Abstract
The breast and ovarian cancer susceptibility protein BRCA1 is evolutionarily conserved and functions in DNA double-strand break (DSB) repair through homologous recombination, but its role in meiosis is poorly understood. By using genetic analysis, we investigated the role of the Caenorhabditis elegans BRCA1 orthologue (brc-1) during meiotic prophase. The null mutant in the brc-1 gene is viable, fertile and shows the wild-type complement of six bivalents in most diakinetic nuclei, which is indicative of successful crossover recombination. However, brc-1 mutants show an abnormal increase in apoptosis and RAD-51 foci at pachytene that are abolished by loss of spo-11 function, suggesting a defect in meiosis rather than during premeiotic DNA replication. In genetic backgrounds in which chiasma formation is abrogated, such as him-14/MSH4 and syp-2, loss of brc-1 leads to chromosome fragmentation suggesting that brc-1 is dispensable for crossing over but essential for DSB repair through inter-sister recombination.
Keywords: Caenorhabditis elegans, meiosis, DNA repair
Introduction
DNA interruptions, including double-strand breaks (DSBs), are caused by endogenous (DNA synthesis, recombination) and exogenous (genotoxic agents) factors. In all organisms, imprecise repair of such discontinuities leads to an assortment of genetic alterations, such as point mutations, deletions and rearrangements. There are two main DSB repair pathways—homologous recombination (HR) and non-homologous end joining (NHEJ) (West, 2003; Wyman & Kanaar, 2006). Homologous recombination is an accurate repair pathway that uses an intact sister/homologue as a template, whereas NHEJ involves direct religation of the DSB and is prone to errors. Failure to repair DSBs accurately is associated with several cancer-predisposition syndromes, such as Fanconi anaemia (FANCD2), Bloom syndrome (BLM) or breast and ovarian cancer susceptibility (BRCA1, BRCA2/FANCD1), which are affected for DSB repair by homologous recombination (Venkitaraman, 2002; D'Andrea, 2003; Wu & Hickson, 2003; Mankouri & Hickson, 2004). As many of the genes in the homologous recombination pathway, including breast and ovarian cancer susceptibility genes BRCA1 and BRCA2/FANCD1 (Martin et al, 2005; Petalcorin et al, 2006), have been conserved during metazoan evolution, their roles and interactions can be investigated in model systems that are suited for the study of DSB repair and meiosis.
The U-shaped gonad of Caenorhabditis elegans contains germ cells, the physical location of which correlates with their stage in meiotic progression, which facilitates the study of the effect of gene depletions at various stages of meiotic prophase (for reviews, see Couteau et al, 2004; Colaiacovo, 2006; Garcia-Muse & Boulton, 2007). During meiotic prophase, DSBs are induced by the conserved meiotic protein SPO-11 (Keeney et al, 1997; Dernburg et al, 1998). The repair of at least one meiotic DSB to generate a crossover between each pair of homologous chromosomes is essential for accurate chromosome segregation at the first meiotic division (Brenner, 1974; Hawley, 1988; Hillers & Villeneuve, 2003). Failure to generate the obligate crossover results in chromosome non-disjunction and aneuploidy in the next generation. Mutants affecting homologous recombination repair of meiotic DSBs show increased embryonic lethality owing to aneuploidy, high incidence of males owing to X-chromosome non-disjunction, anomalies in the number and shape of chromosomes at diakinesis, and altered levels and distribution of RAD-51 foci at the pachytene stage.
A battery of mitotically silent genes, including him-14/MSH4, msh-5, syp-1, syp-2 and him-3, have been described in C. elegans that are involved in directing recombination between homologous chromosomes during meiosis (Zalevsky et al, 1999; Zetka et al, 1999; Kelly et al, 2000; MacQueen et al, 2002; Colaiacovo et al, 2003). Null mutants in these genes show 12 well-structured univalents at diakinesis instead of the 6 bivalents in wild-type (representing homologous chromosomes held together by chiasmata). In these mutants, meiotic DSBs are believed to be repaired using non-crossover pathways, but the factors that promote this type of homologous recombination repair are not known (Colaiacovo et al, 2003).
Studies in mammalian cells have established a role for BRCA1 and its heterodimeric partner BARD1 in DSB repair by homologous recombination (Scully et al, 1997; Moynahan et al, 1999). A role for BRCA1 in meiosis is also suggested from knockout mice that are infertile as a result of pachytene arrest (Xu et al, 2003). Recent studies have shown that BRCA1 has a specialized meiotic role in the XY body that is maintained in a transcriptionally silent state through meiotic sex chromosome inactivation (MSCI; Turner et al, 2004). BRCA1 is required for the localization of ATR and subsequent phosphorylation of H2AX at the XY body, which is important for the establishment of MSCI (Turner et al, 2004). It is clear that BRCA1 functions in both mitotic and meiotic cells, but its contribution to meiotic DSB repair is less well understood. Here, we investigate the contribution of BRCA1 of C. elegans (brc-1) during meiotic recombination by exploiting specific aspects of C. elegans meiosis: meiotic DSBs induced by SPO-11 are not a prerequisite for the assembly of the synaptonemal complex (Dernburg et al, 1998), and axis morphogenesis and synaptonemal complex formation are not required for loading RAD-51 onto DSBs (Colaiacovo et al, 2003). Our data indicate that BRC-1 contributes to meiotic DSB repair by a non-crossover pathway.
Results
Apoptosis and RAD-51 foci increase in the brc-1 mutant
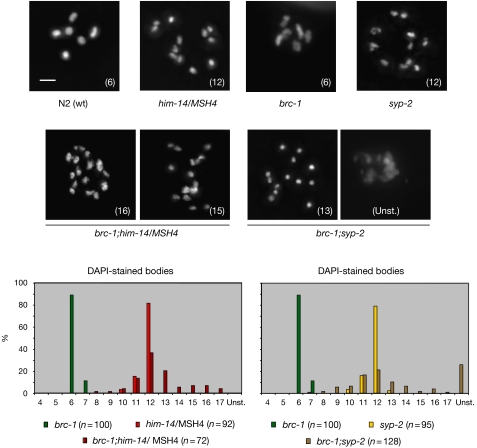
Previous studies have shown that the brc-1 mutant is viable and fertile, but shows a weak meiotic phenotype: the incidence of males (Him phenotype), reflecting the frequency of X-chromosome non-disjunction in C. elegans meiosis, is elevated in the brc-1 mutant (∼2% versus ∼0.1% in wild type; Boulton et al, 2004). However, the levels of embryonic lethality (0.38%) are not significantly increased in the absence of brc-1, indicating that the segregation of autosomes during meiosis occurs as normal. Most of the diakinetic nuclei in brc-1 mutants show the wild-type complement of six 4,6-diamidino-2-phenylindole (DAPI)-stained bivalents at diakinesis, representing homologous chromosomes joined by chiasmata (Fig 1). However, approximately 12% of oocyte nuclei show seven DAPI-stained bodies, which might account for the Him phenotype. Thus, brc-1 is largely dispensable for crossover recombination on the autosomes.
Figure 1.
Increased number of DAPI-stained structures at diakinesis in brc-1;him-14/MSH4 and in brc-1;syp-2 double mutants. Representative images of DAPI-stained oocyte nuclei at diakinesis in the indicated genotypes double mutants. The number in parenthesis within each image indicates the number of DAPI-stained bodies that were detectable through the z stack of the nucleus. Histograms represent quantification of the DAPI-stained bodies. The number (n) of observed nuclei is indicated next to each genotype. The y axis represents the percentage of nuclei in each class and the x axis indicates the number of DAPI-stained bodies. The difference in DAPI-stained bodies between brc-1;him-14/MSH4 and him-14/MSH4, and between brc-1;syp-2 and syp-2 are statistically significant, (see supplementary Table A online). Scale bar, 2 μm. DAPI, 4,6-diamidino-2-phenylindole; Unst, nuclei with unstructured chromatin; wt, wild type.
A possible meiotic defect is also suggested by findings that brc-1 mutants show elevated basal levels of apoptosis of pachytene nuclei (Boulton et al, 2004). To determine whether this apoptotic phenotype is dependent on meiotic DSB formation, we generated a brc-1;spo-11 double mutant. Eliminating meiotic DSB formation with the spo-11 mutation (Dernburg et al, 1998) suppressed the apoptotic phenotype of brc-1 mutants (Table 1). To examine the consequence of eliminating apoptosis in the absence of brc-1, we combined brc-1 with a mutation in ced-3, which is essential for apoptosis (Yuan et al, 1993). A fourfold elevation in embryonic lethality was observed in the brc-1;ced-3 double mutant when compared with the single mutants alone (Table 1), suggesting that in the absence of BRC-1, apoptosis is required to eliminate compromised meiotic cells that are incompatible with producing viable offspring.
Table 1.
Germline apoptosis and embryonic lethality in the brc-1 mutant
| Apoptosis | Lethality | ||||
|---|---|---|---|---|---|
| No. of gonad arms analysed | Apoptotic nuclei | Average apoptotic nuclei/arm | No. of embryos scored | Embryonic lethality (%) | |
| Wild type | 85 | 337 | 3.96 | 1,977 | <0.05 |
| brc-1* | 73 | 563 | 7.71 | 1,048 | 0.38 |
| spo-11 | 82 | 267 | 3.26 | ND | ND† |
| brc-1;spo-11 | 65 | 238 | 3.66 | ND | ND† |
| cep-1/p53 | 89 | 273 | 3.07 | 1,032 | 0.19 |
| brc-1;cep-1/p53 | 94 | 337 | 3.58 | 1,339 | 0.22 |
| ced-3 | 64 | 6 | 0.09 | 1,240 | 1.94 |
| brc-1;ced-3 | 83 | 7 | 0.08 | 1,369 | 9.72 |
| *brc-1 is statistically different from wild type, brc-1;spo-11 and brc-1;cep-1/p53 (Student's t-test; P-value <0.0001). †spo-11 and brc-1;spo-11 embryonic lethality has not been scored as is more than 99% due to progeny aneuploidy. ND, not determined. | |||||
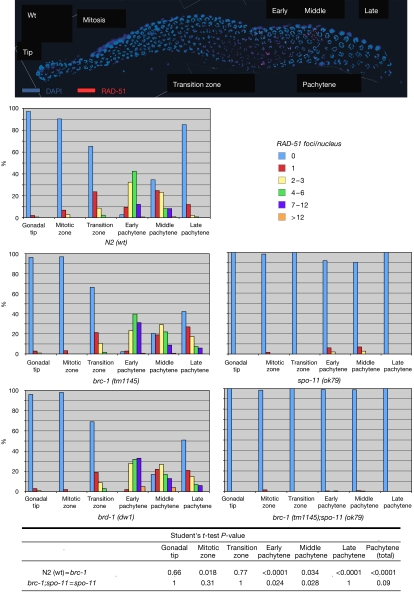
To analyse further a potential meiotic DSB repair defect in brc-1 mutants, we examined RAD-51 foci, which mark homologous recombination events (Alpi et al, 2003; Colaiacovo et al, 2003). The loading of RAD-51 at meiotic DSBs in the early stages of meiotic prophase occurs as normal in brc-1 mutants. However, brc-1 mutants show abnormally high levels of RAD-51 foci in early pachytene nuclei, a phenotype that is suppressed by the spo-11 mutation (Fig 2). A similar elevation in RAD-51 foci is also seen in the meiotic nuclei of brd-1 mutants, which lack the heterodimeric partner protein of BRC-1 (Fig 2; Boulton et al, 2004; Polanowska et al, 2006). These data indicate a role for brc-1 in the efficient repair of a subset of SPO-11-dependent meiotic DSBs.
Figure 2.
brc-1 mutants show spo-11-dependent elevation of RAD-51 foci. Representative image of a wild-type germline immunostained with anti-RAD-51 (red) and DNA counterstained with DAPI (blue). Histograms represent quantification of RAD-51 foci in germlines of animals of the indicated genotypes. The y axis represents the percentage of nuclei with the indicated number of foci. The x axis represents the position (zone) along the germline. Statistical analysis was carried out using the Student's t-test. DAPI, 4,6-diamidino-2-phenylindole; wt, wild type.
BRC-1 acts in DSB repair in the absence of crossovers
In C. elegans, one crossover per homologue pair is necessary and sufficient to generate interhomologue connections irrespective of the length of the chromosome (Hawley, 1988; Edgley & Riddle, 1993; Hillers & Villeneuve, 2003), therefore, it follows that additional DSBs generated by SPO-11 must be repaired by non-crossover pathways. The bias towards the homologue as a homologous recombination template is regulated by pro-crossover factors such as the HIM-14/MSH4–MSH-5 complex and the synaptonemal complex. Mutants in either of these complexes are achiasmate and show 12 intact univalents at diakinesis (Zalevsky et al, 1999; MacQueen et al, 2002). To analyse the potential role of brc-1 in non-crossover DSB repair pathways, we examined the effects of combining the brc-1 mutant with (i) the him-14/MSH4 mutant, in which the synaptonemal complex is present, but crossing over is abolished, and (ii) the syp-2 mutant, in which both synaptonemal complex formation and crossing over are absent (Fig 1). Combining the brc-1 mutation with either him-14/MSH4 or syp-2 mutants resulted in a statistically significant increase in DAPI-stained bodies at diakinesis, which is indicative of chromosome fragmentation (Fig 1; supplementary Table A online). Unlike the brc-1;him-14/MSH4 double mutant, approximately 25% of the observed brc-1;syp-2 nuclei at diakinesis resolve in misshapen, poorly condensed chromatin (Fig 1). This phenotype is similar to that observed in brc-2- and rad-51-deficient strains, which are compromised for repair of all meiotic DSBs through the homologous recombination pathway (Rinaldo et al, 2002). These data indicate that in the absence of crossing over, BRC-1 contributes to meiotic DSB repair by using the sister chromatid as a template.
It is possible that the fragmentation observed in brc-1;him-14/msh-4 and brc-1;syp-2 double mutants might result from premature loss of chromosome cohesion or from a DSB repair defect. To distinguish between these two possibilities, brc-1;him-14/MSH4 and brc-1;syp-2 diakinetic nuclei were immunostained for the meiosis-specific cohesin REC-8 (Pasierbek et al, 2001). In both double mutants, DAPI-stained bodies retained REC-8, indicating that chromosome fragmentation arises as a result of a repair defect rather than the premature loss of cohesion (supplementary Fig A online).
If a non-crossover pathway of homologous recombination is impaired in the absence of brc-1, this defect might be compensated by an elevation in the frequency of crossovers. Therefore, we assayed the frequency of recombination in the brc-1 mutant in two different chromosomal intervals: dpy-5 unc-29 on chromosome I, which spans a region with a low level of recombination (about 1 cM/Mb), and unc-60 dpy-11 on chromosome V, where the frequency of recombination is higher (3.7 cM/Mb). In both intervals tested, the frequency of recombination (3.52% and 19.64%, respectively) was comparable with that observed in the wild type and with the map units reported previously (Edgley & Riddle, 1993). Therefore, brc-1 does not significantly influence either recombination frequency or crossover distribution.
DSBs can be repaired by homologous recombination or by NHEJ. NHEJ is generally believed to act in somatic cells before S phase, but might contribute to meiotic DSB repair when homologous recombination is compromised. To assess the latter, we compared the meiotic outcome of him-14/MSH4;lig-4 (C. elegans DNA ligase-4; Martin et al, 2005) and brc-1;him-14/MSH4 double mutants. In contrast to brc-1;him-14/MSH4, chromosome fragmentation was not observed in him-14/MSH4; lig-4 double mutants. Rather, the diakinetic nuclei in him-14/MSH4;lig-4 showed a pattern similar to that of him-14/MSH4 single mutants (supplementary Fig B online). These data indicate that brc-1 and lig-4 function in different DSB repair pathways. Furthermore, NHEJ has little or no role in meiotic DSB repair in wild-type C. elegans.
Collectively, our results are consistent with a role for BRC-1 in a non-crossover homologous recombination pathway of meiotic DSB repair through inter-sister recombination.
Discussion
Recent evidence indicates that germ cells switch between specific DSB repair pathways depending on their temporal and spatial position in the C. elegans germ line (Hayashi et al, 2007). In the case of meiotic DSB repair, a subset of DSBs per nucleus are selected for repair through crossover recombination, which ultimately produce chiasmata between homologous chromosomes. As the number of SPO-11-induced DSBs created at the onset of meiotic prophase exceed the number of final crossovers, these extra breaks must be repaired using alternative pathways. It is assumed that in the presence of an intact synaptonemal complex and the HIM-14/MSH4-MSH5 complex, excess DSBs are repaired as inter-homologue non-crossovers. Evidence suggests that the bias towards inter-homologue repair is released before the end of pachytene, to ensure that any remaining DSBs are repaired before the first meiotic division. This is based on the observation that mutants that lack the synaptonemal complex, for example, syp-2 mutants, show persistent meiotic DSBs that are ultimately repaired late in meiotic prophase in a rad-51- and rec-8-dependent manner, giving rising to 12 intact univalents (Colaiacovo et al, 2003).
The data presented here support a role for BRC-1 in meiotic DSB repair through inter-sister homologous recombination. This is suggested by the fact that BRC-1 is dispensable for crossover formation, but brc-1 mutants show abnormally high levels of apoptosis and meiotic RAD-51 foci, which are both SPO-11 dependent. In the absence of the synaptonemal complex (syp-2 mutant) where inter-homologue recombination is eliminated, repair of meiotic DSBs can only proceed by inter-sister recombination. The observation that syp-2 mutants show 12 intact univalents, yet brc-1;syp-2 double mutants show a large number of diakinetic nuclei with uncondensed, misshapen chromatin, similar to that previously observed in the HR-deficient mutants rad-51 and brc-2 (Chin & Villeneuve, 2001; Rinaldo et al, 2002; Colaiacovo et al, 2003; Martin et al, 2005), strongly suggests that BRC-1 is required for meiotic DSB repair by inter-sister recombination. Given the subtle meiotic phenotype of the brc-1 single mutant, our data suggest that once the single obligate crossover per homologue pair is generated in C. elegans, most remaining meiotic DSBs are repaired by inter-homologue non-crossover recombination, rather than by inter-sister recombination. A specific role for BRC-1 in DSB repair using the sister chromatid as a template is consistent with the known function of BRCA1 in mitotic cells, where the synaptonemal complex is absent and crossover-promoting genes are not expressed.
Similar to its human counterpart, BRC-1 is recruited to sites of DSBs (Scully et al, 1997; Polanowska et al, 2006), but how BRC-1/BRCA1 promotes DSB repair in mitotic or meiotic cells remains unclear. BRCA1 deficiency in both C. elegans and humans is associated with a DSB repair defect (Moynahan et al, 1999; Bhattacharyya et al, 2000; Boulton et al, 2004). However, one important difference is that BRC-1 is dispensable for RAD-51 recruitment to sites of DSBs (Polanowska et al, 2006). This difference, coupled with our present finding that BRC-1 is required for inter-sister recombination, raises the possibility that BRCA1 might have additional functions in DSB repair independent of its role in recruiting Rad51 to DSBs. As BRC-1/BRCA1 have E3-ubiquitin ligase activity, which is stimulated by DSBs, identification of substrates for this activity might provide important insights into the control of DSB repair (Morris & Solomon, 2004; Polanowska et al, 2006). So far, CtIP and H2AX are the only definitive BRCA1 substrates identified in mitotic cells, whereas substrates in meiotic cells have yet to be found (Yu et al, 2006; Zhao et al, 2007). Recent studies have also revealed connections between BRCA1 and the Fanconi anaemia proteins during the DNA-damage response (Wang, 2007). It will be important to assess possible interactions between BRC-1 and CeFANCD2 during C. elegans meiosis (Collis et al, 2006). These studies might provide mechanistic insight into the function of BRCA1 and FANCD2 that could have important implications for the understanding of genome stability and tumorigenesis.
Methods
DAPI analysis and immunostaining. DAPI-staining, immunostaining and analysis of meiotic nuclei were carried out as described by Colaiacovo et al (2003). Quantitative analysis of RAD-51 foci was carried out on z series of images acquired using a Leica DM5000 fluorescence microscope, Leica DC 350 FX camera under the control of Leica LAS AF 6000 software. Optical sections were collected at 0.25 μm increments. Quantitative analysis of DAPI-staining bodies in diakinetic nuclei was carried out on z series of images (optical sections 0.50 μm increments). The numbers of nuclei scored for genotype are indicated in Fig 1 next to the coloured bar codes.
Apoptosis assay. Germline apoptosis was visualized with SYTO12 (Invitrogen, Molecular Probes, San Giuliano Milanese, Italy) as described by Gumienny et al (1999).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Information
Acknowledgments
We thank S. Mitani and the Caenorhabditis Genetics Center for strains, J. Loidl for REC-8-antibody, A. Sollo for technical support, and P. Bazzicalupo and A. Storlazzi for discussions. This work was supported by grant Telethon GGP04010 to A.L.V. and Cancer Research UK to S.J.B. N.S. is recipient of a predoctoral fellowship from Ministero dell'Università e della Ricerca (Fondo Sostegno Giovani); this work is in partial fulfillment of the requirements for his Doctoral degree in Genetics and Molecular Medicine at the University ‘Federico II'.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alpi A, Pasierbek P, Gartner A, Loidl J (2003) Genetic and cytological characterization of the recombination protein RAD-51 in Caenorhabditis elegans. Chromosoma 112: 6–16 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK (2000) The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem 275: 23899–23903 [DOI] [PubMed] [Google Scholar]
- Boulton SJ, Martin JS, Polanowska J, Hill DE, Gartner A, Vidal M (2004) BRCA1/BARD1 orthologs required for DNA repair in Caenorhabditis elegans. Curr Biol 14: 33–39 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin GM, Villeneuve AM (2001) C. elegans mre-11 is required for meiotic recombination and DNA repair but is dispensable for the meiotic G(2) DNA damage checkpoint. Genes Dev 15: 522–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaiacovo MP (2006) The many facets of SC function during C. elegans meiosis. Chromosoma 115: 195–211 [DOI] [PubMed] [Google Scholar]
- Colaiacovo MP, MacQueen AJ, Martinez-Perez E, McDonald K, Adamo A, La Volpe A, Villeneuve AM (2003) Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev Cell 5: 463–474 [DOI] [PubMed] [Google Scholar]
- Collis SJ, Barber LJ, Ward JD, Martin JS, Boulton SJ (2006) C. elegans FANCD2 responds to replication stress and functions in interstrand cross-link repair. DNA Repair 5: 1398–1406 [DOI] [PubMed] [Google Scholar]
- Couteau F, Goodyer W, Zetka M (2004) Finding and keeping your partner during meiosis. Cell Cycle 3: 1014–1016 [PubMed] [Google Scholar]
- D'Andrea AD (2003) The Fanconi road to cancer. Genes Dev 17: 1933–1936 [DOI] [PubMed] [Google Scholar]
- Dernburg AF, McDonald K, Moulder G, Barstead R, Dresser M, Villeneuve AM (1998) Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94: 387–398 [DOI] [PubMed] [Google Scholar]
- Edgley ML, Riddle DL (1993) The Nematode Caenorhabditis elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Garcia-Muse T, Boulton SJ (2007) Meiotic recombination in Caenorhabditis elegans. Chromosome Res 15: 607–621 [DOI] [PubMed] [Google Scholar]
- Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO (1999) Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126: 1011–1022 [DOI] [PubMed] [Google Scholar]
- Hawley RS (1988) Exchange and Chromosomal Segregation in Eucaryotes, pp 497–527. Washington, DC: American Society for Microbiology [Google Scholar]
- Hayashi M, Chin GM, Villeneuve AM (2007) C. elegans germ cells switch between distinct modes of double-strand break repair during meiotic prophase progression. PLoS Genet 3: e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillers KJ, Villeneuve AM (2003) Chromosome-wide control of meiotic crossing over in C. elegans. Curr Biol 13: 1641–1647 [DOI] [PubMed] [Google Scholar]
- Keeney S, Giroux CN, Kleckner N (1997) Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88: 375–384 [DOI] [PubMed] [Google Scholar]
- Kelly KO, Dernburg AF, Stanfield GM, Villeneuve AM (2000) Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 156: 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen AJ, Colaiacovo MP, McDonald K, Villeneuve AM (2002) Synapsis-dependent and -independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev 16: 2428–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankouri HW, Hickson ID (2004) Understanding the roles of RecQ helicases in the maintenance of genome integrity and suppression of tumorigenesis. Biochem Soc Trans 32: 957–958 [DOI] [PubMed] [Google Scholar]
- Martin JS, Winkelmann N, Petalcorin MI, McIlwraith MJ, Boulton SJ (2005) RAD-51-dependent and -independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol Cell Biol 25: 3127–3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JR, Solomon E (2004) BRCA1:BARD1 induces the formation of conjugated ubiquitin structures, dependent on K6 of ubiquitin, in cells during DNA replication and repair. Hum Mol Genet 13: 807–817 [DOI] [PubMed] [Google Scholar]
- Moynahan ME, Chiu JW, Koller BH, Jasin M (1999) Brca1 controls homology-directed DNA repair. Mol Cell 4: 511–518 [DOI] [PubMed] [Google Scholar]
- Pasierbek P, Jantsch M, Melcher M, Schleiffer A, Schweizer D, Loidl J (2001) A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev 15: 1349–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petalcorin MI, Sandall J, Wigley DB, Boulton SJ (2006) CeBRC-2 stimulates D-loop formation by RAD-51 and promotes DNA single-strand annealing. J Mol Biol 361: 231–242 [DOI] [PubMed] [Google Scholar]
- Polanowska J, Martin JS, Garcia-Muse T, Petalcorin MI, Boulton SJ (2006) A conserved pathway to activate BRCA1-dependent ubiquitylation at DNA damage sites. EMBO J 25: 2178–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C, Bazzicalupo P, Ederle S, Hilliard M, La Volpe A (2002) Roles for Caenorhabditis elegans rad-51 in meiosis and in resistance to ionizing radiation during development. Genetics 160: 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston DM (1997) Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell 88: 265–275 [DOI] [PubMed] [Google Scholar]
- Turner JM, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GV, Barrett JC, Burgoyne PS, Deng CX (2004) BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol 14: 2135–2142 [DOI] [PubMed] [Google Scholar]
- Venkitaraman AR (2002) Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108: 171–182 [DOI] [PubMed] [Google Scholar]
- Wang W (2007) Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat Rev Genet 8: 735–748 [DOI] [PubMed] [Google Scholar]
- West SC (2003) Molecular views of recombination proteins and their control. Nat Rev Mol Cell Biol 4: 435–445 [DOI] [PubMed] [Google Scholar]
- Wu L, Hickson ID (2003) The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature 426: 870–874 [DOI] [PubMed] [Google Scholar]
- Wyman C, Kanaar R (2006) DNA double-strand break repair: all's well that ends well. Annu Rev Genet 40: 363–383 [DOI] [PubMed] [Google Scholar]
- Xu X, Aprelikova O, Moens P, Deng CX, Furth PA (2003) Impaired meiotic DNA-damage repair and lack of crossing-over during spermatogenesis in BRCA1 full-length isoform deficient mice. Development 130: 2001–2012 [DOI] [PubMed] [Google Scholar]
- Yu X, Fu S, Lai M, Baer R, Chen J (2006) BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP. Genes Dev 20: 1721–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR (1993) The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 β-converting enzyme. Cell 75: 641–652 [DOI] [PubMed] [Google Scholar]
- Zalevsky J, MacQueen AJ, Duffy JB, Kemphues KJ, Villeneuve AM (1999) Crossing over during Caenorhabditis elegans meiosis requires a conserved MutS-based pathway that is partially dispensable in budding yeast. Genetics 153: 1271–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetka MC, Kawasaki I, Strome S, Muller F (1999) Synapsis and chiasma formation in Caenorhabditis elegans require HIM-3, a meiotic chromosome core component that functions in chromosome segregation. Genes Dev 13: 2258–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GY et al. (2007) A critical role for the ubiquitin-conjugating enzyme Ubc13 in initiating homologous recombination. Mol Cell 25: 663–675 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information