Abstract
Identification of the molecular targets for post-translational modifications is an important step for explaining the regulated pathways. The ubiquitin-like molecule NEDD8 is implicated in the regulation of cell proliferation, viability and development. By combining proteomics and in vivo NEDDylation assays, we identified a subset of ribosomal proteins as novel targets for the NEDD8 pathway. We further show that the lack of NEDDylation in cells causes ribosomal protein instability. Our studies identify a novel and specific role of the NEDD8 pathway in protecting a subset of ribosomal proteins from destabilization.
Keywords: NEDD8, proteomics, proteasome, ribosomal proteins
Introduction
Despite the close relationship between ubiquitin-like molecules and ubiquitin, modification of proteins by ubiquitin-like molecules has distinct biological effects (Kerscher et al, 2006). Genetic experiments have shown a clear role of the ubiquitin-like molecule NEDD8 in cell proliferation, viability and development. At the molecular level, the most well-characterized substrate for the NEDD8 pathway is the cullin family of proteins (Pan et al, 2004). Cullins are scaffold components of the SCF ubiquitin ligase (Skip-1, cullin, F-box), which controls the ubiquitination and proteasomal degradation of proteins involved in cell-cycle regulation (p27 and cyclin E), transcriptional regulation and signal transduction (IκBα, β-catenin), O2 regulation (HIF1α), and centrosome and cytoskeletal regulation (Pan et al, 2004). NEDDylation of cullins modulates the ubiquitin-ligase activity of the complex, resulting in increased ubiquitination and proteasomal degradation of the substrates (Pan et al, 2004). Recently, the MDM2 oncogene product, the p53 tumour suppressor protein and its homologue p73, the von Hippel–Lindau protein, the epidermal growth factor receptor and the breast-cancer-associated protein 3 were identified as substrates for NEDDylation (Stickle et al, 2004; Xirodimas et al, 2004; Gao et al, 2006; Li et al, 2006; Oved et al, 2006; Watson et al, 2006). This indicates that proteins other than cullins might be targets of the NEDD8 conjugation pathway. Therefore, identification of new NEDD8 substrates will greatly advance our knowledge of the molecular pathways that are regulated by the NEDD8 modification machinery. We performed a proteomic analysis to identify new NEDD8 substrates, and our data show that, among other proteins, 36 small and large ribosomal proteins are potential NEDDylated substrates. Using in vivo and in vitro approaches, we show the covalent modification of five out of six of the ribosomal proteins tested. By inhibiting NEDDylation in cells and blocking the proteasome function, we show that the NEDD8 pathway specifically modulates the stability of ribosomal proteins in cells.
Results And Discussion
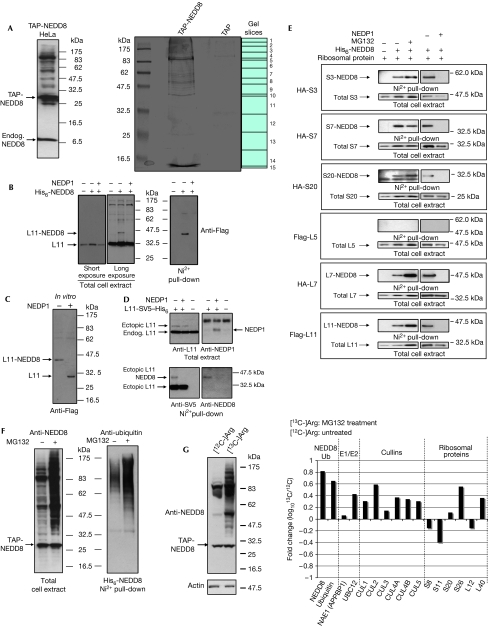
To allow the proteomic identification of proteins modified with NEDD8, we generated stably transformed HeLa cells expressing a tandem affinity purification (TAP)-tagged version of NEDD8 (Fig 1A). We used TAP, which has the advantage of two sequential purification steps, thus providing extra specificity (Rigaut et al, 1999). TAP-NEDD8 conjugates were purified as described in Methods, and a Coomassie blue-stained SDS gel of this purification is shown in Fig 1A. The whole gel track was cut into 15 individual gel slices and each gel slice was analysed for peptide identification by mass spectrometry (MS/MS; see Methods). In this way, we could identify proteins in a defined molecular weight range. As a control, we performed purification using HeLa cells stably expressing the TAP tag only (Fig 1A). Proteins that were present in the TAP-NEDD8 analysis but not in the TAP-only control were selected. From the list of proteins, a range of cullin family proteins and free NEDD8 protein were identified (Table 1). Other components of the NEDD8 conjugation pathway, such as the E2 conjugating enzyme UBC12 and the regulatory subunit of the E1-activating enzyme (NAE1/APPBP1), were also in the list of identified proteins (Table 1). This shows the validity of the purification approach and indicates that the rest of the identified proteins are likely to be NEDD8 substrates.
Figure 1.
Ribosomal proteins are modified with NEDD8 in vivo. (A) HeLa cells stably expressing tandem affinity purification (TAP)-NEDD8 were lysed in 2 × SDS sample buffer and analysed by western blotting with NEDD8 antibodies (left panel). Coomassie blue staining of eluates from either TAP-NEDD8 or TAP-alone stable HeLa cells are shown (right panel). Endog., endogenous. (B) Cells were transfected with Flag-L11, NEDP1 and His6-NEDD8 constructs, as indicated. Twenty per cent of the cells were lysed in 2 × SDS sample buffer and equal amounts of protein were analysed (total extract). The rest of the cells were used for purification of His6-NEDDylated proteins (Ni2+ pull-down). (C) H1299 cells were transfected with His6-NEDD8 and Flag-L11 expression constructs as before. His6-NEDD8 proteins were purified and treated with recombinant NEDP1 protease as described in Methods. L11 was detected with Flag antibodies. (D) H1299 cells were transfected as described in Methods. Cell extracts (upper panel) or Ni2+ pull-down eluates (lower panel) were analysed by western blotting with L11, NEDP1, SV5 or NEDD8 antibodies. (E) H1299 cells were transfected with the indicated expression constructs. Total cell extracts and His6-NEDDylated proteins were analysed as in (B) by western blotting with the appropriate antibodies. HA, haemagglutinin. (F) TAP-NEDD8 HeLa cells were untreated or treated with MG132 before they were collected. Cells were lysed in 2 × SDS sample buffer and analysed by western blotting with NEDD8 antibodies (left panel). A similar experiment performed but transfecting H1299 cells with His6-NEDD8 expression constructs. NEDDylated proteins were purified with Ni2+ agarose and western blotted with ubiquitin antibodies (right panel). (G) Anti-NEDD8 western blot of extracts from TAP-NEDD8 HeLa cells labelled with either [12C]arginine (untreated) or [13C]arginine (MG132) (left panel). Fold change in NEDD8 conjugation after MG132 treatment (right panel). CUL, cullin; NAE1 (APPBP1), NEDD8-activating enzyme E1 subunit 1.
Table 1.
Ribosomal proteins are targets of the NEDD8 pathway
| Identified proteins | Protein score | Queries matched | Acc. no. |
|---|---|---|---|
| Cullin 4A | 2976 | 55 | gi∣5565655 |
| Cullin 4B | 2180 | 42 | gi∣55977850 |
| Cullin 1 | 748 | 37 | gi∣19863257 |
| Cullin 2 | 1736 | 44 | gi∣74744599 |
| Cullin 3 | 534 | 36 | gi∣12643396 |
| Cullin 5 | 530 | 22 | gi∣14917099 |
| NEDD8 | 562 | 6 | gi∣2833270 |
| UBC12 | 297 | 10 | gi∣46577655 |
| NAE1/APPBP1 | 42 | 5 | gi∣50400302 |
| Ribosomal protein L5 | 99 | 8 | gi∣81175191 |
| Ribosomal protein L6 | 73 | 4 | gi∣16753227 |
| Ribosomal protein L7 | 87 | 12 | gi∣15431301 |
| Ribosomal protein L7a | 133 | 7 | gi∣54039239 |
| Ribosomal protein L8 | 127 | 4 | gi∣51702823 |
| Ribosomal protein L9 | 47 | 2 | gi∣15431303 |
| Ribosomal protein L10a | 43 | 3 | gi∣51702773 |
| Ribosomal protein L11 | 137 | 3 | gi∣15431290 |
| Ribosomal protein L12 | 147 | 6 | gi∣4506597 |
| Ribosomal protein L13 | 47 | 4 | gi∣15431297 |
| Ribosomal protein L14 | 54 | 2 | gi∣7513316 |
| Ribosomal protein L17 | 216 | 6 | gi∣36126 |
| Ribosomal protein L18 | 165 | 5 | gi∣4506607 |
| Ribosomal protein L21 | 50 | 3 | gi∣984143 |
| Ribosomal protein L23 | 92 | 6 | gi∣51338639 |
| Ribosomal protein L24 | 169 | 5 | gi∣4506619 |
| Ribosomal protein L26 | 36 | 2 | gi∣47117765 |
| Ribosomal protein L27 | 64 | 8 | gi∣4506623 |
| Ribosomal protein L29 | 41 | 2 | gi∣4506629 |
| Ribosomal protein L30 | 92 | 5 | gi∣4506631 |
| Ribosomal protein L31 | 53 | 1 | gi∣4506633 |
| Ribosomal protein L35a | 49 | 2 | gi∣22002061 |
| Ribosomal protein S2 | 37 | 2 | gi∣15055539 |
| Ribosomal protein S3 | 198 | 14 | gi∣417719 |
| Ribosomal protein S4 | 58 | 9 | gi∣50403628 |
| Ribosomal protein S6 | 143 | 6 | gi∣17158044 |
| Ribosomal protein S7 | 167 | 6 | gi∣551251 |
| Ribosomal protein S8 | 136 | 6 | gi∣50403622 |
| Ribosomal protein S11 | 79 | 9 | gi∣4506681 |
| Ribosomal protein S13 | 40 | 7 | gi∣4506685 |
| Ribosomal protein S14 | 41 | 3 | gi∣5032051 |
| Ribosomal protein S15a | 52 | 5 | gi∣50403624 |
| Ribosomal protein S16 | 48 | 5 | gi∣4506691 |
| Ribosomal protein S20 | 125 | 3 | gi∣46397703 |
| Ribosomal protein S23 | 78 | 2 | gi∣50403755 |
| Ribosomal protein S26 | 73 | 2 | gi∣296452 |
| NAE1/APPBP1, NEDD8-activating enzyme E1 subunit 1. | |||
The complete list of proteins (supplementary Fig S1 online) shows that NEDD8 modification could have a role in processes such as messenger RNA splicing, DNA replication and repair and proteasomal degradation, suggesting a more diverse than previously thought proteome for NEDD8 conjugation. Out of the 75 proteins identified, 36 were small and large ribosomal proteins (Table 1). Ribosomal and NEDD8 protein peptides were identified in gel slices much higher than their predicted molecular weight (slice 1; Fig 1A), further indicating the post-translational modification of ribosomal proteins, possibly with NEDD8. However, the purification method used for the isolation of TAP-NEDDylated proteins cannot exclude the possibility that non-covalent interactors with NEDD8 or with NEDDylated proteins are co-purified. To validate these identifications and to distinguish between covalently modified substrates and possible non-covalent interactors with NEDD8, we used an in vivo NEDDylation assay. This involves coexpression of the protein of interest with a His6-tagged version of NEDD8. After purification of His6-NEDDylated species, modification of the target protein is monitored by western blotting. The purification is performed under highly denaturing conditions to protect the modified substrates from de-conjugation and also prevent any non-covalent interactions. Using this approach, we show in Fig 1B (Ni2+ pull-down) the covalent modification of ribosomal protein L11 with NEDD8. This modification could also be detected in total cell extracts, representing a small fraction of the total pool of L11. Modification of proteins with ubiquitin and ubiquitin-like molecules is a reversible/regulated process due to the action of proteases, which remove the attached molecule from the substrate. Structural and biological studies have shown that NEDP1/DEN1/SENP8 is a highly specific de-NEDDylating enzyme and does not result in deconjugation of ubiquitin or SUMO1 from substrates in vivo (Mendoza et al, 2003; Xirodimas et al, 2004; Reverter et al, 2005; Shen et al, 2005; this study; supplementary Fig S2 online). This provides a highly specific tool to study the role of a conjugation pathway in a biological process. Expression of NEDP1 resulted in the loss of L11 immunoreactivity in the Ni2+ pull-down and of the modified form observed in the total cell extract (Fig 1B). To confirm further that the modified form of L11 isolated in the Ni2+ pull-down is NEDDylated L11, we purified His6-NEDD8 eluates from cells transfected with constructs expressing His6-NEDD8 and Flag-L11 ribosomal protein. These eluates, which potentially contain only the NEDDylated form of Flag-L11, were treated with high-purity recombinant NEDP1. As shown in Fig 1C, treatment with NEDP1 resulted in deconjugation of modified L11, as measured from the shift to the unmodified state. To detect the modification of L11 with endogenous NEDD8, we tagged L11 at its carboxyl terminus with SV5 and His6 (L11-SV5-His6) to enable Ni2+ purification of L11 under highly denaturing conditions, which would favour the detection of the modified form of L11. As shown in Fig 1D (upper panel), under these experimental conditions L11-SV5-His6 was expressed as a small fraction of the endogenous L11. Western blot analysis of the Ni2+-purified L11 with SV5 and NEDD8 antibodies shows L11 modification with endogenous NEDD8 (Fig 1D, lower panel). Coexpression of NEDP1 reduced the levels of the modified form of L11. The above data show the covalent modification of L11 with ectopic and endogenous NEDD8.
Using the in vivo NEDDylation assay and the NEDP1 protease, we tested the NEDDylation of small ribosomal proteins S3, S7 and S20 and large ribosomal proteins L5 and L7, all of which were present in our list of identified proteins. All tested ribosomal proteins with the exception of L5 are modified with NEDD8 (Fig 1E). This strongly suggests that most of our identified proteins are genuine NEDD8 substrates. The lack of NEDDylation for L5 also shows that the list of proteins generated by the TAP-NEDD8 purification might represent not only the covalently modified NEDD8 substrates, but also possibly the non-covalent interactors with NEDD8 or with NEDDylated substrates.
Recent quantitative proteomic studies on the nucleolus showed that the localization of ribosomal proteins is dynamic, responding to different growth conditions. In particular, significant changes in the nucleolar localization of a subset of ribosomal proteins were observed after treatment with transcription inhibitors such as actinomycin D (ActD) and the proteasome inhibitor MG132 (Andersen et al, 2005). Our previous studies showed that inhibition of the proteasome function by MG132 resulted in increased modification of p53 with NEDD8 (Xirodimas et al, 2004). Therefore, we tested whether NEDDylation of ribosomal proteins is also subject to similar regulation. Treatment of cells expressing TAP-NEDD8 with MG132 resulted in a general increase of NEDDylated substrates (Fig 1F). Within this global increase, we observed an increase of NEDDylation for all tested ribosomal proteins with the exception of S7, in which MG132 caused a small decrease (Fig 1E). This indicates a potential link between the levels of NEDDylation of ribosomal proteins and proteasome-mediated degradation and that NEDD8 might also have diverse roles in controlling the function of ribosomal proteins. This potential link between NEDDylation and proteasomal degradation is supported by the observation that, among our identified proteins, ubiquitin was present with a high confidence score. We validated this observation by purifying NEDDylated proteins and western blotting for ubiquitin. As shown in Fig 1F, ubiquitin is present in the NEDDylated eluates and, as with NEDD8, is increased after inhibition of the proteasome with MG132. This is consistent with our previous findings in which NEDDylated p53 was also modified with ubiquitin and suggests that modification of NEDDylated proteins with ubiquitin might be more common than originally suspected. These observations are also consistent with the idea that NEDD8 and ubiquitin might co-regulate proteasomal degradation of a subset of proteins.
The above qualitative changes to ribosomal protein NEDDylation detected by western blotting were also confirmed by quantitative proteomics. We used stable isotope labelling with amino acids in cell culture as a method to quantify changes in the NEDD8 proteome. We metabolically labelled the TAP-NEDD8 HeLa cells with either [12C]arginine or [13C]arginine and these cells were either untreated (12C) or treated with MG132 (13C). Western blot analysis confirmed the global increase in NEDDylation after MG132 treatment (Fig 1G). The samples were then mixed in a 1:1 ratio, and TAP-NEDD8 purification and proteomic analysis were performed as before. Quantification of the data confirmed that both NEDD8 and ubiquitin conjugation are increased after MG132 treatment. An increase in the components of the pathway, such as UBC12, and of the cullin proteins was also observed. However, MG132 had a differential effect on ribosomal protein NEDD8 modification. For the ribosomal proteins presented, for which we obtained peptides in both the 12C and 13C states, MG132 treatment caused an increase in NEDD8 modification for some ribosomal proteins, whereas for some it caused a decrease (Fig 1G). Therefore, the combination of western blot analysis and quantitative proteomics shows that MG132 can differentially affect NEDD8 modification of ribosomal proteins.
The data from the MS/MS analysis can also be analysed to identify modified lysine residues. Trypsin digestion results in peptides in which the modified lysines are linked to a di-glycine (-GG), which is regarded as a modification signature (Kirkpatrick et al, 2005). Our search resulted in the identification of a NEDD8 peptide with a -GG signature on lysine 48 (Lys 48; supplementary Fig S3 online), showing that Lys 48 in NEDD8 can be used for the formation of chains. In ubiquitin, linkage through Lys 48 is regarded as the signal for targeting the modified substrate to the 26S proteasome. However, because NEDD8, ubiquitin and ISG15 all produce the same -GG signature, our data cannot indicate which molecule is attached to NEDD8 Lys 48.
The data presented in Fig 1E suggest a link between NEDD8 conjugation and stability of ribosomal proteins. To gain further insights into the biological role of the endogenous NEDD8 pathway in the stability of ribosomal proteins, we used NEDP1 as a tool to specifically prevent modification of substrates with endogenous NEDD8. As shown in Fig 2A, expression of NEDP1 decreased the levels of ribosomal protein L11. Treatment of cells with MG132 increased the basal levels of L11 and inhibited the decrease caused by NEDP1 expression. In an independent experiment, we addressed the specificity of NEDP1 expression on L11 protein levels by testing the effect of SENP6, a SUMO-specific protease that belongs to the same family of proteases as NEDP1. As shown in Fig 2B, SENP6 (and SENP2 protease; data not shown) had no effect on L11 levels, whereas NEDP1, as before, resulted in reduced levels of L11. To test further the biological significance of the NEDD8 pathway on the levels of L11, we used a well-established biological system (Chinese hamster ovary (CHO), TS-41 cells) in which the APPBP1 gene (its product is one of the components of the NEDD8 E1-activating enzyme) carries a temperature-sensitive mutation. As shown in Fig 2C, growing the TS-41 but not the parental CHO cells at the restrictive temperature (39°C), at which the NEDD8 pathway is defective, resulted in decreased levels of endogenous L11. A similar reduction in L11 levels was observed after knockdown of APPBP1 with short interfering RNA (siRNA; Fig 2D). The above results indicate that prolonged de-NEDDylation in cells results in decreased levels of L11.
Figure 2.
Lack of NEDDylation decreases the levels of L11 ribosomal protein. (A) Flag-L11 and NEDP1 were expressed in H1299 cells, as indicated. Cells were lysed in 2 × SDS sample buffer and equal amounts of protein were analysed by western blotting. Transfection efficiency and loading were monitored by β-gal antibodies. (B) The experiment was performed as above using the SENP6 expression construct. Expression of NEDP1 and SENP6 was monitored with NEDP1 or SV5 antibodies, respectively. (C) TS-41 or the parental Chinese hamster ovary (CHO) cells were grown at 32°C. Fifteen hours before cells were collected, some cells were transferred to 39°C. Extracts prepared in 2 × SDS sample buffer were analysed for endogenous L11 ribosomal protein. (D) H1299 cells were transfected with 20 nM of the indicated siRNA. At 48 h after transfection, cells were lysed in 2 × SDS sample buffer and equal amounts of protein were analysed by western blotting. Endog., endogenous; NAE1/APPBP1, NEDD8-activating enzyme E1 subunit 1; siRNA, short interfering RNA.
To determine whether the reduction of L11 protein levels under these conditions was due to changes in the protein stability of L11, we measured the half-life of L11 using cycloheximide. As shown in Fig 3A, expression of NEDP1 decreased the half-life of L11. We also performed the same experiment for the ribosomal protein L5, which is not modified with NEDD8. Our data show that L5 is a more stable protein than L11 and that expression of NEDP1 did not decrease its half-life (Fig 3A). Similar results were obtained when we measured the half-life of L11 and L5 by [35S]methionine pulse chase (supplementary Fig S4 online). Consistent with the protective role of NEDD8 in ribosomal protein stability, comparison of the half-life of the NEDDylated form with that of the unmodified form of L11 showed that the modified form is more stable (Fig 3B). This also indicates that direct modification with NEDD8 controls the stability of L11.
Figure 3.
De-NEDDylation decreases the half-life of L11. (A) H1299 cells were transfected as indicated. A measure of 30 μg/ml of cycloheximide (CHX) was added and cells were lysed in 2 × SDS sample buffer at the indicated time points. Equal amounts of protein were analysed by western blotting. The signals were quantified and presented as percentage difference in intensity. (B) Flag-L11, His6-NEDD8 and β-gal constructs were transfected and an experiment with CHX was performed as above. Cells were collected and used for either Ni2+ pull-down or direct western blotting. Quantification of signals from five independent experiments was performed as above.
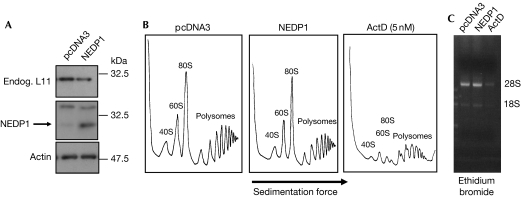
Our findings indicate that the lack of NEDDylation in cells causes protein instability in a subset of ribosomal proteins. To test whether the lack of NEDDylation caused ribosome biogenesis stress, which might decrease ribosomal protein levels, we performed ribosome profiling under de-NEDDylating conditions. For this, we transfected cells with the NEDP1 protease using Fugene to ensure high transfection efficiency (>90%). Consistent with our previous results, expression of NEDP1 reduced the levels of endogenous L11 (Fig 4A). However, under these conditions, we did not observe any significant changes in ribosome profiling (Fig 4B). Similar results were obtained using TS-41 cells at the permissive and restrictive temperature (supplementary Fig S5 online). This is consistent with previous studies in which reduction of the levels of L11 by siRNA did not have any effect on ribosome production (Bhat et al, 2004). As a control, we treated cells with low doses (5 nM) of ActD. At these doses, ActD is believed to specifically inhibit RNA polymerase I and ribosomal RNA production and is predicted to cause ribosome biogenesis stress. As shown in Fig 4B,C, ActD inhibited both rRNA and ribosome subunit production. Therefore, the reduction in the levels of L11 under de-NEDDylating conditions is not due to ribosome biogenesis stress. These data also suggest that, at least for the experimental time frame used in this study, the levels of ribosomal proteins are not a limiting factor for the production of ribosomes.
Figure 4.
NEDP1 does not cause ribosomal biogenesis stress. (A) H1299 cells were transfected with 2 μg of NEDP1 construct or pcDNA3 vector with Fugene. After 36 h, cells were collected and 10% of the cells were used for 2 × SDS lysis and western blotting. Endog., endogenous. (B) Cytoplasmic extracts from the remaining cells were isolated and used for ribosome profiling as described in the Methods (Strezoska et al, 2000). H1299 cells were treated with 5 nM actinomycin D (ActD) overnight before analysis. (C) Cells transfected as in (A) or treated with ActD as in (B) were used for RNA isolation and samples were analysed in an ethidium bromide agarose gel.
Ribosome biogenesis is an active process and it occurs in the nucleolus. Ribosomal proteins are imported from the cytoplasm to the nucleolus, where they form a complex with rRNA to construct ribosome subunits as part of the mature ribosome (Olson & Dundr, 2005; Hernandez-Verdun, 2006). Studies on the dynamics of nucleolar proteins showed that ribosomal proteins are shuttling between the nucleolus and the nucleoplasm (Chen & Huang, 2001). In more recent studies, by combining real-time microscopy and quantitative mass spectrometry, it was shown that the highly mobile fraction of ribosomal proteins in the nucleoplasm is unstable and possibly degraded by the 26S proteasome. It was also shown that, compared with other ribosomal proteins, L5 was stable and showed a slow accumulation in the nucleolus (Lam et al, 2007). Our data support a model in which the NEDD8 pathway protects a group of ribosomal proteins from degradation, possibly through the 26S proteasome. An opposite role for NEDD8 in controlling protein stability is proposed for cullins, in which the lack of NEDDylation protects cullins from degradation (Wu et al, 2006). Our studies suggest that the NEDD8 pathway can have differential roles in regulating the stability of its molecular targets. Identification of the components and signals that control NEDDylation of ribosomal proteins will provide insights into the mechanism by which the NEDD8 pathway protects ribosomal proteins from destabilization.
Methods
In vivo NEDD8 assay. The assay was performed as described previously (Xirodimas et al, 2004). Unless otherwise stated, H1299 cells were transfected with 5 μg of plasmids expressing HA-S3, HA-S7, HA-S20, Flag-L5, HA-L7, Flag-L11, NEDP1 or SENP6, as indicated. A 2 μg portion of His6-NEDD8 was used. His6-NEDDylated proteins were purified as described previously (Xirodimas et al, 2001), and modified target proteins were detected by western blotting using the appropriate antibodies. Where indicated, MG132 was applied at 30 μM for 4 h before cells were collected. For the modification of L11-SV5-His6 with endogenous NEDD8, 4 × 10 cm dishes of 80% confluent H1299 cells were transfected using Fugene 6 with or without 1 μg/plate of L11-SV5-His6 or NEDP1, as indicated. Twenty per cent of the cells were lysed with 2 × SDS sample buffer and the rest were used for Ni2+ purification of L11-SV5-His6.
Knockdown experiments. H1299 cells in 24-well plates were transfected with 20 nM of the indicated siRNA (Dharmacon, Lafayette, CO, USA; ON-TARGETplus SMART pool) with oligofectamine according to the manufacturer's instructions. At 48 h after transfection, cells were lysed in 2 × SDS sample buffer and protein analysis was performed as above.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figures and Information
Acknowledgments
We are grateful to K. Vousden, H. Horn and S. Lain for providing us with valuable reagents. This project was funded by the Biotechnology and Biological Sciences Research Council and the Association for International Cancer Research (AICR). D.P.X. is an AICR Research Fellow.
Footnotes
The authors declare that they have no conflict of interest.
References
- Andersen JS, Lam YW, Leung AK, Ong SE, Lyon CE, Lamond AI, Mann M (2005) Nucleolar proteome dynamics. Nature 433: 77–83 [DOI] [PubMed] [Google Scholar]
- Bhat KP, Itahana K, Jin A, Zhang Y (2004) Essential role of ribosomal protein L11 in mediating growth inhibition-induced p53 activation. EMBO J 23: 2402–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Huang S (2001) Nucleolar components involved in ribosome biogenesis cycle between the nucleolus and nucleoplasm in interphase cells. J Cell Biol 153: 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Cheng J, Shi T, Yeh ET (2006) Neddylation of a breast cancer-associated protein recruits a class III histone deacetylase that represses NFκB-dependent transcription. Nat Cell Biol 8: 1171–1177 [DOI] [PubMed] [Google Scholar]
- Hernandez-Verdun D (2006) Nucleolus: from structure to dynamics. Histochem Cell Biol 125: 127–137 [DOI] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R, Hochstrasser M (2006) Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 22: 159–180 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick DS, Denison C, Gygi SP (2005) Weighing in on ubiquitin: the expanding role of mass-spectrometry-based proteomics. Nat Cell Biol 7: 750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Lamond AI, Mann M, Andersen JS (2007) Analysis of nucleolar protein dynamics reveals the nuclear degradation of ribosomal proteins. Curr Biol 17: 749–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Santockyte R, Shen RF, Tekle E, Wang G, Yang DC, Chock PB (2006) A general approach for investigating enzymatic pathways and substrates for ubiquitin-like modifiers. Arch Biochem Biophys 453: 70–74 [DOI] [PubMed] [Google Scholar]
- Mendoza HM, Shen LN, Botting C, Lewis A, Chen J, Ink B, Hay RT (2003) NEDP1, a highly conserved cysteine protease that deNEDDylates Cullins. J Biol Chem 278: 25637–25643 [DOI] [PubMed] [Google Scholar]
- Olson MO, Dundr M (2005) The moving parts of the nucleolus. Histochem Cell Biol 123: 203–216 [DOI] [PubMed] [Google Scholar]
- Oved S et al. (2006) Conjugation to Nedd8 instigates ubiquitylation and down-regulation of activated receptor tyrosine kinases. J Biol Chem 281: 21640–21651 [DOI] [PubMed] [Google Scholar]
- Pan ZQ, Kentsis A, Dias DC, Yamoah K, Wu K (2004) Nedd8 on cullin: building an expressway to protein destruction. Oncogene 23: 1985–1997 [DOI] [PubMed] [Google Scholar]
- Reverter D, Wu K, Erdene TG, Pan ZQ, Wilkinson KD, Lima CD (2005) Structure of a complex between Nedd8 and the Ulp/Senp protease family member Den1. J Mol Biol 345: 141–151 [DOI] [PubMed] [Google Scholar]
- Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B (1999) A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol 17: 1030–1032 [DOI] [PubMed] [Google Scholar]
- Shen LN, Liu H, Dong C, Xirodimas D, Naismith JH, Hay RT (2005) Structural basis of NEDD8 ubiquitin discrimination by the deNEDDylating enzyme NEDP1. EMBO J 24: 1341–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickle NH, Chung J, Klco JM, Hill RP, Kaelin WG Jr, Ohh M (2004) pVHL modification by NEDD8 is required for fibronectin matrix assembly and suppression of tumor development. Mol Cell Biol 24: 3251–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strezoska Z, Pestov DG, Lau LF (2000) Bop1 is a mouse WD40 repeat nucleolar protein involved in 28S and 5.8S rRNA processing and 60S ribosome biogenesis. Mol Cell Biol 20: 5516–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson IR, Blanch A, Lin DC, Ohh M, Irwin MS (2006) Mdm2-mediated NEDD8 modification of TAp73 regulates its transactivation function. J Biol Chem 281: 34096–34103 [DOI] [PubMed] [Google Scholar]
- Wu JT, Chan YR, Chien CT (2006) Protection of cullin-RING E3 ligases by CSN-UBP12. Trends Cell Biol 16: 362–369 [DOI] [PubMed] [Google Scholar]
- Xirodimas D, Saville MK, Edling C, Lane DP, Lain S (2001) Different effects of p14ARF on the levels of ubiquitinated p53 and Mdm2 in vivo. Oncogene 20: 4972–4983 [DOI] [PubMed] [Google Scholar]
- Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP (2004) Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell 118: 83–97 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Information