/50 µl/infusion) and were subsequently extinguished in the absence of cocaine or previously cocaine-paired cues (light+tone). Following extinction, rats experienced cue-, cocaine-, and yohimbine-induced reinstatement testing. No significant differences were seen between groups for lever responding during the maintenance phase and during extinction. Likewise, reinstatement of cocaine-seeking did not vary across groups for cue-, cocaine-, or yohimbine-induced reinstatement. Under these specific parameters, operant training prior to cocaine self-administration and/or dietary restriction do not significantly alter reinstatement of cocaine-seeking. The results arguably support the approach of not using prior lever training with a non-drug reinforcer and to limit the use of dietary restriction only to the acquisition phase of drug self-administration.
Keywords: Cocaine, Food, Reinstatement, Relapse, Self-administration
1. Introduction
A central feature of addictive disorders is the recurrent relapse to drug-seeking behavior after periods of abstinence from prolonged drug use. Clinical and pre-clinical studies indicate that exposure to drug-associated environmental cues (Childress et al., 1993; See, 2002), stressful stimuli (Shaham et al., 2000; Sinha et al., 2000), and the drug itself (de Wit and Stewart, 1981; Jaffe et al., 1989) are sufficient to produce craving in abstinent addicts and reinstatement of drug-seeking in experimental animals. The extinction/reinstatement paradigm is a preclinical animal model that has been developed to investigate the underlying neurobiological substrates of relapse (Meil and See, 1997; Neisewander et al., 2000; Weiss et al., 2000) and to test putative anti-relapse medications (Baker et al., 2003;Feltenstein et al., 2007; Heidbreder, 2005). In this model, animals repeatedly self-administer a commonly abused substance (e.g., cocaine) over a number of days. Once a steady level of drug self-administration is achieved, drug-seeking behavior is subsequently extinguished through active dissociation between operant responses made to obtain the drug and drug reinforcement. Upon presentation of drug-associated environmental cues, stressful stimuli, or the drug itself, animals will robustly reinstate previously extinguished drug-seeking as measured by increases in responding on the previously drug reinforced lever (Feltenstein and See, 2006; Fuchs et al., 2004b; Meil and See, 1996; Moffett and Goeders, 2007).
Although the reinstatement model is widely used to model various aspects of relapse in humans, a number of possibly critical methodological distinctions exist between laboratories. One issue concerns the procedures for operant training prior to drug self-administration. For instance, prior to i.v. drug self-administration, animals are often initially trained to respond for non-drug reinforcers (e.g., food or sucrose pellets) in order to facilitate the acquisition of operant responding for the drug (Ahmed et al., 2000; Baker et al., 2003; Di Ciano et al., 2001; Meil and See, 1996; Sutton et al., 2003; Weiss et al., 2000). Following a limited training period to respond for food reward, i.v. drug self-administration begins after various intervals. Since the animal has experienced reinforcement for both non-drug and drug, it is possible that lever responses made during subsequent reinstatement testing cannot exclusively be attributed to drug-seeking. Thus, operant training for non-drug reinforcers prior to drug self-administration may confound reinstatement effects under certain circumstances.
An additional methodological issue involves the use of dietary restriction as a method to promote appetitive drug-seeking in the operant context. It has been shown that acute and chronic food restriction (approximately 30 – 40% of free feeding daily ration) augments oral and intravenous drug intake in experimental animals (Carroll et al., 1981; Carroll and Meisch, 1979), possibly through the enhancement of the centrally rewarding pathways that underlie commonly abused drugs (Cabeza de Vaca and Carr, 1998; Carr et al., 2000). In addition to food restriction enhancement of drug-taking, food restriction can also promote enhanced responding during extinction as compared to conditions of no food restriction (Comer et al., 1995). Furthermore, acute (1 day) food restriction and/or deprivation (no food) can act as a stressor to facilitate the reinstatement of cocaine- and heroin-seeking behavior (Carroll, 1985; Shaham et al., 2003; Shalev et al., 2001). It is possible that the intrinsic motivation driven by interoceptive cues to seek food may confound the ability to discern motivation to seek drug after a period of abstinence.
While it has been experimentally demonstrated that food restriction augments drug-taking and drug-seeking, the effects of dietary restriction in conjunction with prior operant training for non-drug reinforcement on reinstatement behavior have not been directly compared. Therefore, the current study assessed whether food restriction and/or prior operant training produced measurable differences in reinstatement to drug-seeking produced by cocaine-paired cues (See, 2002), acute non-contingent cocaine injections (Kippin et al., 2006), or the pharmacological stressor yohimbine, an α2 noradrenergic receptor antagonist which reliably produces reinstatement of drug-seeking (Feltenstein and See, 2006; Shepard et al., 2004). We utilized yohimbine since it robustly increases norepinepherine release in neural structures implicated in stress (Khoshbouei et al., 2002), and potently increases drug-seeking in rats with a prior history of methamphetamine (Shepard et al., 2004), cocaine (Feltenstein and See, 2006), or alcohol (Le et al., 2005) self-administration. Additionally, stress activation by yohimbine has clinical correlates in that it induces craving in drug-dependent human subjects (Stine et al., 2002).
2. Methods
2.1. Subjects
A total of 51 adult male Long Evans rats (Charles River Laboratories, Wilmington, MA) weighing 250–275 g at the beginning of the experiment were used. Upon arrival, rats were handled and given 4–5 days to acclimate to their environment. All animals were individually housed in a climate controlled reverse light/dark cycle room (lights on: 7 am, lights off: 7 pm) and given access to ad libitum food and water until experimental procedures began. All experimental procedures occurred during the dark phase of the cycle. Housing and care of the rats were carried out in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996).
2.2. Experimental groups
Before drug self-administration began, animals were randomly assigned to one of four experimental groups: Untrained/ad libitum diet (UT/AL; n=14), Untrained/restricted diet (UT/R; n=13), Food trained/ad libitum diet (FT/AL; n=12), and Food trained/restricted diet (FT/R; n=12). Animals in the FT/AL and FT/R groups experienced lever response training for food pellets prior to cocaine self-administration and were maintained on either a restricted diet for the duration of the experiment (FT/R), or until levels of responding for cocaine met a pre-set criterion (FT/AL), at which point they were switched to an ad libitum diet for the remainder of the experiment. Animals in the UT/R group did not experience lever training prior to cocaine self-administration, but were maintained on a restricted diet throughout the duration of the experiment. Finally, animals in the UT/AL group did not experience lever training prior to self-administration, and once cocaine-taking behavior was acquired, animals were maintained on an ad libitum diet throughout the duration of the experiment.
2.3. Lever response training for food reinforcement
For those animals that were trained to lever press for food reinforcement (FT/AL and FT/R groups), training occurred in standard self-administration chambers (30×20×20 cm) controlled by a computerized data collection program (MED-PC, Med Associates Inc., St. Albans, VT, USA). Chambers were equipped with two retractable levers, a stimulus light above each lever, a food pellet dispenser between the levers, a speaker linked to a programmable tone generator (ANL-926, Med Associates), and a house light on the wall opposite the levers. Each chamber was contained within a sound-attenuating cubicle equipped with a ventilation fan. For all phases of the study in all four groups, the house light signaled the initiation of the session and remained illuminated throughout the entire session. Rats were food deprived overnight and trained to lever press on a fixed ratio (FR) 1 schedule of reinforcement (45 mg pellets; Noyes, Lancaster, NH, USA) during a 15-h overnight training session in the absence of explicit conditioned stimulus (CS) presentation. Lever presses on an inactive lever were recorded, but had no programmed consequences. Following lever response training, food dispensers were permanently removed from the test chambers.
Additionally, 6 rats that were not trained to lever press for food pellets underwent a 15-h overnight exposure to the self-administration chamber before surgery in order to determine whether the initial context exposure that is involved in operant training affected the outcome of the experimental procedures. The data for these animals was compared to the data of untrained animals that were not exposed to the self-administration chamber for 15-h prior to surgery.
2.4. Surgical procedures
Twenty four h before surgery, all rats were food restricted, but given access to ad libitum water. Rats were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively, IP) followed by equithesin (0.5 ml/kg with a solution of 9.72 mg/ml pentobarbital sodium, 42.5 mg/ml chloral hydrate, and 21.3 mg/ml magnesium sulfate heptahydrate dissolved in a 44% propylene glycol, 10% ethanol solution, IP). Surgical procedures were conducted using aseptic techniques. Catheters were constructed using previously described methods (Fuchs et al., 2004a) and consisted of external guide cannulae with screw-type connectors (Plastics One Inc., Roanoke, VA, USA), Silastic tubing (10 cm; i.d.=0.64 mm; o.d.=1.19 mm; Dow Corning, Midland, MI, USA), prolite polypropylene monofilament mesh (2 cm diameter, Atrium Medical Corporation, Hudson, NH, USA), and cranioplastic cement. The end of the catheter was inserted into the right jugular vein and secured to surrounding tissue with suture. The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades. To maintain patency, catheters were flushed once daily for 4 days after surgery with 0.1 ml each of an antibiotic solution of cefazolin (10 mg/ml; Schein Pharmaceuticals, Florham Park, NJ, USA) dissolved in heparinized saline (70 U/ml; Elkins-Sinn, Cherry Hill, NJ, USA) and heparinized saline. For the duration of the experiment, each rat then received 0.1 ml of heparinized saline (10 U/ml) immediately prior to self-administration sessions and the cefazolin and 70 U/ml heparinized saline regimen following each session. In order to verify catheter patency, rats periodically received a 0.12 ml infusion of methohexital which causes rapid loss of muscle tone when administered intravenously.
2.5. Dietary restriction
Following 4 days of recovery from surgery, and before self-administration procedures began, all rats were given a restricted diet of 20–25 g standard rat chow/day (Harlan, Indianapolis, IN, USA). We selected this restricted diet (which produced approximately 80% free-feeding body weight), since we have previously utilized similar approaches in past reinstatement studies (Feltenstein and See, 2006; Fuchs et al., 2006; Fuchs et al., 2004b; Kippin et al., 2006). Animals in the UT/AL and FT/AL groups were maintained on this diet until they reached a set criterion of 10 infusions of cocaine/session for at least 2 sessions. Most animals reached the pre-set criterion no more than 4 days after the first self-administration session. Once the pre-set criterion was met, UT/AL and FT/AL animals were switched to an ad libitum diet for the remainder of the experiment (i.e., maintenance, extinction, and reinstatement). Rats in the UT/R and FT/R groups were maintained on the restricted diet (20–25 g rat chow/day) for the duration of the experiment.
2.6. Cocaine self-administration
All rats self-administered cocaine (cocaine hydrochloride dissolved in 0.9% sterile saline; cocaine provided by the National Institute on Drug Abuse, Research Triangle Park, NC, USA) during daily 2 h sessions according to an FR 1 schedule of reinforcement. At the start of each session, the catheter was connected to a swivel (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One Inc., Roanoke, VA, USA). The house light signaled the initiation of the session and remained illuminated throughout the entire session across all phases of the study. Lever presses on the active lever resulted in a 2 s activation of the infusion pump (0.2 mg/50 µl/infusion) and a 5 s presentation of a stimulus complex, consisting of activation of the white stimulus light above the active lever and the tone generator (78 dB, 4.5 kHz). After each infusion, responses on the active lever were recorded, but had no consequences during a 20 s time-out period. All responses on the inactive lever were also recorded, but had no programmed consequences. Daily cocaine self-administration continued until each rat had obtained the self-administration criterion of 10 sessions with at least 10 infusions per session.
2.7 Extinction
Following chronic cocaine self-administration and before the first reinstatement test, all rats underwent daily 2 h extinction sessions for a minimum of 7 days. During extinction, rats were returned to the self-administration chamber, but animals were not attached to the leash and the pump was not operated. Responses on both the active and inactive levers were recorded, but had no programmed consequences. Extinction criterion levels were met when rats showed ≤15 active lever responses/session for two sessions or longer. The same extinction criteria applied between reinstatement tests.
2.8. Reinstatement testing
All animals underwent the same reinstatement tests in the same order using a within subjects design. Each reinstatement test was followed by at least 2 days of extinction in which animals met the pre-set criterion of ≤15 active lever responses/session for 2 consecutive sessions or longer before the subsequent reinstatement test occurred.
Conditioned cue-induced reinstatement
Following extinction, rats were returned to the test chamber for a 2 h test session. Responses on the active lever resulted in a 5 s presentation of the previously cocaine-paired light and tone (78 dB, 4.5 kHz); however, no cocaine infusions were received. Active lever responses were followed by a 20 s timeout period where responses were recorded, but did not result in presentation of the stimulus complex.
Cocaine-primed reinstatement
Immediately prior to the test session, rats were injected with a single priming dose of cocaine (10 mg/kg; i.p. dissolved in sterile saline) and placed into the chamber for a 2 h session. Responses on the levers were recorded, but had no programmed consequences.
Yohimbine-induced reinstatement
Yohimbine hydrochloride (1.25 mg/kg and 2.5 mg/kg; i.p. dissolved in ddH2O; Sigma–Aldrich, St. Louis, MO, USA) was administered 30 min prior to reinstatement testing. Doses were chosen based on previous reinstatement studies (Shepard et al. 2004; Feltenstein and See 2006). Thirty minutes post-injection, animals were then placed into the chamber for a 2 h session where responses on both levers were recorded, but had no programmed consequences.
2.9. Data analysis
A two-way repeated measures ANOVA (time × group) was performed to determine differences in responding during the different phases of self-administration and extinction between the four groups. If there was a significant F value for the interaction, Tukey’s test was used for post hoc analyses of differences in responding between separate groups. One-way ANOVAs were used to determine differences in responding between groups for each reinstatement test and paired t-tests were used to measure differences in responding between extinction and reinstatement within subjects. All data was calculated with SigmaStat 3.1 software with alpha set at p <0.05.
3. Results
3.1. Effects of diet restriction on animal weights compared to free feeding animals
Animals in the UT/R and FT/R groups were maintained on a restricted diet of 20–25g of rat chow per day while animals in the UT/AL and FT/AL groups were maintained on an ad libitum diet following acquisition of cocaine self-administration, which occurred no later than days 4 and 5 for most animals. The average weights of food restricted animals vs ad libitum diet animals are represented in Figure 1 across the experiment in 5 day increments. Two way repeated measures ANOVA (time × group) revealed a significant difference in weights between restricted diet and ad libitum diet animals (F8,369=19.10; p<0.001). Tukey’s post hoc analyses revealed significant difference in weights between groups for each timepoint beginning on day 5 (p<0.05), and these differences in weight persisted throughout the duration of the experiment (p<0.01).
Fig. 1.
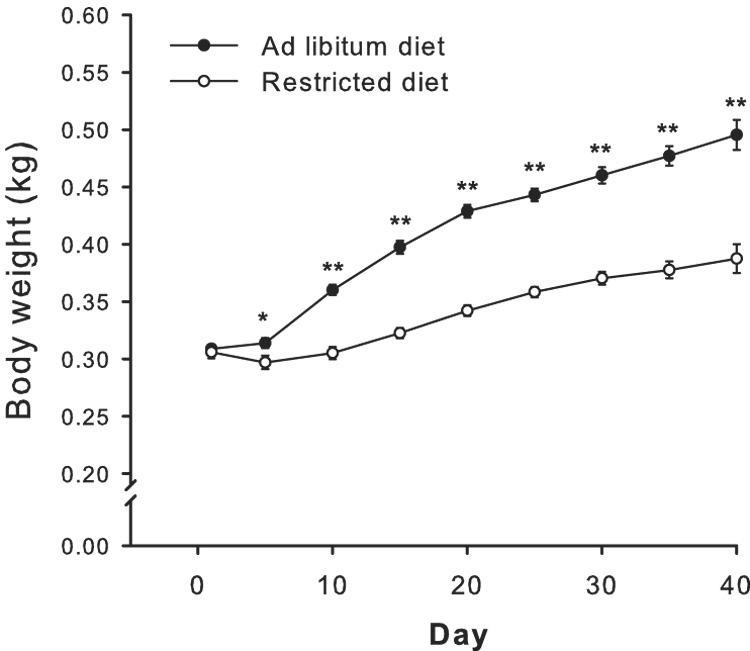
Body weights of food restricted (N=25) and ad libitum (N=26) diet animals across the study. On Day 5 of cocaine self-administration, ad libitum animals demonstrated slightly higher weights than restricted diet animals (*p<0.05). Between days 10–40 of the study (maintenance, extinction, and reinstatement), ad libitum animals maintained substantially greater weights than food restricted animals (**p<0.01). Approximate days of experimental time points are as follows: Day 0, pre self-administration; Days 5–10, maintenance; Days 10–20, Extinction; Days 20–40, reinstatement testing and intervening extinction sessions.
3.2. Effects of prior operant training and dietary restriction on lever responding and total cocaine intake (mg/kg) during self- administration
Acquisition
Prior operant training promoted enhanced responding and cocaine intake (mg/kg) during the acquisition phase (days 1–4) of cocaine self-administration (Fig. 2A and 2B). During this acquisition phase, all groups were maintained on the same restricted diet. Two-way repeated measures ANOVA revealed a significant time × group interaction for cocaine intake (F9,188=2.33, p<0.05), but not for active lever responding (F9,188=1.41, p=0.19). Tukey’s post-hoc analyses revealed significant differences (p<0.05) in cocaine intake between the UT/AL or UT/R groups as compared to the FT/R and FT/AL groups for the first 2 to 3 days of self-administration.
Fig. 2.
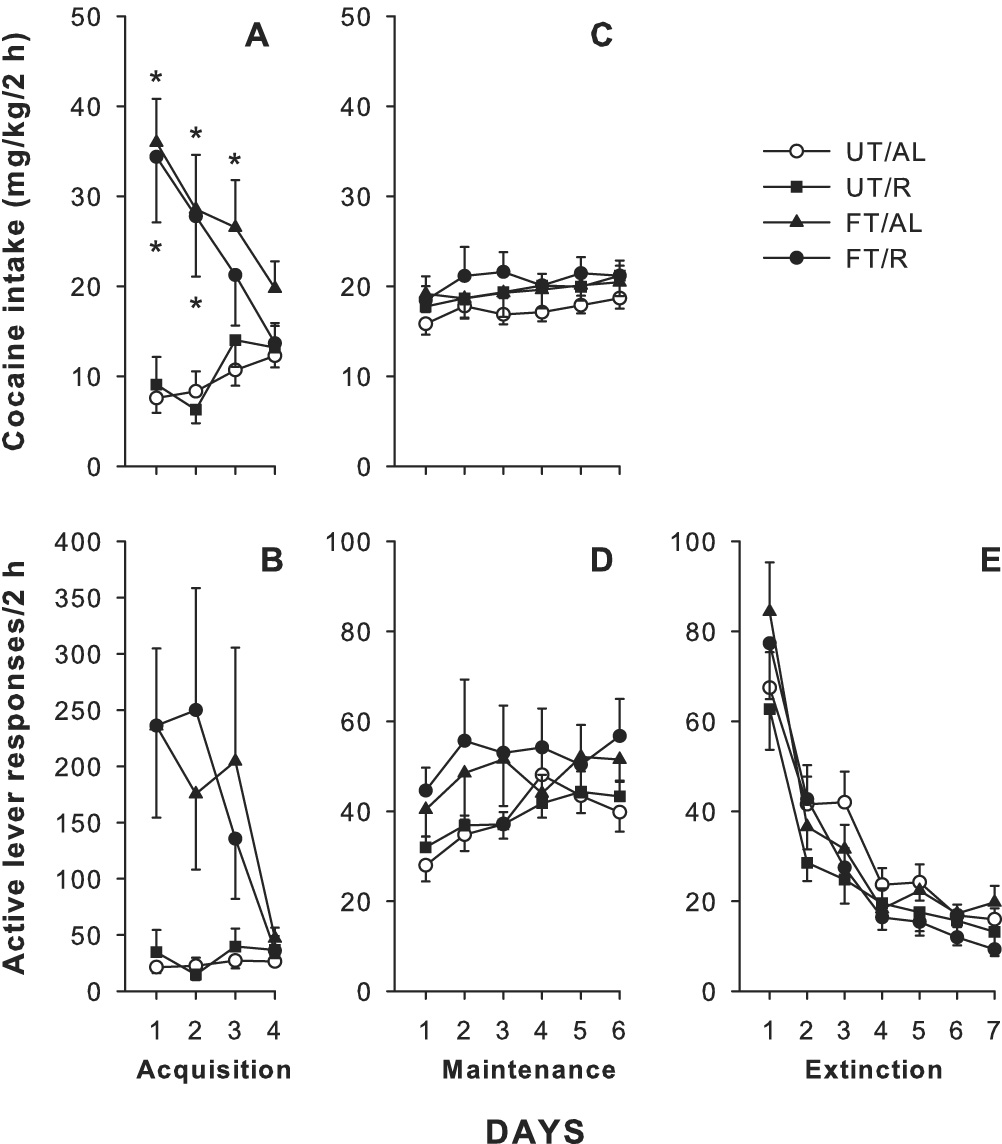
Active lever responding and cocaine intake (mg/kg) during acquisition, maintenance, and extinction. A) Daily cocaine intake during the acquisition phase of cocaine self-administration (Days 1–4). Significant differences between animals previously trained to lever press for food pellets (FT) and those that were untrained (UT) are indicated (*p<0.05). B) Active lever responding during acquisition of cocaine self-administration (Days 1–4). C) Daily cocaine intake during the maintenance phase of cocaine self-administration (last 6 days of cocaine self-administration for each animal). D) Active lever responding during the maintenance phase of cocaine self-administration (last 6 days of cocaine self-administration for each animal). E) Active lever responding during the first 7 days of extinction (note: some animals required additional days to meet extinction criteria which are not represented in this figure).
Maintenance
For the current results, maintenance was defined as the last 6 days of cocaine self-administration for each rat, when relatively stable levels of lever responding and cocaine intake are observed. There were no significant differences in the amount of time spent in the maintenance phase of cocaine self-administration between groups, and all animals included in this study completed cocaine self-administration between days 10 – 14. During the maintenance phase and all subsequent sessions, the ad libitum groups were maintained on a free feeding diet. In contrast to the acquisition sessions, prior operant training and dietary restriction had no significant effects on the maintenance phase of cocaine self-administration after animals had achieved a steady level of responding for cocaine. Rats exhibited stable lever responding (Fig. 2D) and cocaine intake (Fig. 2C), with no significant time × group interaction for active lever responding (F15,269=0.39, p=0.98) or total cocaine intake (F15,269=0.19, p=1.0).
3.3. Effects of prior operant training and dietary restriction on extinction responding
Active lever responding for all groups showed a typical pattern of high responding on the first day of extinction that rapidly dropped over sessions (Fig. 2E). A two-way repeated measures ANOVA revealed that there was no significant time × group interaction during extinction (F18,294=0.910, p=0.57). Extinction criterion for reinstatement testing was met when animals pressed the active lever ≤15 times for at least 2 days. Extinction responding at criterion prior to the first reinstatement test was as follows: UT/AL (11.31±0.96), UT/R (10.04±1.15), FT/AL (10.2±0.97), and FT/R (12.73±0.75). Additionally, there were no significant differences between groups in the number of sessions required to reach extinction criterion (UT/AL=8.17±0.32, UT/R=8.09±0.49, FT/AL=7.67±0.28, and FT/R=8.2±0.42 sessions).
3.4. Effects of prior operant training and dietary restriction on cue-induced, cocaine-primed, and stress-induced reinstatement
Following extinction, rats were exposed to drug-associated cues (light+tone), an acute cocaine priming injection (10 mg/kg; i.p.), and the pharmacological stressor yohimbine (1.25 mg/kg and 2.5 mg/kg, i.p.) in separate tests for reinstatement of extinguished cocaine-seeking. All three modalities produced significant reinstatement of cocaine-seeking, with levels of responding that approximated those seen during the maintenance phase of self-administration (Fig. 3). Paired comparisons revealed that rats in all four groups showed reinstatement of cocaine-seeking relative to their respective extinction levels of responding across all reinstatement stimuli (p<0.01). Furthermore, there were no significant differences in reinstatement responding between untrained animals exposed to the context for 15-h and untrained animals that were not exposed to the context. Importantly, there were no significant differences in lever responding between groups for any of the reinstatement tests conducted (all ANOVAs, p>0.05).
Fig. 3.
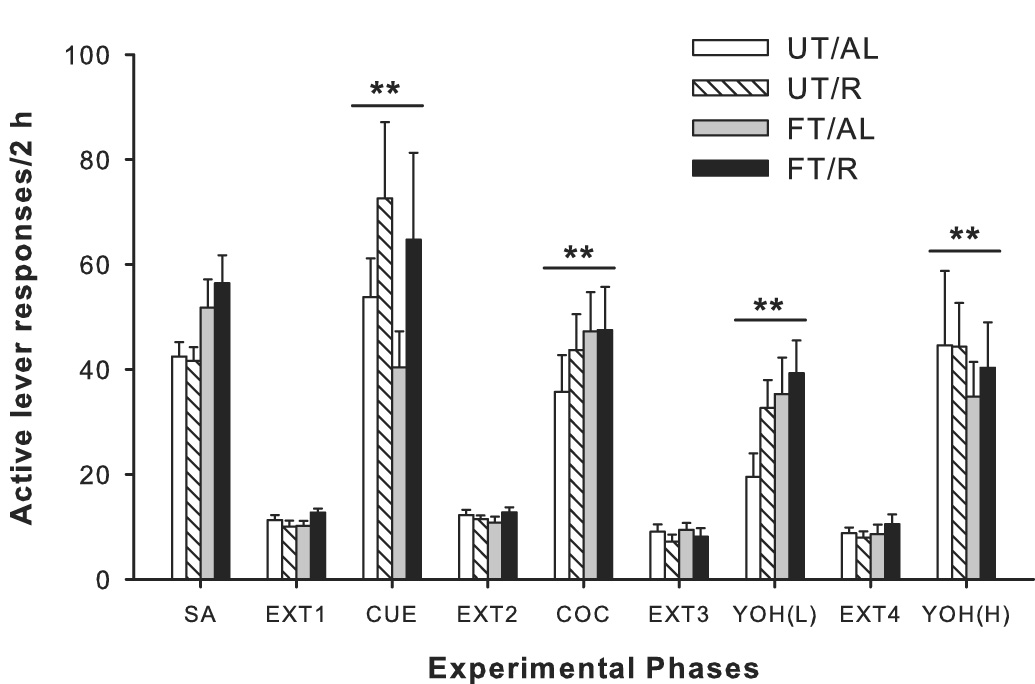
Responding on the previously cocaine-paired lever during reinstatement testing following chronic cocaine self-administration (SA) and extinction (EXT). Values shown for SA are the average active lever responses over the last 3 days of cocaine self-administration. Values shown for EXT represent the last 2 days of extinction training, when the animal met the pre-set criterion. All groups showed significant reinstatement of cocaine-seeking over extinction levels (**p<0.01) after exposure to previously cocaine-paired cues, a cocaine (COC) priming injection (10 mg/kg), and both low (1.25 mg/kg) or high (2.5 mg/kg) doses of yohimbine (YOH). Notably, there were no significant differences in the magnitude of reinstatement between the four groups for any of the reinstatement tests.
4. Discussion
The extinction/reinstatement paradigm is widely used to model various aspects of human relapse (Alleweireldt et al., 2003; See, 2002; Shaham et al., 2003). However, methodological distinctions that exist between different laboratories may influence the outcome of reinstatement behavior. As specifically examined in this study, these distinctions include operant training for food reinforcement prior to drug self-administration and the use of dietary restrictions. Our results showed that operant training for food pellets prior to cocaine self-administration and/or maintained dietary restriction failed to produce significant differences in responding for cocaine under stable maintenance conditions, during extinction, and at the time of reinstatement produced by explicitly cocaine-paired cues, cocaine itself, and the pharmacological stressor, yohimbine. We utilized experimental parameters in the current study that were quite comparable to many previous reinstatement studies (e.g., bolus dose of cocaine, FR1 schedule, duration of self-administration, etc.). It should be emphasized that while reinstatement did not differ between groups in the current study, we cannot rule out the importance of these two variables (prior operant training and maintained dietary restriction) under other conditions of reinstatement (e.g., using other drugs of abuse, other forms of cues or contexts, different schedules of reinforcement, different withdrawal periods, etc.). However, the results suggest that prior operant training with a non-drug reinforcer or maintaining dietary restrictions do not necessarily affect the magnitude of reinstatement of drug-seeking.
The present study utilized rats divided into four different groups: food trained/diet restricted (FT/R), food trained/ad libitum diet (FT/AL), untrained/diet restricted (UT/R), and untrained/ad libitum diet (UT/AL). During the first two days of cocaine self-administration, animals with prior operant training experience for food pellets not surprisingly showed higher rates of active lever responding than the untrained animals (Kruzich et al., 1999). In contrast, the UT/R and the UT/AL groups did not significantly differ from each other, since both groups underwent a similar degree of food restriction during the first 3–4 days of cocaine self-administration. Previous studies have reported enhanced cocaine self-administration under conditions of dietary restriction (Carroll et al., 1981). However, the enhanced cocaine seeking previously reported under dietary restriction was seen when animals had 24-h access to cocaine, and the differences were not clearly evident until the 8th hour of the session (Carroll et al., 1981; Carroll and Meisch, 1981). Our study may have seen no effects of food deprivation on cocaine consumption since we used discrete 2-h self-administration sessions. In line with this possibility, our results are consistent with later studies which have reported that differences in the amount of cocaine that is self-administered during a 2-h session under different satiation states are undetectable (Comer et al., 1995). Therefore, under the specific parameters investigated, operant training prior to cocaine self-administration exerts the greatest influence during the early phases of acquisition of drug self-administration and dietary restriction alone does not seem to greatly influence maintenance of cocaine-taking behavior. It should be emphasized that all groups in this study underwent dietary restriction during early self-administration as previously utilized in self-administration studies (Alleweireldt et al., 2006; Carroll et al., 1981; Zavala et al., 2007). Short term food restriction facilitates exploratory activity in the self-administration chamber and acquisition of drug self-administration. Notably, the most important finding in regards to cocaine-taking was the lack of differences between food-restricted and non-restricted groups during the later phase of cocaine self-administration (stable maintenance). However, while cocaine intake and average active lever responding across the last 6 days of cocaine self-administration was not significantly different between groups, there was a trend towards greater cocaine self-administration in animals with previous lever training experience. Thus, it is possible that prior operant training may exert a continued influence over ongoing cocaine self-administration.
Following daily cocaine self-administration, animals from all four groups showed typical extinction of lever responding over time. Since extinction responding is a measure of drug-seeking, these results show that the various prior regimens in the four groups did not significantly alter drug-seeking under conditions of no reinforcement. While our results showed no effect of prior operant training or food restriction on extinction responding, Comer and colleagues (1995) did report a significant difference in extinction responding among animals with different satiation states. Possible reasons for the differential outcomes between these studies include the major differences in design (a single 7-h within session design of cocaine self-administration, extinction, and cocaine-primed reinstatement on one day vs. daily 2-h sessions spread out over weeks) and the use of different strains of rats (Wistar vs. Long Evans).
Once the responding in the animals met extinction criteria, they were exposed to different stimuli that have been well established to reliably produce reinstatement to drug-seeking, including previously cocaine-paired cues (Lu et al., 2005; Meil and See, 1996; Weiss et al., 2000), a priming injection of cocaine (de Wit and Stewart, 1981; McFarland and Kalivas, 2001), and the á-2 receptor antagonist, yohimbine, which invokes stress-induced reinstatement of cocaine-seeking (Feltenstein and See, 2006; Lee et al., 2004). All rats showed significant reinstatement of cocaine-seeking during all three forms of reinstatement, regardless of prior operant training for food or dietary restriction patterns. Importantly, there were no significant differences between groups in previously cocaine-paired lever responding during any of the reinstatement tests. Therefore, under these specific parameters, differences in operant training prior to cocaine self-administration and/or maintained dietary restriction have no effect on an animal’s propensity to reinstate cocaine-seeking in the presence of cocaine-associated cues, cocaine priming injections, or the pharmacological stressor, yohimbine. It should be noted that Comer and colleagues (1995) found that rats fed after a cocaine prime-induced reinstatement test responded at significantly higher rates than those who were fed before the cocaine prime was administered. The differences with the present results may be related to several paradigm differences, including the histories of the different comparison groups, different rat strains, the immediate feeding schedule around the test time in the earlier study, and the different routes of administration for the cocaine prime (intravenous vs intraperitoneal).
In conclusion, prior operant training and dietary restriction did not influence the outcome of reinstatement of cocaine-seeking behavior under the parameters utilized in this study. While these factors did not affect the outcome of the reinstatement tests, it remains unknown as to whether prior operant training for a non-drug reinforcer and moderate dietary restriction throughout all experimental phases may influence the magnitude of reinstatement responding under different parameters (e.g., under different schedules of reinforcement, different drug doses, other drugs of abuse, different rat strains, etc.). Nonetheless, our manipulations did not produce any significant differences in the maintenance of cocaine self-administration, extinction responding, or reinstatement of cocaine-seeking produced by multiple stimuli. Therefore, we suggest that the best approach in extinction/reinstatement paradigms is to avoid using prior operant training for non-drug reinforcement and to limit the use of dietary restriction to the first few days of drug self-administration (i.e., acquisition). Such an approach reduces the possible confounds of interpretation for responding at the time of reinstatement.
Acknowledgements
We thank Alisha Henderson for providing excellent technical assistance. This research was supported by National Institute on Drug Abuse grants DA016511 (RES), DA010462 (RES), and NIH grant C06 RR015455.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL. Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology. 2006;31:363–374. doi: 10.1038/sj.npp.1300794. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Kirschner KF, Blake CB, Neisewander JL. D1-receptor drugs and cocaine-seeking behavior: investigation of receptor mediation and behavioral disruption in rats. Psychopharmacology (Berl) 2003;168:109–117. doi: 10.1007/s00213-002-1305-x. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Kim GY, Cabeza de Vaca S. Chronic food restriction in rats augments the central rewarding effect of cocaine and the delta1 opioid agonist, DPDPE, but not the delta2 agonist, deltorphin-II. Psychopharmacology (Berl) 2000;152:200–207. doi: 10.1007/s002130000523. [DOI] [PubMed] [Google Scholar]
- Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Depend. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217:241–247. [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Determinants of increased drug self-administration due to food deprivation. Psychopharmacology (Berl) 1981;74:197–200. doi: 10.1007/BF00427092. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Effects of food deprivation on etonitazene consumption in rats. Pharmacol Biochem Behav. 1979;10:155–159. doi: 10.1016/0091-3057(79)90182-5. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Curtis LK, Carroll ME. Food deprivation affects extinction and reinstatement of responding in rats. Psychopharmacology (Berl) 1995;121:150–157. doi: 10.1007/BF02245624. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Changes in dopamine efflux associated with extinction, CS-induced and d- amphetamine-induced reinstatement of drug-seeking behavior by rats. Behav Brain Res. 2001;120:147–158. doi: 10.1016/s0166-4328(00)00373-9. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Altar CA, See RE. Aripiprazole blocks reinstatement of cocaine seeking in an animal model of relapse. Biol Psychiatry. 2007;61:582–890. doi: 10.1016/j.biopsych.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004a;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004b;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder C. Novel pharmacotherapeutic targets for the management of drug addiction. Eur J Pharmacol. 2005;526:101–112. doi: 10.1016/j.ejphar.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA. Behavioral reactivity to stress: amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol Biochem Behav. 2002;71:407–417. doi: 10.1016/s0091-3057(01)00683-9. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2006;187:60–67. doi: 10.1007/s00213-006-0386-3. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Grimm JW, Rustay NR, Parks CD, See RE. Predicting relapse to cocaine-seeking behavior: a multiple regression approach. Behav Pharmacol. 1999;10:513–521. doi: 10.1097/00008877-199909000-00009. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self- administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–180. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]


