Abstract
Apoptosis of VSMCs (vascular smooth-muscle cells) leads to features of atherosclerotic plaque instability. We have demonstrated previously that plaque-derived VSMCs have reduced IGF1 (insulin-like growth factor 1) signalling, resulting from a decrease in the expression of IGF1R (IGF1 receptor) compared with normal aortic VSMCs [Patel, Zhang, Siddle, Soos, Goddard, Weissberg and Bennett (2001) Circ. Res. 88, 895–902]. In the present study, we show that apoptosis induced by oxidative stress is inhibited by ectopic expression of IGF1R. Oxidative stress repressed IGF1R expression at multiple levels, and this was also blocked by mutant p53. Oxidative stress also induced p53 phosphorylation and apoptosis in VSMCs. p53 negatively regulated IGF1R promoter activity and expression and, consistent with this, p53−/− VSMCs demonstrated increased IGF1R expression, both in vitro and in advanced atherosclerotic plaques in vivo. Oxidative-stress-induced interaction of endogenous p53 with TBP (TATA-box-binding protein) was dependent on p53 phosphorylation. Oxidative stress also increased the association of p53 with HDAC1 (histone deacetylase 1). Trichostatin A, a specific HDAC inhibitor, or p300 overexpression relieved the repression of IGF1R following oxidative stress. Furthermore, acetylated histone-4 association with the IGF1R promoter was reduced in cells subjected to oxidative stress. These results suggest that oxidative-stress-induced repression of IGF1R is mediated by the association of phosphorylated p53 with the IGF1R promoter via TBP, and by the subsequent recruitment of chromatin-modifying proteins, such as HDAC1, to the IGF1R promoter–TBP–p53 complex.
Keywords: apoptosis, atherosclerosis, histone deacetylase (HDAC), insulin-like growth factor 1 receptor (IGF1R), p53, vascular smooth-muscle cell (VSMC)
Abbreviations: ApoE, apolipoprotein E; ChIP, chromatin immunoprecipitation; DMEM, Dulbecco's modified Eagle's medium; FCS, foetal calf serum; HDAC, histone deacetylase; IGF, insulin-like growth factor; IGFBP, IGF binding protein; IGF1R, IGF1 receptor; IGF1R-YF, kinase-dead mutant of IGF1R; ROS, reactive oxygen species; SMA, smooth-muscle actin; TBP, TATA-box-binding protein; T-(BuOOH), t-butyl hydroperoxide; TFIID, transcription factor IID; TSA, trichostatin A; VSMC, vascular smooth-muscle cell
INTRODUCTION
Atherosclerosis is a complex chronic arterial disease and is a major cause of morbidity and mortality. VSMCs (vascular smooth- muscle cells) are the principle source of collagen and extracellular matrix required in order to maintain the tensile strength of atherosclerotic plaques. Apoptosis of VSMCs decreases the synthesis of collagen and extracellular matrix, and induces features of plaque instability [1]. Rupturing of atherosclerotic plaques can result in thrombotic complications, leading to stroke, myocardial infarction and sudden death.
Oxidative stress is increasingly implicated in the development of atherosclerosis [2]. Increased oxidative damage and elevated levels of DNA strand breaks occur in human atherosclerotic plaques [3]. Furthermore, apoptosis in plaques co-localizes with macrophages, which are a major source of ROS (reactive oxygen species), and oxidative stress can induce VSMC apoptosis in cell-culture conditions [4–6]. Understanding how ROS regulates apoptosis of VSMCs may therefore help to identify new treatments for advanced atherosclerosis.
We demonstrated previously that human plaque-derived VSMCs exhibit reduced IGF1 (insulin-like growth factor 1)-dependent survival compared with VSMCs derived from normal vessels [7]. IGF1R (IGF1 receptor) activation initiates signalling pathways involved in cell proliferation, survival, differentiation and transformation [8]. IGF1R-dependent signalling is crucial for the survival of many cell types, including VSMCs [9,10]. IGF1R activity in VSMCs is mediated by a number of intracellular and extracellular factors [11–14], and its expression is also regulated at the level of transcription [15]. However, the role of oxidative stress on IGF1R gene expression in VSMCs is not clear. In the present study, we show, for the first time, that oxidative stress represses IGF1R expression via an increase in the association of p53 with the IGF1R promoter and recruitment of HDAC1 (histone deacetylase 1). Thus oxidative stress may contribute to plaque instability by repression of IGF1R.
EXPERIMENTAL
Cell culture
Normal and plaque VSMCs were isolated from aortas of cardiac transplant patients or following carotid endarterectomies performed in patients with recent transient ischaemic episodes respectively. Informed consent and approval of the local Ethics Committee was obtained in all cases. Pup rat (WKY12-22) aortic VSMCs, rat aortic VSMCs and VSMCs from aortas of wild-type or p53−/− knockout mice (C57Bl6/J background) were also used. Cells were cultured in DMEM (Dulbecco's modified Eagle's medium) (Sigma–Aldrich) or Waymouth's medium (Sigma–Aldrich) supplemented with 10 units/ml penicillin, 10 μg/ml streptomycin, 5 μg/ml L-glutamine, and 10% (v/v) FCS (foetal calf serum). Transient transfection was performed using FuGENE6 transfection reagent (Roche Applied Science). Cells were treated with T-(BuOOH) (t-butyl hydroperoxide) (Sigma–Aldrich) after being cultured to 70–90% confluence. For luciferase assays, Renilla luciferase activity, generated by pRL-TK (Promega), was used as an internal control to normalize transfection efficiency. For studies involving TSA (trichostatin A; Cayman Chemical Company), cells were treated with various concentrations of TSA and T-(BuOOH), and were subsequently harvested 24 h after treatment. For experiments involving H-1356 (Bachem), an IGF1 peptide analogue, rat VSMCs were treated with 10 μg/ml H-1356 for 6 h in the presence of DMEM containing 10% (v/v) FCS. Cells were subsequently harvested for analysis by Western blotting.
Plasmid constructs
The rat IGF1R promoter construct (−476/+640 Luc) [16], and wild-type and YF mutant form of IGF1R [17] have been reported previously. pCMV-p53 and pCMV-p53G135A were purchased from Clontech. p300 CMVβ was purchased from Upstate Biotechnology, and CMVβ was generated by restriction enzyme digest and removal of p300 cDNA.
Generation of stable cell lines
The wild-type (IGF1R) or kinase-dead (IGF1R-YF) cDNAs [17] were cloned into the retroviral vector pBMN IRES-puro [18]. The resulting plasmids were transfected into the ecotropic packaging cell line Bosc23; and the infectious replication-deficient virus produced was used to infect rat VSMCs in the presence of 8 μg/ml polybrene. After selection with 5 μg/ml puromycin, IGF1R expression was demonstrated by immunoblotting with an antibody against IGF1Rβ.
Cell viability and apoptosis
Cells were washed in PBS, harvested using trypsin/EDTA and viable cells were determined using the Trypan Blue exclusion assay. For apoptosis assays, VSMCs were treated with T-(BuOOH) for 24 h prior to cell harvesting. Apoptotic cells were quantified using an Annexin V–FITC kit (Bender MedSystems) following the manufacturer's instructions. Annexin-V-positive/propidium-iodide-negative cells were analysed by flow cytometry (FACSCalibur™; Becton Dickinson).
Quantitative PCR
Total RNA was isolated using STAT-60 (Tel-Test) following the manufacturer's instructions. cDNA generated from 5 μg of RNA was analysed by quantitative PCR (absolute quantification) using the Rotor-Gene 3000 (Corbett Research) and Taqman gene expression assay systems for IGF1R, p53 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase), as specified by the manufacturer (Applied Biosystems).
Western blot analysis
Equal amounts of protein lysate, prepared in Laemmli sample buffer [62.5 mM Tris/HCl, pH 6.8, 25% (v/v) glycerol, 2% (w/v) SDS and 0.01% (w/v) Bromophenol Blue], were resolved by SDS/PAGE, transferred on to Immobilon-P PVDF membranes (Millipore) and incubated with a rabbit anti-p53 antibody (BD Biosciences Pharmingen), mouse anti-[phospho-p53 (Ser392)] antibody (Cell Signaling Technology), rabbit anti-IGF1Rβ antibody (Santa Cruz Biotechnology), mouse anti-HDAC1 antibody (Santa Cruz Biotechnology), mouse anti-(β-actin) antibody (Sigma–Aldrich), rabbit anti-Akt antibody (Cell Signaling Technology) or rabbit anti-[phospho-Akt (Ser473)] antibody (Cell Signaling Technology). Primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (sheep anti-mouse and donkey anti-rabbit antibodies) and ECL® (enhanced chemiluminescence) (GE Healthcare).
Immunoprecipitation studies
VSMCs were harvested in immunoprecipitation buffer [20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.1% (v/v) Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate and 1 mM sodium orthovanadate] and 600 μg of pre-cleared protein lysate was incubated with 2.5 μg of rabbit anti-TBP [TATA-box-binding protein, also known as TFIID (transcription factor IID)] antibody (Santa Cruz Biotechnology) or mouse anti-p53 antibody (Santa Cruz Biotechnology) for 16 h at 4 °C. In some instances, the pre-cleared lysate was incubated with 20 units of calf intestinal phosphatase for 30 min at 37 °C prior to the addition of the anti-TBP antibody. Washed Protein G–Sepharose beads were added to the lysate/antibody mixture and incubated for a further 2 h. Samples were washed extensively with the immunoprecipitation buffer, resolved by SDS/PAGE and subjected to Western blotting.
ChIP (chromatin immunoprecipitation) analysis
Cell lysates were prepared as described previously [19], and immunoprecipitated using 2.5 μg of rabbit anti-TBP antibody, rabbit anti-p53 antibody or mouse anti-(acetylated histone-4) antibody (Upstate Biotechnology). Co-precipitated IGF1R promoter was amplified by PCR using the primers indicated (forward: 5′-CCGGGGCATTGTTTTT-3′ and reverse: 5′-CTCAGCGAGTTAATGCTGGT-3′) under the following PCR conditions: 91 °C for 1 min, followed by 35 or 37 cycles of 91 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min; with a final extension of 72 °C for 4 min. The resultant PCR product was verified by DNA sequencing.
Immunohistochemistry
Paraffin-embedded sections of brachiocephalic arteries from p53+/+/ApoE (apolipoprotein E)−/− and p53−/−/ApoE−/− mice, maintained on a high-fat diet [20], were sectioned at 5 μm intervals. Specimens were de-waxed, microwaved in 120 mM sodium citrate buffer, pH 6.0 and endogenous peroxidase activity was blocked using 3% (v/v) H2O2. Sections were incubated in 10% (w/v) BSA in PBS for 1 h at room temperature (22 °C) and then incubated overnight at 4 °C with a mouse anti-SMA (smooth-muscle actin) antibody (Dako) or rabbit anti-IGF1Rβ antibody (Santa Cruz Biotechnology), followed by biotinylated secondary antibodies (goat anti-mouse and goat anti-rabbit antibodies) and horseradish-peroxidase-conjugated streptavadin using the mouse-on-mouse or ABC hits (Vector) respectively. Peroxidase activity was detected with DAB (diaminobenzidene).
Statistical analysis
Parametric tests were employed, and comparison between three or more unpaired groups was made using ANOVA.
RESULTS
IGF1R inhibits the death of VSMCs in response to oxidative stress
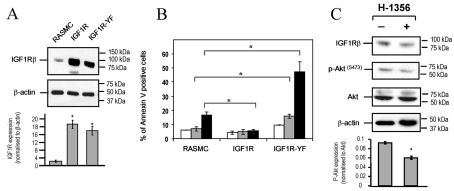
To investigate the role of IGF1R signalling in VSMC survival, we generated rat VSMCs stably overexpressing either wild-type IGF1R or IGF1R-YF [17] (Figure 1A) and tested their sensitivity to oxidative stress. T-(BuOOH), a membrane-permeant analogue of H2O2, induced profound apoptosis in VSMCs expressing IGF1R-YF (Figure 1B), suggesting that IGF1R-YF expression interferes with endogenous IGF1R present in VSMCs. Consistent with a pro-survival role, ectopic expression of wild-type IGF1R protected cells from apoptosis induced by oxidative stress (Figure 1B). To demonstrate the existence of a continuously active IGF1 signalling circuit in VSMCs, we used the IGF1R antagonist H-1356. Rat VSMCs had reduced IGF1R and phospho-Akt (Ser473) protein expression following treatment with H-1356 (Figure 1C). In contrast, there was no change in total Akt levels (Figure 1C). These findings show that suppression of IGF1R-dependent signalling occurs in the absence of exogenous IGF1.
Figure 1. IGF1R inhibits oxidative-stress-induced apoptosis in VSMCs.
(A) Western blot analysis demonstrating ectopic expression of wild-type IGF1R or mutant (IGF1R-YF) compared with control rat VSMCs (RASMC, rat aortic smooth-muscle cells). Lower panel shows IGF1R expression normalized to β-actin (arbitrary densitometric units). Results are means±S.E.M. (n=2). (B) Flow cytometry of Annexin-V-positive/propidium-iodide-negative control, IGF1R- or IGF1R-YF-expressing VSCMs (RASMC) treated with T-(BuOOH) for 24 h. Untreated, white bars; 10 μM T-(BuOOH), grey bars; 20 μM T-(BuOOH), black bars. Results are means±S.E.M. (n=3). *P<0.05. (C) Western blot analysis demonstrating expression of IGF1R, total Akt and phospho-Akt (Ser473) [p-Akt(S473)] following treatment with H-1356 for 6 h in DMEM containing 10% (v/v) FCS. β-Actin staining demonstrates unbiased loading. Lower panel shows phospho-Akt normalized to total Akt (arbitrary densitometry units). Results are means±S.E.M. (n=2).
Oxidative stress represses IGF1R gene expression
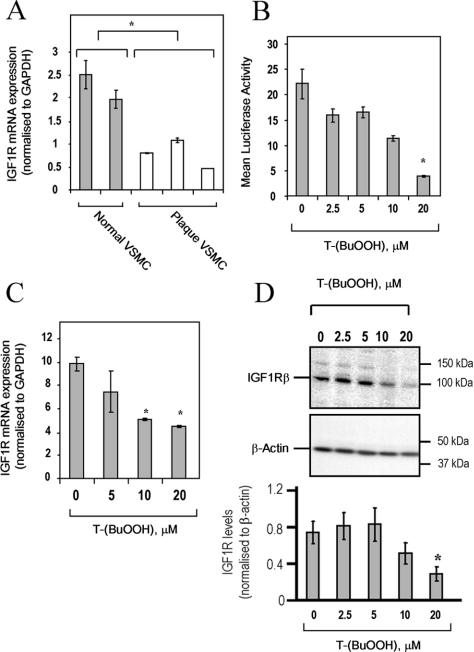
IGF1R protein is expressed at markedly lower levels in human plaque VSMCs compared with VSMCs from normal aorta [7], consistent with reduced IGF1R mRNA levels (Figure 2A). Since atherosclerotic plaques show evidence of oxidative stress [3,21], we investigated whether this might be responsible for the observed decrease in IGF1R expression. Normal human VSMCs demonstrated a dose-dependent reduction in IGF1R promoter activity (Figure 2B), mRNA (Figure 2C) and protein (Figure 2D) after 24 h treatment with T-(BuOOH), demonstrating that oxidative stress can repress IGF1R gene expression in VSMCs.
Figure 2. Oxidative stress represses IGF1R gene expression.
(A) IGF1R mRNA expression in two normal human aortic VSMC isolates and three plaque-derived VSMC isolates, as determined by quantitative PCR. Results are means±S.D. (n=3). *P<0.05. (B) IGF1R promoter activity is reduced by T-(BuOOH). WKY12-22 rat VSMCs were transfected with the −476/+640 IGF1R promoter–luciferase (1 μg per well in a six-well plate) for 6 h, followed by T-(BuOOH) treatment for 18 h. Luciferase activity was normalized to Renilla luciferase activity (pRL-TK). Results are means±S.E.M. (n=3). *P<0.05 compared with untreated control. (C) Expression of IGF1R mRNA in normal human VSMCs following T-(BuOOH) treatment for 24 h, as determined by quantitative PCR. Results are means±S.E.M. (n=3). *P<0.05 compared with untreated control. (D) Expression of IGF1Rβ protein in normal human VSMCs following T-(BuOOH) treatment for 24 h, as determined by Western blotting. Lower panel shows IGF1R expression normalized to β-actin (arbitrary densitometric units). Results are means±S.E.M. (n=2). *P<0.05 compared with untreated control.
p53 is associated with reduced IGF1R expression and mediates oxidative-stress-induced apoptosis
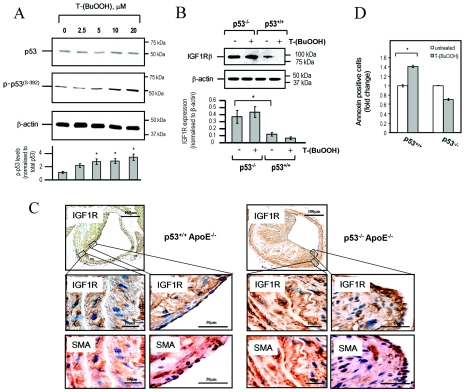
Oxidative stress induces the expression and activity of p53 [22]. Post-translational modifications of p53 can regulate its transcriptional activity, and this is essential for the response to many stimuli, such as oxidative stress and DNA damage [22,23]. Since atherosclerotic plaques are associated with evidence of oxidative stress, elevated p53 levels (including increased p53 phosphorylation) and apoptosis [3,24], we investigated whether p53 is involved in oxidant-induced repression of IGF1R. Although no change in total p53 protein levels was observed 24 h after oxidant stress (Figure 3A), p53 mRNA expression was induced by T-(BuOOH) at earlier time points (2–4 h after stress) (results not shown). In addition, we observed increased phosphorylation of p53 at Ser392 in human VSMCs treated with T-(BuOOH) (Figure 3A), suggesting that oxidative stress activates p53 in VSMCs. In support of this, T-(BuOOH) also induced p21 (results not shown), a direct transcriptional target of p53 that mediates growth arrest [25].
Figure 3. p53 mediates T-(BuOOH)-induced apoptosis of VSMCs in vitro and is associated with reduced IGF1R in atherosclerotic plaques.
(A) Western blot analysis of total p53 and phospho-p53 [p-p53(S−392)] in normal human VSMCs following T-(BuOOH) treatment for 24 h. β-Actin staining demonstrates even protein loading. Lower panel illustrates phospho-p53 expression normalized to total p53 (arbitrary densitometric units). Results are means±S.D. (n=2). *P<0.05 compared with untreated controls. (B) Western blot analysis of IGF1R protein in wild-type and p53−/− mouse VSMCs untreated or treated with 20 μM T-(BuOOH). Lower panel shows IGF1R expression normalized to β-actin (arbitrary densitometric units). Results are means±S.D. (n=2). *P<0.05. (C) Expression of IGF1R in atherosclerotic plaques of p53+/+/ApoE−/− and p53−/−/ApoE−/− mice, as determined by immunohistochemistry. Presence of α-SMA was used to identify VSMCs. Middle and bottom panels show high-power views of areas indicated in the top panel. (D) Flow cytometric analysis of Annexin-V-positive/propidium-iodide-negative wild-type and p53−/− mouse VSMCs following treatment with 20 μM T-(BuOOH) for 24 h. Results are means±S.D. (n=3). *P<0.05.
To examine whether p53 regulates IGF1R expression in VSMCs, we derived VSMCs from aortae of p53−/− or p53+/+ mice. p53−/− VSMCs exhibited elevated levels of IGF1R protein compared with p53+/+ cells (Figure 3B). The quantity of IGF1R protein in p53+/+ cells was reduced by T-(BuOOH), but not in VSMCs lacking p53 (Figure 3B), suggesting that IGF1R expression is at least partly regulated by p53. Immunohistochemical analysis indicated that IGF1R expression was reduced in both medial and fibrous cap VSMCs from atherosclerotic plaques in brachiocephalic arteries of p53+/+/ApoE−/− mice compared with p53−/−/ApoE−/− mice (Figure 3C). Moreover, p53+/+ VSMCs had increased levels of apoptosis when treated with T-(BuOOH) in vitro, a finding not observed in p53−/− VSMCs (Figure 3D). These results suggest that p53 negatively regulates IGF1R expression in VSMCs from atherosclerotic plaques, and this regulation is most likely to be in response to oxidative stress.
Repression of IGF1R by T-(BuOOH) is mediated by p53
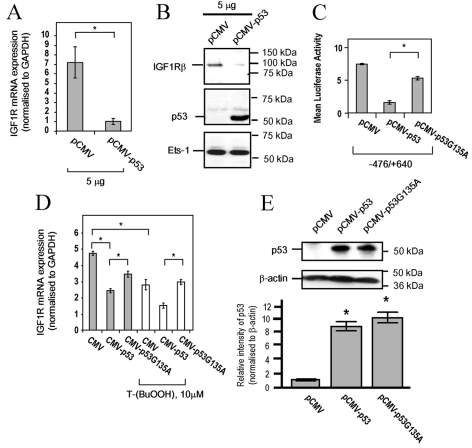
To determine whether p53 directly regulates IGF1R in VSMCs, we transfected wild-type p53 into rat VSMCs. Exogenous expression of p53 significantly reduced IGF1R mRNA (Figure 4A) and protein (Figure 4B) levels, compared with the empty vector control pCMV. In addition, a firefly luciferase reporter, containing approx. 1 kb of the IGF1R promoter (−476/+640 Luc), was inhibited by wild-type p53, but not by an inactive mutant, p53G135A (Figure 4C). The wild-type and p53G135A mutant proteins were expressed at equivalent levels (Figure 4E). These findings suggest that IGF1R gene expression in VSMCs is regulated, in part, by p53. We also investigated whether IGF1R repression in response to T-(BuOOH) is mediated by p53. Although the inhibition of IGF1R mRNA (Figure 4D) and promoter activity (results not shown) in response to treatment with 10 μM T-(BuOOH) was enhanced by the expression of wild-type p53, no repression was observed in the presence of p53G135A. These findings suggest that oxidative-stress-induced repression of IGF1R is mediated by p53.
Figure 4. Repression of IGF1R by T-(BuOOH) is mediated by p53.
(A) IGF1R mRNA expression in control and p53-transfected WKY12-22 rat VSMCs, as determined by quantitative PCR. Results are means±S.E.M. (n=3). *P<0.05. (B) Western blot analysis of IGF1R, p53 and Ets-1 (loading control) in control and p53-transfected WKY12-22 rat VSMC lysates. (C) IGF1R promoter activity in WKY12-22 rat VSMCs co-transfected with −476/+640 IGF1R promoter–luciferase (1 μg/well in a six-well plate) and 0.5 μg/well of pCMV, pCMV-p53 or pCMV-p53G135A, as determined with the dual luciferase assay system. Results are means±S.E.M. (n=3). *P<0.05. (D) IGF1R mRNA expression in WKY12-22 rat VSMCs transfected with CMV, CMV–p53 or CMV–p53G135A in the presence or absence of 10 μM T-(BuOOH) for 24 h, as determined by quantitative PCR. Results are means±S.E.M. (n=3). *P<0.05. (E) p53 protein expression in WKY12-22 rat VSMCs transfected with 5 μg of pCMV, pCMV-p53 and pCMV-p53G135A. Lower panel shows p53 expression normalized to β-actin (arbitrary densitometric units). Results are means±S.E.M. (n=2). *P<0.05.
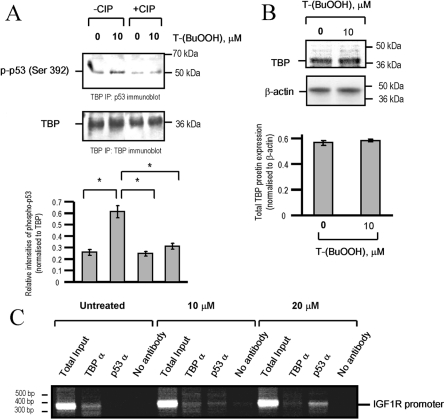
T-(BuOOH) stimulates the interaction of p53 with TBP
p53-dependent repression can be mediated by binding to cis-acting elements and also by binding to the transcriptional machinery [26]. The IGF1R promoter does not contain p53-consensus-binding sites, and p53 does not bind the IGF1R promoter directly [16]. In VSMCs, endogenous p53 was shown to bind TBP, the TFIID component of the basal transcriptional machinery (Figure 5A), and this interaction was increased following the addition of 10 μM T-(BuOOH) (Figure 5A). The p53–TBP interaction was phosphorylation-dependent, since treatment with phosphatase reduced the proportion of p53 that co-precipitated with TBP to basal levels (Figure 5A). No change in TBP protein expression was observed after treatment with 10 μM T-(BuOOH) (Figure 5B). To determine whether p53 is recruited to the IGF1R promoter in vivo following oxidative stress, we treated VSMCs with 10 and 20 μM T-(BuOOH) for 24 h and prepared them for ChIP studies. We were able to amplify the IGF1R promoter using antibodies against TBP, suggesting that TBP binds to the endogenous IGF1R promoter in VSMCs (Figure 5C). In contrast, we were only able to detect an IGF1R amplicon following immunoprecipitation with an antibody directed against p53 in cells treated with T-(BuOOH) (Figure 5C). Concomitant with this, detection of an IGF1R amplicon following treatment with 10 μM T-(BuOOH) and immunoprecipitation with TBP was reduced, but not abolished (Figure 5C). Our results suggest that, in response to oxidative stress, increased binding of p53 to TBP mediates the down-regulation of the IGF1R promoter.
Figure 5. T-(BuOOH)-dependent repression of IGF1R is associated with increased p53-TBP interaction.
(A) Co-immunoprecipitation of phospho-p53 [p-p53 (Ser 392)] after pretreatment of cell lysates in the absence or presence of 20 units of CIP (calf-intestinal phosphatase). The blot was stripped and re-probed for TBP. The intensity of phospho-p53 relative to TBP (lower panel) was determined by densitometry. Results are means±S.E.M. (n=2). *P<0.05. (B) T-(BuOOH) has no effect on TBP protein expression. The blot was stripped and re-probed for β-actin. The intensity of TBP relative to β-actin was determined by densitometry (lower panel). Results are means±S.E.M. (n=2). *, P<0.05. (C) Association of TBP and p53 on the IGF1R promoter was determined by ChIP. WKY12-22 rat VSMCs were treated with 10 or 20 μM T-(BuOOH) or left untreated for 24 h and cross-linked protein–DNA complexes were immunoprecipitated with anti-TBP (TBPα) or anti-p53 (p53α) antibodies prior to PCR amplification of the IGF1R promoter (n=2). Total Input, pre-immunoprecipitated DNA.
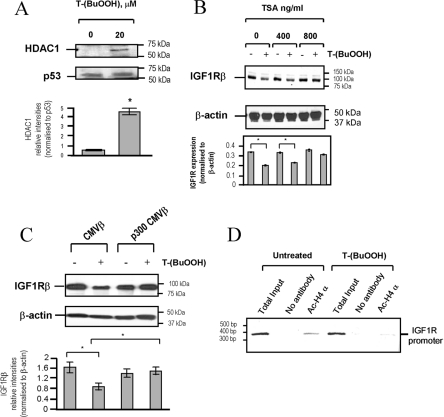
Repression of IGF1R by T-(BuOOH) involves histone deacetylation and p53–HDAC1 association
In eukaryotic cells, transcriptional regulation also involves chromatin remodelling by histone acetyltransferases and HDACs [27]. Repressed chromatin is associated with deacetylated histones. In contrast, acetylation of histones can enhance transcriptional activity. Since HDACs associate with p53, either directly or by co-localization [28,29], we next investigated whether T-(BuOOH)-activated p53 could recruit HDAC1 in VSMCs. Figure 6(A) demonstrates an increased association of p53 with HDAC1 following T-(BuOOH) treatment (20 μM) compared with untreated cells. In order to determine whether oxidative-stress-dependent repression of the IGF1R promoter requires HDAC activity, we used TSA, a specific HDAC inhibitor. The T-(BuOOH)-dependent reduction in IGF1R protein was inhibited by 800 ng/ml TSA compared with the control (Figure 6B). Moreover, forced expression of p300, a histone acetyltransferase, relieved the IGF1R repression induced by T-(BuOOH) (Figure 6C), confirming a role for chromatin remodelling in oxidant-stress-induced repression of IGF1R. Consistent with this, ChIP analysis demonstrated that there was reduced amplification of the IGF1R promoter in T-(BuOOH)-treated VSMCs immunoprecipitated using an anti-(acetylated histone-4) antibody (Figure 6D). These findings suggest that histone-4 is deacetylated in VSMCs after treatment with T-(BuOOH), and that chromatin remodelling and recruitment of gene silencing complexes to the IGF1R promoter regulates oxidative-stress-induced repression of IGF1R expression.
Figure 6. Repression of IGF1R by T-(BuOOH) involves chromatin remodelling.
(A) T-(BuOOH) promotes p53–HDAC1 association. p53 was immunoprecipitated, and associated HDAC1 was detected by Western blot analysis. WKY12-22 rat VSMCs were treated with T-(BuOOH) for 24 h. Lower panel shows HDAC1 expression normalized to p53 (arbitrary densitometric units). Results are means±S.E.M. (n=2). *P<0.05. (B) Western blot analysis of IGF1R and β-actin showing that TSA treatment dose-dependently inhibits repression of IGF1R protein expression in human VSMCs induced by 20 μM T-(BuOOH). Lower panel shows IGF1R expression normalized to β-actin (arbitrary densitometric units). Results are means±S.E.M. (n=2). *P<0.05. (C) Western blot analysis of IGF1R and β-actin showing that p300 overexpression relieves T-(BuOOH)-induced repression of IGF1R. WKY12-22 rat VSMCs were transfected with 3 μg of p300 CMVβ or the empty vector control CMVβ, and treated with 20 μM T-(BuOOH) for 24 h. Lower panel shows IGF1Rβ expression normalized to β-actin (arbitrary densitometric units). Results are means±S.E.M. (n=3). *P<0.05. (D) Oxidative stress reduces the association of acetylated histone-4 (ac-H4 α) with the IGF1R promoter. WKY12-22 rat VSMCs were treated with 20 μM T-(BuOOH) for 24 h and cross-linked protein–DNA complexes were immunoprecipitated with anti-(acetylated histone-4) antibodies (Ac-H4 α), prior to PCR amplification of the IGF1R promoter (n=2).
DISCUSSION
Atherosclerotic plaques are associated with the up-regulation of p53 and phospho-p53 [3,21], and exhibit DNA damage, oxidative stress and increased apoptosis [3,21,24]. IGF1R mRNA and protein levels are reduced in human carotid plaque-derived VSMCs compared with VSMCs derived from normal aorta [7]. Given that VSMCs from the thoracic aortic and coronary arteries behave comparably in vitro, both differing from that of plaque VSMCs from coronary atherectomies [30], and that VSMCs are cultured for several weeks prior to experiments, we conclude that differences in IGF1R expression are unlikely to arise from differences in the vascular bed or from prior medication of the patient. Consistent with our studies, which show a reduction in the expression of IGF1R following oxidative stress in vitro, a reduction in IGF1R protein expression has been reported in sections from advanced human atherosclerotic tissue [31]. Since down-regulation of IGF1R in VSMCs correlates with an increased sensitivity to apoptosis [7], loss of IGF1 signalling may be an important determinant of cell fate within the atherosclerotic plaque. Establishing the mechanism of IGF1R repression in response to oxidative stress in VSMCs is, therefore, vital to understanding advanced atherosclerosis.
Following exposure to oxidative stress and DNA-damaging agents, p53 expression and activity is induced [22]. Although we see no change in total p53 protein levels 24 h after oxidant stress, p53 mRNA expression is induced by T-(BuOOH) at earlier time points, consistent with previous reports demonstrating an increase in protein expression of p53 within 6 h of treatment by H2O2 [32]. p53 phosphorylation occurs at several sites by multiple kinases, including casein kinase I, casein kinase II, Cdc2 and PKC (protein kinase C) (reviewed in [22,23]). These post-translational modifications activate p53, allowing p53 to accumulate in the nucleus and alter the transcription of target genes. In particular, phosphorylation of p53 at Ser392 stabilizes p53 tetramer formation and activates p53 as a transcription factor in response to DNA damage [33]. Oxidant stress can exert multiple effects in atherosclerosis, including inactivation of nitric oxide, oxidation of DNA and proteins, lipid oxidation (and therefore retention) and apoptosis of vascular cells [21]. Our studies provide further insight into the complexity of gene regulation by p53 in response to oxidant stress.
The role of oxidative stress in regulating IGF1R expression in VSMCs is not clear. For example, exposure of VSMCs to ROS, either by H2O2 or ox-LDL (oxidized-low-density lipoprotein), has been reported to either increase [12] or decrease [13] IGF1R expression. The molecular and transcriptional mechanisms of IGF1R expression in response to oxidative stress have not been examined. We demonstrate that exposure of VSMCs to oxidative stress results in repression of the IGF1R as a result of epigenetic modifications involving the phosphorylation of p53 and its increased association with TBP and HDAC1. p53+/+ VSMCs display reduced IGF1R expression compared with p53−/− cells in vitro, suggesting that basal regulation of IGF1R expression in this cell type is mediated by p53, a finding supported by previous studies [15,16,34]. Consistent with this, overexpression of wild-type p53, but not mutant p53, negatively regulates IGF1R expression.
p53 does not bind the IGF1R promoter directly, but interacts with TBP [16]. Although some p53 associates with TBP under basal conditions, this association is increased by T-(BuOOH) and is dependent on p53 phosphorylation. Increased binding of p53 to TBP may interfere with the ability of the anti-TBP antibody to recognize its epitope, which may account for the reduced TBP–IGF1R promoter association observed following oxidative stress (Figure 5C). For example, p53-dependent alterations in the conformation of the transcriptional complex, or recruitment of additional factors to the complex, such as co-repressors, may mask the TBP epitope. Indeed, we observed an increase in HDAC1 associated with p53 following T-(BuOOH) treatment. Moreover, T-(BuOOH) treatment resulted in a reduction in the proportion of acetylated histone-4 associating with the IGF1R promoter. In contrast, both TSA and forced expression of p300 significantly block oxidative-stress-induced repression of IGF1R protein expression. These findings suggest that chromatin remodelling and epigenetic modifications take place on the IGF1R promoter following oxidative stress. The fact that TSA markedly exacerbates atherogenesis in Ldlr (low-density lipoprotein receptor)−/− mice [35] indicates that histone acetylation (and deacetylation) is an important regulator of atherosclerosis. Our findings support a model whereby oxidative-stress-dependent p53 recruitment of HDAC1 and subsequent chromatin modification contributes to transcriptional repression of the IGF1R promoter. A similar mechanism has been described for repression of the α-fetoprotein gene [36].
The role of p53-dependent apoptosis in atherosclerotic plaques remains controversial. Under certain circumstances, p53 can afford protection against apoptosis, for example, by activation of TIGAR (TP53-induced glycolysis and apoptosis regulator) [37]. Different experimental conditions and protocols may account for the reported variation in the rate of apoptosis between p53+/+ VSMCs and p53−/− VSMCs [20,38,39]. In the present study, we have demonstrated that p53 is required for oxidative-stress-induced apoptosis of VSMCs, since T-(BuOOH) is unable to induce apoptosis in p53−/− cells in vitro. It is also likely that p53 contributes to the apoptosis of VSMCs by increasing the transcription of IGFBPs (IGF binding proteins) [40–42], and we have previously discovered increased expression of IGFBP2, 3 and 4 in plaque VSMC cultures compared with normal aortic VSMCs [7]. Although we have demonstrated a reduction in IGF1R expression in VSMCs from p53+/+/ApoE−/− mice compared with p53−/−/ApoE−/− mice, owing to the small number of apoptotic VSMCs observed in atherosclerotic plaques [20,38,39] we have not been able to correlate a reduction in IGF1R expression with apoptosis in vivo. However, the fact that ectopic expression of IGF1R inhibits oxidative-stress-induced (and p53-dependent) apoptosis in vitro suggests that p53-mediated repression of IGF1R may contribute to the apoptosis of VSMCs in atherosclerotic plaques.
In conclusion, we have demonstrated that p53 represses IGF1R gene expression in VSMCs following oxidative stress. This repression is mediated by epigenetic modifications and an increased association of p53 with HDAC1 and TBP. We propose a model in which oxidative-stress-dependent activation of p53 recruits HDACs to the IGF1R promoter, resulting in the repression of gene expression. These studies may help delineate the complex pathways regulating atherosclerosis and atherosclerotic plaque instability.
Acknowledgments
This work was supported by British Heart Foundation (Grant PG/04/005/16497). M. M. K. was supported by a C. J. Martin Fellowship from the National Health and Medical Research Council of Australia (ID 300587). We would like to thank Mr Fernando Santiago for his technical advice on ChIP studies.
References
- 1.Clarke M. C., Figg N., Maguire J. J., Davenport A. P., Goddard M., Littlewood T. D., Bennett M. R. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat. Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 2.Stocker R., Keaney J. F., Jr New insights on oxidative stress in the artery wall. J. Thromb. Haemostasis. 2005;3:1825–1834. doi: 10.1111/j.1538-7836.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 3.Martinet W., Knaapen M. W., De Meyer G. R., Herman A. G., Kockx M. M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927–932. doi: 10.1161/01.cir.0000026393.47805.21. [DOI] [PubMed] [Google Scholar]
- 4.Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J. C., Pouzet C., Samadi M., Elbim C., et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song H. J., Lee T. S., Jeong J. H., Min Y. S., Shin C. Y., Sohn U. D. Hydrogen peroxide-induced extracellular signal-regulated kinase activation in cultured feline ileal smooth muscle cells. J. Pharmacol. Exp. Ther. 2005;312:391–398. doi: 10.1124/jpet.104.074401. [DOI] [PubMed] [Google Scholar]
- 6.Sandberg E. M., Sayeski P. P. Jak2 tyrosine kinase mediates oxidative stress-induced apoptosis in vascular smooth muscle cells. J. Biol. Chem. 2004;279:34547–34552. doi: 10.1074/jbc.M405045200. [DOI] [PubMed] [Google Scholar]
- 7.Patel V. A., Zhang Q. J., Siddle K., Soos M. A., Goddard M., Weissberg P. L., Bennett M. R. Defect in insulin-like growth factor-1 survival mechanism in atherosclerotic plaque-derived vascular smooth muscle cells is mediated by reduced surface binding and signaling. Circ. Res. 2001;88:895–902. doi: 10.1161/hh0901.090305. [DOI] [PubMed] [Google Scholar]
- 8.Valentinis B., Baserga R. IGF-I receptor signalling in transformation and differentiation. Mol. Pathol. 2001;54:133–137. doi: 10.1136/mp.54.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen R. T., Krueger K. D., Dhume A., Agrawal D. K. Sustained Akt/PKB activation and transient attenuation of c-jun N-terminal kinase in the inhibition of apoptosis by IGF-1 in vascular smooth muscle cells. Apoptosis. 2005;10:525–535. doi: 10.1007/s10495-005-1882-3. [DOI] [PubMed] [Google Scholar]
- 10.Vincent A. M., Feldman E. L. Control of cell survival by IGF signaling pathways. Growth Horm. IGF Res. 2002;12:193–197. doi: 10.1016/s1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 11.Du J., Brink M., Peng T., Mottironi B., Delafontaine P. Thrombin regulates insulin-like growth factor-1 receptor transcription in vascular smooth muscle: characterization of the signaling pathway. Circ. Res. 2001;88:1044–1052. doi: 10.1161/hh1001.090840. [DOI] [PubMed] [Google Scholar]
- 12.Du J., Peng T., Scheidegger K. J., Delafontaine P. Angiotensin II activation of insulin-like growth factor 1 receptor transcription is mediated by a tyrosine kinase-dependent redox-sensitive mechanism. Arterioscler. Thromb. Vasc. Biol. 1999;19:2119–2126. doi: 10.1161/01.atv.19.9.2119. [DOI] [PubMed] [Google Scholar]
- 13.Higashi Y., Peng T., Du J., Sukhanov S., Li Y., Itabe H., Parthasarathy S., Delafontaine P. A redox-sensitive pathway mediates oxidized LDL-induced downregulation of insulin-like growth factor-1 receptor. J. Lipid. Res. 2005;46:1266–1277. doi: 10.1194/jlr.M400478-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Maile L. A., Clemmons D. R. Integrin-associated protein binding domain of thrombospondin-1 enhances insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Circ. Res. 2003;93:925–931. doi: 10.1161/01.RES.0000101754.33652.B7. [DOI] [PubMed] [Google Scholar]
- 15.Werner H., Hernandez-Sanchez C., Karnieli E., Leroith D. The regulation of IGF-I receptor gene expression. Int. J. Biochem. Cell. Biol. 1995;27:987–994. doi: 10.1016/1357-2725(95)00074-y. [DOI] [PubMed] [Google Scholar]
- 16.Werner H., Karnieli E., Rauscher F. J., LeRoith D. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8318–8323. doi: 10.1073/pnas.93.16.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S., Ferber A., Miura M., Baserga R. Mitogenicity and transforming activity of the insulin-like growth factor-I receptor with mutations in the tyrosine kinase domain. J. Biol. Chem. 1994;269:32558–32564. [PubMed] [Google Scholar]
- 18.Garton K. J., Ferri N., Raines E. W. Efficient expression of exogenous genes in primary vascular cells using IRES-based retroviral vectors. BioTechniques. 2002;32:830–843. doi: 10.2144/02324rr01. [DOI] [PubMed] [Google Scholar]
- 19.Rafty L. A., Santiago F. S., Khachigian L. M. NF1/X represses PDGF A-chain transcription by interacting with Sp1 and antagonizing Sp1 occupancy of the promoter. EMBO J. 2002;21:334–343. doi: 10.1093/emboj/21.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercer J., Figg N., Stoneman V., Braganza D., Bennett M. R. Endogenous p53 protects vascular smooth muscle cells from apoptosis and reduces atherosclerosis in ApoE knockout mice. Circ. Res. 2005;96:667–674. doi: 10.1161/01.RES.0000161069.15577.ca. [DOI] [PubMed] [Google Scholar]
- 21.Martinet W., Knaapen M. W., Guido R. Y., De Meyer G. R., Arnold G. H., Kockx M. M. Oxidative DNA damage and repair in experimental atherosclerosis are reversed by dietary lipid lowering. Circ. Res. 2001;88:733–739. doi: 10.1161/hh0701.088684. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y. Regulation of p53 responses by post-translational modifications. Cell Death Differ. 2003;10:400–403. doi: 10.1038/sj.cdd.4401182. [DOI] [PubMed] [Google Scholar]
- 23.Brooks C. L., Gu W. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr. Opin. Cell. Biol. 2003;15:164–171. doi: 10.1016/s0955-0674(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 24.Lutgens E., de Muinck E. D., Kitslaar P. J., Tordoir J. H., Wellens H. J., Daemen M. J. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc. Res. 1999;41:473–479. doi: 10.1016/s0008-6363(98)00311-3. [DOI] [PubMed] [Google Scholar]
- 25.el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 26.Seto E., Usheva A., Zambetti G. P., Momand J., Horikoshi N., Weinmann R., Levine A. J., Shenk T. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc. Natl. Acad. Sci. U.S.A. 1992;89:12028–12032. doi: 10.1073/pnas.89.24.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allfrey V. G., Faulkner R., Mirsky A. E. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl. Acad. Sci. U.S.A. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juan L.-J., Shia W. J., Chen M. H., Yang W. M., Seto E., Lin Y. S., Wu C. W. Histone deacetylases specifically down-regulate p53-dependent gene activation. J. Biol. Chem. 2000;275:20436–20443. doi: 10.1074/jbc.M000202200. [DOI] [PubMed] [Google Scholar]
- 29.Basile V., Mantovani R., Imbriano C. DNA damage promotes histone deacetylase 4 nuclear localization and repression of G2/M promoters, via p53 C-terminal lysines. J. Biol. Chem. 2005;281:2347–2357. doi: 10.1074/jbc.M507712200. [DOI] [PubMed] [Google Scholar]
- 30.Bennett M. R., Evan G. I., Schwartz S. M. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J. Clin. Invest. 1995;95:2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okura Y., Brink M., Zahid A. A., Anwar A., Delafontaine P. Decreased expression of insulin-like growth factor-1 and apoptosis of vascular smooth muscle cells in human atherosclerotic plaque. J. Mol. Cell. Cardiol. 2001;33:1777–1789. doi: 10.1006/jmcc.2001.1441. [DOI] [PubMed] [Google Scholar]
- 32.McNeill-Blue C., Wetmore B. A., Sanchez J. F., Freed W. J., Merrick B. A. Apoptosis mediated by p53 in rat neural AF5 cells following treatment with hydrogen peroxide and staurosporine. Brain Res. 2006;1112:1–15. doi: 10.1016/j.brainres.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi K., Sakamoto H., Lewis M. S., Anderson C. W., Erickson J. W., Appella E., Xie D. Phosphorylation of serine 392 stabilizes the tetramer formation of tumor suppressor protein p53. Biochemistry. 1997;36:10117–10124. doi: 10.1021/bi970759w. [DOI] [PubMed] [Google Scholar]
- 34.Nahor I., Abramovitch S., Engeland K., Werner H. The p53-family members p63 and p73 inhibit insulin-like growth factor-I receptor gene expression in colon cancer cells. Growth Horm. IGF Res. 2005;15:388–389. doi: 10.1016/j.ghir.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Choi J. H., Nam K. H., Kim J., Baek M. W., Park J. E., Park H. Y., Kwon H. J., Kwon O. S., Kim D. Y., Oh G. T. Trichostatin A exacerbates atherosclerosis in low density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:2404–2409. doi: 10.1161/01.ATV.0000184758.07257.88. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen T. T., Cho K., Stratton S. A., Barton M. C. Transcription factor interactions and chromatin modifications associated with p53-mediated, developmental repression of the alpha-fetoprotein gene. Mol. Cell. Biol. 2005;25:2147–2157. doi: 10.1128/MCB.25.6.2147-2157.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bensaad K., Tsuruta A., Selak M. A., Vidal M. N., Nakano K., Bartrons R., Gottlieb E., Vousden K. H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 38.Guevara N. V., Kim H. S., Antonova E. I., Chan L. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat. Med. 1999;5:335–339. doi: 10.1038/6585. [DOI] [PubMed] [Google Scholar]
- 39.van Vlijmen B. J., Gerritsen G., Franken A. L., Boesten L. S., Kockx M. M., Gijbels M. J., Vierboom M. P., van Eck M., van de Water B., van Berkel T. J., Havekes L. M. Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3-Leiden transgenic mice. Circ. Res. 2001;88:780–786. doi: 10.1161/hh0801.089261. [DOI] [PubMed] [Google Scholar]
- 40.Grimberg A., Liu B., Bannerman P., El-Deiry W. S., Cohen P. IGFBP-3 mediates p53-induced apoptosis during serum starvation. Int. J. Oncol. 2002;21:327–335. [PMC free article] [PubMed] [Google Scholar]
- 41.Grimberg A., Coleman C. M., Shi Z., Burns T. F., MacLachlan T. K., Wang W., El-Deiry W. S. Insulin-like growth factor factor binding protein-2 is a novel mediator of p53 inhibition of insulin-like growth factor signaling. Cancer Biol. Ther. 2006;5:1408–1414. doi: 10.4161/cbt.5.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimberg A., Coleman C. M., Burns T. F., Himelstein B. P., Koch C. J., Cohen P., El-Deiry W. S. p53-dependent and p53-independent induction of insulin-like growth factor binding protein-3 by deoxyribonucleic acid damage and hypoxia. J. Clin. Endocrinol. Metab. 2005;90:3568–3574. doi: 10.1210/jc.2004-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]