Abstract
Mature vertebrate oocytes typically undergo programmed arrest at the second meiotic cell cycle until they are signalled to initiate embryonic development at fertilization. Here, we describe the underlying molecular mechanisms of this second meiotic arrest and release in Xenopus, and compare and contrast them with their counterparts in mice.
Keywords: metaphase II, Emi2, cytostatic factor, vertebrate meiosis, fertilization
Introduction
Mature oocytes of vertebrates such as Xenopus laevis (African clawed frog) and Mus musculus (mouse) are arrested at the second metaphase (mII) of the meiotic cell cycle. Second meiotic progression is restrained by cytostatic factor (CSF), the presence of which was originally inferred from the ability of mII oocyte extracts from Rana pipiens (the northern leopard frog) to induce cleavage arrest when injected into dividing blastomeres (Masui & Markert, 1971). CSF activity sustains mII arrest in fertilizable oocytes to prevent parthenogenesis. Here, we compare the maintenance of, and exit from, mII arrest in the oocytes of frogs and mice (Table 1); a composite schematic summarizing the molecular interactions implicated in these processes is shown in Fig 1.
Table 1.
Some disparities between the oocytes and early embryos of Xenopus and Mus
| Property | Xenopus | Mus | References | |
|---|---|---|---|---|
| Mature oocyte volume | 1 μl | 270 pl | – | |
| Spindle assembly checkpoint requirement for mII arrest | Yes | No | Tunquist et al, 2003; Tsurumi et al, 2004 | |
| Intact spindle requirement for maturation promoting factor destruction | No | Yes | Kubiak et al, 1993; Clute & Masui, 1995 | |
| Activation by pricking in Ca2+-containing medium | Yes | No | Wolf, 1974 | |
| Protein synthesis inhibition induces mII exit | No | Yes | Zhang & Masui, 1992; Siracusa et al, 1978 | |
| Cytostatic factor activity demonstrated by injecting cleaving blastomeres with (≥12% of their volume) mII ooplasm | Yes | No | Shibuya & Masui, 1988; Shoji et al, 2006 | |
| Oocytes support nuclear transfer cloning of adults from adult-derived somatic cells | No | Yes | Gurdon, 1960; Wakayama et al, 1998 | |
| Full chromatin decondensation requires activation | Yes | No | Yoshida et al, 2007 | |
| Activation accompanied by an increase in intracellular pH | Yes | No | Webb & Nuccitelli, 1981; Phillips & Baltz, 1996 | |
| [Ca2+]i oscillations induced by fertilization | No | Yes | Runft et al, 2002 | |
| Zygotic gene activation | 4,000-cell stage | Late 1-cell stage | Aoki et al, 1997; Newport & Kirschner, 1982 |
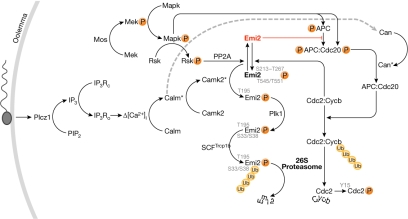
Figure 1.
Composite schematic of endogenous meiotic inhibitor 2 regulation in vertebrates. Details of selected interactions are described in the text. APC, anaphase promoting complex; Calm, calmodulin; Camk2, calmodulin-dependent kinase 2; Can, calcineurin; Cdc2/20, cell division cycle 2/20 homologues, respectively; Cdc2:Cycb, maturation promoting factor; Cycb, cyclin B; Emi2, endogenous meiotic inhibitor 2; IP3, inositol 1,4,5-trisphosphate; IP3Rc, closed IP3 receptor; IP3Ro, open IP3 receptor; Mapk, mitogen-activated protein kinase; Mek, mitogen-activated protein kinase kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; Plcz1, phospholipase C-zeta; Plk1, polo-like kinase 1; PP2A, protein phosphatase 2A; Rsk, ribosomal s6 kinase; SCF, Skp1– Cullin– F-box-containing E3 ubiquitin ligase complex; Trcp1b, F-box protein β-transducin repeat containing protein 1b; Ub, ubiquitin; P, phosphate; *, active form; Δ[Ca2+]i, increase in the intracellular free calcium ion concentration.
An overview of metaphase II arrest
Metaphase correlates with the kinase activity of maturation promoting factor (MPF), which is a heterodimer of cyclin B (Cycb) and the cyclin-dependent kinase, Cdc2 (also known as Cdk1; Masui & Markert, 1971; Gautier et al, 1989, 1990). MPF is indirectly stabilized by active CSF and its substrates include the linker histone H1 (Gautier et al, 1989). Although MPF is active in both mitotic and meiotic cell cycles, stabilization by CSF is unique to mII; CSF compensates for the negative feedback by MPF that typically predominates in mitotic cells, in which the activation of MPF leads to its own inactivation (Félix et al, 1990; Kubiak et al, 1993).
MPF activity is inhibited in prophase I—that is, in immature oocytes—through the phosphorylation of Cdc2 residues Thr 14 and Tyr 15. This reflects the net activities of the MPF-activating phosphatase, xCdc25c (Cdc25b in the mouse), and the MPF-inhibitory kinases xWee1a (Tyr 15 phosphorylation) and xMyt1 (Thr 14 and Tyr 15 phosphorylation), which are supplanted by Wee1b in the mouse (Leise & Mueller, 2002; Han et al, 2005). In Xenopus, germinal vesicle breakdown is prevented by the action of cAMP-dependent protein kinase A (PKA), which phosphorylates Ser 287 of xCdc25 until meiosis is resumed (Duckworth et al, 2002). However, there is no firm evidence in either the mouse or Xenopus that the pathways involving PKA, Cdc25, Wee1 or Myt mediate mII maintenance or exit. In Xenopus, CSF-induced metaphase arrest occurs only when the spindles are correctly attached to the kinetochores—the spindle assembly checkpoint (SAC); the SAC-mediating protein, xMad1, is required to maintain mII (Tunquist et al, 2003). By contrast, the SAC is not required to establish or to maintain mII arrest in the mouse (Tsurumi et al, 2004).
Exit from mII occurs when the destruction box motif of Cycb directs ubiquitination by an E3 ubiquitin ligase called the anaphase promoting complex (APC), a complex of at least 12 protein subunits (Peters, 2006). Ubiquitination targets Cycb for 26S proteasomal hydrolysis to eliminate MPF (Glotzer et al, 1991; Peters, 2006). The main effect of CSF is therefore to stabilize MPF by abrogating APC activity towards Cycb. In prevailing models, APC inhibition involves the oocyte-restricted endogenous meiotic inhibitor 2 (Emi2), the activity of which is essential for CSF arrest. The events after gamete fusion that precede syngamy are referred to as oocyte activation (Runft et al, 2002).
Emi2 and Mos in meiosis II
Xenopus endogenous meiotic inhibitor 2 (xEmi2, also known as Emi1-related protein 1, xErp1) is a 651-residue protein that mediates mII establishment and/or maintenance (Fig 1; Schmidt et al, 2005; Rauh et al, 2005). During CSF arrest, xEmi2 apparently negates APC activity by binding to the core of the APC, one component of which is xCdc27 (Wu et al, 2007a). This interaction reflects the balance of xEmi2 phosphorylation by xCycb–xCdc2 (MPF; Wu et al, 2007a,b), and the antagonistic removal of these phosphates (Nishiyama et al, 2007a; Inoue et al, 2007; Wu et al, 2007a).
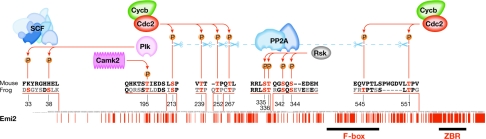
The search for the CSF-promoting contribution to this balance effectively began with classical experiments in which xMos complementary RNA (cRNA) injected into the blastomeres of Xenopus two-cell embryos induced cleavage arrest (Sagata et al, 1989). xMos immunodepletion reduced the ability of mature oocyte extracts to induce CSF arrest (Sagata et al, 1989), and depletion of xMos by morpholino injection of intact Xenopus oocytes prevented mitogen-activated protein kinase (Mapk) activation and induced parthenogenesis (Dupré et al, 2002). Therefore, meiotic arrest in Xenopus involves an xMos–xMek–xMapk (xErk1/2) pathway. Pharmacological inhibition of xMek with U0126 induces mII exit (Wu et al, 2007a) in a manner that can be bypassed by the expression of constitutively active xRsk, a target of xMapk signalling (Gross et al, 2000). Indeed, xRsk phosphorylates xEmi2 primarily at Ser 335 and Thr 336, but also at Ser 342 and Ser 344 (Fig 2), with phosphorylation at Ser 335 and Thr 336 facilitating the binding of xEmi2 to protein phosphatase 2A (xPP2A; Wu et al, 2007a). In turn, xPP2A binding promotes dephosphorylation of xEmi2 residues Ser 213, Thr 239, Thr 252, Thr 267, Thr 545 and Thr 551. Dephosphorylation of Thr 545/Thr 551 enhances the binding of the xEmi2 carboxy-terminal zinc-binding region to the APC core, thereby inhibiting APC (Wu et al, 2007a). Dephosphorylation of the Ser 213–Thr 267 cluster, which is conserved or conservatively substituted in the mouse alignment (Fig 2), stabilizes xEmi2 (Wu et al, 2007b). Phosphorylation of xEmi2 Ser 213–Thr 267 and Thr 545/Thr 551 clusters, which tends to negate CSF activity, is mediated by MPF through a balancing negative feedback loop (Wu et al, 2007a,b).
Figure 2.
Pairwise alignment of mouse (Mus, top) and frog (Xenopus, bottom) endogenous meiotic inhibitor 2 predicted amino-acid sequences, showing identities as red bars beneath. The alignment was based on one produced by BLAST. Key portions of the alignment (described in the text) are expanded, showing residues identical to the mouse sequence in black, non-identical ones in grey and those subject to phosphorylation in red, with their corresponding kinases and/or phosphatases. Information directing mII establishment and/or maintenance apparently resides in the carboxy-terminal 70%, with the amino-terminal 30% containing elements that direct mII exit. The F-box and the zinc-binding region (ZBR) are as delineated by Schmidt et al (2005). Abbreviations are as for Fig 1.
xMos is a crucial Mapkkk that acts upstream from xRsk (Inoue et al, 2007; Nishiyama et al, 2007a), and exogenous xMos fails to induce metaphase arrest in interphase extracts from which xRsk2 has been immunodepleted (Bhatt & Ferrel, 1999). Therefore, xPP2A activity towards xEmi2 is stimulated by xMos through xRsk, to promote mII arrest. The xMos–xMapk–xRsk cascade permanently disappears after mII exit, whereas xEmi2 is destroyed during Xenopus mII exit and then reappears in pre-blastula cell cycles but without its CSF activity; the xMos–xMapk–xRsk pathway might therefore be required for the CSF activity of xEmi2 (Inoue et al, 2007). However, CSF extracts that are depleted of xRsk2 (in which it is approximately 16-fold more abundant than xRsk1) remain arrested in mII for at least 25 min (Bhatt & Ferrell, 1999), which is consistent with a role for xRsk-independent events to sustain MPF activity.
In the mouse, homozygously targeted Mos−/− oocytes fail to activate the Mapk pathway and pause at mII for 2–4 h, with initially normal MPF activity (Colledge et al, 1994; Choi et al, 1996; Verlhac et al, 1996). Oocytes that lack Mos contain anomalously long, interphase-like microtubules during the mI–mII and mII–mIII transitions (Verlhac et al, 1996). Coordination of the cell cycle and microtubule dynamics could involve the microtubule-stabilizing Mapk targets, Miss and Doc1r, although neither contributes directly to CSF activity (Lefebvre et al, 2002; Terret et al, 2003). However, a deficiency of Mos perturbs meiosis I, and subsequent phenotypes might reflect this.
In the mouse, constitutively active Rsk fails to stabilize mII arrest in Mos−/− oocytes, and oocytes homozygous for deletions in the three expressed Rsk genes (Rsk1, Rsk2, Rsk3 triple-null mice) present a stable CSF arrest with classical barrel-shaped spindles (Dumont et al, 2005). Thus, Rsk-independent signalling pathways might stabilize mII in both the mouse and Xenopus.
Maturing and mII mouse oocytes also contain Emi2 (Shoji et al, 2006), which exhibits 44% amino-acid identity with xEmi2 over its 541-residue alignment (Fig 2). Emi2 is also expressed at high levels in the mouse testis, suggesting an as-yet-undetermined role in male meiosis (Shoji et al, 2006).
Emi2 functions by interfering with the APC activator, Cdc20. A C-terminal fragment of Xenopus xEmi2 inhibits xCdc20-dependent ubiquitination of a Cycb fragment in vitro (Schmidt et al, 2005). Cdc20 depletion in intact mouse oocytes prevents mII exit induced by Emi2-targeted RNA interference, and Emi2 and Cdc20 have been shown to interact in vitro (Shoji et al, 2006). However, Xenopus xEmi2 interacts only weakly with xCdc20, if at all (Schmidt et al, 2005; Wu et al, 2007a), raising the possibility that mouse oocyte Cdc20–Emi2 interactions are also weak. Cdc20 removal completely blocks mII exit induced by sperm or the parthenogenetic agent SrCl2 in situ (Shoji et al, 2006), indicating that in the mouse, either all pathways regulating mII exit involve Cdc20, or that others are secondary to those that do.
Exit from mII: the inducing sperm signal
Sperm–oocyte fusion in Xenopus induces an increase in intracellular ‘free' calcium ([Ca2+]i) that propagates across the oocyte before returning after 5 min to its resting level (Runft et al, 1999, 2002); there are no [Ca2+]i oscillations. Mouse gamete fusion stimulates a marked [Ca2+]i increase from the point of sperm–oocyte contact within 3 min (Lawrence et al, 1997). The [Ca2+]i then returns to its resting level (∼100 nM), but is followed by a series of oscillations lasting several hours in which [Ca2+]i spikes to approximately 1 μM for about 1 min every 5–15 min (Runft et al, 2002).
The murine activity that induces oocyte [Ca2+]i oscillations includes the phospholipase C (Plc) isoform, Plc-ζ (Plcz1; Saunders et al, 2002). Injection of Plcz1 cRNA into mouse oocytes induces [Ca2+]i oscillations and pronucleus formation (Saunders et al, 2002). Sperm protein profiling showed that Plcz1 correlates with a physiological oocyte activating factor (Fujimoto et al, 2004). Plcz1 orthologues are conserved among mammals but are unusual among Plcs because they lack plekstrin or Src homology (PH or SH) domains; proceeding from its amino- to C-termini, Plcz1 comprises four EF-hand domains, X and Y domains and a C-terminal C2 domain (Saunders et al, 2002). No Xenopus Plcz1 counterpart has been confirmed and although the hypothetical protein NP_001090146.1 exhibits 42% identity along a 628-residue alignment with mouse Plcz1 (NP_473407; 647 residues), its expression pattern and activity have not been reported. Orthologues of Plc-β and Plc-γ isoforms are also present in Xenopus oocytes but are dispensable for activation (Runft et al, 1999).
Calcium signalling is relayed through xCamk2 in Xenopus
How is intracellular calcium signalling detected to induce mII exit? Xenopus egg extracts supplemented with a calmodulin-binding peptide prevent exogenous calcium from triggering xCycb degradation (Lorca et al, 1991). This implicit calmodulin-dependent regulation involves calmodulin kinase II (xCamk2), as challenging Xenopus eggs or their extracts with constitutively active rat Camk2a (the α-isoform; residues 1–290, lacking regulatory and subunit association domains, referred to here as Camk2aΔct) inactivates CSF (Hanson & Schulman, 1992; Lorca et al, 1993).
Native Camk2 activity in newly fertilized mouse eggs shadows [Ca2+]i oscillations (Markoulaki et al, 2004) and injection of mouse mII oocytes with cRNA encoding Camk2aΔct results in meiotic progression without these oscillations (Knott et al, 2006). However, the Camk2 antagonist, myristoylated autocamtide 2-related inhibitory peptide, myrAIP, does not block early [Ca2+]i oscillations or polyspermy, and incompletely prevents meiotic resumption after sperm–oocyte fusion (Markoulaki et al, 2004; Gardner et al, 2007).
Xenopus xCamk2 signals mII exit through xEmi2
In Xenopus oocyte extracts, xCamk2 phosphorylates xEmi2 at Thr 195 of its canonical Camk2 phosphorylation motif, RXST, in a manner facilitated by the preceding serine residue, Ser 194; xEmi2T195A is resistant to degradation in Ca2+-supplemented CSF extracts (Rauh et al, 2005). A peptide corresponding to the Camk2 regulatory domain (Camk2218–309) reduces Ca2+-induced xEmi2 degradation, which supports a direct interaction between xEmi2 and xCamk2 (Rauh et al, 2005; Liu & Maller, 2005).
xEmi2 phosphorylated at Thr 195 is an improved substrate for the polo-like kinase, xPlk1 (also referred to as Plx1), and neither S194A nor T195A mutants interact with xPlk1 (Rauh et al, 2005). Immunodepletion of xPlk1 from CSF extracts prevents meiotic progression on addition of Ca2+ (Descombes & Nigg, 1998) and a marked (10- to 15-, but not twofold) excess of xPlk1 induces meiotic progression in the absence of Ca2+ (Liu & Maller, 2005). xEmi2 regulation involves the polo-box domain (PBD) of xPlk1, because a xPlk1 dominant-negative PBD stabilizes xEmi2 and inhibits CSF release in Xenopus extracts supplemented with Ca2+ (Schmidt et al, 2005).
The binding of xPlk1 to xEmi2 primed by Thr 195 phosphorylation facilitates the secondary phosphorylation of xEmi2 by xPlk1 at Ser 33 and Ser 38 within the phosphodegron motif DSGX3S (Schmidt et al, 2005; Rauh et al, 2005). After these secondary DSGX3S phosphorylations have occurred, xEmi2 is rapidly targeted by the Skp1–Cullin–F-boxTrcpb-containing E3 ubiquitin ligase complex, resulting in its 26S proteasomal hydrolysis (Schmidt et al, 2005).
Depletion of Emi2 from intact mouse mII oocytes causes meiotic release, and Emi2 degradation during oocyte activation precedes Cycb destruction (Shoji et al, 2006; Madgwick et al, 2006). Mouse Emi2 lacks the canonical RXXT/S motif corresponding to the Camk2 phosphorylation target (which includes Thr 195) in its alignment with xEmi2 (Rauh et al, 2005). However, the arginine is conservatively substituted (by Lys 173 in mouse); the resultant KXXT/S motif is phosphorylated by Camk2 in the smooth muscle contractility regulator, caldesmon (Hanson & Schulman, 1992). Although xEmi2 is phosphorylated by xPlk1 in its Ser 33/Ser 38 phosphodegron, the corresponding region is poorly conserved in the mouse and other mammals (Schmidt et al, 2005), and there is no canonical substitute nearby (Fig 2).
Calcineurin and mII exit in Xenopus
In addition to the involvement of xCamk2, the Ca2+/calmodulin-dependent protein phosphatase, calcineurin (xPpp3c; referred to here as Can) has recently been implicated in Xenopus mII exit (Mochida & Hunt, 2007; Nishiyama et al, 2007b). Can comprises a catalytic A subunit, Cana, tightly bound to a regulatory B subunit, Canb, which is bound to Ca2+/calmodulin in active Can (Klee et al, 1998). When Ca2+ is added to CSF extracts, the APC core subunit xCdc27 (also known as xAPC3) becomes rapidly and transiently dephosphorylated in an xCamk2-independent manner that is inhibited by the indirect Can inhibitors, cyclosporin A (CsA) or FK506 (Mochida & Hunt, 2007). CsA also inhibits xCycb destruction, and depletion of Can reduces xCdc2 kinase inactivation in response to Ca2+ (Mochida & Hunt, 2007; Nishiyama et al, 2007b). Can apparently dephosphorylates xCdc27 and xCdc20; dephosphorylation of xCdc20 is rapid and might ‘unlock' it to contribute to APC activation (Mochida & Hunt, 2007).
Little is known about Can or its relationship to Camk2-induced mII progression in mouse oocytes. They might be part of the same pathway, as Can is a substrate of Camk2 (Hanson & Schulman, 1992), or Can-mediated reactions immediately triggered by a rise in [Ca2+]i might be independent of Camk2, as seems to be the case in frogs (Nishiyama et al, 2007b). In the mouse, Camk2aΔct only weakly establishes the block to polyspermy and does not fully induce cortical granule exocytosis (Gardner et al, 2007); these are early events of activation in which Can might have a significant role. Can is activated in response to small changes in [Ca2+]i, which might explain why Xenopus oocytes are readily activated by the influx of external calcium ions that often accompanies injection or pricking (Wolf, 1974); mouse oocytes are not activated in this manner.
A low-resolution model of mII homeostasis
We propose a simple model of meiosis II in which Mos activity senses and modulates correct spindle behaviour to potentiate the stable establishment of mII arrest by Emi2 (Fig 3).
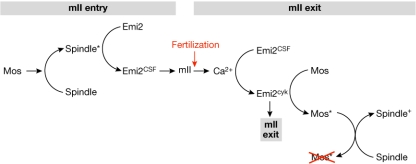
Figure 3.
Low-resolution model of second metaphase homeostasis. The model links spindle modulation by Mos to the establishment and maintenance of, and exit from, Emi2-mediated mII arrest. The establishment of Emi2 as cytostatic factor (Emi2CSF) is coordinated by mII spindle formation (spindle*) and Mos activity. Following fertilization, Emi2CSF is replaced by Emi2cyk activity which modulates Mos function (Mos*) to ensure that the spindle is formatted for productive cytokinesis (spindle+).
In the model, Mos-mediated microtubule adjustments during the transition from meiosis I to II result in a Mapk-utilizing, Rsk-independent signal to Emi2 to establish mII (Bodart et al, 2005). The features of Emi2, Mos and microtubules are linked in several ways. Emi2 localizes to spindles in both Xenopus and mouse maturing oocytes (Madgwick et al, 2006; Ohe et al, 2007). Mouse Mapk regulates microtubule organization and asymmetrical division (Yu et al, 2007), and the absence of Mos perturbs Mapk signalling, inducing microtubule elongation between meiotic divisions (Verlhac et al, 1996). A further indication that Mos and spindle dynamics are linked comes from the world of microRNA (miRNA); in mouse oocytes, a functional miRNA network is required both for spindle maintenance and the regulation of Mos mRNA (Tang et al, 2007).
Mouse Cycb degradation and mII exit also require an intact spindle; microtubule disruption prevents meiotic progression (Winston et al, 1995). This suggests that signalling through Camk2, Can or other activating pathways is insufficient to guarantee mII exit and is subordinate to at least one independently regulated mechanism involving microtubules, possibly through Plk1, which is dynamically associated with mII spindles (Tong et al, 2002). Mos might also modulate Emi2 activity during mII exit, as Mos−/− mII oocytes often collapse to mIII following exogenous activation (Araki et al, 1996). Mos and Emi2 could be associated with macromolecular assemblies that proximate elements of the Mapk pathway, Cycb/Cdc2, Plk1, tubulin and motors required for spindle positioning with respect to cortical actin. It remains to be seen whether Mos or Emi2 are constituents of a macromolecular assembly in either Xenopus or the mouse.
Our appreciation of CSF largely derives from work using Xenopus oocyte extracts, which is a powerful system to analyse biochemical interactions and signalling pathways. Research on intact mouse oocytes suggests that regulation of the spindle—a pivotal macromolecular assembly—might be essential for mII maintenance and exit. In the future, it will be important to characterize the interactions between microtubules, Emi2 and the Mos–Mapk pathway. In particular, we should learn whether the spindle directs local Mos–Mapk activation and if so, the extent to which this modulates Emi2 activity and other processes that are significant for mII homeostasis.

Anthony C.F. Perry

Marie-Hélène Verlhac
References
- Aoki F, Worrad DM, Schultz RM (1997) Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev Biol 181: 296–307 [DOI] [PubMed] [Google Scholar]
- Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, Sato E (1996) Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod 55: 1315–1324 [DOI] [PubMed] [Google Scholar]
- Bhatt RR, Ferrell JE (1999) The protein kinase p90 rsk as an essential mediator of cytostatic factor activity. Science 286: 1362–1365 [DOI] [PubMed] [Google Scholar]
- Bodart JF, Baert FY, Sellier C, Duesbery NS, Flament S, Vilain JP (2005) Differential roles of p39Mos–Xp42Mpk1 cascade proteins on Raf1 phosphorylation and spindle morphogenesis in Xenopus oocytes. Dev Biol 283: 373–383 [DOI] [PubMed] [Google Scholar]
- Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF (1996) The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci USA 93: 7032–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute P, Masui Y (1995) Regulation of the appearance of division asynchrony and microtubule-dependent chromosome cycles in Xenopus laevis embryos. Dev Biol 171: 273–285 [DOI] [PubMed] [Google Scholar]
- Colledge WH, Carlton MB, Udy GB, Evans MJ (1994) Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature 370: 65–68 [DOI] [PubMed] [Google Scholar]
- Descombes P, Nigg EA (1998) The polo-like kinase Plx1 is required for M phase exit and destruction of mitotic regulators in Xenopus egg extracts. EMBO J 17: 1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth BC, Weaver JS, Ruderman JV (2002) G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc Natl Acad Sci USA 99: 16794–16799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J, Umbhauer M, Rassinier P, Hanauer A, Verlhac MH (2005) p90Rsk is not involved in cytostatic factor arrest in mouse oocytes. J Cell Biol 169: 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré A, Jessus C, Ozon R, Haccard O (2002) Mos is not required for the initiation of meiotic maturation in Xenopus oocytes. EMBO J 21: 4026–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félix MA, Labbe JC, Doree M, Hunt T, Karsenti E (1990) Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase. Nature 346: 379–382 [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Yoshida N, Fukui T, Amanai M, Isobe T, Itagaki C, Izumi T, Perry ACF (2004) Mammalian phospholipase Cζ induces oocyte activation from the sperm perinuclear matrix. Dev Biol 274: 370–383 [DOI] [PubMed] [Google Scholar]
- Gardner AJ, Knott JG, Jones KT, Evans JP (2007) CaMKII can participate in but is not sufficient for the establishment of the membrane block to polyspermy in mouse eggs. J Cell Physiol 212: 275–280 [DOI] [PubMed] [Google Scholar]
- Gautier J, Matsukawa T, Nurse P, Maller J (1989) Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature 339: 626–629 [DOI] [PubMed] [Google Scholar]
- Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL (1990) Cyclin is a component of maturation-promoting factor from Xenopus. Cell 60: 487–494 [DOI] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW (1991) Cyclin is degraded by the ubiquitin pathway. Nature 349: 132–138 [DOI] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL (2000) The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk). Curr Biol 10: 430–438 [DOI] [PubMed] [Google Scholar]
- Gurdon JB (1960) The developmental capacity of nuclei taken from differentiating endoderm cells of Xenopus laevis. J Embryol Exp Morphol 8: 505–526 [PubMed] [Google Scholar]
- Han SJ, Chen R, Paronetto MP, Conti M (2005) Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol 15: 1670–1676 [DOI] [PubMed] [Google Scholar]
- Hanson PI, Schulman H (1992) Neuronal Ca2+/calmodulin-dependent protein kinases. Ann Rev Biochem 61: 559–601 [DOI] [PubMed] [Google Scholar]
- Inoue D, Ohe M, Kanemori Y, Nobui T, Sagata N (2007) A direct link of the Mos–MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature 446: 1100–1104 [DOI] [PubMed] [Google Scholar]
- Klee CB, Ren H, Wang X (1998) Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem 273: 13367–13370 [DOI] [PubMed] [Google Scholar]
- Knott JG, Gardner AJ, Madgwick S, Jones KT, Williams CJ, Schultz RM (2006) Calmodulin-dependent protein kinase II triggers mouse egg activation and embryo development in the absence of Ca2+ oscillations. Dev Biol 296: 388–395 [DOI] [PubMed] [Google Scholar]
- Kubiak JZ, Weber M, de Pennart H, Winston NJ, Maro B (1993) The metaphase II arrest in mouse oocytes is controlled through microtubule-dependent destruction of cyclin B in the presence of CSF. EMBO J 12: 3773–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence Y, Whitaker M, Swann K (1997) Sperm–egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development 124: 233–241 [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Terret ME, Djiane A, Rassinier P, Maro B, Verlhac M-H (2002) Meiotic spindle stability depends on MAPK-interacting and spindle-stabilizing protein (MISS), a new MAPK substrate. J Cell Biol 157: 603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leise WF III, Mueller PR (2002) Multiple Cdk1 inhibitory kinases regulate the cell cycle during development. Dev Biol 249: 156–173 [DOI] [PubMed] [Google Scholar]
- Liu J, Maller JL (2005) Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol 15: 1–11 [DOI] [PubMed] [Google Scholar]
- Lorca T, Galas S, Fesquet D, Devault A, Cavadore JC, Doree M (1991) Degradation of the proto-oncogene product p39mos is not necessary for cyclin proteolysis and exit from meiotic metaphase: requirement for a Ca2+-calmodulin dependent event. EMBO J 10: 2087–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T, Cruzalegui FH, Fesquet D, Cavadore JC, Mery J, Means A, Dorée M (1993) Calmodulin-dependent protein kinase II mediates inactivation of MPF and CSF upon fertilization of Xenopus eggs. Nature 366: 270–273 [DOI] [PubMed] [Google Scholar]
- Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT (2006) Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol 174: 791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoulaki S, Matson S, Ducibella T (2004) Fertilization stimulates long-lasting oscillations of CaMKII activity in mouse eggs. Dev Biol 272: 15–25 [DOI] [PubMed] [Google Scholar]
- Masui Y, Markert CL (1971) Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 177: 129–145 [DOI] [PubMed] [Google Scholar]
- Mochida S, Hunt T (2007) Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449: 336–340 [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M (1982) A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell 30: 687–696 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Ohsumi K, Kishimoto T (2007a) Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature 446: 1096–1099 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Yoshizaki N, Kishimoto T, Ohsumi K (2007b) Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis. Nature 449: 341–345 [DOI] [PubMed] [Google Scholar]
- Ohe M, Inoue D, Kanemori Y, Sagata N (2007) Erp1/Emi2 is essential for the meiosis I to meiosis II transition in Xenopus oocytes. Dev Biol 303: 157–164 [DOI] [PubMed] [Google Scholar]
- Peters JM (2006) The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7: 644–656 [DOI] [PubMed] [Google Scholar]
- Phillips KP, Baltz JM (1996) Intracellular pH change does not accompany egg activation in the mouse. Mol Reprod Dev 5: 52–60 [DOI] [PubMed] [Google Scholar]
- Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU (2005) Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature 437: 1048–1052 [DOI] [PubMed] [Google Scholar]
- Runft LL, Watras J, Jaffe LA (1999) Calcium release at fertilization of Xenopus eggs requires type I IP3 receptors, but not SH2 domain-mediated activation of PLCγ or Gq-mediated activation of PLCβ. Dev Biol 214: 399–411 [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM (2002) Egg activation: where it all begins. Dev Biol 245: 237–254 [DOI] [PubMed] [Google Scholar]
- Sagata N, Watanabe N, Vande Woude GF, Ikawa Y (1989) The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 342: 512–518 [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA (2002) PLCζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129: 3533–3544 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Duncan PI, Rauh NR, Sauer G, Fry AM, Nigg EA, Mayer TU (2005) Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev 19: 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya EK, Masui Y (1988) Stabilization and enhancement of primary cytostatic factor (CSF) by ATP and NaF in amphibian egg cytosols. Dev Biol 129: 253–264 [DOI] [PubMed] [Google Scholar]
- Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, Perry ACF (2006) Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. EMBO J 25: 834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa G, Whittingham DG, Molinaro M, Vivarelli E (1978) Parthenogenetic activation of mouse oocytes induced by inhibitors of protein synthesis. J Embryol Exp Morphol 43: 157–166 [PubMed] [Google Scholar]
- Tang F, Kaneda M, O'Carroll D, Hajkova P, Barton SC, Sun YA, Lee C, Tarakhovsky A, Lao K, Surani MA (2007) Maternal microRNAs are essential for mouse zygotic development. Genes Dev 21: 644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terret ME, Lefebvre C, Djiane A, Rassinier P, Moreau J, Maro B, Verlhac M-H (2003) DOC1R: a MAP kinase substrate that control microtubule organization of metaphase II mouse oocytes. Development 130: 5169–5177 [DOI] [PubMed] [Google Scholar]
- Tong C, Fan HY, Lian L, Li SW, Chen DY, Schatten H, Sun QY (2002) Polo-like kinase-1 is a pivotal regulator of microtubule assembly during mouse oocyte meiotic maturation, fertilization, and early embryonic mitosis. Biol Reprod 67: 546–554 [DOI] [PubMed] [Google Scholar]
- Tsurumi C, Hoffmann S, Geley S, Graeser R, Polanski Z (2004) The spindle assembly checkpoint is not essential for CSF arrest of mouse oocytes. J Cell Biol 167: 1037–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi C, Hoffmann S, Geley S, Graeser R, Polanski Z (2004) The spindle assembly checkpoint is not essential for CSF arrest of mouse oocytes. J Cell Biol 167: 1037–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunquist BJ, Eyers PA, Chen LG, Lewellyn AL, Maller JL (2003) Spindle checkpoint proteins Mad1 and Mad2 are required for cytostatic factor-mediated metaphase arrest. J Cell Biol 163: 1231–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Weber M, Geraud G, Colledge WH, Evans MJ, Maro B (1996) Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development 122: 815–822 [DOI] [PubMed] [Google Scholar]
- Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R (1998) Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 394: 369–374 [DOI] [PubMed] [Google Scholar]
- Webb D, Nuccitelli R (1981) Direct measurement of intracellular pH changes in Xenopus eggs at fertilization and cleavage. J Cell Biol 91: 562–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston NJ, McGuinness O, Johnson MH, Maro B (1995) The exit of mouse oocytes from meiotic M-phase requires an intact spindle during intracellular calcium release. J Cell Sci 108: 143–151 [DOI] [PubMed] [Google Scholar]
- Wolf DP (1974) The cortical response in Xenopus laevis ova. Dev Biol 40: 102–115 [DOI] [PubMed] [Google Scholar]
- Wu Q et al. (2007a) A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr Biol 17: 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Hansen DV, Guo Y, Wang MZ, Tang W, Freel CD, Tung JJ, Jackson PK, Kornbluth S (2007b) Control of Emi2 activity and stability through Mos-mediated recruitment of PP2A. Proc Natl Acad Sci USA 104: 16564–16569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Brahmajosyula M, Shoji S, Amanai M, Perry ACF (2007) Epigenetic discrimination by mouse metaphase II oocytes mediates asymmetric chromatin remodeling independently of meiotic exit. Dev Biol 301: 464–477 [DOI] [PubMed] [Google Scholar]
- Yu LZ et al. (2007) MEK1/2 regulates microtubule organization, spindle pole tethering and asymmetric division during mouse oocyte meiotic maturation. Cell Cycle 6: 330–338 [DOI] [PubMed] [Google Scholar]
- Zhang SC, Masui Y (1992) Activation of Xenopus laevis eggs in the absence of intracellular Ca activity by the protein phosphorylation inhibitor, 6 dimethylaminopurine (6-DMAP). J Exp Zool 262: 317–329 [DOI] [PubMed] [Google Scholar]