Abstract
In the present study, the BCAAs (branched-chain amino acids) leucine and valine caused a significant suppression in the loss of body weight in mice bearing a cachexia-inducing tumour (MAC16), producing a significant increase in skeletal muscle wet weight, through an increase in protein synthesis and a decrease in degradation. Leucine attenuated the increased phosphorylation of PKR (double-stranded-RNA-dependent protein kinase) and eIF2α (eukaryotic initiation factor 2α) in skeletal muscle of mice bearing the MAC16 tumour, due to an increased expression of PP1 (protein phosphatase 1). Weight loss in mice bearing the MAC16 tumour was associated with an increased amount of eIF4E bound to its binding protein 4E-BP1 (eIF4E-binding protein 1), and a progressive decrease in the active eIF4G–eIF4E complex due to hypophosphorylation of 4E-BP1. This may be due to a reduction in the phosphorylation of mTOR (mammalian target of rapamycin), which may also be responsible for the decreased phosphorylation of p70S6k (70 kDa ribosomal S6 kinase). There was also a 5-fold increase in the phosphorylation of eEF2 (eukaryotic elongation factor 2), which would also decrease protein synthesis through a decrease in translation elongation. Treatment with leucine increased phosphorylation of mTOR and p70S6k, caused hyperphosphorylation of 4E-BP1, reduced the amount of 4E-BP1 associated with eIF4E and caused an increase in the eIF4G–eIF4E complex, together with a reduction in phosphorylation of eEF2. These changes would be expected to increase protein synthesis, whereas a reduction in the activation of PKR would be expected to attenuate the increased protein degradation.
Keywords: branched-chain amino acid (BCAA), cachexia, double-stranded-RNA-dependent protein kinase (PKR), eukaryotic elongation factor 2 (eEF2), eukaryotic-initiation factor 4E (eIF4E), eukaryotic-initiation factor 4E-binding protein 1 (4E-BP1), leucine
Abbreviations: Ang II, angiotensin II; BCAA, branched-chain amino acid; DMEM, Dulbecco's modified Eagle's medium; eEF, eukaryotic elongation factor; eIF, eukaryotic initiation factor; 4E-BP1, eIF4E-binding protein; FCS, fetal calf serum; HS, horse serum; mTOR, mammalian target of rapamycin; m7GTP, 7-methyl-GTP; NF-κB, nuclear factor κB; p70S6k, 70 kDa ribosomal protein S6 kinase; PIF, proteolysis-inducing factor; PKR, double-stranded-RNA-dependent protein kinase; PP1, protein phosphatase 1
INTRODUCTION
Muscle protein loss in cancer cachexia is considered to arise from a combination of hypoanabolism together with an increase in catabolism. The primary mechanism for the increase in catabolism has been attributed to an increase in expression and activity of the ubiquitin–proteasome proteolytic pathway [1], but until recently the mechanism underlying the failure to maintain protein synthesis was largely unknown. However, a single mechanism has been proposed to explain the depression in protein synthesis and the increased degradation of myofibrillar proteins in cachexia through the activation, by autophosphorylation, of PKR (double-stranded-RNA-dependent protein kinase) [2]. Activation of PKR by agents such as PIF (proteolysis-inducing factor) and Ang II (angiotensin II) induces phosphorylation of eIF2α (eukaryotic initiation factor 2α), leading to inhibition of translation initiation, through competition with the guanine-nucleotide-exchange factor, eIF2B, preventing the conversion of eIF2 from its GDP-bound state into the active GTP-bound form [3]. Activation of PKR also induces protein degradation in muscle through the induction of the expression and activity of the ubiquitin–proteasome pathway in an NF-κB (nuclear factor κB)-mediated process [2]. Agents such as IGF-1 (insulin-like growth factor-1), which attenuate protein degradation in muscle, also attenuate the activation of PKR [4], suggesting an avenue for therapeutic development in the treatment of muscle-wasting diseases.
Protein synthesis also requires a correctly balanced mixture of amino acids, and a number of studies have noted widespread decreases in the plasma levels of free amino acids in patients with cachexia [5]. The maximum decreases are often found for the BCAAs (branched-chain amino acids) leucine, isoleucine and valine [6]. BCAAs, as well as being integral components of skeletal muscle proteins, are uniquely able to initiate signal transduction pathways that modulate translation initiation [7]. Of the BCAAs, leucine is most potent in stimulating muscle protein synthesis, whereas isoleucine and valine are much less effective [8]. The mechanism for stimulation is through activation of the mRNA-binding steps in translation initiation through hyperphosphorylation of 4E-BP1 (eIF4E-binding protein 1), resulting in the release of eIF4E from the inactive 4E-BP1–eIF4E complex [9]. The freed eIF4E then associates with eIF4G to form the active eIF4F complex. Leucine also stimulates the phosphorylation and thus the activation of p70S6k (70 kDa ribosomal S6 kinase), which also stimulates protein synthesis [8]. The effect of BCAAs on activation of PKR has not been investigated previously, although administration of leucine to rats, which had been fasted for 18 h, did not cause significant alterations in either the activity of eIF2B or the phosphorylation of eIF2α [9].
The aim of the present study was to investigate the effect of BCAAs on muscle wasting and to examine the mechanism, both in vitro, using catabolic stimuli such as PIF, and in vivo, in mice bearing a cachexia-inducing tumour.
EXPERIMENTAL
Materials
FCS (fetal calf serum), HS (horse serum) and DMEM (Dulbecco's modified Eagle's medium) were purchased from Life Technologies. L-[2,6-3H]Phenylalanine (specific radioactivity, 2.0 TBq/mmol), Hybond A nitrocellulose membranes, m7GTP (7-methyl-GTP)-Sepharose 4B and ECL® (enhanced chemiluminescence) development kits were from Amersham Biosciences. Rabbit polyclonal antisera to phospho-eIF2α (Ser51) was from Abcam, and rabbit polyclonal antisera to total eIF2α was from Santa Cruz Biotechnology. Rabbit polyclonal antisera to 4E-BP1–eIF4E, phospho-eIF4E (Ser209), total eIF4G, phospho-eIF4G (Ser1108), phospho-eEF2 (eukaryotic elongation factor 2) (Thr56), phospho-mTOR (Ser2448), phospho-p70S6k (Thr389) and total p70S6k and rabbit monoclonal antibodies to phospho-4E-BP1 (Thr37/46) and total PKR were from New England Biolabs. A mouse monoclonal antibody to PP1 (protein phosphatase 1) was purchased from Autogen Bioclear. Peroxidase-conjugated goat anti-rabbit and peroxidase-conjugated rabbit anti-mouse antibodies were purchased from Dako. PIF was purified by affinity chromatography from solid MAC16 tumours, as described previously [10]. PhosphoSafe™ extraction reagent and the PKR inhibitor were from Merck. The protease inhibitor cocktail was purchased from Sigma–Aldrich.
Animals
Pure strain NMRI mice (mean weight, 25 g) were obtained from our own inbred colony, and were fed on a rat and mouse breeding diet (Special Diet Services) and water ad libitum. Tumour fragments, obtained from donor animals with established weight loss, were implanted subcutaneously into the flank by means of a trocar, as described previously [11]. Weight loss occurred 12–15 days after tumour transplantation, and animals were entered into the study when they had lost approx. 5% of their starting body weight. Animals were randomized into groups of six to receive solvent (PBS), leucine, isoleucine or valine (BCAAs all at 1 g/kg of body weight) administered daily per os by gavage. Both tumour volume and body weight were monitored daily. Animals were killed by cervical dislocation when the body weight loss reached 20% of their starting weight, and all animal experiments followed a strict protocol approved by the British Home Office, and the ethical guidelines that were followed meet the standards required by the UKCCR (United Kingdom Co-ordinating Committee on Cancer Research) guidelines [12]. The soleus and gastrocnemius muscles were quickly dissected out and maintained in isotonic ice-cold saline before determination of protein synthesis and degradation respectively.
Cell culture
C2C12 murine myoblasts were routinely passaged in DMEM supplemented with 10% (v/v) FCS, glutamine and 1% penicillin/streptomycin under an atmosphere of 10% CO2 in air at 37 °C. When the myoblasts reached confluence, they were allowed to fuse to form myotubes by replacing the propagation medium with DMEM containing 2% (v/v) HS, with medium changes every 2 days. Differentiation was complete within 5–7 days.
Measurement of protein synthesis and degradation in vitro
Experiments were conducted in six-well dishes in 2 ml of DMEM without Phenol Red. PIF or Ang II was added at the concentrations indicated in the Figures, followed by 2 μl (370 kBq) of L-[2,6-3H]phenylalanine (specific radioactivity, 2 TBq/mmol) in 8 μl of sterile PBS. The plates were then incubated for 4 h at 37 °C under an atmosphere of 10% CO2 in air. After this time, the myotubes were washed three times with 1 ml of 0.2 mol/l ice-cold sterile PBS, followed by 1 ml of ice-cold perchloric acid, and were incubated at 4 °C for 20 min. The perchloric acid was replaced by 1 ml of 0.3 mol/l NaOH per well, and incubation was continued for 30 min at 4 °C, followed by a further incubation at 37 °C for 20 min. The NaOH extract was removed and combined with a further 1 ml wash from each well, and 0.5 ml of 0.2 mol/l perchloric acid was added and left on ice for 20 min. The extract was centrifuged at 700 g for 5 min at 4 °C and the protein-containing pellet was dissolved in 1 ml of 0.3 mol/l NaOH. A portion (0.5 ml) of the solution was counted for radioactivity, after mixing with 8 ml of Ultima Gold XR scintillation fluid. To measure the intracellular amino acid pool, the initial perchloric acid extract of the myotubes was neutralized with 0.2 mol/l KOH, and the insoluble potassium perchlorate was removed by centrifugation (4500 g for 10 min). The radioactivity of the supernatant was determined as above.
Protein degradation in myotubes was determined by the release of L-[2,6-3H]phenylalanine from cells prelabelled for 24 h as described previously [13]. After labelling, cells were washed extensively and pre-incubated in fresh medium for 2 h to allow degradation of short-lived proteins. Protein degradation was determined over a 24 h period in medium containing 2 mmol/l unlabelled phenylalanine to prevent re-incorporation of the radiolabel. The radioactivity released into the medium was calculated as a fraction of the total radioactivity incorporated into the myotubes.
Protein synthesis and degradation in muscle
The method for the determination of protein synthesis in muscle has been described previously [14]. Protein synthesis was measured by the incorporation of L-[2,6-3H]phenylalanine into acid-insoluble material during a 2 h period in which soleus muscles were incubated at 37 °C in RPMI 1640 without Phenol Red and saturated with 95% O2 and 5% CO2. After incubation, muscles were rinsed in non-radioactive medium, blotted and homogenized in 4 ml of 2% (v/v) perchloric acid. The rate of protein synthesis was calculated by dividing the amount of protein-bound radioactivity by the amount of acid-soluble radioactivity.
For protein degradation, gastrocnemius muscle was incubated in 3 ml of oxygenated (95% O2 and 5% CO2) Krebs–Henselit buffer (pH 7.4), containing 5 mmol/l glucose and 0.5 mmol/l cycloheximide. The rate of protein degradation was determined by the release of tyrosine [15] over a 2 h period.
Western blot analysis
Samples (approx. 10 mg) of gastrocnemius muscle were homogenized in 500 μl of PhosphoSafe™ extraction reagent and centrifuged at 15000 g for 15 min. Samples of cytosolic protein (10 μg) were resolved by SDS/PAGE (10% gels, 6% gels for eIF2α or 15% gels for 4E-BP1), and transferred on to 0.45 μm nitrocellulose membranes, which had been blocked with 5% (w/v) non-fat dried milk (Marvel) in PBS (pH 7.5) at 4 °C for 1–2 h, and then washed for 15 min in 0.1% Tween 20-buffered saline or PBS/Tween 20, prior to adding the primary antibodies. The primary antibodies were used at a dilution of 1:1000, except for phospho-eIF2α (1:500 dilution) and actin (1:200 dilution) antibodies. The secondary antibodies were used at a dilution of 1:1000. Incubation was for 1 h at room temperature (for actin) or overnight, and development was by ECL®. Blots were scanned by a densitometer to quantify differences.
m7GTP-Sepharose chromatography
The extent of phosphorylation of 4E-BP1 and the association of 4E-BP1 and eIF4G with eIF4E was determined by Western blotting when eIF4E was extracted from the muscle samples by m7GTP-Sepharose 4B-affinity binding. Muscle samples were homogenized in lysis buffer [150 mmol/l NaCl, 1% Nonidet P40, 50 mM Tris/HCl (pH 7.4), 0.25% sodium deoxycholate, 2 mmol/l EGTA, 1 mmol/l EDTA, 0.2 mmol/l sodium orthovanadate, 20 mmol/l NaF and 1% protease inhibitor mixture] at 4 °C and left for a further 10 min at room temperature with occasional vortex-mixing. The homogenate was centrifuged for 15 min at 15000 g, and the bound material (approx. 350 μg of protein) was used for Western blotting of eEF2; the remainder was added to a microcentrifuge tube containing 80 μl of m7GTP-Sepharose 4B at 4 °C, and incubation was continued overnight. After low-speed centrifugation (13000 g) and three washes with 1 ml of lysis buffer, the bound material was resuspended in 1×SDS sample buffer (50 μl) and subjected to SDS/PAGE on 10 or 15% gels, followed by Western blotting as described above.
Statistical analysis
Results are presented as means±S.E.M. Differences in means between groups were determined by one-way ANOVA, followed by Tukey–Kramer multiple comparison test. P values <0.05 were considered significant.
RESULTS
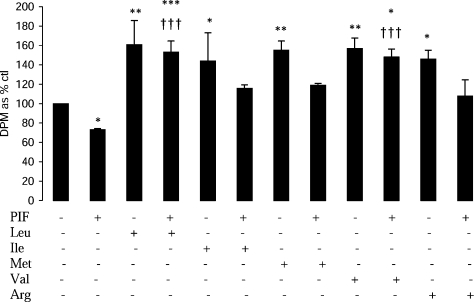
The BCAAs leucine and valine attenuated the depression of protein synthesis in murine myotubes in response to PIF, whereas methionine and arginine had no effect (Figure 1). This suggests that these amino acids have a specific effect on the depression of protein synthesis induced by PIF.
Figure 1. Effect of PIF alone or in combination with amino acids on protein synthesis in murine myotubes after 4 h of incubation.
The amino acids (2 mmol/l) were added 2 h prior to PIF (4.2 nmol/l). The concentration of the amino acids in DMEM was: 0.8 mmol/l for Leu, Ile and Val; 0.4 mmol/l for Phe; 0.34 mmol/l for Arg; and 0.2 mmol/l for Met. The results are means±S.E.M. of three separate determinations. *P<0.05, **P<0.01 or ***P<0.001 compared with control. †††P<0.001 compared with PIF alone.
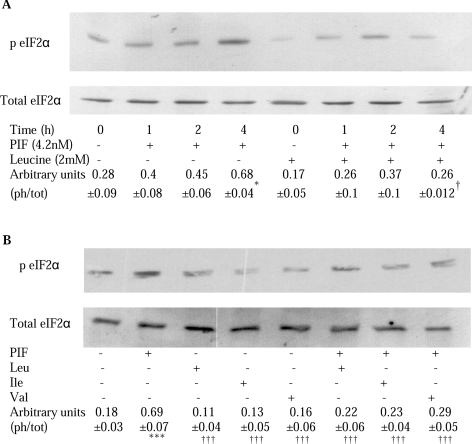
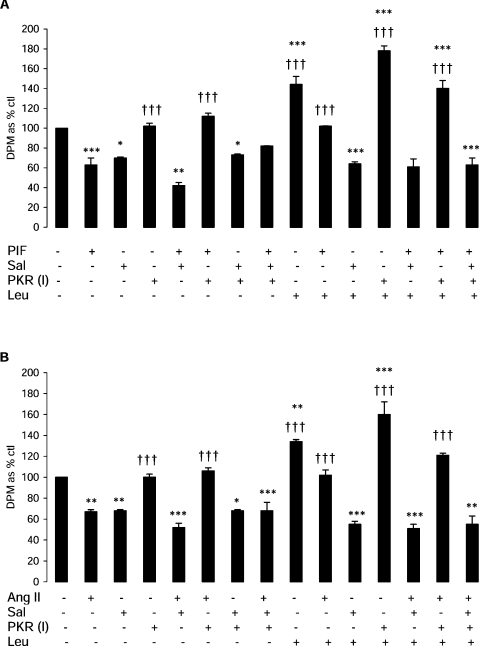
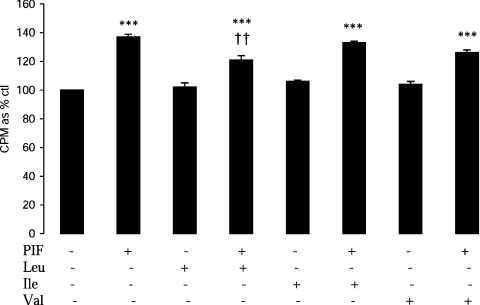
A previous study [2] has suggested that PIF inhibits protein synthesis in myotubes through activation of PKR, causing an increased phosphorylation of eIF2α, which leads to inhibition of translation initiation by blocking the action of the guanine-nucleotide-exchange factor eIF2B [3]. Figure 2 shows that PIF induced phosphorylation of eIF2α in myotubes, which was significantly increased above control values after incubation for 4 h (Figure 2A), and this was completely attenuated by leucine, isoleucine and valine (Figure 2B). This effect is important in the attenuation, by leucine, of protein synthesis inhibition by PIF, since the effect was completely attenuated by co-treatment with salubrinal, a specific inhibitor of eIF2α dephosphorylation by the phosphatase [16] (Figure 3A). There was no significant effect of salubrinal on protein synthesis inhibition by PIF alone (Figure 3A). Stimulation of protein synthesis by leucine in the absence of PIF was also attenuated by salubrinal, suggesting that this was due, at least in part, to inhibition of eIF2α phosphorylation. This effect appears to be due to attenuation of the activity of PKR, since the ability of leucine to reverse the inhibition of protein synthesis by PIF was enhanced in the presence of a PKR inhibitor [17] up to the value of leucine alone (Figure 3A). Similar results were obtained when Ang II was used to depress protein synthesis (Figure 3B). These results suggest that leucine acts to attenuate activation of PKR by PIF and Ang II. Leucine, but not valine or isoleucine, also partially attenuated the increase in protein degradation induced by PIF (Figure 4). Such an effect would be predicted from its ability to attenuate activation of PKR [2].
Figure 2. Effect of PIF alone or in combination with BCAAs on phosphorylated and total eIF2α.
(A) Western blot analysis of phospho-eIF2α (p EIF2α) in relation to total eIF2α in cytosolic extracts of murine myotubes after incubation with PIF (4.2 nmol/l) for various periods of time, either alone or in the presence of leucine (2 mmol/l). (B) Effect of the BCAA (2 mmol/l) alone or together with PIF on the phosphorylation of eIF2α after 4 h incubation determined by Western blotting. The amino acids were added to the myotubes 2 h prior to PIF. The densitometric analysis represents the means±S.E.M. for three separate Western blots, and is presented as the ratio of the phosphorylated to total eIF2α (ph/tot). *P<0.05 and ***P<0.001 compared with control; †P<0.05 and †††P<0.001 compared with PIF alone.
Figure 3. Effect of PIF (A) or Ang II (B) alone or in combination with leucine, salubrinal and a PKR inhibitor on protein synthesis in murine myotubes after 4 h of incubation.
Both the inhibitors and leucine (2 mmol/l) were added to the myotubes 2 h prior to PIF (4.2 nmol/l) or Ang II (0.5 μmol/l). Salubrinal (Sal; 15 μmol/l) is a phospho-eIF2α phosphatase inhibitor. PKR (I), PKR inhibitor {210 nmol/l; 8-[1-(1H-imidazol-4-yl)-meth-(Z)-yl idene]-6,8-dihydrothiazol[5,4-e]indol-7-one}. *P<0.05, **P<0.01 and ***P<0.001 compared with control; †††P<0.001 compared with PIF or Ang II alone.
Figure 4. Effect of PIF alone or in combination with BCAAs on protein degradation in myotubes after 24 h of incubation.
Details of the procedure are given in the Experimental section. The amino acids (2 mmol/l) were added 2 h prior to PIF (4.2 nmol/l). Results are means±S.E.M. of three separate determinations. ***P<0.001 compared with control; ††P<0.01 compared with PIF alone.
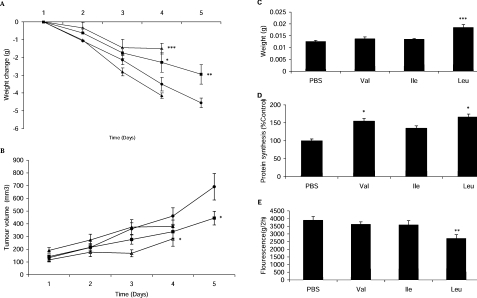
To investigate the effect of BCAAs on muscle loss in cachexia, the individual BCAAs were administered daily to mice bearing the MAC16 tumour, and the effects on weight loss and tumour growth were evaluated (Figure 5). Of the three BCAAs, only leucine and valine caused a significant suppression of body weight loss (Figure 5A), whereas only leucine caused an increase in soleus muscle wet weight (Figure 5C). Leucine and valine also produced a significant increase in protein synthesis (Figure 5D), whereas only leucine produced a decrease in protein degradation in skeletal muscle (Figure 5E). Interestingly, leucine and valine produced a small, but significant, inhibition of tumour growth (Figure 5B), an effect also seen with a low-molecular-mass PKR inhibitor [18].
Figure 5. Effect of administration of BCAAs on muscle loss in cachexia in mice bearing the MA16 tumour.
Effect of daily per os administration of leucine (■), isoleucine (▲) and valine (△) (all at 1g/kg of body weight) in comparison with PBS controls (●) on body weight change (A) and tumour volume (B) of mice bearing the MAC16 tumour. At the end of the experiment, mean weight of the soleus muscles (C), the rate of protein synthesis in gastrocnemius muscles (D) and the rate of protein degradation in soleus muscles (E) were determined. The details of the experiment are given in the Experimental section. Values are means±S.E.M. of six animals per treatment. *P<0.05, **P<0.01 and ***P<0.001 compared with control.
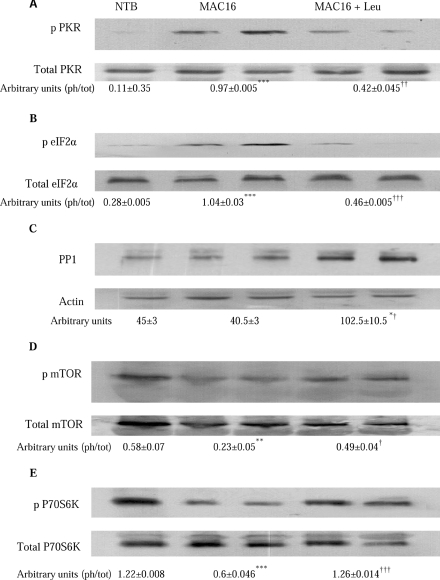
We have shown previously [2] that weight loss induced by the MAC16 tumour is associated with an increased phosphorylation of PKR and eIF2α in gastrocnemius muscle, which contribute to the depression of protein synthesis and protein degradation. The results shown in Figures 6(A) and 6(B) indicate that leucine attenuated the increased phosphorylation of both PKR and eIF2α, almost to values found in non-tumour-bearing animals, whereas levels of total PKR and eIF2α remained unchanged. The decreased phosphorylation of PKR is accompanied by a 2.5-fold increase in expression of PP1 (Figure 6C) in muscles of mice bearing the MAC16 tumour which were treated with leucine. PKR is a known substrate of PP1 [19].
Figure 6. Effect on phosphorylated and total PKR, eIF2α, mTOR and p70S6k and total PP1 in gastrocnemius muscles from non-tumour-bearing mice, and mice bearing the MAC16 tumour treated with either PBS or leucine.
Western blots of phospho-PKR (p PKR) and total PKR (A), phospho-eIF2α (p eIF2α) and total eIF2α (B), PP1 with actin loading control (C), phospho-mTOR (p mTOR) and total mTOR (D) and phospho-p70S6k (P70S6K) and total p70S6k (E) in gastrocnemius muscle of non-tumour-bearing mice (NTB), and mice bearing the MAC16 tumour treated with either PBS (MAC16) or leucine (1 g/kg of body weight) (MAC16+Leu). Samples were taken at the end of the experiment shown in Figure 5. Representative Western blots are shown, and the densitometric analysis shown underneath the blots represents means±S.E.M. for three separate Western blots. The values represent the ratio of the phosphorylated (ph) to total (tot) forms. *P<0.05 and ***P<0.001 compared with non-tumour-bearing mice; †P<0.05, ††P<0.01 and †††P<0.001 compared with mice bearing the MAC16 tumour treated with PBS.
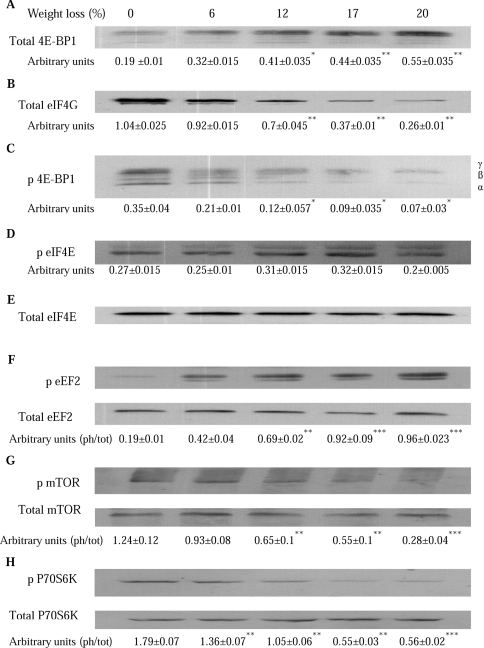
The effect of weight loss on the amount of eIF4E available for active eIF4G–eIF4E complex formation in gastrocnemius muscle is shown in Figure 7. There was a progressive increase in the amount of 4E-BP1 associated with eIF4E with increasing weight loss (Figure 7A), and a progressive decrease in the formation of the eIF4G–eIF4E complex (Figure 7B), which had an 80% decrease in animals with a weight loss of 20%, compared with those with no weight loss. The decrease in the availability of eIF4E for binding eIF4G resulted from hypophosphorylation of 4E-4BP1. There was a 5-fold reduction in 4E-BP1 phosphorylation in animals with a 20% weight loss compared with those without weight loss (Figure 7C). There was no effect of weight loss on phosphorylation of eIF4E (Figure 7D), but a 5-fold increase in phosphorylation of eEF2, with no change in the total amount (Figure 7E), indicating that global protein synthesis was also reduced through a decrease in translation elongation.
Figure 7. Effect of weight loss on the amount of eIF4E available for active eIF4G–eIF4E complex formation.
Total amounts of 4E-BP1 (A) and eIF4G (B) associated with eIF4E, and phosphorylation states of 4E-BP1 (p 4E-BP1) (C), eIF4E (p eIF4E) (D), eEF2 (p eEF2) (E), mTOR (p mTOR) (F) and p70S6k (p P70S6K) (G) in gastrocnemius muscle of mice bearing the MAC16 tumour with increasing levels of weight loss, were determined by Western blotting. In (C), the amount of 4E-BP1 in the γ-phosphorylated form is shown as a percentage of the total 4E-BP1. The γ-form is the most highly phosphorylated and has the slowest electrophoretic mobility. Representative Western blots are shown, and the densitometric analysis, shown underneath, represents the means±S.E.M. for three separate Western blots. The ratio of phospho (ph) to total (tot) forms is shown in (F), (G) and (H). *P<0.05, **P<0.01 and ***P<0.001 compared with animals without weight loss.
Treatment of mice bearing the MAC16 tumour with leucine (Figure 5) reversed these changes in gastrocnemius muscle towards that seen in animals without weight loss (Figure 8). Thus leucine treatment caused a reduction in the amount of 4E-BP1 associated with eIF4E (Figure 8A), and an increase in the eIF4G–eIF4E complex above the value found in non-tumour-bearing animals (Figure 8B). This was due to the hyperphosphorylation of 4E-BP1 (Figure 8C), without an effect on the phosphorylation of eIF4E (Figure 8D). Leucine treatment also reduced the phosphorylation of eEF2 (Figure 8E). These changes would explain the ability of leucine to stimulate protein synthesis in the gastrocnemius muscle.
Figure 8. Effect on phosphorylated and total 4E-BP1, eIF4E and eEF2 from non-tumour-bearing mice, and mice bearing the MAC16 tumour treated with either PBS or leucine.
Western blots showing the amount of 4E-BP1 (A) and eIF4G (B) associated with eIF4E, and phosphorylation states of 4E-BP1 (p 4E-BP1) (C), eIF4E (p eIF4E) (D) and eEF2 (p eEF2) (E) in gastrocnemius muscle of non-tumour-bearing mice (NTB), and in mice bearing the MAC16 tumour treated with PBS (MAC16) or leucine (MAC16+Leu). The details of the experiment are given in the Experimental section and in the legend to Figure 5. Samples were taken at the end of the experiment depicted in Figure 5. Representative Western blots are shown, and the densitometric analysis, shown underneath, represents the means±S.E.M. for three separate Western blots. The ratio of phospho (ph) to total (tot) forms is shown in (F). *P<0.05 and **P<0.01 compared with non-tumour-bearing animals; †P<0.05 and †††P<0.001 compared with mice bearing the MAC16 tumour treated with PBS.
The effect of weight loss on the expression of the phosphorylated forms of mTOR and p70S6k in the gastrocnemius muscle is shown in Figure 7. As with 4E-BP1 (Figure 7C), there was a progressive reduction in phosphorylation of both mTOR and p70S6k with increasing weight loss, with values at 20% weight loss being only 30% for mTOR (Figure 7F) and 40% for p70S6k (Figure 7G) of the values found in animals without weight loss, without a change on total mTOR or p70S6k. Leucine treatment of mice bearing the MAC16 tumour increased levels of phosphorylated mTOR (Figure 6D) and phosphorylated p70S6k (Figure 6E) in the gastrocnemius muscle up to values found in non-tumour-bearing animals.
DISCUSSION
BCAAs comprise 14–18% of the total amino acids in muscle proteins [20], and function not only as building blocks, but also as modulators of protein synthesis. Although BCAAs are known to stimulate protein synthesis and inhibit protein degradation in the skeletal muscle of cachectic rats [21], there have been no studies on the mechanisms involved in this effect. In starvation, the stimulatory effect of leucine on protein synthesis has been attributed to hyperphosphorylation of 4E-BP1 and p70S6K [9], with little effect on the phosphorylation of eIF2α or on the activity of eIF2B. In cachexia, the mechanism appears to be similar to that seen in starvation, with hyperphosphorylation of 4E-BP1 and p70S6k, but in addition leucine has been shown to inhibit phosphorylation of eIF2α through a direct effect on the activation of PKR by phosphorylation.
A previous study [2] has shown an increase in phosphorylation of PKR and eIF2α in gastrocnemius muscle of weight-losing mice bearing the MAC16 tumour. Such a change is concordant with the ability of hyperphosphoylated PKR to inhibit protein synthesis through an effect on translation initiation and an increase in protein degradation through up-regulation of the ubiquitin–proteasome proteolytic pathway [2]. Muscle atrophy in mice bearing the MAC16 tumour has been shown to be due to a depression of up to 60% in protein synthesis accompanied by a 240% increase in protein degradation [14]. In the present study, leucine has been shown to attenuate both the increase in phosphorylation of PKR and eIF2α in skeletal muscle of mice bearing the MAC16 tumour, as well as in murine myotubes exposed to PIF. The concentration of leucine employed in vitro (2 mmol/l) is similar to that reported previously [8] in serum of rats when leucine was administered at 1.35 g/kg of body weight. The effect of leucine on the phosphorylation of PKR appears to arise from an increase in the expression of PP1, which has been shown to bind to the N-terminal regulatory region of PKR and inhibit autophosphorylation [19]. The present study is the first report to show that leucine increased the expression of PP1, although insulin, EGF (epidermal growth factor) and PDGF (platelet-derived growth factor) have been shown to stimulate expression [22]. The ability of leucine to attenuate activation of PKR would explain, at least in part, the ability to increase protein synthesis in skeletal muscle and attenuate protein degradation, as shown from in vitro experiments with PIF. Inhibition of PKR may also explain the small, but significant, inhibition of tumour growth by leucine, since inhibition of PKR by a low-molecular-mass inhibitor also inhibits tumour growth [18].
Stimulation of protein synthesis in the skeletal muscle of cachetic mice by leucine is also due to increases in the eIF4F complex, which promotes the migration and recruitment of the 43S pre-initiation complex to the mRNA, enhancing peptide-chain initiation [23]. The eIF4F complex is made up of three subunits: eIF4E, eIF4G and eIF4A, with eIF4E being considered to be rate-limiting in binding on mRNA to ribosomes, since it is the least abundant in muscle [24]. The assembly of the eIF4E complex is in part controlled by 4E-BP1, which sequesters eIF4E, preventing it from interacting with eIF4G [24]. Phosphorylation of 4E-BP1 in response to growth factors, or BCAAs, results in the release of eIF4E from the inactive 4E-BP1–eIF4E complex, allowing it to interact with eIF4G [9]. In the gastrocnemius muscle of weight-losing mice bearing the MAC16 tumour, there was a reduction in the phosphorylation of 4E-BP1 with increasing weight loss, resulting in an increase in the amount of eIF4E associated with 4E-BP1, and a decrease in the levels associated with eIF4G. The activity of eIF4E can also be regulated by phosphorylation, with the phosphorylated form having an increased affinity for the cap structure [25]; however, phosphorylation of eIF4E was not as affected by weight loss as it was by cellular stress [26]. Treatment of weight-losing mice with leucine attenuated the depression in protein synthesis, and this was associated with an increase in phosphorylation of 4E-BP1, resulting in a reduced binding of eIF4E with 4E-BP1, and an increased association with eIF4G. Recent results [27] show an increased expression of eIF4E and eIF4G in the gastrocnemius muscle of pregnant rats bearing the Walker 256 tumour and treated with a leucine-rich diet.
The decreased levels of the eIF4E complex with increasing weight loss may be due to decreases in plasma insulin levels [11], since insulin has been shown to stimulate phosphorylation of 4E-BP1, decreasing its association with eIF4E and increasing the active eIF4F complex [28]. Changes in 4E-BP1 phosphorylation modulated by insulin and leucine occur through the mTOR pathway, which also results in activation of p70S6k [29]. Phosphorylation of p70S6k is associated with its activation causing selective translation of mRNAs that contain a 5′-polypyrimidine tract, which codes for proteins that are generally involved in the translation apparatus [30]. Levels of both phosphorylated forms of p70S6k and mTOR in skeletal muscle of mice bearing the MAC16 tumour were found to decrease with increasing weight loss, and these were restored to values found in mice without weight loss after treatment with leucine. Previous studies in food-deprived rats have shown that leucine stimulates phosphorylation of p70S6k independently of increases in serum insulin [9]. Although mTOR can directly phosphorylate the N-terminal threonine sites in 4E-BP1 in vitro, the effect is most likely to arise from repression of the inhibitory action of TSC1–TSC2 (where TSC is tuberous sclerosis complex) on mTOR signalling [31].
Increasing weight loss was also associated with an increased phosphorylation of the elongation factor eEF2, which would result in an inhibition of elongation by decreasing its affinity for the ribsome 10–100 times [32]. This would reduce global protein synthesis. Treatment of cachectic mice with leucine decreased the phosphorylation of eEF2 in gastrocnemius muscle to levels found in animals without weight loss, and this would attenuate the depression of protein synthesis. Leucine starvation has been shown previously [33] to increase phosphorylation of eEF2 in murine myotubes, whereas insulin induces dephosphorylation of eEF2 via mTOR [34]. Since leucine has been shown to stimulate the mTOR pathway, it may act by a similar mechanism.
Some of the effects of leucine on protein synthesis in vivo may be due to its ability to transiently stimulate insulin secretion [35]. Both increased leucine and insulin are required for sustained stimulation of protein synthesis in skeletal muscle of food-deprived rats. Somatostatin, an inhibitor of pancreatic hormone release, prevented the leucine-induced changes in serum insulin and protein synthesis and attenuated the leucine-induced changes in the 4E-BP1 and p70S6k phosphorylation in skeletal muscle. However, it had no effect on eIF4G–eIF4E assembly, which by itself is insufficient to stimulate the rates of protein synthesis in skeletal muscle. Another study [36] found the rates of protein synthesis in diabetic rats were higher when they were fed leucine than when they remained food-deprived, suggesting that a portion of the protein synthetic response must occur through an insulin-independent pathway and in the absence of changes in phosphorylation of 4E-BP1 and p70S6k. Leucine was also shown to attenuate the depression in protein synthesis and the increase in protein degradation in murine myotubes in response to PIF, which has been attributed to its ability to attenuate activation of PKR and the subsequent phosphorylation of eIF2α, and it is possible that this may be important in the diabetic state. Leucine also stimulated the basal rate of protein synthesis in myotubes, but had no effect on the basal rate of protein degradation, because both are independent of PKR activation in the absence of a stimulus (PIF). Leucine could stimulate the basal rate of protein synthesis through an increase in the eIF4G–eIF4E complex and a reduced phosphorylation of eEF2. Although PKR has been shown to increase expression of the ubiquitin–proteasome proteolytic pathway [2], basal protein degradation has been shown [37] not to occur through this pathway, but through a Ca2+-dependent proteolytic system (calpains). Thus, although leucine can attenuate the induced (PKR-dependent) protein degradation, it would not be expected to influence the basal rate.
Thus BCAAs, and leucine in particular, have a protective effect on skeletal muscle atrophy in cancer cachexia. Leucine acts to stimulate protein synthesis and reduce protein degradation. The effect on protein synthesis is likely to arise from multiple effects, including dephosphorylation of eIF2α, an increase in eIF4F complex formation, increased activity of p70S6k and decreased phosphorylation of eEF2. The effect on protein degradation is most likely to arise from attenuation of the phosphorylation of PKR through increased expression of PP1.
This could be employed as a therapeutic regime to treat cachectic cancer patients, since oral supplementation with leucine at much lower levels (0.052 g/kg of body weight) than employed in the murine study have been reported to stimulate muscle protein synthesis in elderly men [38].
Acknowledgments
This work has been supported by a grant from Novartis Medical Nutrition. We thank Mr M. Wynter and Mr W. Fleary for performing the animal experiment.
References
- 1.Khal J., Hine A. V., Fearon K. C. H., Dejong C. H. C., Tisdale M. J. Increased expression of proteasome subunits in skeletal muscle of cancer patients with weight loss. Int. J. Biochem. Cell. Biol. 2005;37:2196–2206. doi: 10.1016/j.biocel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Eley H. L., Tisdale M. J. Skeletal muscle atrophy: a link between depression of protein synthesis and increase in degradation. J. Biol. Chem. 2007;282:7087–7097. doi: 10.1074/jbc.M610378200. [DOI] [PubMed] [Google Scholar]
- 3.Rowlands A. G., Panniers R., Henshaw E. The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J. Biol. Chem. 1988;263:5526–5533. [PubMed] [Google Scholar]
- 4.Russell S. T., Eley H., Tisdale M. J. Mechanism of attenuation of angiotensin II-induced protein degradation by insulin-like growth factor I (IGF-I) Cell. Signalling. 2007;19:1797–1806. doi: 10.1016/j.cellsig.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Norton J. A., Gorschboth C. M., Wesley R. A. Fasting plasma amino acid levels in cancer patients. Cancer. 1985;56:1181–1186. doi: 10.1002/1097-0142(19850901)56:5<1181::aid-cncr2820560535>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Beck S. A., Tisdale M. J. Nitrogen excretion in cancer cachexia and its modification by a high fat diet in mice. Cancer Res. 1989;49:3800–3804. [PubMed] [Google Scholar]
- 7.Yoshizawa F. Regulation of protein synthesis by branched-chain amino acids in vivo. Biochem. Biophys. Res. Commun. 2004;313:417–422. doi: 10.1016/j.bbrc.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Anthony J. C., Yoshizawa F., Anthony T. G., Vary T. C., Jefferson L. S., Kimball S. R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 9.Anthony J. C., Anthony T. G., Kimball S. R., Vary T. C., Jefferson L. S. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J. Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 10.Todorov P. T., McDevitt T. M., Cariuk P., Coles B., Deacon M., Tisdale M. J. Induction of muscle protein degradation and weight loss by a tumour product. Cancer Res. 1996;56:1256–1261. [PubMed] [Google Scholar]
- 11.Bibby M. C., Double J. A., Ali S. A., Fearon K. C. H., Brennan R. A., Tisdale M. J. Characterisation of a transplantable adenocarcinoma of the mouse producing cachexia in recipient animals. J. Natl. Cancer Inst. 1987;78:539–546. [PubMed] [Google Scholar]
- 12.Workman P., Twentyman P., Balkwill F., Balmain A., Chaplin D., Double J., Embelton J., Newell D., Raymond R., Stables J., Wallace J. United Kingdom Co-ordinating Committee on Cancer Research (UKCCR). Guidelines for the welfare of animals with experimental neoplasia (second edition) Br. J. Cancer. 1998;77:1–10. doi: 10.1038/bjc.1998.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitehouse A. S., Tisdale M. J. Increased expression of the ubiquitin-proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-κB. Br. J. Cancer. 2003;89:1116–1122. doi: 10.1038/sj.bjc.6601132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith K. L., Tisdale M. J. Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br. J. Cancer. 1993;67:680–685. doi: 10.1038/bjc.1993.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waalkes T. P., Udenfriend S. A. A fluorimetric method for the estimation of tyrosine in plasma and tissues. J. Lab. Clin. Med. 1957;50:733–736. [PubMed] [Google Scholar]
- 16.Boyce M., Bryant K. F., Jousse C., Long K., Harding H. P., Scheuner D., Kaufman R. J., Ma D., Coen D. M., Ron D., Yuan J. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 17.Jammi N. V., Whitby L. R., Beal P. A. Small molecule inhibitors of the RNA-dependent protein kinase. Biochem. Biophys. Res. Commun. 2003;308:50–57. doi: 10.1016/s0006-291x(03)01318-4. [DOI] [PubMed] [Google Scholar]
- 18.Eley H. L., Russell S. T., Tisdale M. J. Attenuation of muscle atrophy in a murine model of cachexia by inhibition of the dsRNA-dependent protein kinase (PKR) Br. J. Cancer. 2007;96:1216–1222. doi: 10.1038/sj.bjc.6603704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan S.-L., Tareen S. U., Melville M. W., Blakely C. M., Katze M. G. The direct binding of the catalytic subunit of protein phosphatase 1 to the PKR protein kinase is necessary but not sufficient for inactivation and disruption of enzyme dinner formation. J. Biol. Chem. 2002;277:36109–36117. doi: 10.1074/jbc.M205109200. [DOI] [PubMed] [Google Scholar]
- 20.Riazi R., Wykes L. J., Ball R. O., Pencharz P. B. The total branched-chain amino acid requirement in young healthy adult men determined by indicator amino acid oxidation by use of L-[1-13C] phenylalanine. J. Nutr. 2003;133:1383–1389. doi: 10.1093/jn/133.5.1383. [DOI] [PubMed] [Google Scholar]
- 21.Busquets S., Alvarez B., Lopez-Soriano F. J., Argiles J. M. Branched-amino acids: a role in skeletal muscle proteolysis in catabolic states? J. Cell. Physiol. 2002;19:283–289. doi: 10.1002/jcp.10097. [DOI] [PubMed] [Google Scholar]
- 22.Chan C. P., McNall S. J., Krebs E. G., Fischer E. H. Stimulation of protein phosphatase activity by insulin and growth factors in 3T3 cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6257–6261. doi: 10.1073/pnas.85.17.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pain V. M. Initiation of protein synthesis in eukaryotic cells. Eur. J. Biochem. 1997;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- 24.Duncan R., Milburn S. C., Hershey J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF4F suggest a role in translational control. Heat shock effect of eIF4F. J. Biol. Chem. 1987;262:380–388. [PubMed] [Google Scholar]
- 25.Minich W. B., Balasta M. L., Goss D. J., Rhoads R. C. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF4E: increased cap affinity of the phosphorylated form. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7668–7672. doi: 10.1073/pnas.91.16.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser C. S., Pain V. M., Mordey S. J. Cellular stress in Xenopus kidney cells enhances the phosphorylation of eukaryotic translation initiation factor (eIF)4E and the association of eIF4F with poly(A) binding protein. Biochem. J. 1999;342:519–526. [PMC free article] [PubMed] [Google Scholar]
- 27.Ventrucci G., Mello M. A. R., Gomes-Marcondes M. C. C. Leucine-rich diet alters the eukaryotic translation initiation factors expression in skeletal muscle of tumour-bearing rats. BMC Cancer. 2007;7:42–53. doi: 10.1186/1471-2407-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen W.-H., Boyle D. W., Wisniowski P., Bade A., Liechty E. A. Insulin and IGF-I stimulate the formation of the eukaryotic initiation factor 4F complex and protein synthesis in C2C12 myotubes independent of availability of external amino acids. J. Endocrinol. 2005;185:275–289. doi: 10.1677/joe.1.06080. [DOI] [PubMed] [Google Scholar]
- 29.Hara K., Yonezawa K., Weng Q-P., Kozlowski M. T., Belham C., Avruch J. Amino acid sufficiency and mTOR regulate p70S6kinase and eIF-4EBP1 through a common effector mechanism. J. Biol. Chem. 1998;273:14484–14494. doi: 10.1074/jbc.273.23.14484. [DOI] [PubMed] [Google Scholar]
- 30.Duffner A., Thomas G. Ribosomal S6 kinase signalling and the control of translation. Exp. Cell Res. 1999;253:100–109. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Corradetti M. N., Inoki K., Gunn K.-L. TSC2: filling the GAP in the mTOR signalling pathway. Trends Biochem. Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Carlberg U., Nilsson A., Nygard O. Functional properties of phosphorylated elongation factor 2. Eur. J. Biochem. 1990;191:639–645. doi: 10.1111/j.1432-1033.1990.tb19169.x. [DOI] [PubMed] [Google Scholar]
- 33.Talvas J., Obled A., Fafournoux P., Mordier S. Regulation of protein synthesis by leucine starvation involves distinct mechanisms in mouse C2C12 myoblasts and myotubes. J. Nutr. 2006;136:1466–1471. doi: 10.1093/jn/136.6.1466. [DOI] [PubMed] [Google Scholar]
- 34.Wang X., Li W., Williams M., Terada N., Alessi D. R., Proud C. G. Regulation of elongation factor 2 kinase by p90RSK1 and p70, S6K kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anthony J. C., Lang C. H., Crozier S. J., Anthony T. G., MacLean D. A., Kimball S. R., Jefferson L. S. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am. J. Physiol. Endocrinol. Metab. 2002;282:E1092–E1101. doi: 10.1152/ajpendo.00208.2001. [DOI] [PubMed] [Google Scholar]
- 36.Anthony J. C., Reiter A. K., Anthony T. G., Crozier S. J., Lang C. H., MacLean D. A., Kimball S. R., Jefferson L. S. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes. 2002;51:928–936. doi: 10.2337/diabetes.51.4.928. [DOI] [PubMed] [Google Scholar]
- 37.Whitehouse A. S., Smith H. J., Drake J. L., Tisdale M. J. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res. 2001;61:3604–3609. [PubMed] [Google Scholar]
- 38.Rieu I., Balage M., Sornet C., Giraudet C., Pujos E., Grizard J., Mosoni L., Dardavet D. Leucine supplementation improves muscle protein synthesis independently of hyperaminoacidaemia. J. Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]