This Cold Spring Harbor meeting on Neurobiology of Drosophila took place between 3 and 7 October 2007, in New York, USA, and was organized by A. Kolodkin and A. Sehgal.
Introduction
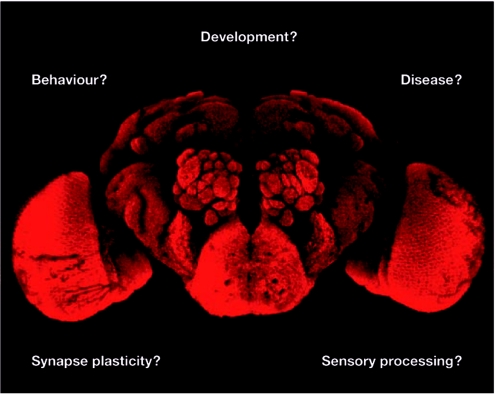
Studies of the tiny fruit fly, Drosophila melanogaster, have heralded remarkable discoveries ranging from the genetic basis of heredity to the development of the animal body plan. An outstanding mystery that drives neuroscience research today is how the brain encodes cognition and behaviour. The beautifully organized brain architecture, the relatively small number of neurons, and the molecular, genetic, cellular and behavioural approaches available to dissect brain development and processing in Drosophila, argue that the fly brain could be the place to find some answers (Fig 1).
Figure 1.
Questions in Drosophila neuroscience. This meeting presented findings on many of the interesting questions shown above. A whole-mount image of the adult Drosophila central brain region, counterstained with the nc82 antisera to visualize different lobes is shown.
At the 2007 ‘Neurobiology of Drosophila' meeting at Cold Spring Harbor Laboratory, New York, USA, approximately 425 fly neurobiologists gathered to brainstorm. As in the past, continuing advances in the field addressed the molecular mechanisms underlying cell fate decisions, axon guidance, and synapse formation and function. In addition, this year marked an intensification of interest in the molecular and genetic bases of behaviour and sensory processing, and in the use of Drosophila to model human neurodegenerative disorders.
Brain development: axon guidance, synapses and glia
The elaborate and precise patterns of neural connectivity that arise during development dictate brain function and behaviour. How correct connections are formed and maintained—but allow for plasticity—are crucial questions in neural development. The larval neuromuscular junction (NMJ), the midline of the embryo and the developing visual system are being used to study connectivity and function, and the signalling pathways mediating these processes are being revealed in detail.
Identification of the molecular components involved in axonal pathfinding is essential for understanding how connectivity is coordinated to form the Drosophila nervous system. At the larval NMJ, motor neurons synapse in a stereotypical fashion on muscle fibres of the body wall. Several molecules have a role in synapse targeting, including Capricious, a leucine-rich repeat protein involved in homophilic interactions (Shishido et al, 1998). K. Zinn (Pasadena, CA, USA) presented data suggesting a role for Tartan, another leucine-rich repeat protein, in correct synaptic targeting of axons. The developing visual system has also proven to be a useful model for studying axon guidance. The R7 and R8 photoreceptors terminate in different layers of the medulla, with R8 arriving first. The transcription factor Sequoia is necessary for this targeting event (Brenman et al, 2001). M. Petrovic (Münster, Germany) presented data showing that Sequoia is expressed at different times in R7 and R8 cells, which corresponds to the period when axonal targeting occurs.
The larval NMJ has also been an attractive model system to study the details of synaptic growth and plasticity. Development of NMJ synapses is controlled by several signalling cascades, including the bone morphogenetic protein (BMP) pathway, which promotes synaptic growth, and the Nervous wreck (Nwk) and Highwire (Hiw) pathways, both of which inhibit synaptic growth (Collins & DiAntonio, 2007). Data presented by K. O'Connor-Giles (Madison, WI, USA) suggest that Nwk interacts with members of the BMP pathway and inhibits BMP signalling. By contrast, the Hiw pathway negatively restricts synaptic growth by reducing the levels of Wallenda, acting through the c-Jun amino-terminal kinase (JNK) pathway to regulate d-Fos expression (Collins et al, 2006). C. Wu (St Louis, MO, USA) presented data that the F-box protein DFsn is a Hiw-binding protein required for maintaining appropriate growth (Wu et al, 2007).
Research on glial development has also become prominent in recent years. In the embryo, specification of all glial cells—except midline glia—is dependent on the transcription factor Glial cells missing (Gcm; Hosoya et al, 1995; Jones et al, 1995; Vincent et al, 1996). The mechanisms underlying post-embryonic gliogenesis are just beginning to be elucidated. A. Giangrande (Strasbourg, France) showed that ectopic expression of Gcm triggers gliogenesis at the expense of neurogenesis and suggested that epigenetic mechanisms underly this change in cell identity. T. Awasaki (Worcester, MA, USA) examined the mechanism of post-embryonic gliogenesis during normal development. He proposed that glial precursors proliferate locally during larval development and migrate throughout the brain during pupation to give rise to adult glia. Understanding how post-embryonic gliogenesis occurs and whether it is mechanistically similar to embryonic gliogenesis will reveal how neuronal and glial development is coordinated throughout the Drosophila life cycle.
Sensory systems from detection to behaviour
Sensory neurons translate the detection of a cue in the external environment into a neural code that is processed by the brain to produce behaviour. In flies, as in mammals, the visual system has historically been the focus of much research, providing fundamental insights into photoreceptor development, signal transduction and learning. More recently, the powerful molecular tools available in Drosophila have been brought to bear on the olfactory, gustatory and mechanosensory systems. Research presented at the meeting was largely focused in these areas, with olfaction dominant. Two main areas of active investigation are the molecular mechanisms of sensory detection and the initial events in sensory processing.
The molecules that sensory neurons use to detect features of the outside world define and limit the sensations of an animal. The discovery of large families of Drosophila olfactory and gustatory receptors in the past decade has provided insight into how receptor cells detect chemical cues. In the gustatory system, the gustatory receptor Gr5a marks sugar-sensing sensory neurons (Marella et al, 2006). Work presented by A. Dahanukar (New Haven, CT, USA) showed that many gustatory receptors are expressed in sugar-sensing neurons, and highlighted Gr64a and Gr5a as essential for sugar detection (Dahanukar et al, 2007), which is consistent with recent studies (Jiao et al, 2007; Slone et al, 2007). Pheromones in Drosophila are also detected by members of the olfactory and gustatory receptor families. Recent work has shown that the odorant-binding protein, Lush, and the olfactory receptor, Or67d, act together to detect the pheromone cis-vaccenyl acetate (cVA) involved in courtship and aggregation (Ha & Smith, 2006; Kurtovic et al, 2007). Research presented by D. Smith (Dallas, TX, USA), and recent studies (Benton et al, 2007), now identify a CD36 homologue, Sensory neuron membrane protein (Snmp), as acting with Lush and Or67d for cVA detection. These studies suggest that many proteins function, in addition to odorant receptors, for ligand detection.
How do receptor–ligand interactions lead to the detection and discrimination of sensory cues? Studies in the olfactory system of larvae and adults are beginning to yield some surprising answers. Molecular genetic approaches have been used to create larvae and flies with few functional classes of olfactory receptor neurons (Fishilevich et al, 2005). Behavioural studies of these flies and larvae were presented at the meeting. Previous studies have shown that larvae have robust chemotaxis (Fishilevich et al, 2005), and the new research shows that adults can discriminate two odours fairly well (S. DasGupta, Worcester, MA, USA). These studies argue that small numbers of olfactory neurons are sufficient to perform sophisticated olfactory behaviours to several odours.
As our understanding of sensory detection in the periphery rapidly advances, efforts to understand higher-order sensory processing are continuing to emerge. The olfactory system, with its beautifully organized primary relay, the antennal lobe, and well-described sensory and second-order projections, lends itself to studies of sensory processing. V. Bhandawat (Boston, MA, USA) presented electrophysiological data showing that second-order neurons are more broadly tuned to odours compared with the tuning of sensory neurons, and suggested that this transformation enhances odour discrimination (Bhandawat et al, 2007). Although connectivity is less well understood in the gustatory system, M. Gordon (Berkeley, CA, USA) presented a neural silencing behavioural screen combined with functional calcium imaging that enabled the isolation of candidate motor neurons involved in taste behaviours. Functional imaging studies in mushroom body neurons argue that they respond to mechanical stimuli in addition to olfactory cues (A. Mamiya; Cold Spring Harbor, NY, USA), suggesting that this brain region participates in multimodal sensory processing, similar to other insects. These studies provide a glimpse of an exciting future integrating electrophysiological and molecular studies to characterize neurons in a defined network to study the brain and behaviour.
Molecules and neurons mediating behaviours
Behavioural genetics is an ever-expanding field that was strongly represented at the meeting. The talks focused on a wide variety of innate behaviours, including courtship behaviour, feeding behaviours, aggression, egg-laying and circadian rhythms. In addition, conditioned behaviours involving learning and memory were discussed.
K. Furukubo-Tokunaga (Tsukaba, Japan) presented his work on a larval model for olfactory learning, which confirmed a requirement for the dopaminergic and octopaminergic systems in aversive and appetitive conditioning, respectively. This was one of the few presentations on learning and memory at this year's meeting, although this is a long-standing field in Drosophila neurobiology.
A behavioural system that enjoyed plenty of exposure was the circadian clock. Y. Fang (Philadelphia, PA, USA) described a new role for Protein phosphatase 1 (Pp1) in regulating the activity of both Period and Timeless (Tim), showing that Tim is a direct substrate of Pp1 dephosphorylation (Fang et al, 2007). Y. Huang (Boston, MA, USA) examined the control of physiological and behavioural outputs of the cellular clock by studying a crucial intermediary—the Lark RNA-binding protein. By using a biochemical approach, Huang identified Lark target RNAs and showed that one target, the E74 gene, links the circadian clock to the timing of eclosion (Huang et al, 2007). Neurons expressing the Pigment dispersing factor (Pdf) neuropeptide are also crucial in regulating the sleep–wake cycle, and M. Ceriani (Buenos Aires, Argentina) showed that mutants in the potassium channel Slowpoke (Slo) affected both the distribution of Pdf and the anatomy of the source neurons (Fernandez et al, 2007). Together, these studies show significant interest in characterizing the molecules and neurons underlying circadian behaviours.
One clear theme that emerged from the behavioural studies is that the activation of neural subsets is a powerful complement to genetics and neural silencing in studying circuits that control behaviour. Channelrhodopsin-2 and the purinoreceptor P2X2 have been used successfully in flies for light activation (Lima & Miesenbock, 2005; Schroll et al, 2006) and D. Bucher (Wurzburg, Germany) showed that photoactivated adenylyl cyclase is also a potent inducible activator. In separate talks, light activation of specific neurons was shown to control several behaviours, from larval nociception (Hwang et al, 2007) to proboscis extension (M. Gordon, Berkeley, CA, USA). These results show that behavioural programmes can be elicited by artificially activating discrete neural populations. This approach will undoubtedly lead to further understanding of how information flows through neural circuits and drives particular behaviours.
Drosophila as a model for neurodegenerative diseases
The cellular events underlying neurodegeneration are of great interest, given their causal role in devastating conditions such as Alzheimer, Parkinson and Huntington diseases. Flies have become an effective model system for studying these processes owing to the conservation of crucial molecules involved and the toxicity of human disease genes in Drosophila neurons. The presentations at this meeting showed that flies continue to provide insight into molecular pathways controlling the transition between normal and diseased states in the nervous system.
Neural degeneration can result from protein toxicity or from loss-of-function mutations. Polyglutamine diseases comprise a significant class of conditions in which CAG repeat expansion in the disease protein confers toxicity. Overexpression of the causal protein in spinocerebellar ataxia type 3 disease produces neurodegeneration in the fly (Warrick et al, 2005). J. Jung (Philadelphia, PA, USA) extended this disease model by showing that triplet repeat expansion increases in successive generations and is promoted by transcription of the disease gene (Jung & Bonini, 2007). L. Keegan (Edinburgh, UK) presented his work on the RNA-editing protein Adenosine deaminase acting on RNA (Adar). Mutations in the fly version of this gene lead to neurodegeneration and severe behavioural deficits (Palladino et al, 2000). This pathology can be rescued by the expression of human forms of the Adar gene, and the Edinburgh group used this system to uncover functional differences between three human isoforms and identify crucial RNA substrates of Adar. J. Mast (Stanford, CA, USA) used a loss-of-function mutation in a mitochondrial enzyme to induce mild mitochondrial dysfunction. This resulted in a neurodegeneration phenotype and Mast presented evidence that the build-up of reactive oxygen species has a role in this pathology.
In addition to processes intrinsically affecting neurons, there was also interest in studying the role that central nervous system glia can have in disease pathology. For example, during developmentally programmed or injury-induced neuronal cell death, glia clear apoptotic fragments (Hoopfer et al, 2006; MacDonald et al, 2006). The engulfment receptor Draper is known to mediate this process and E. Kurant (New York, NY, USA) presented evidence that a related receptor is also required. Glia can also have a protective role, which includes the formation of a blood–brain barrier to limit access of haemolymph-borne molecules to the central nervous system. R. Bainton (San Francisco, CA, USA) presented his work on an ABC-B transporter required for the function of the blood–brain barrier, which revealed similarities to the molecules that are involved in the vertebrate blood–brain barrier.
Conclusions
Drosophila neurobiology continues to advance our understanding of the molecules that mediate brain development and function. Many of these genes are conserved from flies to humans, arguing that these studies will have a significant impact on our understanding of human health and disease. Although the molecular mechanisms underlying processes as diverse as axon guidance, sensory detection and circadian rhythms are rapidly being revealed, an understanding of the assembly and function of neural circuits is incomplete. A shift from studies of single genes to studies of neural ensembles in the future might provide a more holistic understanding of how the underlying neural architecture of the fly brain arises in development and allows for a sophisticated repertoire of behaviours.
From left: Michael Gordon, Andrea Manzo & Kristin Scott
Acknowledgments
We thank A. Kolodkin and A. Sehgal for organizing the meeting. We thank the participants who allowed us to discuss unpublished findings, and apologize to those we could not cite because of space limitations. The meeting was funded in part by the National Institute of Neurological Disorders and Stroke. M.D.G. is supported by the Damon Runyon Cancer Research Foundation. K.S. is supported by a grant from the National Institute on Deafness and other Communication Disorders (NIDCD), a BurroughsWellcome Fund Career Award, a McKnight Scholar Award and a John Merck Award.
References
- Benton R, Vannice KS, Vosshall LB (2007) An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 450: 289–293 [DOI] [PubMed] [Google Scholar]
- Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI (2007) Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci 10: 1474–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Gao FB, Jan LY, Jan YN (2001) Sequoia, a tramtrack-related zinc finger protein, functions as a pan-neural regulator for dendrite and axon morphogenesis in Drosophila. Dev Cell 1: 667–677 [DOI] [PubMed] [Google Scholar]
- Collins CA, DiAntonio A (2007) Synaptic development: insights from Drosophila. Curr Opin Neurobiol 17: 35–42 [DOI] [PubMed] [Google Scholar]
- Collins CA, Wairkar YP, Johnson SL, DiAntonio A (2006) Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron 51: 57–69 [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR (2007) Two gr genes underlie sugar reception in Drosophila. Neuron 56: 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Sathyanarayanan S, Sehgal A (2007) Post-translational regulation of the Drosophila circadian clock requires protein phosphatase 1 (PP1). Genes Dev 21: 1506–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MP, Chu J, Villella A, Atkinson N, Kay SA, Ceriani MF (2007) Impaired clock output by altered connectivity in the circadian network. Proc Natl Acad Sci USA 104: 5650–5655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich E, Domingos AI, Asahina K, Naef F, Vosshall LB, Louis M (2005) Chemotaxis behaviour mediated by single larval olfactory neurons in Drosophila. Curr Biol 15: 2086–2096 [DOI] [PubMed] [Google Scholar]
- Ha TS, Smith DP (2006) A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci 26: 8727–8733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O'Leary DD, Luo L (2006) Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron 50: 883–895 [DOI] [PubMed] [Google Scholar]
- Hosoya T, Takizawa K, Nitta K, Hotta Y (1995) Glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell 82: 1025–1036 [DOI] [PubMed] [Google Scholar]
- Huang Y, Genova G, Roberts M, Jackson FR (2007) The LARK RNA-binding protein selectively regulates the circadian eclosion rhythm by controlling E74 protein expression. PLoS ONE 2: e1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD (2007) Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol 17: 2105–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Montell C (2007) A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci USA 104: 14110–14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Fetter RD, Tear G, Goodman CS (1995) Glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell 82: 1013–1023 [DOI] [PubMed] [Google Scholar]
- Jung J, Bonini N (2007) CREB-binding protein modulates repeat instability in a Drosophila model for polyQ disease. Science 315: 1857–1859 [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ (2007) A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 446: 542–546 [DOI] [PubMed] [Google Scholar]
- Lima SQ, Miesenbock G (2005) Remote control of behaviour through genetically targeted photostimulation of neurons. Cell 121: 141–152 [DOI] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR (2006) The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 50: 869–881 [DOI] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K (2006) Imaging taste responses in the fly brain reveals a functional map of taste category and behaviour. Neuron 49: 285–295 [DOI] [PubMed] [Google Scholar]
- Palladino MJ, Keegan LP, O'Connell MA, Reenan RA (2000) dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. RNA 6: 1004–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll C et al. (2006) Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol 16: 1741–1747 [DOI] [PubMed] [Google Scholar]
- Shishido E, Takeichi M, Nose A (1998) Drosophila synapse formation: regulation by transmembrane protein with Leu-rich repeats, CAPRICIOUS. Science 280: 2118–2121 [DOI] [PubMed] [Google Scholar]
- Slone J, Daniels J, Amrein H (2007) Sugar receptors in Drosophila. Curr Biol 17: 1809–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S, Vonesch J, Giangrande A (1996) Glide directs glial fate commitment and cell fate switch between neurones and glia. Development 122: 131–139 [DOI] [PubMed] [Google Scholar]
- Warrick JM, Morabito LM, Bilen J, Gordesky-Gold B, Faust LZ, Paulson HL, Bonini NM (2005) Ataxin-3 suppresses polyglutamine neurodegeneration in Drosophila by a ubiquitin-associated mechanism. Mol Cell 18: 37–48 [DOI] [PubMed] [Google Scholar]
- Wu C, Daniels RW, Diantonio A (2007) DFsn collaborates with Highwire to down-regulate the Wallenda/DLK kinase and restrain synaptic terminal growth. Neural Develop 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]