Abstract
Purpose
The addition of anionic charge on denture base resins has been shown to inhibit Candida albicans adhesion and to facilitate adsorption of salivary defense molecules. The aim of this study was to evaluate the physical properties of a modified denture base resin for denture fabrication.
Materials and Methods
Specimens made from heat polymerizing resin Lucitone 199 were used as the control group. The two experimental groups, E-10 and E-20, had 10% and 20%, respectively, of the monomer substituted with an experimental phosphate-containing monomer. Flexural strength and modulus, water sorption, solubility, and color stability tests were conducted to ensure compliance with ADA specification No. 12. Water diffusion coefficient into the resins and stainability were also assessed. ANOVA and Scheffe tests were performed for statistical significance.
Results
There was an overall decline in all properties with the addition of the experimental phosphate compound. The flexural strength and modulus, water sorption and solubility for E-10, as well as the control were, however, within the ADA specifications. The diffusion coefficients were significantly different (p < 0.05) for the three groups. Staining and color specimens showed no significant difference (p > 0.05) among the three groups.
Conclusions
Within the limitations of this study, the physical properties of the phosphate denture base resin at 10% should be suitable for denture fabrication based on the properties assessed.
INDEX WORDS: Candida albicans, PMMA, denture material, phosphate
The use of a dental prosthesis is indispensable for functional and esthetic rehabilitation of edentulous patients. Despite concerns about the decline in the need for prosthodontic services due to delayed edentulism, the actual unmet need for these services will rise steadily in the next two decades.1 Douglass and Watson calculated that the unmet need for prosthodontic services will increase from 488 million hours in 2005 to 560 million hours in 2020.1 It has been estimated that the need for dentures will rise to 37.9 million adults in 2020.2 Synthetic acrylics resins have a long, clinically proven history of use for dentures since they exhibit adequate physical, mechanical, and esthetic properties; however, they are susceptible to microbial adhesion, leading to denture stomatitis, which is the most common infectious disease affecting the palatal mucosa, and is highly prevalent in denture wearers.3,4
Denture stomatitis is a common clinical entity characterized by inflammation of oral tissues and colonization of the intaglio surface of prostheses by pathogenic organisms. It has a multi-factorial etiology reportedly consisting of either an ill-fitting prosthesis causing mechanical irritation or poor hygiene leading to chronic infection. Rarely, allergic response to the resin is also seen. Regardless of the initiating mechanism, denture stomatitis is characterized by the presence of a yeast biofilm on the prosthesis, primarily associated with Candida albicans.5–7 According to a report by Reichart on a group of Germans aged 65 to 74, there was an 18.3% prevalence of denture stomatitis.3 Others have shown prevalence rates near 60% in a denture-wearing institutionalized population.4
The treatment for denture stomatitis is as complex as the disease itself. Traditional treatment modalities include the use of antifungal agents and modification of the prosthesis to receive a temporary soft tissue reliner. Oral antimycotic agents seem helpful, but recurrence is rapid and assured unless the denture tissue surface is modified to eliminate candidal hyphae.8 Using either systemic fluconazole or topical amphotericin antifungal treatments for denture stomatitis, Bissell et al documented a 50% relapse in stomatitis after 12 weeks using both mycologic and clinical criteria independent of the type of therapy used.9 Additionally, compliance with antifungal regimens can be compromised by a patient’s non-perception of need for medication for a relatively painless condition. Therefore, there has been a tendency toward the incorporation of antimicrobial agents into the denture liners or the resin itself and other similar intraoral drug delivery systems.10–15
A previous study showed substantial differences in the protein compositions of the acquired enamel pellicle in comparison to denture pellicle.16 The acquired enamel pellicle contains antimicrobial peptides like histatins along with other constituents such as IgA, amylase, salivary statherins, and mucins. In contrast, the denture pellicle lacks salivary statherins and histatin. The absence of these important salivary defense molecules on the denture base has been attributed to the lack of anionic charge in polymethyl methacrylate (PMMA) polymers.16 Thus, this lack of surface charge may be responsible for the decreased protective function of the acquired pellicle on the denture. Moreover, reduction of candidal adherence has been demonstrated using a novel PMMA made with increasing amounts of methacrylic acid in place of methyl methacrylate monomer during processing. The new polymer is more hydrophilic and showed a significant correlation between the amount of methacrylic acid in the polymer and decrease in adhesion of C. albicans.17
This formed the rationale for the hypothesis that denture base resins with a phosphate-based anionic charge might also inhibit C. albicans adhesion, and should theoretically provide an enhanced biomimetic surface for the salivary cationic antimicrobials, such as the histatins. The protective role of these histatins would be a potentially viable treatment modality for denture stomatitis; however, any new denture base material must meet standards for clinical acceptability.
Therefore, the purpose of this study was to evaluate the physical properties of denture resins with different concentrations of phosphate substitutions in the monomer. These resins would be compared against each other and the control resin, Lucitone 199 (Dentsply International Inc., York, PA), for suitability for denture fabrication. ISO 1567 for denture base polymers and ADA specification No. 12 for denture base polymers were followed in setting up the study design. The null hypothesis was that there would be no difference in physical properties of Lucitone 199 and the other experimental resins.
Materials and Methods
Three groups of acrylic were investigated in this study — a control group, and two experimental groups. Acrylic denture base material, Lucitone 199®, identified as L199, was used as the control. This material is supplied as a powder and liquid system. The main component of the powder is the pre-polymer, PMMA and the main component of the liquid is the monomer, methyl-methacrylate (MMA). The experimental groups, identified as E-10 and E-20, contained two concentrations of a phosphate-containing monomer (10% and 20% by volume) substituted into the liquid. This substitution was performed in the authors’ laboratory with no attempt made to restore the original levels of cross-linker found in the monomer. Each group was tested for flexural strength and modulus, water sorption, solubility, color stability, and stainability.
Flexural Strength and Modulus
The specimens were prepared by mixing powder and liquid in a 3:1 ratio and packing into rectangular stainless steel molds (65 × 40 × 5 mm) in accordance with ISO 1567 for denture base resins. The plates were then polymerized in a curing unit (Hanau Teledyne, Buffalo, NY) at 73°C for 90 minutes and 100°C for 30 minutes. This process was repeated to obtain 24 acrylic plates, 8 for each group. Each processed acrylic plate was cut lengthwise on a milling machine under wet conditions to prevent overheating, yielding three specimens each (64 × 10 × 3.3 mm). The specimens were visually examined to ensure they were free of voids, with 16 samples from each group chosen for testing.
The specimens were stored in distilled water at 37°C for 50 hours prior to testing for flexural strength. Immediately after removal from water, the specimen was placed on the supports of the three-point load fixture immersed in a water bath at 37°C mounted on the loading cell of the universal testing machine (Model 55R1114, Instron Corp, Canton, MA). The distance between the specimen supports was 50 mm. The loading force was applied to the specimen at a cross-head speed of 5 mm/min until the specimen fractured. Flexural strength and modulus were calculated using the recorded data values and specimen geometric measurements.
Water Sorption and Solubility and Diffusion Coefficient
Using two circular stainless steel molds, ten specimens with a diameter of 50 mm and thickness of 1 mm were made for each of the three groups investigated. Due to the difficulty in fabricating 0.5 mm thick specimens described by ISO 1567, the thickness of the specimens was increased to 1 mm. The specimens were first conditioned to a constant mass. This consisted of placing them in a rack inside a dessicator with 0.5 kg of freshly dried silica gel (Sigma Aldrich, Milwaukee, WI). The dessicator was placed in an oven at 37°C for 23 hours. Following removal from the dessicator, the specimens were placed in a second dessicator containing freshly dried silica gel at 23°C for 60 minutes. The conditioned mass was reached when the difference between two successive readings was less than 0.2 mg. At this point, volume was calculated using an average of three diameter readings and five thickness readings. Next, the specimens were immersed in water at 37°C for 7 days. To obtain the data necessary to compute diffusion coefficients, weight measurements were made at intervals of 1 hour for 8 hours on the first day and at the conclusion of the immersion period of 7 days. After the final weighing on the seventh day of soaking, the specimens were reconditioned to a constant mass following the same protocol as for conditioning the specimens prior to water immersion. The amount of water sorption and water solubility was calculated as in ISO 1567. The diffusion coefficient (D), which gives a relation of water sorption over immersion time, was calculated using the following formula derived from Fick’s second equation: D = S2πL2/4, where L is half the thickness of the sample, and S is the slope of the linear portion of the Ms/Meq versus Time1/2 curve, where Ms equals 1 minus the mass uptake at a specific immersion time, and Meq is the mass uptake at equilibrium (7 days).
Color Stability
Specimens similar to those used for water sorption (50 mm diameter × 1 mm thickness) were prepared for the color measurements. Five CIE L*a*b* measurements were made on each half of the circular specimen (pre-exposure measurements) using a colorimeter (Chroma Meter CR-300, Minolta, Langenhagen, Germany). One-aluminum foil, and the specimen was exposed to UV light for 24 hours using an apparatus (Sabri Dental Enterprises, Inc., Downers Grove, IL) described by a previous version of ADA specification No. 80 for color stability. Five CIE L*a*b* measurements on each half of the specimen were made again (post-exposure measurements). Except during the colorimeter measurements and prior to and after exposure to UV light, the specimens were kept entirely covered to prevent exposure to light.
The total color difference between readings, ΔE*, was calculated by the following equation: ΔE* ={(ΔL*)2+(Δa*)2+(Δb*)2}1/2, where ΔL*, Δa*, and Δb* are differences in measured values on the specimens. Using the above methodology allows several color difference comparisons to be made. ΔE*homogeneous, given by the difference in L*a*b* readings from the pre-exposure UV-exposed side and pre-exposure non-exposed side, gives a measure of the color homogeneity of the specimen. ΔE*UVexposure, given by the difference in L*a*b* readings from the pre-exposure UV-exposed side and post-exposure UV-exposed side, gives a direct measure of color change due to UV exposure. ΔE*ADA, given by the difference in L*a*b* readings from the post-exposure exposed side and post-exposure non-exposed side, gives a measure of color change representative of how these specimens are subjectively evaluated by observers as specified in ISO 7491.
Staining
Five specimens (64 × 10 × 3.3 mm) per group were sectioned to make five test specimens and five matched controls. The five test specimens of each group were placed in a concentrated coffee solution (10 g coffee in 200 ml distilled water) at 43°C for 90 hours. The other five specimens were placed in 200 ml of distilled water at 43°C for 90 hours to serve as the controls. After the end of 90 hours, the specimens were rinsed and dried. A visual comparative examination of each specimen pair was made by two observers. The visual scale used to rate comparative stain level was: 0 = no staining, 1 = very light staining, 2 = light staining, 3 = moderate staining, 4 = heavy staining, 5 = very heavy staining.
Statistical Analysis
The data for flexural strength, flexural modulus, water sorption, solubility, diffusion coefficient, and color stability were analyzed using 1-way analysis of variance (ANOVA), and an additional post-hoc Scheffé multiple comparison and range test was done when indicated (SPSS Inc., Chicago, IL). Level of significance was set at p < 0.05. For stainability, a Kruskal-Wallis test was used to determine significance.
Results
Flexure Strength and Modulus
The means and standard deviations for flexural strength and flexural modulus are displayed in Table 1. One-way ANOVA showed a significant difference (p < 0.05) in flexural strength and flexural modulus among the three groups. Scheffé’s multiple comparison and range test showed that L199 had the highest flexural strength and modulus. There was a 5.25% decrease in flexural strength and 12% decrease in flexural modulus associated with the addition of 10% phosphate to the monomer of L199, while a 10.4% decrease in flexural strength and a 19% decrease in flexural modulus were observed with the addition of 20% phosphate to the monomer of L199. The decrease in flexural strength and modulus is significant in both the cases; however, the flexural strength and modulus values for E-10 comply with the minimum values (65 MPa and 2000 MPa, respectively) set forth by ADA specification No.12.
Table 1.
Mean (Standard Deviation) Values for the Physical Properties Tested
| Property/Measure | Units | L199 | E-10 | E-20 |
|---|---|---|---|---|
| Flexural strength | MPa | 69.4 A (4.9) | 65.8 B (2.8) | 61.9 C (3.0) |
| Flexural modulus | MPa | 2420 A (89) | 2128 B (77) | 1949 C (83) |
| Water sorption | μg/mm3 | 20.3 A (1.9) | 27.5 B (2.4) | 32.8 C (3.7) |
| Water solubility | μg/mm3 | 3.02 A (0.81) | 3.55 A (1.00) | 3.91 A (0.60) |
| Diffusion coefficient | cm2/s | 1.64 × 10−8A (2.3 × 10−9) | 1.33 × 10−8B (1.7 × 10−9) | 1.12 × 10−8C (3.3 × 10−9) |
For each test, different superscript letters (within row) indicate the mean is statistically different (p < 0.05).
Water Sorption and Solubility and Diffusion Coefficient
The values for water sorption, solubility, and diffusion coefficient are also shown in Table 1. ANOVA and post hoc Scheffé test showed significant differences (p < 0.05) among all three groups for water sorption and diffusion coefficient. Despite an increase in the mean solubility with phosphate concentration, ANOVA showed no significant differences (p > 0.05). Overall, E-20 exhibited the largest water sorption and water solubility, although both L199 and E-10 were within the acceptable range specified by ADA and ISO. The average absorption curves of the three groups tested are shown in Figure 1.
Figure 1.
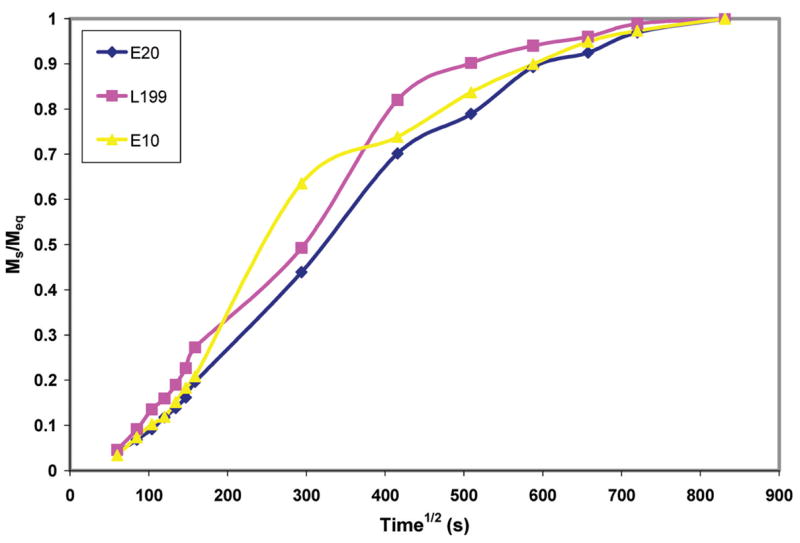
Absorption curves (water sorption ratio, Ms/Meq, vs. immersion time) for the three groups tested.
Color Stability
Results from the three color difference (ΔE*) comparisons are shown in Table 2. There were no significant differences in ΔE*homogeneous among the three groups, indicating the specimens did not differ in color homogeneity. For both ΔE*UVexposure and ΔE*ADA, the color change increased with phosphate concentration, and there were significant differences (p < 0.05) between L199 and E-20.
Table 2.
Mean (Standard Deviation) ΔE* Color Differences for the Three Groups
| ΔE*homogeneous | ΔE*UVExposure | ΔE*ADA | |
|---|---|---|---|
| L199 | 0.82 A (0.51) | 0.94 A (0.41) | 0.97 A (0.34) |
| E-10 | 1.48 A (0.82) | 1.18 AB (0.72) | 1.56 AB (0.68) |
| E-20 | 1.09 A (0.71) | 1.88 B (0.84) | 2.51 B (1.10) |
For each ΔE*, different superscript letters (within column) indicate the mean is statistically different (p < 0.05).
Staining
The specimens for the staining test showed no significant difference (p > 0.05; Kruskal-Wallis test) among the three groups. The vast majority (82%) of observations were denoted as very light staining when compared with the controls.
Discussion
Flexural Strength and Modulus
The flexural strength values for L199 were lower than the manufacturer’s claims (69.4 vs. 90 MPa), but the values obtained for flexural modulus were comparable (2420 vs. 2510 MPa). The results for flexural strength were slightly lower than those reported by Smith et al (72 MPa)18 and Williamson et al (73.3 MPa).19 L199 exhibited the highest strength and modulus, while E-20 exhibited the lowest, indicating that the addition of the experimental phosphate compound lowers the flexural strength and modulus in a dose-related manner. The flexural modulus was similar to values (2441 MPa) reported by Phoenix et al.20 Compared with another method used to modify the anionic charge on resin, namely the substitution of monomer with methacrylic acid, the addition of phosphate appears to cause less decline in flexural strength.21
The decrease in flexural strength and modulus can perhaps be associated with the dilution of other components of the liquid, such as the crosslinking agent ethylene glycol dimethacrylate (EGDMA). Denture base resins, specifically Lucitone 199, are primarily composed of pre-polymerized PMMA powder, MMA monomer, and crosslinking agent EGDMA. During the process of polymerization, the monomer penetrates the polymer and partially dissolves it. After polymerization, a new larger molecular weight polymer is formed, and the crosslinking agent aids in this process. EGDMA is chemically and structurally similar to MMA and may be incorporated in the growing chain itself, facilitating the interlinking of two adjacent chains. This assures a greater interlinking of polymer chains and increases the strength and stiffness of the resultant resin. Usually crosslinking agent is incorporated in the resins to a concentration of 5% to 6% by weight. Arima et al22 showed the interrelationship between increasing concentrations of crosslinking agent and increased flexural strength and modulus, as well as decreased water sorption and solubility. Also, the experimental resins exhibit greater water sorption, and since all the specimens were soaked in water for 24 hours, the plasticizing effect of increased water sorption for E-10 and E-20 may partially be responsible for lower values of flexural strength and modulus. Further studies are underway to assess whether the addition of crosslinking agent can offset the increased water solubility and decreased flexural strength. Preliminary data does suggest addition of EGDMA to the experimental formulations limits the decline of flexural strength and modulus.
Water Sorption, Solubility, and Diffusion Coefficient
The values obtained for water sorption indicate that E-20 exhibited the greatest sorption, followed by E-10 and L199. When expressed as a percent increase from original mass, the water sorption for L199 (1.93%), and E-10 (2.39%) are within the range described by Murphy et al23 (1.34% to 2.56%) and Anderson et al24 (0.92% to 2.53%) for Lucitone 199. The trend toward an increase in water solubility of the resin with the addition of phosphate compound indicates that the experimental groups are more hydrophilic. The absorption of water into the resin is influenced by the polarity of the PMMA molecules and diffusion of water molecules into the interstitial spaces between polymer chains. The addition of phosphate into the monomer increases the polarity of the resin, causing increased water sorption. This causes two major effects on the resin, namely a decrease in mechanical properties and an expansion due to the pushing apart of the resin polymers by the water molecules. The limited absorption of water over prolonged use can cause expansion of the resin, which in some measure helps to offset the polymerization shrinkage, but extensive absorption of water can be detrimental. Greater affinity for water may result in a long-term plasticizing effect on the resin after prolonged use. Increased water absorption may also prevent the intermeshing of polymer chains, causing them to be progressively more mobile, resulting in relaxation of built-up internal polymerization stresses.25
As the physical and mechanical properties of denture base resins are adversely affected by water, the diffusion coefficient of water for denture base resin also merits consideration. There was an increase in water sorption but an overall decrease in the diffusion coefficient values with the addition of a phosphate group. These results appear to be contradictory, but on closer look the decrease in diffusion coefficient can be explained. This is due to the technique involved for calculation of diffusion coefficient, which involves measuring the slope of the linear portion of the absorption curve. The absorption curve reflects a ratio of the original mass over the equilibrium mass of the specimen. Since the water sorption was greater for E-10 and E-20, smaller diffusion coefficients would be obtained by dividing the original mass with an increasing denominator.
Though the trend toward an increase in water solubility of the resin with addition of phosphate (17.43% and 29.47% increase in water solubility compared with the control for E-10 and E-20, respectively) did not reach statistical significance, it may indicate a greater leaching out of the monomer over time and thus, perhaps, a lesser degree of conversion of the monomer to fully polymerized polymer chains. Thus, it could be hypothesized that the addition of phosphate into the monomer prevents optimal crosslinking of the polymers. It remains to be seen whether this mechanism is due to the phosphate occupying a terminal position on the polymer chain, thus causing premature chain termination, or perhaps the answer is much simpler, and the dilution of crosslinking agent is responsible. ADA specification No. 12 describes the procedure for measuring residual monomer using gas chromatography, which could provide greater insights into the cause of increased solubility with increased addition of phosphate.
Color Stability
Color is an important property for the esthetic evaluation of denture base resins, as the denture base resins are exposed to the oral environment and many colorants in food and beverages. It is essential for the denture base resin to maintain its color despite the onslaught of everyday colorants. The three groups were tested for color stability, and changes in L*a*b* values were observed after exposure to UV radiation for 24 hours. As initially found by Kuehni and Marcus26 and subsequently quoted by other authors,27,28 under controlled laboratory conditions, a color difference of 1 ΔE unit would be judged a perfect match by more than 50% of the observers. Johnston and Kao29 have stated that color differences of 3.7 ΔE units were unnoticeable in the oral environment, and Doray et al30 stated that a color change is visually perceptible at 3.3 ΔE units. Although L199 showed the greatest color stability (lowest ΔE*UVexposure and ΔE*ADA) followed by E-10 and then E-20, it is important to note that none of the denture base resins showed ΔEs that would be clinically discernible.
Staining
One of the concerns that arose during the study was that if the denture base resin attracts salivary defense molecules, does it also attract other agents responsible for odor and staining? Though staining is not a test required by ADA specification No. 12, it was performed to evaluate the staining potential of the resin. No observable difference was found among the three groups in relation to the staining potential.
The limitations of this study were the negation of the crosslinking agent by its effective dilution with the addition of phosphate to the monomer. It is not clearly understood what effects, if any, it has on the physical and mechanical properties of the resin investigated. Thus, addition of crosslinking agent as another control for the study is an area that needs further investigation. Though previous studies with methacrylic acid instead of phosphate provide us an insight into the role of surface modification of resins for anticandidal activity,17,31 further areas of investigation with these denture base resins should include cytotoxicity tests for tissue and also adherence of Candida albicans to the resin. Also, in-depth evaluation of the degree of conversion of the resin and contact angle analysis would help to provide definitive answers to the polar nature of these resins. Lastly, this was an in vitro study with a limited sample size, and much larger clinical trials are needed to translate the impact such resins would have in the prevention or treatment of fungal infections.
Conclusions
Based on the results and limitations of this study, the following conclusions can be drawn:
L199 exhibited the highest strength and modulus while E-20 exhibited the lowest, indicating that the addition of the experimental phosphate compound may lower the flexural strength and modulus in a dose-related manner.
L199 showed the lowest water sorption and solubility values. E-20, which had the highest sorption and solubility, did not meet the criteria set forth by ADA specification No. 12 and ISO 1567. E-10 and L199 showed water sorption and solubility values less than the maximum permissible values.
For color stability, all three resins showed visually non-perceptible changes in ΔE values, though it is noteworthy that L199 had the smallest increase in ΔE values, while E-20 had the highest increase.
For staining, there was no statistically significant difference within the three groups tested.
Within the limitations of this study, the preliminary tests show promising results for phosphate resins at 10% having the threshold physical properties required for denture base usage.
Acknowledgments
The authors wish to thank Dr. Tiba, CAPT Ehrlich, and the Naval Institute for Dental and Biomedical Research, Great Lakes, IL, for use of the colorimeter. Part of this work was presented at the International Association for Dental Research meeting, Baltimore, MD, March, 2005 and funded by a grant provided by the American College of Prosthodontists and Procter & Gamble, 2003-04, as well as DE016925 (ARD) from NIDCR.
References
- 1.Douglass CW, Watson AJ. Future needs for fixed and removable partial dentures in the United States. J Prosthet Dent. 2002;87:9–14. doi: 10.1067/mpr.2002.121204. [DOI] [PubMed] [Google Scholar]
- 2.Douglass CW, Shih A, Ostry L. Will there be a need for complete dentures in the United States in 2020? J Prosthet Dent. 2002;87:5–8. doi: 10.1067/mpr.2002.121203. [DOI] [PubMed] [Google Scholar]
- 3.Reichart PA. Oral mucosal lesions in a representative cross-sectional study of aging Germans. Community Dent Oral Epidemiol. 2000;28:390–398. doi: 10.1034/j.1600-0528.2000.028005390.x. [DOI] [PubMed] [Google Scholar]
- 4.Moskona D, Kaplan I. Oral lesions in elderly denture wearers. Clin Prev Dent. 1992;14:11–14. [PubMed] [Google Scholar]
- 5.Bastiaan RJ. Denture sore mouth. Aetiological aspects and treatment. Aust Dent J. 1976;21:375–382. doi: 10.1111/j.1834-7819.1976.tb05091.x. [DOI] [PubMed] [Google Scholar]
- 6.Olsen I, Birkeland JM. Denture stomatitis-yeast occurrence and the pH of saliva and denture plaque. Scand J Dent Res. 1977;85:130–134. doi: 10.1111/j.1600-0722.1977.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 7.Budtz-Jorgensen E. Clinical aspects of Candida infection in denture wearers. J Am Dent Assoc. 1978;96:474–479. doi: 10.14219/jada.archive.1978.0088. [DOI] [PubMed] [Google Scholar]
- 8.Kulak Y, Arikan A, Delibalta N. Comparison of three different treatment methods for generalized denture stomatitis. J Prosthet Dent. 1994;72:283–288. doi: 10.1016/0022-3913(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 9.Bissell V, Felix DH, Wray D. Comparative trial of fluconazole and amphotericin in the treatment of denture stomatitis. Oral Surg Oral Med Oral Pathol. 1993;76:35–39. doi: 10.1016/0030-4220(93)90290-k. [DOI] [PubMed] [Google Scholar]
- 10.Addy M. In vitro studies into the use of denture base and soft liner materials as carriers for drugs in the mouth. J Oral Rehabil. 1981;8:131–142. doi: 10.1111/j.1365-2842.1981.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 11.Quinn DM. The effectiveness, in vitro, of miconazole and ketoconazole combined with tissue conditioners in inhibiting the growth of Candida albicans. J Oral Rehabil. 1985;12:177–182. doi: 10.1111/j.1365-2842.1985.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 12.Mirth DB, Bartkiewicz A, Shern RJ, et al. Development and in vitro evaluation of an intraoral controlled-release delivery system for chlorhexidine. J Dent Res. 1989;68:1285–1288. doi: 10.1177/00220345890680081401. [DOI] [PubMed] [Google Scholar]
- 13.Schneid TR. An in vitro analysis of a sustained release system for the treatment of denture stomatitis. Spec Care Dentist. 1992;12:245–250. doi: 10.1111/j.1754-4505.1992.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 14.Pesci-Bardon C, Fosse T, Madinier I, et al. In vitro new dialysis protocol to assay the antiseptic properties of a quartenary ammonium compound polymerized with denture acrylic resin. Lett Appl Microbiol. 2004;39:226–231. doi: 10.1111/j.1472-765X.2004.01569.x. [DOI] [PubMed] [Google Scholar]
- 15.Etienne O, Gasnier C, Taddei C, et al. Antifungal coating by biofunctionalized polyelectrolyte multilayered films. Biomaterials. 2005;26:6704–6712. doi: 10.1016/j.biomaterials.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 16.Edgerton M, Levine MJ. Characterization of acquired denture pellicle from healthy and stomatitis patients. J Prosthet Dent. 1992;68:683–691. doi: 10.1016/0022-3913(92)90387-p. [DOI] [PubMed] [Google Scholar]
- 17.Park SE, Periathamby AR, Loza JC. Effect of surface-charged poly(methyl methacrylate) on the adhesion of Candida albicans. J Prosthodont. 2003;12:249–254. doi: 10.1016/s1059-941x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 18.Smith LT, Powers JM, Ladd D. Mechanical properties of new denture resins polymerized by visible light, heat, and microwave energy. Int J Prosthodont. 1992;5:315–320. [PubMed] [Google Scholar]
- 19.Williamson DL, Boyer DB, Aquilino SA, et al. Effect of polyethylene fiber reinforcement on the strength of denture base resins polymerized by microwave energy. J Prosthet Dent. 1994;72:635–638. doi: 10.1016/0022-3913(94)90296-8. [DOI] [PubMed] [Google Scholar]
- 20.Phoenix RD, Mansueto MA, Ackerman NA, et al. Evaluation of mechanical and thermal properties of commonly used denture base resins. J Prosthodont. 2004;13:17–27. doi: 10.1111/j.1532-849X.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- 21.Park SE, Chao M, Raj PA, et al. Mechanical properties of surface-charged resin polymers as denture base. J Dent Res. 2003;82 doi: 10.1155/2009/841431. abstract 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arima T, Hamada T, McCabe JF. The effects of cross-linking agents on some properties of HEMA-based resins. J Dent Res. 1995;74:1597–1601. doi: 10.1177/00220345950740091501. [DOI] [PubMed] [Google Scholar]
- 23.Murphy WM, Bates JF, Huggett R, et al. A comparative study of 3 denture base materials. Br Dent J. 1982;152:273–276. doi: 10.1038/sj.bdj.4804796. [DOI] [PubMed] [Google Scholar]
- 24.Anderson GC, Schulte JK, Arnold TG. Dimensional stability of injection and conventional processing of denture base acrylic resin. J Prosthet Dent. 1988;60:394–398. doi: 10.1016/0022-3913(88)90292-2. [DOI] [PubMed] [Google Scholar]
- 25.Ristic B, Carl L. Water sorption by denture acrylic resin and consequent changes in vertical dimension. J Prosthet Dent. 1987;58:689–693. doi: 10.1016/0022-3913(87)90420-3. [DOI] [PubMed] [Google Scholar]
- 26.Kuehni RG, Marcus RT. An experiment in visual scaling of small color difference. Color Res Appl. 1979;4:83–91. [Google Scholar]
- 27.Shotwell JL, Johnston WM, Swarts RG. Color comparisons of denture teeth and shade guides. J Prosthet Dent. 1986;56:31–34. doi: 10.1016/0022-3913(86)90278-7. [DOI] [PubMed] [Google Scholar]
- 28.Seghi RR, Hewlett ER, Kim J. Visual and instrumental colorimetric assessments of small color differences on translucent dental porcelain. J Dent Res. 1989;68:1760–1764. doi: 10.1177/00220345890680120801. [DOI] [PubMed] [Google Scholar]
- 29.Johnston WM, Kao EC. Assessment of appearance match by visual observation and clinical colorimetry. J Dent Res. 1989;68:819–822. doi: 10.1177/00220345890680051301. [DOI] [PubMed] [Google Scholar]
- 30.Doray PG, Li D, Powers JW. Color stability of provisional restorative materials after accelerated aging. J Prosthodont. 2001;10:212–216. doi: 10.1111/j.1532-849x.2001.00212.x. [DOI] [PubMed] [Google Scholar]
- 31.Edgerton M, Raj PA, Levine MJ. Surface-modified poly(methyl methacrylate) enhances adsorption and retains anticandidal activeties of salivary histatin 5. J Biomed Mater Res. 1995;29:1277–1286. doi: 10.1002/jbm.820291015. [DOI] [PubMed] [Google Scholar]


