Abstract
We have discovered that some weak uncouplers (typified by butylated hydroxytoluene) have a dynamic range of more than 106 in vitro: the concentration giving measurable uncoupling is less than one millionth of the concentration causing full uncoupling. They achieve this through a high-affinity interaction with the mitochondrial adenine nucleotide translocase that causes significant but limited uncoupling at extremely low uncoupler concentrations, together with more conventional uncoupling at much higher concentrations. Uncoupling at the translocase is not by a conventional weak acid/anion cycling mechanism since it is also caused by substituted triphenylphosphonium molecules, which are not anionic and cannot protonate. Covalent attachment of the uncoupler to a mitochondrially targeted hydrophobic cation sensitizes it to membrane potential, giving a small additional effect. The wide dynamic range of these uncouplers in isolated mitochondria and intact cells reveals a novel allosteric activation of proton transport through the adenine nucleotide translocase and provides a promising starting point for designing safer uncouplers for obesity therapy.
Keywords: adenine nucleotide translocase (ANT); butylated hydroxytoluene (BHT); carboxyatractylate (CAT); 2,4-dinitrophenol (DNP); mitochondrion; obesity; uncoupling
Abbreviations: ANT, adenine nucleotide translocase; BHA, butylated hydroxyanisole; BHT, butylated hydroxytoluene; CAT, carboxyatractylate; CCCP, carbonylcyanide m-chlorophenylhydrazone; DNP, 2,4-dinitrophenol; FCCP, carbonyl cyanide p-trifluoromethoxyphenylhydrazone; TPMP, triphenylmethylphosphonium; TPP, triphenylphosphonium
INTRODUCTION
Weight gain, leading to obesity, occurs when energy intake consistently exceeds energy expenditure. In principle, obesity can be treated by reducing caloric intake, by increasing expenditure, or by both approaches simultaneously. Current pharmacological therapies using sibutramine or orlistat [1] or, more recently, rimonabant [2] act primarily to reduce energy intake, and energy expenditure has been relatively neglected as a target for obesity therapy [3]. One way to increase energy expenditure is to use uncouplers to weaken the coupling between fuel oxidation and ATP production. Uncouplers work by transporting protons across the mitochondrial inner membrane, short-circuiting the normal pathway of oxidative ATP synthesis driven by proton flow and causing the loss of calories as heat. Uncouplers are usually lipophilic weak acids that pick up a proton, diffuse across the mitochondrial inner membrane into the matrix, deprotonate and then exit as anions before repeating the catalytic cycle.
The notable success of the uncoupler DNP (2,4-dinitrophenol) as a treatment for human obesity in the 1930s provided an important proof-of-concept and showed that the beneficial effect of uncoupling on energy expenditure is not overwhelmed by compensatory increases in caloric intake. The early literature on treatment of humans and previous studies in rats suggest that DNP matches or outperforms modern drug candidates at causing weight loss [1,4–6]. However, the narrow therapeutic window of DNP and other conventional uncouplers led to the abandonment of their official use in treatment of obesity. Increases (3–10-fold) above the minimum effective dose result in too much uncoupling, leading to compromised ATP production, hyperthermia and death. Concern over this narrow therapeutic window was one of the primary reasons that DNP was withdrawn from the market in 1938 [1,5–7].
For uncouplers to work safely, they should cause uncoupling that increases very little as their concentration rises, potentially widening the difference between therapeutic and toxic doses and giving a wide therapeutic window. Knowing which features of uncouplers to manipulate to give the desired wide dynamic range (ratio of concentrations giving maximum and minimum observable uncoupling) is crucial to the rational design of uncouplers that may be safe for use in obesity treatment. In the present study we investigated uncouplers with an extraordinarily wide dynamic range of greater than 106-fold, and analyse the mechanisms by which this is achieved.
MATERIALS AND METHODS
Mitochondria
Rats were housed (and killed under schedule 1) following Home Office Guidelines for the Care and Use of Laboratory Animals (U.K.). Rat liver mitochondria were isolated from female Wistar rats (4–8 weeks old) in medium comprising 0.25 M sucrose, 5 mM Tris/HCl and 2 mM EGTA (pH 7.4 at 4 °C) [8]. Rat skeletal muscle was dissected from the hind limbs and placed in ice-cold medium comprising 100 mM KCl, 2 mM EGTA and 50 mM Tris/HCl (pH 7.4 at 4 °C). Skeletal muscle mitochondria were isolated at 4 °C as described previously [9]. Mitochondrial protein was assayed using the biuret method, with BSA as a standard.
Rat thymocytes
Thymocytes were isolated from the thymus of female Wistar rats (4–6 weeks old) as described previously [10]. Cells were stored for up to 4 h in plastic flasks at 37 °C and regularly gassed with CO2/air (1:19). The cell concentration was determined using a haemocytometer. The viability of freshly isolated cells was greater than 95% as determined by Trypan Blue exclusion. Isolation and incubations were performed in RPMI medium (supplemented with 2 mM glutamine and without glucose).
Oxygen consumption
Mitochondria (0.5 mg of protein/ml) were incubated at 37 °C in an oxygen electrode (Rank Brothers) connected to a Powerlab data analysis system (Powerlab Chart 5, ADInstruments) in ‘reaction medium’ comprising 120 mM KCl, 5 mM KH2PO4, 3 mM Hepes and 1 mM EGTA (pH 7.2), supplemented with 5 μM rotenone, 1 μg/ml oligomycin and 80 ng/ml nigericin. Uncouplers were added in 1 μl of DMSO using DMSO stocks prepared at different uncoupler concentrations. Where appropriate, malonate was present at concentrations from 0 to 2 mM to give different membrane potentials. Respiration was initiated by adding 4 mM succinate. At the end of the run, 0.3 μM FCCP (carbonyl cyanide p-trifluoromethoxyphenylhydrazone) was added to uncouple the mitochondria fully. The reaction volume was 3.5 ml [1 ml when the membrane potential was measured in parallel using radiolabelled TPMP (triphenylmethylphosphonium)]. To eliminate any problems of carry-over of uncouplers, we always checked that titrations from high to low concentrations gave the same results as titrations from low to high concentrations.
Thymocyte respiration was measured in the same system for 10 min at 37 °C in 1 ml of RPMI medium in the presence of 160 ng/ml oligomycin and uncoupler (from DMSO stock). Oligomycin was absent in the experiments using gramicidin (Figures 6F and 6G).
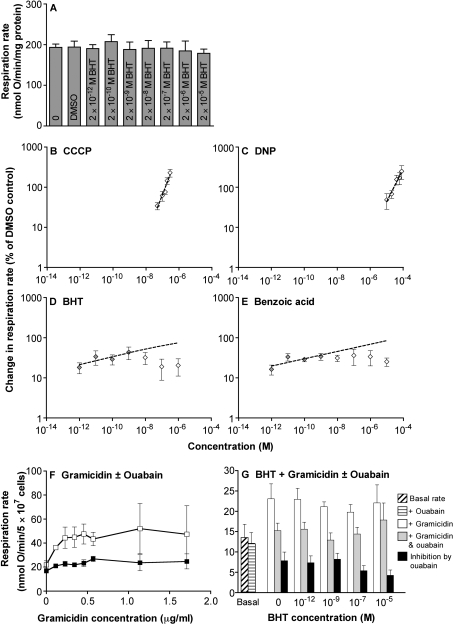
Figure 6. Effect of different uncouplers on mitochondrial ATP synthesis and the respiration of rat thymocytes.
(A) State 3 respiration in rat liver mitochondria with DMSO (vehicle) and at different BHT concentrations in the presence of 50 μM p1,p5-di(adenosine-5′)pentaphosphate (an adenylate kinase inhibitor). State 3 respiration rates were measured after the addition of succinate and 0.1 mM ADP. State 3 respiration rates without DMSO are included for comparison. Values are means±S.D. for two independent experiments. (B–E) Double logarithmic plots of uncoupling of thymocyte respiration by CCCP, DNP, BHT or benzoic acid. Values are means±S.E.M. for three or four independent experiments. Lines were fitted by regression. The slopes are (B) 1.07±0.15, (C) 0.78±0.20, (D) 0.11±0.07 and (E) 0.10±0.04. For BHT and benzoic acid, lines were fitted only between 10−12 and 10−9 M (shaded diamonds). (F) Gramicidin titration of thymocyte respiration. Gramicidin was sequentially added to the cells after a steady baseline was achieved in the presence (filled squares) or absence (open squares) of 1 mM ouabain. Values are means±S.E.M. for four experiments. (G) Ouabain-sensitive respiration driving the Na+/K+-ATPase at different BHT concentrations. Gramicidin at 0.57 μg/ml and ouabain at 1 mM were added before BHT, and the cellular respiration rate was measured for 10 min. Basal cell respiration rates with and without ouabain are included for comparison. Values are means±S.E.M. for three to five independent experiments. Rates and differences were not significantly affected by BHT (as analysed by ANOVA).
Membrane potential
Mitochondria (0.5 mg of protein in 1 ml) were incubated in microcentrifuge tubes containing reaction medium supplemented with 1 μM TPMP+, 0.2 μCi/ml 3[H]TPMP+ (American Radio-labeled Chemicals), 4 mM succinate and uncoupler (from DMSO stock) at 37 °C for 2 min. Where appropriate, malonate was present at concentrations from 0 to 2 mM to give different membrane potentials. The mitochondria were pelleted by centrifugation [8000 g for 2 min at room temperature (25 °C)] and 3[H]TPMP+ in the pellet and supernatant was determined by scintillation counting [11]. The membrane potential was calculated assuming a mitochondrial volume of 0.8 μl/mg of protein and a TPMP binding correction of 0.4 [11]. Runs were carried out in triplicate.
Novel uncouplers
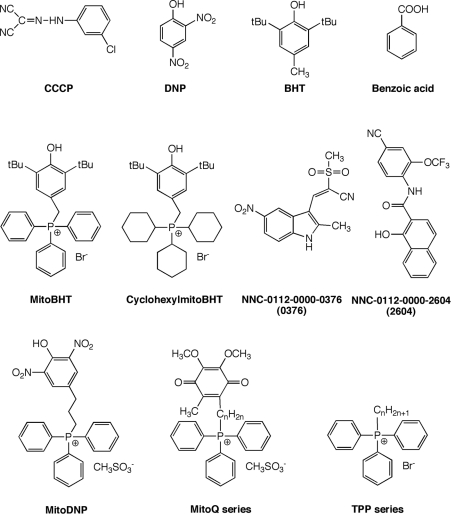
BHT (butylated hydroxytoluene) linked to TPP (triphenylphosphonium) [‘mitoBHT’; (3,5-di-tert-butyl-4-hydroxybenzyl)-triphenylphosphonium], tricyclohexylphosphonium [‘cyclohexy-lmitoBHT’; (3,5-di-tert-butyl-4-hydroxybenzyl)-tricyclohexylphosphonium], and compounds 0376 [NNC-0112-0000-0376; 1-hydroxynaphthalene-2-carboxylic acid(4-cyano-2-trifluoromethoxyphenyl)amide] and 2604 [NNC-0112-0000-2604; 2-methanesulfonyl-3-(2-methyl-5-nitro-1H-indol-3-yl)-acrylonitrile] were prepared according to [12]. MitoDNP and mitoQ were prepared as described in [13–16].
Statistics and regression analysis
Values are given as means±S.E.M. or means±S.D. where appropriate. GraphPad Prism (version 4) was used to find the best-fit linear regression (log–log plots), deduce the slopes and differences between slopes, and determine the significance of differences between means by ANOVA. P values <0.05 were taken to be significant.
RESULTS
Uncouplers with a wide dynamic range of activity
Figure 1 gives the structures of the uncouplers used. They are all lipophilic weak acids except for the mitoQ and TPP series.
Figure 1. Structures of compounds used.
‘n’ denotes the number of alkyl chain carbon atoms. tBu, tertiary butyl.
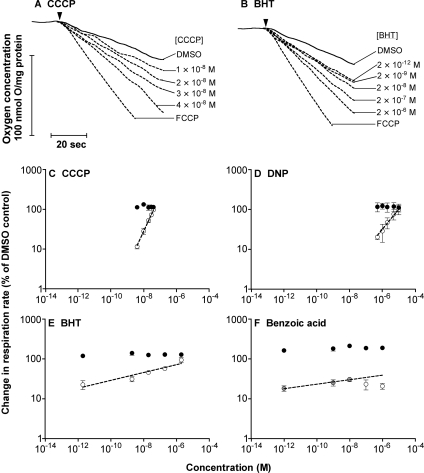
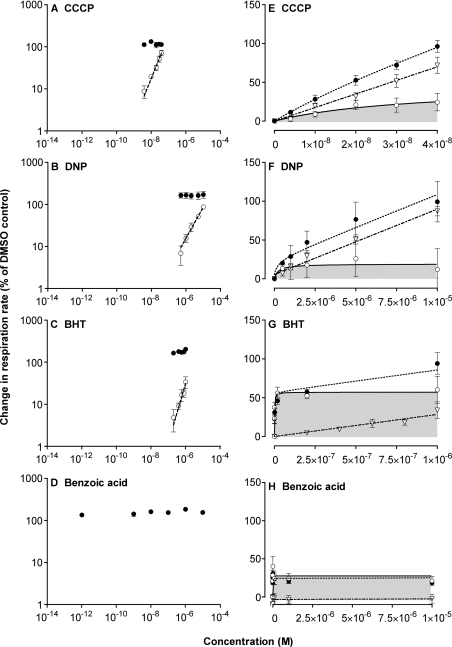
Figure 2 shows oxygen consumption by isolated rat liver mitochondria. The low, fully coupled rate occurred in the presence of vehicle (DMSO) alone, whereas the fully uncoupled rate after addition of excess chemical uncoupler (FCCP) was approx. 3-fold higher, reflecting normal respiratory control. Figure 2(A) shows the result of titrating respiration with a conventional uncoupler, CCCP (carbonylcyanide m-chlorophenylhydrazone). The titration gave the conventional result: respiration was markedly stimulated by 10−8 M CCCP, and increased proportionally as the CCCP concentration was raised 4-fold.
Figure 2. Effect of different uncouplers on the respiration of rat liver mitochondria.
(A and B) Representative traces of oxygen consumption in the presence of (A) CCCP or (B) BHT. Succinate (4 mM) was added to initiate respiration (indicated by the arrowhead). DMSO, different concentrations of CCCP or BHT, or excess (0.3 μM) FCCP were present where indicated. (C–F) Double logarithmic plots of uncoupling. Open or grey circles, titration of respiration rate with CCCP, DNP, BHT or benzoic acid. Closed circles, the respiration rate at each uncoupler concentration after subsequent addition of 0.3 μM FCCP, giving the maximum respiratory capacity of the mitochondria. Values are means±S.E.M. for four independent experiments. Lines were fitted by regression [to the grey points in (F)].
2,6-Bis(1,1-dimethylethyl)-4-methylphenol, more commonly known as BHT, has been reported to be a conventional but weak uncoupler [17–19]. The molecule is lipid-soluble, but the pK of the phenol group is rather high (pK=10), so uncoupling should be limited by the low concentration of the anionic form. When we titrated respiration with BHT (Figure 2B), it gave unexpected results. There was marked uncoupling at extremely low concentrations, 2×10−12 M BHT, but only very modest increases in uncoupling as the concentration of BHT was raised a million-fold to 2×10−6 M.
The unusual characteristics of uncoupling by BHT are clearer when the results are plotted on logarithmic axes. Figure 2(C) shows the conventional behaviour exhibited by CCCP: doubling the CCCP concentration doubled its uncoupling effect, so the plot of log change in respiration rate against log CCCP concentration gave a straight line with a slope (Table 1) of approx. 1. Also plotted is the respiration rate at each CCCP concentration after subsequent addition of excess FCCP to fully uncouple, showing that there was no confounding effect on maximum respiratory capacity caused by CCCP addition. The dynamic range of CCCP concentrations from the lowest measured uncoupling to the maximum uncoupling was approx. 10. Rather similar results were obtained with DNP (Figure 2D), which gave a slope of approx. 0.6 and a dynamic range of 10–100. Figure 2(E) shows the same plot for BHT. Doubling the BHT concentration caused only a small increase in uncoupling, and a straight line fit through the points gave a slope of only 0.1, giving a dynamic range of more than 106.
Table 1. Effect of different uncouplers on the respiration of rat liver mitochondria.
Total action, data from Figures 2(C)–2(F), 7(C), 8(A) and 8(B), and similar experiments. K0.5 values were calculated from the linear regressions. Action not through ANT, uncoupling insensitive to CAT, data from Figures 5(A)–5(D), 7(D), 8(A) and 8(B), and similar experiments. Action through ANT, uncoupling sensitive to CAT, data from Figures 5(E)–5(H) and 7(E), and similar experiments. Km and Vmax values were calculated from hyperbolic regressions using GraphPad Prism 4.0. Data from experiments not shown in the Figures are means±S.E.M. for four independent experiments. nm, not measured; −, no value can be sensibly calculated from the data.
| Total action | Action not through ANT | Action through ANT | |||
|---|---|---|---|---|---|
| Uncoupler | Slope in log–log plot | K0.5 (M) | Slope in log–log plot | Vmax (change in respiration rate, % of DMSO control) | Km (M) |
| CCCP | 0.92±0.06 | 2(±0.4)×10−8 | 0.95±0.11 | 46±41 | 3(±5)×10−8 |
| FCCP | 0.71±0.07 | 3(±0.8)×10−9 | nm | nm | nm |
| DNP | 0.55±0.11 | 4(±2)×10−6 | 0.90±0.11 | 19±12 | 2(±10)×10−7 |
| BHT | 0.11±0.02 | 6(±1)×10−8 | 0.93±0.22 | 55±6 | 2(±1)×10−9 |
| BHA | 0.15±0.03 | 5(±1)×10−8 | nm | nm | nm |
| Benzoic acid | 0.06±0.02 | − | − | 28±3 | 3(±5)×10−13 |
| MitoBHT | 0.06±0.02 | 6(±2)×10−9 | 0.38±0.03 | 47±9 | 8(±14)×10−10 |
| Cyclohexyl-mitoBHT | 0.05±0.01 | 4(±1)×10−8 | nm | nm | nm |
| 0376 | 0.05±0.01 | 2(±1)×10−5 | nm | nm | nm |
| 2604 | 0.09±0.02 | 8(±1)×10−9 | nm | nm | nm |
| MitoDNP | 0.08±0.03 | − | nm | nm | nm |
| MitoQ10 | 0.08±0.03 | − | 0.39±0.08 | − | − |
| DecylTPP | 0.23±0.07 | 5(±3)×10−6 | 0.54±0.1 | − | − |
Benzoic acid is another ‘weak’ uncoupler, with an appropriate pK of 4.2 but low solubility of the anionic form in the membrane, which should limit its ability to uncouple. However, at low concentrations, benzoic acid behaved like BHT, with a slope of approx. 0.1. It failed to give full uncoupling at higher concentrations (Figure 2F).
Table 1 reports the slopes of these log–log plots for different uncouplers and the concentrations that gave half-maximal uncoupling. CCCP and FCCP gave slopes near 1, corresponding to the expected narrow dynamic range. DNP gave an intermediate slope. BHT, benzoic acid, the BHT analogue BHA [butylated hydroxyanisole; (1,1-dimethylethyl)-4-methoxyphenol] and a number of novel uncouplers (mitoBHT, cyclohexylmitoBHT, compound 0376, compound 2604, mitoDNP and mitoQ10; see Figure 1 for structures) gave very shallow slopes, corresponding to a very wide dynamic range.
To investigate whether uncouplers like BHT also uncouple mitochondria from cells whose energy metabolism might be more relevant in obesity treatment, similar respiration measurements were performed with CCCP, BHT and benzoic acid in rat skeletal muscle mitochondria (Figures 3A–3C and Table 2). The results were very similar to those observed using liver mitochondria, but the extent of uncoupling in skeletal muscle mitochondria was slightly lower than in liver mitochondria (compare Figures 2C, 2E and 2F with Figures 3A–3C).
Figure 3. Effect of different uncouplers on the respiration of rat skeletal muscle mitochondria.
Double logarithmic plots of uncoupling in skeletal muscle mitochondria by (A) CCCP, (B) BHT, (C) benzoic acid and (D) mitoBHT. Open circles, titration of respiration rate with CCCP, BHT, benzoic acid or mitoBHT. Closed circles, respiration rate at each uncoupler concentration after subsequent addition of 0.3 μM FCCP, giving the maximum respiratory capacity of the mitochondria. Values are means±S.E.M. for four independent experiments. Lines were fitted by regression.
Table 2. Effect of different uncouplers on the respiration of rat skeletal muscle mitochondria.
Data from experiments of Figure 3. K0.5 values were calculated from the linear regressions using GraphPad Prism 4.0. Values are means±S.E.M. for three to four independent experiments. −, no value can be sensibly calculated from the data.
| Uncoupler | Slope in log–log plot | K0.5 (M) |
|---|---|---|
| CCCP | 1.10±0.18 | 2(±1)×10−7 |
| BHT | 0.10±0.02 | − |
| Benzoic acid | 0.01±0.05 | − |
| MitoBHT | 0.15±0.03 | 7(±3)×10−5 |
Potential-dependence contributes little to the wide dynamic range of uncoupling by BHT
One possible explanation for the wide dynamic range of uncoupling by BHT is potential-dependence of the anion translocation step of the uncoupling cycle. If this step strongly limited uncoupling and was steeply dependent on membrane potential, then addition of more BHT might lower the membrane potential sufficiently to attenuate the uncoupling ability of BHT, leading to the observed weak dependence of uncoupling on BHT concentration.
Figure 4(A) shows the kinetic dependence of proton leak rate on membrane potential in liver mitochondria at different concentrations of BHT. The ability of BHT to uncouple by catalysing proton leak was not unusually dependent on the membrane potential compared with other uncouplers such as fatty acids [20], uncoupling proteins [21,22] or the basal proton leak pathway catalysed by the adenine nucleotide carrier [23].
Figure 4. Effect of membrane potential on uncoupling of rat liver mitochondria by BHT.
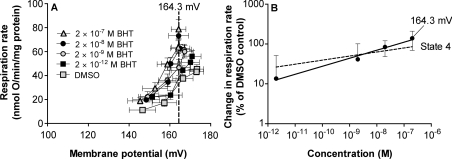
(A) Kinetic response of proton leak rate to membrane potential in the presence of DMSO or different concentrations of BHT as indicated. Proton leak rate was measured as the respiration rate used to drive it; membrane potential was varied by adding different concentrations of malonate. Values are means±S.E.M. for four independent experiments. (B) Uncoupling by BHT. Data taken from (A) at the highest common membrane potential of 164.3 mV (solid line; slope=0.20±0.02; error bars for interpolated points are the weighted means of the error bars on flanking experimental points), or at the highest potential for each uncoupler concentration [broken line; slope=0.13±0.04; points and error bars as in (A) but omitted here for clarity]. The slopes are significantly different; P<0.05.
Any effect of potential-dependence on uncoupling by BHT can be eliminated by comparing proton leak rates at a potential common to all uncoupler concentrations. If unusual potential-dependence caused the wide dynamic range of BHT, at a common potential BHT should behave like CCCP with a slope of 1 in the log–log plots. However, after correction for potential, the slope for BHT remained shallow, with a value of 0.20 (Figure 4B), albeit slightly steeper than the slope of 0.13 obtained from the same dataset without correcting to a common membrane potential.
Thus there is a self-limiting effect of decreasing membrane potential on the ability of BHT to uncouple mitochondria, but the effect is small and it contributes little to the wide dynamic range of uncoupling by BHT.
Role of the ANT (adenine nucleotide translocase) in the uncoupling process
The ANT is an abundant inner membrane protein that imports ADP and exports ATP during oxidative phosphorylation. It can also enhance uncoupling of mitochondria by DNP and several other uncouplers [24–26].
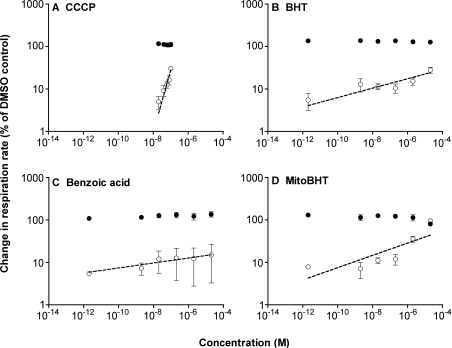
To test whether uncoupling mediated by the ANT was involved in the wide dynamic range of BHT, we examined the effects of CAT (carboxyatractylate), a high-affinity inhibitor of the ANT (Figure 5). Figure 5(C) shows that CAT fully inhibited BHT-induced uncoupling at BHT concentrations below 10−7 M (compare with Figure 2E). It abolished uncoupling induced by benzoic acid (Figure 5D), but had no effect on uncoupling by CCCP (Figure 5A) and only a small effect on uncoupling by DNP (Figure 5B). The effects of CAT on the slopes of the log–log plots are reported in Table 1. In the presence of CAT, the wide dynamic range of BHT was abolished, and CCCP, DNP and BHT all gave slopes near 1. These effects of CAT were mimicked by bongkrekic acid, another specific inhibitor of ANT (results not shown), confirming that they operate through inhibition of this translocase. Thus the wide dynamic ranges of uncoupling by BHT and benzoic acid are completely dependent on the activity of the ANT.
Figure 5. Effect of different uncouplers on the respiration of rat liver mitochondria in the presence of CAT.
(A–D) Double logarithmic plots of uncoupling in the presence of 2 nmol of CAT/mg of protein (added before succinate). Open circles, titration of respiration rate with CCCP, DNP, BHT or benzoic acid. Closed circles, respiration rate at each uncoupler concentration after subsequent addition of 0.3 μM FCCP. Values are means±S.E.M. for four independent experiments. Lines were fitted by regression. (E–H) CAT-sensitive stimulation of mitochondrial respiration rate in linear co-ordinates. CAT-sensitive stimulation of respiration rate (open circles; shaded profile; line fitted as a rectangular hyperbola) was estimated as total uncoupling (filled circles; data from Figures 2C–2F where CAT was absent; line fitted as the sum of the other two lines) minus CAT-insensitive respiration rates (open triangles; data from Figures 5A–5D where CAT was present; linear fit).
The properties of the CAT-sensitive uncoupling by these compounds, re-examined in linear co-ordinates, are shown in Figures 5(E)–5(H). Uncoupling through the ANT, as indicated by CAT-sensitive uncoupling, appeared to follow simple saturation kinetics. Table 1 presents Vmax and Km values for each uncoupler. BHT was a good substrate for the uncoupling activity of the ANT, with a Km in the nanomolar range and a Vmax giving an approx. 50% increase in respiration rate. The translocase had a very low Km for benzoic acid, but at Vmax gave only an approx. 25% increase in respiration, whereas DNP was a relatively poor substrate, with higher Km and lower Vmax values.
Thus the highly-desirable wide dynamic range of uncoupling by BHT is caused by the overlap of two effects: medium-capacity, high-affinity, CAT-sensitive uncoupling through the ANT at low BHT concentrations, and high-capacity, low-affinity, CAT-insensitive uncoupling through other pathways at high BHT concentrations. Surprisingly, CCCP is also a good substrate for the ANT, but since (unlike BHT) the Km for translocase-mediated uncoupling is similar to the K0.5 for CAT-independent uncoupling (Table 1), the two effects overlap and translocase-catalysed uncoupling had no discernable effect on the dynamic range for CCCP.
Competition between uncouplers and nucleotides for the ANT
To test whether the nucleotide substrates of the ANT inhibit uncoupling by BHT, we examined the effect of 1 mM ADP on uncoupling by BHT. ADP lowered the apparent affinity for BHT but, unlike CAT, did not prevent translocase-catalysed uncoupling (results not shown). Presumably, the nanomolar affinity of BHT for the ANT (Table 1) allows it to compete effectively with ADP [Km (ADP)=1–10 μM] [27].
Does BHT prevent nucleotide exchange on the translocase? We found that BHT, at concentrations up to 2×10−5 M, had no significant effect on the state 3 respiration of liver mitochondria (Figure 6A), suggesting that any inhibition is without functional consequences.
Uncoupling with wide dynamic range in rat thymocytes
To directly test whether the wide dynamic range of uncoupling by BHT could still be observed at cellular concentrations of adenine nucleotides and other physiological metabolites, we repeated the measurements of dynamic range in rat thymocytes. Figures 6(B)–6(E) show that the uncoupling effects of CCCP, DNP, BHT and benzoic acid in thymocytes were similar to those observed in isolated liver mitochondria, except that BHT did not give full uncoupling in the higher concentration range. The same pattern of slopes in log–log plots and of dynamic range were observed: CCCP had a steep slope and narrow dynamic range; DNP was similar to CCCP but the slope appeared to be a little shallower; BHT and benzoic acid had very shallow slopes and a much wider dynamic range.
As long as mitochondrial protonmotive force remains sufficiently high to drive ATP synthesis, ATP production in cells will not necessarily be compromised significantly by limited uncoupling of oxidative phosphorylation. This is illustrated by the low control that the proton leak pathway has over ATP production rates in hepatocytes [28] or thymocytes [29]. Nevertheless, we tested empirically whether uncoupling by BHT compromises ATP production in thymocytes, either by lowering the protonmotive force too much, or by preventing adequate ATP/ADP exchange on the ANT. In the presence of low concentrations of gramicidin, to increase the flux of sodium across the plasma membrane, a considerable proportion of respiration is used to make ATP to drive the ouabain-sensitive Na+/K+-ATPase, and the ouabain-sensitive respiration rate is a measure of the rate of this ATP-turnover pathway [30,31]. This is shown in Figure 6(F): concentrations of gramicidin between 0.2 and 1.7 μg/ml doubled the thymocyte respiration rate in the absence but not the presence of ouabain, so that under these conditions about half of the thymocyte respiration rate was used to drive the sodium pump. Figure 6(G) shows this ouabain-sensitive respiration at various concentrations of BHT. There was no significant effect of up to 10−5 M BHT on ATP turnover by the Na+/K+-ATPase in thymocytes, showing that even at the highest concentrations used, 107-fold higher than doses that gave significant uncoupling, there was little or no effect on the ability of thymocytes to make ATP even in response to the extra demand imposed by gramicidin-induced sodium cycling.
Effect of targeting BHT to mitochondria
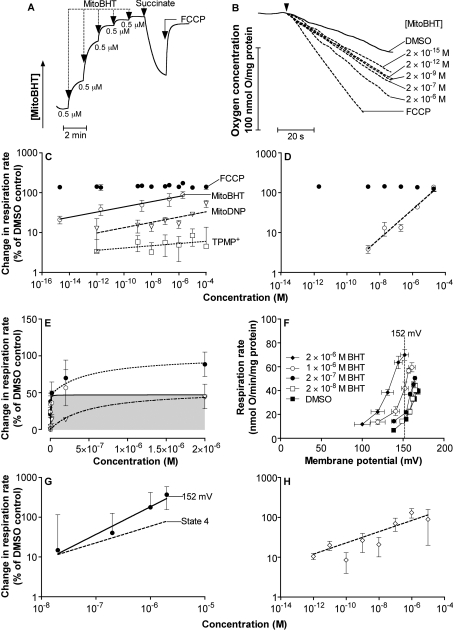
The therapeutic safety of uncouplers might be enhanced by targeting them to mitochondria by covalent linkage to TPP cations, lipophilic molecules that accumulate in mitochondria driven by the membrane potential [32]. In addition, as discussed previously [13], an uncoupler linked to TPP might be strongly self-limiting (because as it uncouples it lowers membrane potential, attenuating further accumulation and therefore attenuating uncoupling), leading to a wider dynamic uncoupling range. DNP linked to TPP (‘mitoDNP’) has been investigated previously [13] but was found to be ineffective at uncoupling. We investigated the uncoupling properties of BHT covalently linked to TPP (‘mitoBHT’) or to tricyclohexylphosphonium (‘cyclohexylmitoBHT’). Figure 7(A) shows that mitoBHT was accumulated approx. 1500–2000-fold into mitochondria in response to the membrane potential set up by succinate oxidation, and released when that potential was fully dissipated by uncoupling with FCCP, consistent with the expected properties of the mitochondrially targeted molecule. CyclohexylmitoBHT was taken up and released in the same way by mitochondria (results not shown). Figure 7(B) shows that, like BHT, mitoBHT is an effective uncoupler, with a dynamic range of at least 109. Figure 7(C) shows the log–log plot of the increase in respiration rate against mitoBHT concentration; the slope was 0.06 (Table 1), similar to or even less than the slope for BHT. However, mitoBHT uncoupled at even lower concentrations than BHT, with a K0.5 of 6 nM for mitoBHT, compared with 60 nM for BHT (Table 1). As a control, TPMP (mitoBHT with BHT replaced by a methyl group) caused much less uncoupling over the same range of concentrations. CyclohexylmitoBHT had similar properties to mitoBHT (Table 1). MitoBHT also uncoupled skeletal muscle mitochondria at very low concentrations (Figure 3D). As with BHT, the extent of this uncoupling was slightly lower in muscle mitochondria than in liver mitochondria (compare Figure 3D with Figure 7C).
Figure 7. Uncoupling effects of mitoBHT in rat liver mitochondria and rat thymocytes.
(A) Uptake and release of mitoBHT. The electrode was calibrated by five additions of 0.5 μM mitoBHT in the presence of mitochondria. Addition of 4 mM succinate induced membrane potential and caused a decrease in the external mitoBHT concentration. The external mitoBHT concentration was largely restored when the membrane potential was dissipated with 0.3 μM FCCP. (B) Representative traces of mitochondrial oxygen consumption in the presence of mitoBHT. DMSO, different concentrations of mitoBHT or 0.3 μM FCCP were present where indicated. (C) Double logarithmic plots of uncoupling in rat liver mitochondria. The titration of respiration rates was with mitoBHT (open circles), mitoDNP (open triangles) or TPMP (squares). Closed circles, respiration rate at each mitoBHT concentration after subsequent addition of 0.3 μM FCCP. Values are means±S.E.M. for four independent experiments. Lines were fitted by regression. (D) Effect of mitoBHT on the respiration of rat liver mitochondria in the presence of CAT. Double logarithmic plots of uncoupling in the presence of 2 nmol of CAT/mg of protein (added before succinate). Open circles, the titration of respiration rate with mitoBHT. Closed circles, respiration rate at each mitoBHT concentration after subsequent addition of 0.3 μM FCCP. Values are means±S.E.M. for four independent experiments. The line was fitted by regression. (E) CAT-sensitive stimulation of mitochondrial respiration rate by mitoBHT in linear co-ordinates. CAT-sensitive stimulation of respiration rate (open circles, shaded profile) was estimated as total uncoupling [filled circles; data from (C) where CAT was absent] minus CAT-insensitive respiration [open triangles; data from (D) where CAT was present]. Lines were fitted as in Figures 5(E)–5(H). (F) Kinetic response of proton leak to membrane potential of rat liver mitochondria incubated with 2 nmol of CAT/mg of protein, in the presence of DMSO and different concentrations of mitoBHT. Values are means±S.E.M. for four or five independent experiments. (G) Potential-independent uncoupling of rat liver mitochondria by mitoBHT. Data taken from (F) at the highest common membrane potential of 152 mV (solid line; slope=0.69±0.10; error bars for interpolated points are the weighted means of the error bars on flanking experimental points), or at the highest potential for each uncoupler concentration [broken line; slope=0.39±0.04; points and error bars as in (F) but omitted here for clarity]. The slopes are significantly different. (H) Effect of mitoBHT on the respiration of rat thymocytes, presented as a double logarithmic plot. Values are means±S.E.M. for three independent experiments. The slope of the regression line is 0.13±0.05.
In the presence of CAT, stimulation of liver mitochondrial respiration rates was inhibited at low mitoBHT concentrations (Figure 7D), indicating that, as with BHT, most of the wide dynamic range for mitoBHT was caused by interactions with the ANT at low uncoupler concentrations. However, unlike BHT, the slope of the log–log plot was not brought to 1 by CAT, but only to approx. 0.4 (Table 1). The CAT-sensitive component showed simple saturation kinetics (Figure 7E) with a nM Km (Table 1).
We tested whether the introduction of the TPP group had rendered mitoBHT more potential-sensitive than BHT, causing the slope of the log–log plot to be less than 1.0 even in the presence of CAT. Mitochondrial proton leak rate in the presence of CAT was compared at a membrane potential common to all mitoBHT concentrations (Figure 7F). After this correction for any effects of membrane potential, the slope was increased from 0.4 to approx. 0.7 (Figure 7G), suggesting that self-limitation of uncoupling through attenuation of membrane potential was a factor in the wide dynamic range of mitoBHT, but much less important than the interaction with the ANT.
MitoBHT uncoupled respiration in thymocytes much as it did in mitochondria (Figure 7H). The slope in log–log plots was shallow (0.1) and the dynamic range was once again very large (more than 106).
Effects of other mitochondrially targeted uncouplers
To investigate why mitoDNP was apparently ineffective even though mitoBHT worked well, we reinvestigated uncoupling by mitoDNP. We found that it behaved in the same way as mitoBHT, but with a much lower Vmax at the translocase (Figure 7C), explaining why the uncoupling effect was missed previously [13].
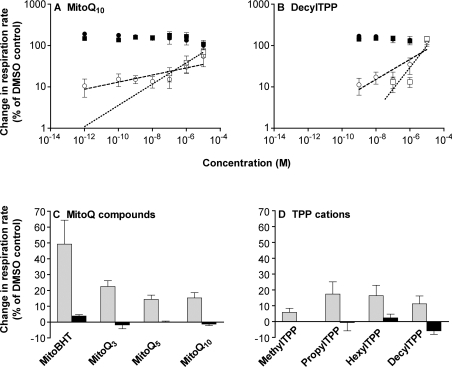
MitoQ10, a derivative of coenzyme Q [14–16], also behaved like mitoBHT, but with a lower Vmax (Figure 8A). Interestingly, decylTPP showed similar behaviour (Figure 8B), even though it lacks a protonatable group. Uncoupling by both mitoQ10 and decylTPP at concentrations below 10−7 M was fully inhibited by CAT (Figures 8A and 8B), showing that uncoupling at these concentrations required translocase activity. Similarly, the slopes of the log–log plots for mitoQ10 and decylTPP were steeper in the presence of CAT (see Table 1). Since the uncoupling activity of the mitoQ compounds was similar to that of the TPP series, but the antioxidant effects of the mitoQ compounds are much greater than those of the TPP series [14–16], the antioxidant effect of mitoQ must be distinct from the uncoupling mechanism through the translocase revealed in the present study.
Figure 8. Effect of mitoQ and TPP compounds on the respiration of rat liver mitochondria.
(A and B) Double logarithmic plots of uncoupling in the presence (squares) and absence (circles) of 2 nmol of CAT/mg of protein (added before succinate). Open symbols, titration of respiration rate with (A) mitoQ10 or (B) decylTPP. In the presence of CAT, concentrations of mitoQ10 or decylTPP below 10−7 M gave a less than 1% increase in respiration and are off-scale. Closed symbols, rate at each mitoQ10 or decylTPP concentration after subsequent addition of 0.3 μM FCCP. Values are means±S.E.M. for three to four independent experiments. Lines (no CAT, dashed line; with CAT, dotted line) were fitted to the points shown by regression. (C and D) Comparison of uncoupling by a series of (C) mitoQ compounds and (D) TPP cations with different numbers of carbon atoms (from three to ten) in the alkyl chain (grey bars). The concentration used for this comparison was 10−9 M. At this concentration, CAT inhibited any uncoupling effect by these compounds (black bars). Data with mitoBHT (Figures 7C and 7D) and TPMP (MethylTPP; Figure 7C) are included for comparison.
Although mitoBHT, at 10−9 M, increased state 4 respiration by approx. 50%, the mitoQ and TPP series of compounds increased it by only 10–20% (Figures 8C and 8D). Since TPP+ (and mitoQ) compounds cannot deprotonate/reprotonate, the uncoupling mechanism for these compounds is not via weak acid cycling. Presumably it is by allosteric activation of a proton leak pathway through the ANT. The greater uncoupling by mitoBHT compared with mitoQ and TPP analogues suggests that there are structural constraints for this allosteric activation.
DISCUSSION
Mitochondrial uncoupling is an effective way to reduce body weight in humans, but using uncouplers such as DNP is problematic because the toxic dose is close to the therapeutic dose. The problem is the steep concentration-dependence of uncoupling by DNP and other conventional uncouplers. Unlike DNP, BHT partially uncoupled mitochondria and cells at extremely low concentrations. This uncoupling was only slightly dependent on concentration, leading to the extraordinarily wide dynamic range of uncoupling by BHT.
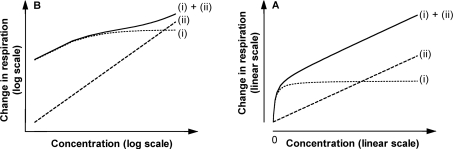
Our experiments show that the main reason for this wide dynamic range is the overlap of two effects as shown in Figure 9: (i) medium-capacity, high-affinity, CAT-sensitive uncoupling through the ANT at low BHT concentrations, and (ii) high-capacity, low-affinity, CAT-insensitive uncoupling through other pathways at high BHT concentrations. Uncouplers such as CCCP and DNP, in which translocase-mediated uncoupling is minor (or has a Km similar to the K0.5 for CAT-insensitive uncoupling), show steep dependence of uncoupling on concentration. Conversely, uncouplers such as BHT, with high translocase-catalysed rates and high affinities relative to the CAT-insensitive ones, show a wide dynamic range and pseudo-linear shallow-slope concentration dependence in log–log plots. Benzoic acid uncouples only through the ANT, explaining its ability to cause only mild uncoupling over a wide range of concentrations. Differences in the involvement of the ANT in the uncoupling mechanism explains the varying concentration-dependence of uncoupling; the bigger the involvement of the translocase, the less the uncoupling effect depends on uncoupler concentration.
Figure 9. Hypothesis for the observed wide dynamic range of uncoupling by BHT and other compounds, illustrated as double logarithmic and linear plots.
Two main effects are responsible: (i) saturable, medium-capacity, high-affinity, CAT-sensitive uncoupling through the ANT at low BHT concentrations (modelled as a rectangular hyperbola with arbitrary Km and Vmax), and (ii) non-saturable, high-capacity, low-affinity, CAT-insensitive uncoupling through other pathways (such as conventional uncoupler cycling) at high BHT concentrations (modelled as a linear relationship). The wide dynamic range (i + ii) is the result of an overlap of these two effects. The right-hand panel shows these effects in linear co-ordinates; the left-hand panel shows the same values plotted in log–log co-ordinates, illustrating how they can combine to give the shallow pseudo-linear overall relationship observed in the other Figures.
How well do the uncouplers work in mitochondria from tissues other than liver? A comparison revealed slightly lower effects in skeletal muscle mitochondria (Figure 3) than in liver mitochondria (Figure 2). ANT1 is the predominant isoform in skeletal muscle, whereas ANT2 predominates in the liver [33]. Differences in the affinities of ANT isoforms for these uncouplers might explain this difference in uncoupling effect. Despite this, uncouplers like BHT or mitoBHT still showed the desirable wide dynamic range of activity in mitochondria from both tissues. This wide dynamic range was also observed at the cellular level using thymocytes, suggesting that there is no major tissue specificity.
At first sight it may appear unlikely that compounds like BHT could uncouple through the ANT in cells, where concentrations of adenine nucleotides are high and might be expected to prevent their action. However, the Km of the ANT for BHT and other wide dynamic range uncouplers is remarkably low (Km<10−8 M), so they can uncouple mitochondria at very low concentrations even in the presence of mM ADP or ATP {Km (ADP)=1–10 μM; Km (ATP)=1–150 μM; [27]}. This is apparent from our measurements of the competition by nucleotides in isolated mitochondria, and from the robust effect of these compounds at low concentrations in cells (Figures 6 and Figure 7H).
The mechanism by which molecules activate uncoupling through the ANT is not known. Previous workers have suggested that the translocase enhances uncoupling by transporting the anionic form of the uncoupler across the inner membrane, catalysing a step that otherwise significantly limits the cycling rate [24–26]. However, we find that CAT-sensitive uncoupling can be activated by mitoBHT and cyclohexylmitoBHT (which are zwitterionic when deprotonated, so should not be driven out through the translocase by the membrane potential). Most importantly, we find CAT-sensitive uncoupling by compounds that cannot uncouple directly via the weak acid cycling mechanism because they have no anionic form and cannot protonate and deprotonate as required for this mechanism: the substituted TPP cations (Figure 8). We therefore prefer a model in which most or all of these hydrophobic CAT-sensitive uncouplers bind to the ANT and allosterically induce net proton transport. Perhaps they do this through the same pathway by which AMP induces proton transport through the translocase [34], and as alkylsulfonates and perhaps fatty acids do in the uncoupling protein family [35,36].
As well as uncoupling, BHT and related compounds like BHA or di-isopropylphenol are reported to inhibit electron transport or ATP synthesis [18,19,37,38]. However, these secondary effects are seen only at relatively high concentrations, 10−3–10−5 M. In isolated mitochondria, there was no inhibition of electron transport capacity at the BHT concentrations we have used in the present study (below 10−5 M) when the mitochondria were fully uncoupled by FCCP (Figures 2E, 2F, 5C, 5D, 7C and 7D), and no inhibition of oxidative phosphorylation. In thymocytes, BHT did not uncouple fully at the higher concentrations (Figure 6D), perhaps indicating secondary inhibition, but this problem was not observed with mitoBHT (Figure 7H). However, 10−5 M BHT did not significantly inhibit the ability of thymocytes to produce ATP for the sodium pump (Figure 6G), suggesting that even at this concentration, 107-fold higher than the BHT concentration that gives significant uncoupling in thymocytes, any secondary effects of BHT on electron transport or ATP synthesis had only minor bioenergetic consequences.
Like BHT, mitoBHT had a wide dynamic range of uncoupling activity, but it caused half-maximal uncoupling at even lower concentrations. This desirable property may be caused partly by potential-sensitive uncoupling, since the concentration-dependence of translocase-independent uncoupling became less steep when the effects of membrane potential were removed (Figure 7G), or simply by greater mitochondrial accumulation because of that potential. It may also be related to differences in hydrophobicity and partitioning in the membrane or binding to the allosteric site on the ANT. Whatever the reasons, the low K0.5, wide dynamic range, relatively large effect and predicted mitochondrial targeting in cells make mitoBHT an attractive candidate for further development.
The wide dynamic range of uncoupling by BHT and benzoic acid provides an excellent starting point for the design of novel uncouplers that could be used to modulate the burning of calories in humans for the treatment of obesity. The production of reactive oxygen species decreases strongly when the mitochondrial protonmotive force is lowered even slightly by uncoupling [39–41]. These uncouplers therefore also present an alternative approach to decreasing radical generation and perhaps treating age-related disorders, particularly since BHT and mitoBHT have antioxidant as well as uncoupling properties. Several improvements could be made to their ability to uncouple. Their properties could be altered to raise or lower the Vmax of their uncoupling through the ANT to fine-tune this component, or to enhance the binding of the uncoupler to the translocase to give even higher specificity. The properties of benzoic acid that allow it to uncouple only through the translocase could be exploited, and it could be used as a starting compound for the design of molecules that have the desired affinity and Vmax for the translocase with no further uncoupling at higher concentrations. More generally, our studies reinforce the importance of the ANT as an important drug target for the identification of molecules that can alter the efficiency and rate of energy expenditure [34].
Acknowledgments
This work was supported by the Medical Research Council U.K. P.H.L. was supported partly by the Cambridge Commonwealth Trust, British Federation of Women Graduates and Novo Nordisk A/S, Denmark.
References
- 1.Harper J. A., Dickinson K., Brand M. D. Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes. Rev. 2001;2:255–265. doi: 10.1046/j.1467-789x.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 2.Van Gaal L. F., Rissanen A. M., Scheen A. J., Ziegler O., Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 3.Halford J. C. Obesity drugs in clinical development. Curr. Opin. Investig. Drugs. 2006;7:312–318. [PubMed] [Google Scholar]
- 4.Cutting W. C., Mehrtens H. G., Tainter M. L. Actions and uses of dinitrophenol. JAMA, J. Am. Med. Assoc. 1933;101:193–195. [Google Scholar]
- 5.Tainter M. L., Stockton A. B., Cutting W. C. Dinitrophenol in the treatment of obesity: final report. JAMA, J. Am. Med. Assoc. 1935;105:332–336. [Google Scholar]
- 6.Parascandola J. Dinitrophenol and bioenergetics: an historical perspective. Mol. Cell. Biochem. 1974;5:69–77. doi: 10.1007/BF01874175. [DOI] [PubMed] [Google Scholar]
- 7.Tainter M. L., Wood D. A. A case of fatal dinitrophenol poisoning. JAMA, J. Am. Med. Assoc. 1934;102:1147–1149. [Google Scholar]
- 8.Chappell J. B., Hansford R. G. Preparation of mitochondria from animal tissue and yeasts. In: Birnie G. D., editor. Subcellular Components: Preparation and Fractionation. London: Butterworths; 1972. pp. 77–91. [Google Scholar]
- 9.Cadenas S., Brand M. D. Effects of magnesium and nucleotides on the proton conductance of rat skeletal-muscle mitochondria. Biochem. J. 2000;348:209–213. [PMC free article] [PubMed] [Google Scholar]
- 10.Buttgereit F., Grant A., Muller M., Brand M. D. The effects of methylprednisolone on oxidative phosphorylation in Concanavalin-A-stimulated thymocytes. Top-down elasticity analysis and control analysis. Eur. J. Biochem. 1994;223:513–519. doi: 10.1111/j.1432-1033.1994.tb19020.x. [DOI] [PubMed] [Google Scholar]
- 11.Brand M. D. Measurement of mitochondrial protonmotive force. In: Brown G. C., Cooper C., editors. Bioenergetics: a Practical Approach. Oxford: IRL Press; 1995. pp. 39–62. [Google Scholar]
- 12.Novo Nordisk A/S. Safe chemical uncouplers for the treatment of obesity. Pat. WO 2004/041256 A3. World Intellectual Property Organization. 2004
- 13.Blaikie F. H., Brown S. E., Samuelsson L. M., Brand M. D., Smith R. A. J., Murphy M. P. Targeting dinitrophenol to mitochondria: limitations to the development of a self-limiting mitochondrial protonophore. Biosci. Rep. 2006;26:231–243. doi: 10.1007/s10540-006-9018-8. [DOI] [PubMed] [Google Scholar]
- 14.Asin-Cayuela J., Manas A. R., James A. M., Smith R. A. J., Murphy M. P. Fine-tuning the hydrophobicity of a mitochondria-targeted antioxidant. FEBS Lett. 2004;571:9–16. doi: 10.1016/j.febslet.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 15.James A. M., Cocheme H. M., Smith R. A. J., Murphy M. P. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J. Biol. Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 16.Kelso G. F., Porteous C. M., Coulter C. V., Hughes G., Porteous W. K., Ledgerwood E. C., Smith R. A. J., Murphy M. P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 17.Pascal M. G., Tennoine T. [Action of an antioxygen food additive, di-tertio-butyl-hydroxy-toluene on the electron transport and on the coupling of oxidation and phosphorylation in the liver mitochondria of rats] C. R. Acad. Sci. Hebd. Seances Acad. Sci. D. 1975;280:1833–1836. [PubMed] [Google Scholar]
- 18.Thompson D., Moldeus P. Cytotoxicity of butylated hydroxyanisole and butylated hydroxytoluene in isolated rat hepatocytes. Biochem. Pharmacol. 1988;37:2201–2207. doi: 10.1016/0006-2952(88)90582-5. [DOI] [PubMed] [Google Scholar]
- 19.Fusi F., Valoti M., Sgaragli G., Murphy M. P. The interaction of antioxidants and structurally related compounds with mitochondrial oxidative phosphorylation. Methods Find. Exp. Clin. Pharmacol. 1991;13:599–603. [PubMed] [Google Scholar]
- 20.Brown G. C., Brand M. D. On the nature of the mitochondrial proton leak. Biochim. Biophys. Acta. 1991;1059:55–62. doi: 10.1016/s0005-2728(05)80187-2. [DOI] [PubMed] [Google Scholar]
- 21.Cadenas S., Echtay K. S., Harper J. A., Jekabsons M. B., Buckingham J. A., Grau E., Abuin A., Chapman H., Clapham J. C., Brand M. D. The basal proton conductance of skeletal muscle mitochondria from transgenic mice overexpressing or lacking uncoupling protein-3. J. Biol. Chem. 2002;277:2773–2778. doi: 10.1074/jbc.M109736200. [DOI] [PubMed] [Google Scholar]
- 22.Echtay K. S., Roussel D., St-Pierre J., Jekabsons M. B., Cadenas S., Stuart J. A., Harper J. A., Roebuck S. J., Morrison A., Pickering S., et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 23.Brand M. D., Pakay J. L., Ocloo A., Kokoszka J., Wallace D. C., Brookes P. S., Cornwall E. J. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem. J. 2005;392:353–362. doi: 10.1042/BJ20050890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andreyev A., Bondareva T. O., Dedukhova V. I., Mokhova E. N., Skulachev V. P., Volkov N. I. Carboxyatractylate inhibits the uncoupling effect of free fatty acids. FEBS Lett. 1988;226:265–269. doi: 10.1016/0014-5793(88)81436-4. [DOI] [PubMed] [Google Scholar]
- 25.Andreyev A., Bondareva T. O., Dedukhova V. I., Mokhova E. N., Skulachev V. P., Tsofina L. M., Volkov N. I., Vygodina T. V. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur. J. Biochem. 1989;182:585–592. doi: 10.1111/j.1432-1033.1989.tb14867.x. [DOI] [PubMed] [Google Scholar]
- 26.Skulachev V. P. Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 27.Vignais P. V. Molecular and physiological aspects of adenine nucleotide transport in mitochondria. Biochim. Biophys. Acta. 1976;456:1–38. doi: 10.1016/0304-4173(76)90007-0. [DOI] [PubMed] [Google Scholar]
- 28.Ainscow E. K., Brand M. D. Top-down control analysis of ATP turnover, glycolysis and oxidative phosphorylation in rat hepatocytes. Eur. J. Biochem. 1999;263:671–685. doi: 10.1046/j.1432-1327.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 29.Krauss S., Buttgereit F., Brand M. D. Effects of the mitogen concanavalin A on pathways of thymocyte energy metabolism. Biochim. Biophys. Acta. 1999;1412:129–138. doi: 10.1016/s0005-2728(99)00058-4. [DOI] [PubMed] [Google Scholar]
- 30.Nobes C. D., Lakin-Thomas P. L., Brand M. D. The contribution of ATP turnover by the Na+/K+-ATPase to the rate of respiration of hepatocytes. Effects of thyroid status and fatty acids. Biochim. Biophys. Acta. 1989;976:241–245. doi: 10.1016/s0005-2728(89)80236-1. [DOI] [PubMed] [Google Scholar]
- 31.Lakin-Thomas P. L., Brand M. D. Stimulation of respiration by mitogens in rat thymocytes is independent of mitochondrial calcium. Biochem. J. 1988;256:167–173. doi: 10.1042/bj2560167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross M. F., Kelso G. F., Blaikie F. H., James A. M., Cocheme H. M., Filipovska A., Da Ros T., Hurd T. R., Smith R. A. J., Murphy M. P. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Moscow) 2005;70:222–230. doi: 10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- 33.Dorner A., Olesch M., Giessen S., Pauschinger M., Schultheiss H. P. Transcription of the adenine nucleotide translocase isoforms in various types of tissues in the rat. Biochim. Biophys. Acta. 1999;1417:16–24. doi: 10.1016/s0005-2736(98)00245-4. [DOI] [PubMed] [Google Scholar]
- 34.Cadenas S., Buckingham J. A., St-Pierre J., Dickinson K., Jones R. B., Brand M. D. AMP decreases the efficiency of skeletal-muscle mitochondria. Biochem. J. 2000;351:307–311. [PMC free article] [PubMed] [Google Scholar]
- 35.Esteves T. C., Brand M. D. The reactions catalysed by the mitochondrial uncoupling proteins UCP2 and UCP3. Biochim. Biophys. Acta. 2005;1709:35–44. doi: 10.1016/j.bbabio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Rial E., Aguirregoitia E., Jimenez-Jimenez J., Ledesma A. Alkylsulfonates activate the uncoupling protein UCP1: implications for the transport mechanism. Biochim. Biophys. Acta. 2004;1608:122–130. doi: 10.1016/j.bbabio.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Ferreira J. Effect of butylated hydroxyanisole on electron transport in rat liver mitochondria. Biochem. Pharmacol. 1990;40:677–684. doi: 10.1016/0006-2952(90)90301-z. [DOI] [PubMed] [Google Scholar]
- 38.Branca D., Roberti M. S., Vincenti E., Scutari G. Uncoupling effect of the general anesthetic 2,6-diisopropylphenol in isolated rat liver mitochondria. Arch. Biochem. Biophys. 1991;290:517–521. doi: 10.1016/0003-9861(91)90575-4. [DOI] [PubMed] [Google Scholar]
- 39.Korshunov S. S., Skulachev V. P., Starkov A. A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 40.Liu S. S. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci. Rep. 1997;17:259–272. doi: 10.1023/a:1027328510931. [DOI] [PubMed] [Google Scholar]
- 41.Miwa S., St-Pierre J., Partridge L., Brand M. D. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radical Biol. Med. 2003;35:938–948. doi: 10.1016/s0891-5849(03)00464-7. [DOI] [PubMed] [Google Scholar]