The European Conference on Cancer and Ageing—SeneCa, senescence and cancer—took place in Warsaw, Poland, between 4 and 6 October 2007, and was organized by E. Sikora and M. Szumowski.
Introduction
What is the relationship between ageing and cancer? Simple—cells need time to accumulate sufficient mutations for carcinogenesis to occur. But of course, nothing is really so simple. If true, short-lived organisms would never get cancer: it could be that this is the case for some animals, but even Caenorhabditis elegans suffers from germ cell tumours (Francis et al, 1995) and the cause of death of short-lived rodents such as mice—in the laboratory at least—is commonly cancer. In fact, cancer incidence rates scale with longevity in mammals in terms of occurrence at the same percentage of lifespan accomplished. We must therefore look elsewhere for the explanation of why cancer rates (for most types of cancer) increase with age in both short- and long-lived animals. The European Conference on Cancer and Ageing brought together a multidisciplinary group of scientists to discuss the multifaceted aspects of this intriguing relationship, which is of even greater importance than thus far appreciated. A. Yashin (Durham, NC, USA) showed that the frequently quoted mere 3.5 years of additional life expectancy that would be gained were cancer to be cured is incorrect because epidemiological studies have evaluated cancer independently of other causes of death. In fact, the impact would be much greater. The sobering statistic that two-thirds of all people reaching 65 years of age are currently alive, and the well-recognized age-associated increase in rates of most cancers, suggests that cancer treatment and cancer deaths will represent an ever-greater burden on health care throughout the world. Indeed, it is estimated that deaths from cancer will continue to exceed even those from tuberculosis, malaria and AIDS combined (P. Boyle, Lyon, France).
Conserved signalling and genome maintenance pathways
Evolutionarily conserved pathways are emerging as the underlying mechanistic explanation for the relationships observed between maintenance of genomic integrity, control of repair and cell death, and cancer and ageing. From work in C. elegans, the central role of the insulin/insulin-like growth factor 1 (IGF1) pathway has become apparent. Long-lived daf-2 (insulin/IGF1 receptor) mutants counteract tumour growth and promote longevity in C. elegans by triggering apoptosis through the Forkhead box, class O (FOXO) transcription factor DAF-16; their murine and human homologues are also known to influence tumorigenesis. The transcriptional targets of DAF-16 were recently found to be involved in extending longevity and include many that influence p53-dependent apoptosis (Pinkston-Gosse & Kenyon, 2007). A loss of p53 results in increased cancer incidence in mice and humans; mice that constitutively overexpress p53 are cancer resistant but short lived (Tyner et al, 2002). A large proportion of human cancer cells express anterior gradient homologue 2 (AGR2), which is an inhibitor of p53 that also might function as a drug-resistance survival factor in cancer (T. Hupp, Edinburgh, UK). However, appropriate control of p53 expression—for example, by differential acetylation of p53 by the histone acetyltransferase HBO1 (also known as MYST2)—can separate these two effects, retaining cancer protection and increasing longevity (H. Scrable, Charlottesville, VA, USA). p53 is a central component in a complex network that is still being analysed. New target molecules are being identified, such as collaborator of alternative reading frame (CARF), which interacts with three members of the p53 system—ARF, human homologue of the murine double minute 2 (MDM2) and p53 itself. Overexpression of CARF induces a senescence-like state in cancer cells and might contribute to the anti-cancer activity of the p53 network (R. Wadhwa, Ibaraki, Japan). A senescence phenotype can also be induced in tumour cells by the combination of topoisomerase II inhibitors and blockage of the ataxia telangiectasia mutated (ATM)/ATM and Rad3-related (ATR) pathways (M. Sabisz, Gdansk, Poland). The possible role of tripeptidyl peptidase II in regulating the cell cycle was underscored by the demonstration that mice lacking this enzyme show a senescent phenotype, shorter lifespan, haematopoietic senescence and accelerated thymic involution (J. Huai, Freiburg, Germany).
The possibility that direct tumour-suppressor activity is not the only anti-cancer mechanism of p53 action was raised by J. Campisi (Berkeley, CA, USA) who showed that p53 also restrains the senescence-associated secretory phenotype (SASP). SASP is a cellular response to severe damage that causes growth arrest and a senescent phenotype characterized by secretion of predominantly proinflammatory and angiogenic factors, resembling a wound-healing response and potentially promoting cancer. Thus, decreased p53 results in enhanced SASP, which can cause different pathologies. Initially, innate immunity eliminates these cells by a cytotoxic mechanism dependent on the ligation of the activating receptor NKG2D on natural killer (NK) cells by the major histocompatibility complex class I-related chain (MIC) on SASP cells. However, immune ‘escape' variants develop, which can no longer be recognized by NK cells because of the excision of MIC from the surface (J. Campisi). It might be no coincidence that a similar phenomenon is known to occur in cancer cells that escape immune surveillance (Doubrovina et al, 2003; Solana et al, 2007).
Recent evidence supports an important role for mitochondrial reactive oxygen species (ROS) in cell lifespan and senescence, and their involvement in cancer and ageing. ROS induce oxidative damage of DNA and proteins, but hydrogen peroxide can also trigger signal transduction pathways involved in apoptosis and proliferation, as well as other changes in gene expression (Giorgio et al, 2007). R. Olinski (Bydgoszcz, Poland) showed a correlation between the degree of oxidative DNA damage and age. Antioxidant status is decreased in centenarians and vitamin supplementation enhances antioxidant defences (K. Kempa, Katowice, Poland). The expression of the mitochondrial nicotinamide adenine dinucleotide phosphate (NADPH) oxidase NOX1 is increased in tumour cells and its overexpression enhances tumorigenicity, supporting a role for ROS production in cancer (M. Kulawiec, Buffalo, NY, USA). Treatments shown to decrease inflammation in mice, such as caloric restriction or a moderate decrease of β-glucuronidase by D-glucarates, can result in increased longevity (Z. Walaszek, San Antonio, TX, USA); the N-glycan profile could therefore be used as a marker of healthy ageing and for monitoring anti-ageing interventions (C. Chen, Ghent, Belgium).
Lower levels of damage that can still be repaired, such as minor DNA damage, do not induce SASP. Clearly, the balance between DNA damage repair, damage limitation and elimination of severely damaged cells is complex but crucial; hence, intense scrutiny of DNA-repair mechanisms is ongoing. There is a close correlation between the severity of compromised DNA repair and the severity and rate of onset of ageing symptoms in both mouse models and human progeroid syndromes (J. Hoeijmakers, Rotterdam, The Netherlands). DNA microarray screening revealed similar gene-expression signatures in normal and premature ageing, including genes involved in increased stress response, inflammation and immunity (perhaps reflecting SASP cells). These signatures were not seen in calorically restricted or long-lived dwarf mice. Moreover, DNA-repair enzyme expression and micro-RNA shifts centred on the IGF axis were observed in ageing, and might also reflect attempts to compensate for increasing damage. Hoeijmakers suggested that insulin/IGF has a central role in metabolic control feedback, whereby increased metabolic activity intrinsically results in increased DNA damage and eventually the trade-off between ageing and controlling cancer.
On the basis of protein–protein interactions, A. Budovsky (Beer-Sheva, Israel) showed that the genes putatively involved in ageing and cancer overlap significantly and that most are involved in signal transduction, DNA repair and energy metabolism. This was a recurring theme: is ageing an evolutionary price paid for protection against tumours? It is an attractive, simple concept, but a closer examination of the logic and evidence supporting the idea that ageing is an evolutionary by-product of anti-cancer protection suggests greater complexity surrounding this hypothesis, which remains open to better definition and testing (T. Kirkwood, Newcastle, UK). At the simplest level, a quick calculation suggests that if replicative senescence exists to prevent cancer development, organisms would have to be able to tolerate the presence of the progeny of the approximately 50 population doublings that normal cells undergo before reaching replicative senescence (L. Hayflick, San Francisco, CA, USA): this corresponds to 1 × 1015 cells, equivalent to approximately the entire number of cells in the human body. The debate goes on.
In any event, there is a lot of evidence for damage-induced senescence-like growth arrest and/or apoptosis even in cells already fully transformed to neoplasia and, indeed, chemotherapeutic drugs commonly act by causing severe DNA damage. This can itself lead to further genetic instability and ‘escape' variants, as in doxorubicin-treated colon cancer cells (E. Sikora, Warsaw, Poland). DNA-repair defects in stem cells might be particularly crucial in ageing, especially in the haematopoietic stem cell (HSC) system. DNA damage accumulates with age in quiescent murine HSCs, which have decreased levels of DNA-repair enzymes (D. Rossi, Stanford, CA, USA). Why such essential cells lose DNA-repair capacity is a puzzle; Rossi suggested that this was a fail-safe mechanism to prevent imperfect repair. If so, this represents another trade-off between cancer protection and the progressive loss of regenerative capacity—that is, ageing. In humans, reports suggest that there are fewer circulating CD34+ HSCs and colony-forming cells in the elderly, perhaps for the same reason, and this is also the case in mice (D. Bonnet, London, UK).
Other age-associated alterations in HSCs include changes in the lineage preference (D. Bryder, Lund, Sweden) and decreased mobilization of progenitors, at least in acute myocardial infarction patients (W. Wojakowski, Katowice, Poland). Bone-marrow-derived mesenchymal stem cells that differentiate into mesoderm-type cells—that is, osteoblasts, chondrocytes and adipocytes—are also affected by ageing as a consequence of genetic and microenvironmental factors (M. Kassem, Odense, Denmark). Age-associated inflammation can be involved in the altered function of these cells (R. Brunauer, Innsbruck, Austria). Nevertheless, it is remarkable that thymic output, measured by T-cell-receptor excision circle (TREC) levels, can still be detected even in elderly individuals (60–99 years of age; W.A. Mitchell, London, UK).
Tumour stroma
As is clear from the above discussion, cancer cells need supportive ‘stroma' for their successful establishment, either as primary or metastatic tumours. Senescent cells, especially SASP cells, might provide a better environment for tumour growth. Indeed, senescent peritoneal mesothelial cells secrete increased amounts of factors such as vascular endothelial growth factor, interleukin-8, monocyte chemoattractant protein 1 and growth-regulated oncogene-α, which can facilitate the progression of ovarian cancer (K. Ksiazek, Poznan, Poland). Given that irradiation and chemotherapy can induce senescent-like states, they could act as double-edged swords and actually facilitate the growth of surviving tumour cells. The mechanism might not only involve the soluble factors mentioned above, but rather seems to depend mainly on cell–cell contact mechanisms involving intercellular adhesion molecule 1 (D. Kletsas, Athens, Greece). Moreover, both irradiated and replicatively senescent fibroblasts support lung cancer growth in severe combined immunodeficiency mice. Remarkably, although non-senescent fibroblasts did not enhance tumour growth, injection of either type of senescent cells significantly promoted tumour formation (D. Kletsas). Indeed, it might be more appropriate to search for the reason for age-related changes in cancer incidence at the level of the response of the stromal cell to carcinogens, rather than merely the tumour cell itself (V. Anisimov, St Petersburg, Russia).
Telomeres and telomerase
Both the extent and the exact location of DNA damage might be of crucial importance in determining outcome. Telomeric repeats might be particularly sensitive ‘monitors' in this regard (von Zglinicki et al, 1995). Moreover, genes in the neighbourhood of telomeres might be similarly affected, as illustrated by the identification of genes next to telomeres that are regulated by telomere length (J. Shay, Dallas, TX, USA). This phenomenon, called the telomere-position effect, has previously been reported in yeast and could be a mechanism to regulate gene expression even before telomere-based replicative senescence occurs.
Telomerase is commonly active in cancer cells to maintain minimal telomere lengths, and can be one of the requirements for the in vitro transformation of normal human cells (Kendall et al, 2006). However, there is no correlation between telomerase activity and longevity in different species, although activity does correlate with body mass in rodents (V. Gorbunova, Rochester, NY, USA). Small, short-lived rodents such as mice express telomerase in many tissues, as do small, long-lived naked mole rats; however, larger rodents such as beavers do not. This could imply that larger animals, which have more cells as targets for neoplastic transformation, benefit from the downregulation of telomerase as an anti-cancer mechanism, at the cost of age-associated changes. Small animals benefit from the effects of telomerase at the cost of dying of cancer—mostly lymphoma in mice—if they live long enough, which of course in the wild is unlikely.
Immune response: facilitation or control of neoplasia?
Are the genetic changes discussed above sufficient per se to account for carcinogenesis? Do tumour cells arise constantly but become eliminated by surveillance mechanisms? Increased rates of cancer with ageing could be explained by age-associated alterations to such putative surveillance systems. Both innate (non-clonotypic, immediate-response or ‘natural') immunity as well as especially adaptive (clonotypic T- and B-cell-dependent) immunity are affected by ageing; therefore, immunosenescence could allow tumours to escape from immunosurveillance (Pawelec et al, 2006). Moreover, assays to establish biomarkers of immunosenescence and assign an ‘immunological age' show that cancer patients are immunologically ‘older' than their chronological age suggests (K. Hirokawa, Tokyo, Japan). However, the extent to which naturally occurring tumours are subject to immune control is controversial. There is overwhelming evidence that cancer cells are immunogenic, and evidence from cancer immunotherapy trials indicates that T cells recognizing tumour antigens can be amplified in patients. Calculations based on the frequency of cells responding to a melanoma antigen family 3 peptide tumour antigen in melanoma patients indicate that 12–27 population doublings are occurring in the clinical setting (P. Coulie, Brussels, Belgium). The high degree of clonal expansion required might contribute to the commonly observed ineffectiveness of anti-cancer responses because of the phenomenon of replicative senescence in T cells (Effros & Pawelec, 1997). Replicative senescence might be involved in the low proportion of effector cytotoxic cells obtained when large numbers of T cells are generated in vitro (R. Tarazona, Caceres, Spain). Nonetheless, Coulie showed that tumours subjected to immunotherapy can regress, often accompanied by the recruitment of T cells recognizing tumour antigens other than the vaccine antigen. In these responders, new T-cell clones against different antigens have been recruited, thereby bypassing senescence of the vaccine antigen-specific cells.
In addition, work in a mouse breast cancer model suggests that the effective anti-cancer immune responses in young animals are predominantly mediated by the adaptive immune system, whereas the less-effective responses in old animals are innate (C. Gravekamp, San Francisco, CA, USA). This could be applicable to other organisms as adaptive immunity is highly susceptible to age-associated deleterious alterations but more ‘primitive' innate responses tend to be well preserved or even enhanced (Aw et al, 2007). The innate response correlates well with the response to vaccines against pathogens (P. Trzonkowski, Gdansk, Poland; Solana et al, 2006), although some aspects of innate immunity that result in an increased susceptibility of leukocytes to vesicular stomatitis virus infection can be altered by ageing (B. Jatczak, Wroclaw, Poland). Indeed, retention of strong inflammatory responses without the beneficial effects of the adaptive responses that they can amplify is likely to enhance immunopathology and carcinogenesis (de Visser & Coussens, 2005). This might also help to explain the genetic component of certain cancers in which inflammation is well established as an amplificatory factor—for example, prostate cancer (C. Caruso, Palermo, Italy) and pancreatic cancer (M. Mirabile, Palermo, Italy). Conversely, tumour progression might be slower in the elderly, as seen in the breast, lung, colon and other sites in very old people and centenarians. This could be due to a selection process, as the ‘successfully aged'—such as centenarians—commonly show decreased inflammatory parameters, which also decreases the ability of the stromal tissue to support tumour growth and angiogenesis (C. Franceschi, Bologna, Italy).
On the basis of these and other data, it is becoming increasingly clear that young and old individuals will require different treatment approaches; this realization might also help to explain some conundrums in cancer therapy. For example, why is cancer in the elderly less responsive to chemotherapy and radiotherapy? The answer could be because the immune system is less effective in the elderly, as recent data suggest that even responses to chemotherapy and radiotherapy require an intact immune system (Apetoh et al, 2007). Therapies tailor-made for the elderly will have a great clinical impact; some immunotherapies have already been developed that are effective in animal models, but only in old and not young individuals (J. Leibovici, Tel-Aviv, Israel).
Conclusions
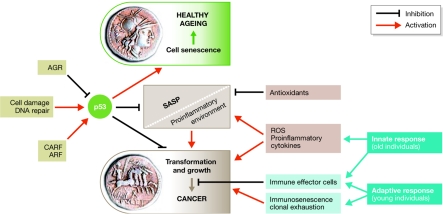
This conference identified cancer and ageing and their interrelationships as one of the most challenging areas of biology, and revealed that they are really two sides of the same coin (Fig 1). Areas of greatest interest for further research were identified that should generate new perspectives for understanding and treating cancer in the aged (Table 1). We hope that this conference will have inspired many of the participants to pursue such an agenda.
Figure 1.
Cancer and ageing and their interrelationships are two sides of the same coin. Cell senescence and tumour transformation are regulated by common preserved pathways in which p53 has a central role. These processes are modulated at several levels and other factors can influence this relationship. The appearance of a SASP as a response to cell damage that can contribute to cancer is also restrained by p53. The immune response, both innate and adaptive, can also have a significant role in cancer development. In certain circumstances it is clearly established that the immune response contributes to the elimination of transformed cells, whereas in other cases, it can produce factors that promote tumour growth. AGR, anterior gradient homologue; ARF, alternative reading frame; CARF, collaborator of alternative reading frame; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype.
Table 1.
Main areas of interest for cancer and ageing research.
| Understanding the deceleration of cancer incidence and mortality at advanced age: is it merely selection, population heterogeneity, remodelling or some other aspect of real ageing physiology? |
| Impact of the ‘old' microenvironment on cancer formation, stem-cell regeneration, proliferation and the senescence-associated secretory phenotype phenomenon |
| Impact of local and systemic inflammation on carcinogenesis and tumour maintenance and the role of the innate immune system therein |
| Impact of antagonistic pleiotropy: ageing as an anti-cancer mechanism? |
| Impact of mitochondrial DNA mutants and polymorphisms |
| Application of more sophisticated systems biology approaches |

Graham Pawelec

Rafael Solana
Acknowledgments
We apologize to all participants whose contributions could not be mentioned due to space restraints. The conference was supported by the European Commission (LSSM-CT-2006-037312).
References
- Apetoh L et al. (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13: 1050–1059 [DOI] [PubMed] [Google Scholar]
- Aw D, Silva AB, Palmer DB (2007) Immunosenescence: emerging challenges for an ageing population. Immunology 120: 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Coussens LM (2005) The interplay between innate and adaptive immunity regulates cancer development. Cancer Immunol Immunother 54: 1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O'Reilly RJ, Dupont B, Vyas YM (2003) Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol 171: 6891–6899 [DOI] [PubMed] [Google Scholar]
- Effros RB, Pawelec G (1997) Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today 18: 450–454 [DOI] [PubMed] [Google Scholar]
- Francis R, Barton MK, Kimble J, Schedl T (1995) gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139: 579–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M, Trinei M, Migliaccio E, Pelicci PG (2007) Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8: 722–728 [DOI] [PubMed] [Google Scholar]
- Kendall SD, Adam SJ, Counter CM (2006) Genetically engineered human cancer models utilizing mammalian transgene expression. Cell Cycle 5: 1074–1079 [DOI] [PubMed] [Google Scholar]
- Pawelec G, Koch S, Griesemann H, Rehbein A, Hahnel K, Gouttefangeas C (2006) Immunosenescence, suppression and tumour progression. Cancer Immunol Immunother 55: 981–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston-Gosse J, Kenyon C (2007) DAF-16/FOXO targets genes that regulate tumor growth in Caenorhabditis elegans. Nat Genet 39: 1403–1409 [DOI] [PubMed] [Google Scholar]
- Solana R, Pawelec G, Tarazona R (2006) Aging and innate immunity. Immunity 24: 491–494 [DOI] [PubMed] [Google Scholar]
- Solana R, Casado JG, Delgado E, DelaRosa O, Marin J, Duran E, Pawelec G, Tarazona R (2007) Lymphocyte activation in response to melanoma: interaction of NK-associated receptors and their ligands. Cancer Immunol Immunother 56: 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner SD et al. (2002) p53 mutant mice that display early ageing-associated phenotypes. Nature 415: 45–53 [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Docke W, Lotze C (1995) Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res 220: 186–193 [DOI] [PubMed] [Google Scholar]