Abstract
The involvement of the left hemisphere occipito-temporal (OT) junction in reading has been established, yet there is current controversy over the region’s specificity for reading and the nature of its role in the reading process. Recent neuroimaging findings suggest that the region is sensitive to orthographic familiarity (Kronbichler et al., 2007), and the present study tested that hypothesis. Using fMRI, the OT region and other regions in the reading network were localized in 28 adult, right-handed participants. The BOLD signal in these regions was measured during a phonological judgment task (i.e., “Does it sound like a word?”). Stimuli included words, pseudohomophones (phonologically familiar yet orthographically unfamiliar), and pseudowords (phonologically and orthographically unfamiliar) that were matched on lexical properties including sublexical orthography. Relative to baseline, BOLD signal in the OT region was greater for pseudohomophones than for words, suggesting that the region is sensitive to orthographic familiarity at the whole-word level. Further contrasts of orthographic frequency within the word condition revealed increased BOLD signal for low- than high-frequency words. Specialization in the OT area for recognition of frequent letter strings may support the development of reading expertise. Additionally, BOLD signal in the OT region correlates positively with reading efficiency, supporting the idea that this region is a skill zone for reading printed words. BOLD signal in the IFG and STG correlate negatively with reading efficiency, indicating that processing effort in these classic phonological regions is inversely related to reading efficiency.
In 1892 Dejerine described a patient with a lesion in the left hemisphere occipito-temporal junction which resulted in a selective deficit for reading letters and words, providing the first functional definition of the putative visual word form area. Subsequent studies on lesioned and non-lesioned individuals have set about determining the functions of the occipito-temporal (OT) region on the fusiform gyrus, which overlaps with Brodmann’s Area 37. The average Talairach Coordinates of the region are -43, -54, -12, according to Cohen et al. (2000). There is consensus that the region is involved in reading - but little agreement regarding its actual function in the reading process and whether or not the region’s functioning extends beyond the domain of reading. The purpose of this paper is to explore the hypothesis that one function of the OT region is to process word specific orthographic information, then to determine how the OT region functions in comparison to other areas in the reading network, and finally to relate BOLD activity in the OT region to cognitive abilities.
Some investigators propose that the OT region functions as an automatic word recognition site, as it is more active for alphabetic stimuli relative to visual baseline (e.g., checkerboards) and for legal letter strings (i.e., words and pseudowords, such as brone, gloop) relative to random consonant strings (Cohen et al., 2002). The region seems to neglect large differences in visual form, as evidenced by invariance to size, case, font or position on the retina (Cohen et al., 2000; 2002; Dehaene et al., 2001), and may relay an abstract representation of the word form to higher areas. Therefore, the name Visual Word Form Area (VWFA; Cohen et al., 2000) has been adopted by some researchers. A recent neurosurgical case (Gaillard et al., 2006) provides direct evidence for the region’s critical role in word recognition. Following resection of a large portion of the left OT region (due to its involvement as a locus of seizures), this particular patient exhibited intact object and face recognition but could only manage to read words in a slow, letter-by-letter fashion.
An alternative view is that the OT region is multimodal and is responsible for several functions, including color naming and picture naming, and even responds to auditory inputs (Price & Devlin, 2003). There are cases of acute damage to the OT region that do not result in impairment to written word comprehension (e.g., Hillis et al., 2005). Some researchers have hypothesized that this area is a “convergence zone” (Damasio & Damasio, 1994) or “interface” (Devlin, Jamison, Gonnerman & Matthews, 2006) whose specific function depends on interactions with other areas. Another interpretation is that the OT region responds preferentially to words, but only under specific task demands - the area shows no preference for words over pictures when words are treated more like objects (e.g. “what is the color of the stimulus?”; Starrfelt & Gerlach, 2007). Ben-Shachar, Dougherty, Deutsch & Wandell (2007) proposed that the region is involved in shape processing, in general, as the responses in OT to both words and line drawings displayed visual hemifield invariance
Due to individual variation in functional neuroanatomy, it is possible that researchers are localizing slightly different (maybe overlapping) neuronal populations in their studies. In a metanalysis, Cohen and Dehaene (2004) reported evidence supporting the hypothesis that the anterior portion of the left OT region (average y = -43) is responsible for processing relatively more abstract and supramodal representations while the posterior portion (average y = -60) responds strictly to the visual properties of words.
Additional consideration of the role played by the OT region in reading is provided by studies of typical reading development and dyslexia. Cross-sectional developmental data has shown that activity in the region correlates with age and word reading skill, suggesting the area develops as a “skill zone” for reading printed words (Shaywitz et al., 2007; Shaywitz et al., 2002; Sandak, Mencl, Frost & Pugh, 2004). Further support for this idea comes from data showing the region is less active for adults with a history of reading disability, who typically endure years of poor decoding skills (Paulesu et al., 2001). It is unclear whether variations in reading skill among non-impaired adult readers are related to OT activity.
For the purposes of the present paper we will assume that the OT region is involved in processing visual word forms. Whether or not the OT region has other functions (i.e., multimodal vs. unimodal), or responds to multiple levels of orthographic structure (letters and graphemes as well as whole words) is not the focus here. Our primary goal was to test the hypothesis that the OT area responds to whole-word orthographic forms as one of its functions (Kronbichler et al., 2004; Kronbichler et al., 2007).
A review by Mechelli, Gorno-Tempini and Price (2003) of studies comparing the BOLD activation to pseudowords and words indicated that words tend to have lower activation than pseudowords in regions neighboring the OT area, suggesting that the area is sensitive to orthographic lexicality. Following on these findings, Kronbichler et al. (2004) contrasted BOLD activation to five levels of letter-string familiarity, including pseudowords and four ascending levels of printed word frequency. They found a negative relationship between familiarity and BOLD activation in the OT region. As frequency is a property of the word as a whole, rather than any of its component letter strings, the authors argued that the OT region responds to whole-word orthographic form. Further support for the whole-word level of functioning is provided by subliminal priming studies in which a syllable-length judgment task revealed that the BOLD activation in OT was lower following priming of the whole word when compared to priming with an anagram (Dehaene et al., 2004). Similarly, Devlin et al. (2006) reported that priming with the same letter string in a different case produces a greater reduction of activation in the OT area for real words than for pseudowords.
Kronbichler et al. (2007) pointed out that a problem with these priming effects, as well as their own frequency effects, is that these designs do not isolate familiarity with the orthographic form of a word from familiarity with its pronunciation and meaning; higher frequency words are more familiar in all respects. Kronbichler et al. employed a simple design to pinpoint the region’s responsiveness to orthographic rather than to phonological familiarity. They asked German-speaking participants to decide whether a letter string sounded like an existing word. Three types of stimuli were presented: words (e.g., taxi), pseudohomophones (PH; taksi) and pseudowords (PW; tazi). All conditions were equated on bigram frequency and number of letters, syllables, and orthographic neighbors. If the OT region responds primarily to the familiarity of whole word orthography, activation elicited by PH and PW should be greater than activation elicited by words. If, on the other hand, OT responds primarily to familiarity at the letter string level and serves primarily as a preprocessor for access to the phonological and semantic lexicon (Cohen et al., 2000; 2002; Dehaene et al., 2001; 2002; Jobard et al., 2003; Mechelli et al., 2003), PH and words should elicit equal activation, and both should be lower than PW, as both PH and words have the same phonological and semantic entries in the lexicon. The results showed clearly that PH elicited greater activation relative to correct spellings of words, supporting the orthographic lexicon function of the area. Activation in the OT region was not significantly different for PH and PW conditions. According to the authors, unfamiliar strings launch a “search” through the lexicon, resulting in longer reaction times and greater processing effort as evidenced by increased BOLD signal.
The present study investigated the properties of the OT region to test the claim that it is sensitive to whole-word orthography. We adopted the design of Kronbichler et al. (2007) with several methodological improvements. In addition, we examined the relationship between BOLD responses in the OT region and measures of reading skill obtained outside the scanner. The first methodological refinement was individual functional localization of a region in the left OT cortex that was sensitive to word and word-like stimuli relative to visual baseline (i.e., patterns of lines). Past studies report coordinates that are averaged across several individuals. However, identification of common stereotaxic coordinates across individuals does not guarantee common function. Localization prior to hypothesis testing increases statistical power, reduces statistical bias and preserves individual variation in terms of functional location (Saxe, Brett & Kanwisher, 2006). The practice of functional localization has become almost universal in vision science but has not yet been widely applied to reading studies.
Findings from Binder, Medler, Westbury, Liebenthal & Buchanan (2006) that the OT region is sensitive to bigram sensitivity stress the importance of controlling for bigram statistics between conditions. Therefore, we improved upon Kronbichler’s design by equating all letter-string type conditions more closely on bigram frequency, in addition to other lexical parameters.
An additional advantage of our design over previous studies was our ability to examine the effect of familiarity assessed by both lexicality and printed word frequency within one experiment. Previous studies have examined these variables individually, raising the possibility that they were localized in different regions. By exploring these two variables in the same study, we were able to examine whether the same region of interest exhibited activation differences due to both orthographic familiarity and printed frequency.
In addition to examining properties of the OT region, we explored response properties of other regions within the reading network. We hypothesized that sensitivity to orthographic familiarity, as reflected in response patterns to the letter-string types, would differ across regions with different functions. The left inferior frontal gyrus (IFG)—Broca’s area—appears to be involved in phonological processing and articulation (Fiez, Balota, Raichle & Petersen, 1999; Jobard et al., 2003; Mechelli et al., 2003). The left posterior superior temporal gyrus (STG), which is also referred to as Wernicke’s area, is a classic area of language comprehension and may also be associated with lexical and/or phonological access (Fiez & Petersen, 1998). McDermott, et al., (2003) reported that lists of semantically related words elicited greater activation in STG than did lists of phonologically related words. The specific functions of both IFG and STG are unclear, but the current study attempts to confirm the phonological functions of both regions. We expected to find universal effects of printed word frequency across regions within the reading network but differential effects of phonological familiarity (e.g., in the contrast between PW and PH/words).
A final goal of the study was to relate cognitive abilities measured outside the scanner, including reading skill, to the BOLD activation in the OT region. Evidence for the region as a skill zone leads to the hypothesis that activation in the region will increase as reading skill increases. Cross-sectional findings from Shaywitz et al. (2004; 2007) suggest a shift of processing from other parts of the reading network to the OT region with age. If this trend can be attributed to increasing skill, and if it continues into adulthood, adult word reading ability should positively correlate with overall intensity of BOLD signal in the OT region.
Materials and Methods
Participants
28 healthy adults (16 females; mean age: 20.5 years; range:18 to 23 years) participated in the present study. All were strongly right-handed, as assessed by the Edinburgh Handedness Questionnaire (Oldfield, 1971; mean laterality quotient: 88.3, SD: 14.6). All participants were monolingual native-English-speakers, and all reported no current reading problems and a negative history for childhood reading disability. Additional screening criteria included normal or corrected-to-normal vision and a negative history of neurological abnormalities. Participants were briefed on scanner safety and gave written consent.
All participants had IQ in the average range (assessed by the Woodcock-Johnson III Tests of Cognitive Abilities [WJ-III, Woodcock, McGrew & Mather, 2001]; Verbal Comprehension mean standard score (SS): 105.1, SD: 9.8; Spatial Relations mean SS: 109.2, SD: 11.4). Reading ability was in the average to above-average range for all participants as assessed by the Reading Fluency subtest of the WJ-III Tests of Achievement (mean SS: 117.9, SD: 13.4), which requires participants to verify the truthfulness of as many single sentences as they can read in 3 minutes. Additionally, 27 of the 28 participants were administered another measure of reading fluency, the Test of Word Reading Efficiency (TOWRE; Torgesen, Wagner, & Rashotte, 1999), and all of them scored within the appropriate age range according to the norms of the tests (mean SS: 107.2, SD: 7.5 [sight word efficiency (SWE)]; 102.2, 10.7 [phonemic decoding efficiency (PDE)]). The TOWRE requires participants to read as many words (SWE) or pseudowords (PDE) aloud as they can in 45 seconds; two lists of each item type are given. The words are generally within the sight word vocabulary of elementary school age readers, and hence would be easily recognized by adult skilled readers. Likewise, the pseudowords range from three to seven letters in length and are generally decodable by the majority of skilled readers. The emphasis in both tasks is on reading accurately with speed. TOWRE standard scores did not result in much variability in this adult skilled reader sample. For purposes of relating reading efficiency with other measures we used the more variable raw scores.
Procedure
Prior to scanning, participants were trained on the two functional MRI tasks with a laptop computer whose display was nearly identical to the experimental display in the scanner. One task was designed to localize regions of interest (ROIs), especially the OT region, for the purpose of further hypothesis testing regarding the response patterns within these regions. Functional data from a phonological lexical decision task served as the means for such testing. The ROI task was a block design which alternated between rhyming and visual baseline (see Figure 1a). Each trial consisted of the following presentation: 1) an individual printed stimulus presented for 700ms, 2) a blank screen for 500ms, 3) a second stimulus for 700ms, 4) a fixation cross for 800ms during which time the participants were instructed to respond, 5) a 300ms inter-trial interval separating the trials. Reaction times are recorded from the onset of the fixation cross. The pairs presented in the rhyming blocks were composed of any combination of the three conditions, words (W), pseudohomophones (PH), and pseudowords (PW); each stimulus type was paired with each other stimulus type (including itself) an equal number of times. Participants were instructed to read silently each pair of words and indicate via a button press with the left hand whether the pair of letter strings rhymed or not (“yes”=index finger; “no”=middle). They were instructed to ignore differences in stimulus type (i.e., W, PH or PW). The visual baseline “barcode matching” block consisted of same-different judgments on pairs of line sequences containing simplified barcodes. Visual barcode stimuli were designed such that verbal encoding was unlikely, and participants were instructed to avoid counting and instead to rely on their visual encoding of the stimulus. On each barcode trial participants were instructed to indicate via a button press with the left hand whether the pair of barcodes matched (“yes”=index finger; “no”=middle). The ROI task consisted of two runs of 60 trials per run, presented in a blocked format with 6 blocks per condition (rhyming/barcodes) and 10 stimulus pairs per block.
Figure 1.
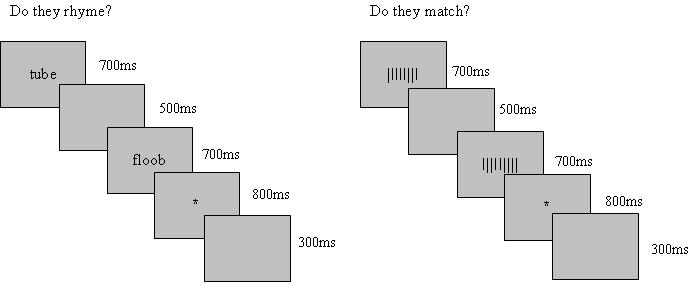
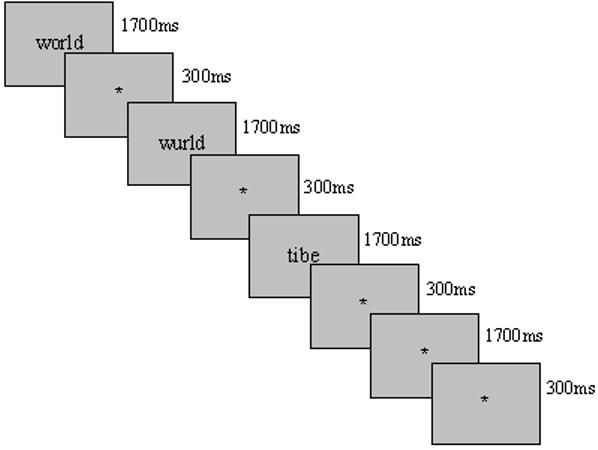
Schematic for a) ROI Localizer task - Block Design Rhyming and Barcode Matching and b) Phonological lexical decision task
In the main experiment, a phonological lexical decision task (PLDT) was used. The task required participants to decide whether an individual item sounded like a real word (see Figure 1b). Items were classified into three conditions: W, PH and PW. Fixation trials were also included. Participants were instructed to respond “yes” to words and PH, “no” to PW, and to rest during fixation. Kronbichler et al. (2007) used the PLDT task and demonstrated (outside the scanner) that participants could distinguish between W and PH in response to the question, “Is this a correctly spelled word?”
Items were presented in an event-related design, and the order of conditions was pseudo-randomized to counterbalance the trial types. The individual stimuli were randomized within each of 6 runs but across runs were constrained such that half of the PH trials were presented before their real word counter-parts and half were presented after. The order of PH/W pairs was balanced across participants (divided into two groups). Each run contained 66 trials; the first two were excluded from analyses yielding 16 trials per condition per run, totaling 384 trials. Half of the 6 runs included low-frequency words, and half included high-frequency words. The order of different frequency levels was counterbalanced (i.e., half of the participants saw high-frequency words first, and half saw low-frequency words first). Due to run duration considerations, it was not possible to include both high- and low-frequency words within a single run.
The order of tasks (localizer and PLDT) was randomized and counterbalanced across all participants. For both tasks, the the stimuli were projected onto a screen at the rear of the scanner, and participants were provided with an adjustable mirror (attached to the head coil) through which they were able to view stimuli. Responses were made via a fiber-optics response button box. Stimulus display was programmed in MATLAB (The MathWorks, Natick, MA, USA) using Psychtoolbox (Brainard, 1997) and controlled by a Macintosh computer.
Materials
The word stimuli contained nouns (all starting with consonants) whose spellings could be altered without affecting pronunciation, thus resulting in pseudohomophones (for example, thirst could be changed to thurst). Words with real homophones were excluded. The low frequency words ranged from 0 to 88 (mean: 25.99, SD: 18.59) occurrences in a corpus of 5,088,721 printed words (American Heritage Word Frequency Book; Carroll, Davies & Richman, 1971). High frequency words ranged from 120 to 8034 (mean: 1021.52, SD: 1329.15). Pseudowords were generated from an online database (MCWord; Medler & Binder, 2005). PW with ambiguous pronunciations were screened out based on pilot participants’ reading aloud, and the list was further reduced based on accuracy and reaction time during pilot performance of the task outside of the scanner by comparable participants - none of whom participated in the scanner portion of the present study. All stimuli were 4-6 letters in length and were matched on lexical and orthographic properties listed in Table 1. It was not possible to match high- and low-frequency words on one property, bigram frequency, but all letter-string type conditions (i.e., words, PH, and PW) were matched on this property. Stimuli from the rhyming decision localizer task matched the stimuli from the phonological lexical decision task on all of the properties listed in Table 1. No stimulus from the training was repeated in the experimental trials, and no item from the rhyming decision task was repeated in the phonological lexical decision task.
Table 1.
Stimuli statistics including mean and standard deviation (in parentheses) for a) word type and b) frequency
| a) | |||||
|---|---|---|---|---|---|
| Words | Pseudohomophones | Pseudowords | Total | F and sig | |
| Letters | 5.26 (.74) | 5.26(.79) | 5.20(.76) | 5.24(.76) | 0.21; ns |
| Syllables | 1.52 (.50) | 1.52(.50) | 1.47(.50) | 1.50(.50) | 0.34; ns |
| Coltheart’s N | 3.65 (3.75) | 3.24(4.15) | 3.96(4.33) | 3.61(4.08) | 0.75; ns |
| Bigram Frequency | 1359.28 (899.74) | 1163.66 (990.04) | 1254.99 (541.81) | 1259.31 (834.27) | 1.33; ns |
| b) | |||
|---|---|---|---|
| Words High | Words Low | F and sig | |
| Letters | 5.35 (.70) | 5.17 (.78) | 1.54; ns |
| Syllables | 1.46(.50) | 1.58 (.50) | 1.50; ns |
| Coltheart’s N | 3.73 (3.95) | 3.56 (3.57) | 0.05; ns |
| Word Frequency | 1021.52 (1329.15) | 25.99 (18.59) | 26.92; p<.001 |
| Bigram Frequency | 1690.24 (991.60) | 1028.32 (655.22) | 14.89 p<.001 |
Image Acquisition
Structural and functional imaging was performed on a Siemens MAGNETOM Trio 3Tesla MRI unit (Siemens Medical Solutions, Malvern, PA) using a CP head coil. Earplugs and sound dampening headphones were employed to shield the participants from acoustic noise. Foam padding was used to minimize head movement.
High resolution structural images were acquired via a T1-weighted MPRAGE sequence (FoV 256 mm; TI 900 ms; TR 2070 ms; TE 4.13 ms; 192 saggital slices). This design allowed us to capture a whole brain image (including cerebellum) with no gaps and 1mm3 isotropic voxels. Functional images sensitive to the blood-oxygen-level-dependent (BOLD) signal were acquired using T2*-weighted echo-planar imaging (EPI) sequence (FoV 224mm; TR 2000; TE 32; 28 axial slices) yielding 3.5 × 3.5 × 4mm voxels with no over sampling.
Data Analysis
Data were subject to online 3D PACE motion correction during acquisition. BrainVoyager QX 1.6.1 (Brain Innovation, Maastricht, the Netherlands) was used to preprocess the data. The functional data images were realigned to the first run with a rigid body transformation and subjected to additional motion correction using trilinear interpolation. Data were spatially smoothed using a 4mm FWHM Gaussian kernel. Temporal filtering included both linear trend removal and a high pass filter with a cutoff of three cycles per timecourse. Slice scan time correction was performed with sinc interpolation. Following preprocessing, data were both automatically (initial) and manually (fine-tuning) aligned to unnormalized structural images. Then the structural and coregistered functional data were normalized into standard stereotaxic space (Talairach & Tournoux, 1988).
ROI Localization
Selection of 3 regions of interest was performed on an individual basis, determined from the rhyming-barcodes contrast map at uncorrected p < 0.000002 (roughly corresponding to a Bonferroni corrected threshold of 0.092) and a cluster size threshold of 25 voxels (See figure 2). To guide our search for regions critical to reading, we constrained our ROI analysis to include regions which had a peak voxel in the left hemisphere and were within 10 Talairach coordinates in each direction of a peak established by previous studies. Coordinates from a study by Cohen et al. (2000) were used to guide our search for the OT region. Localization of other regions of interest was guided by McDermott et al. (2003), which localized frontal (IFG) and posterior (STG) dorsal regions of the language network. Selected ROIs did not exceed a cluster size maximum of 2800 (OT) or 2200 (IFG/STG) voxels. If the number of contiguous activated voxels exceeded the maximum cluster size the ROI was reduced to the maximum size. For participants with multiple peaks in the IFG, the region closest to the coordinates reported by McDermott et al. was chosen because of reports that this more dorsal/posterior region is sensitive to phonology (Poldrack, et al., 1999; McDermott et al., 2003; Vigneau, et al., 2006). Participants for whom there was no peak within 10 Talairach coordinates of the literature-defined coordinates were not included in that particular ROI analysis.
Figure 2.
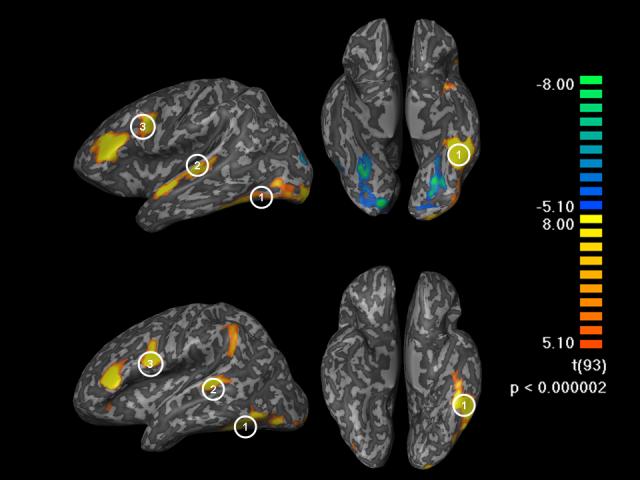
Individual functional data for 2 typical participants, showing activation during localizer task (rhyming minus barcode) on inflated brains.
(Footer) Left column: left hemisphere view; right column: ventral view. Regions of interest, 1: occipito-temporal region, 2: superior temporal gyrus, 3: inferior frontal gyrus. In some individuals two peaks were found on the inferior frontal gyrus. In such cases the peak closest to the one described in McDermott et al. (2003) was used.
For each ROI, GLM analyses were performed on the phonological lexical decision task data using a deconvolution procedure in BrainVoyager QX 1.6.1. The resulting hemodynamic response functions for each condition were used to visualize the time course of activation for 20 seconds following stimulus onset (see Figure 3a). Only the data points at the peak of each plot, occurring around 5 seconds following stimulus onset (average of beta values at points 2 and 3) were used for statistical comparisons. Beta values, indicating percent BOLD signal change averaged across all voxels in the ROI, for each condition relative to baseline were then compiled and averaged across all participants.
Figure 3.
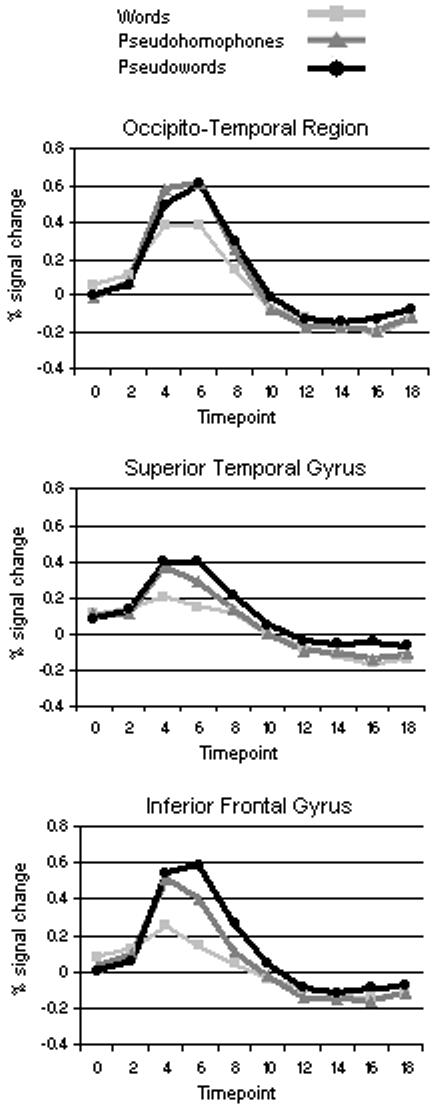
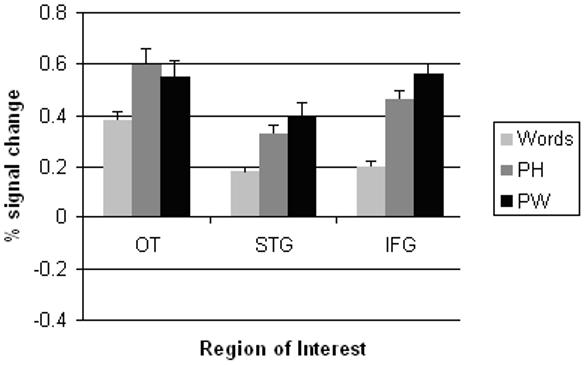
HRF curves for each ROI. Timepoints are in seconds, 0 = stimulus onset. b) Peak BOLD response (average of time points 3 and 4 in the HRF curve) for each ROI. (Footer) OT, occipito-temporal region; STG, superior temporal gyrus; IFG, inferior frontal gyrus.
Results
Task Performance
Accuracy on the localizer task was consistently high and did not significantly differ between conditions (rhyming condition mean: 94%; visual control: 94%; F(1,27) = 0.01; ns; See Table 2a). Median reaction time on correct trials did differ between conditions (rhyming: 321 ms; control: 290 ms; F(1,27) = 16.70, p < 0.001).
Table 2.
Accuracy (means) and reaction times (means of individual medians for excluding error trials) in ms for a) localizer task, b) phonological lexical decision task
| a) | |||
|---|---|---|---|
| Barcode | Rhyming | F; sig | |
| Proportion correct | 0.94(.06) | 0.94(.06) | 0.01; ns |
| Reaction time | 290(90) | 321(91) | 16.70; p<.001 |
| b) | |||||||
|---|---|---|---|---|---|---|---|
| Word | Pseudohomophone | Pseudoword | F; sig | High Word | Low Word | F; sig | |
| Proportion correct* | 0.99(0.02) | 0.93(0.04) | 0.90(0.11) | 53.99; p<.001 | 0.99(.08) | 0.98(.13) | 3.624; p>.05 |
| Reaction time** | 642(184) | 765(224) | 986(237) | 147.63; p<.001 | 662(187) | 622(179) | 28.194; p<.001 |
Word high and word low refer to levels of printed word frequency. F values are calculated by Wilks’ Lambda tests
Pairwise comparisons reveal a significant difference between Words and PH, and words and PW pBonferroni < .001; PH and PW do not differ pBonferroni >.10
Pairwise comparisons reveal a significant difference between every possible combination pBonferroni <.001.
Accuracy and reaction time for the phonological lexical decision task are presented in Table 2b. There was a possibility of response bias toward “yes” responses, given that there were twice as many “yes” as “no” items. However, this seems unlikely, as mean accuracy on each condition was ≥ 90% (see Kronbichler et al., 2007, for a more in-depth explanation). A repeated measures ANOVA performed on accuracy found a main effect of overall letter string type (Wilks’ Lambda F(2,26) = 53.99; p < 0.001), but no main effect of word frequency (Wilks’ Lambda F(1,27) = 3.62; ns). Post-hoc tests revealed a significant difference in proportion correct between words and PH (mean difference = 6%; PBonferroni < 0.001) as well as words and PW (mean difference = 9%; PBonferroni < 0.001). Accuracy did not differ between PH and PW (PBonferroni > 0.10). Reaction time analyses were performed using individual participants’ median values. There was a main effect of letter string type (Huynh-Feldt F(1.21,32.69) = 267.65; p < 0.001), as well as an effect of word frequency (Wilks’ Lambda F(1,27) = 28.19; p < 0.001). Post-hoc tests on letter string type revealed significantly longer reaction times for PH relative to words (mean difference = 125ms; PBonferroni < 0.001), for PW relative to words (mean difference = 350ms; PBonferroni < 0.001) and for PW relative to PH (mean difference = 225ms; PBonferroni < 0.001).
fMRI - Localization
Based on the criteria described in the Methods section for ROI selection, 26 of the 28 participants were included in the OT region analysis, 24 in the STG, and 25 in the IFG. Properties of the three functionally localized ROIs are listed in Table 3. In independent analyses, whole-brain data from all participants’ phonological lexical decision task runs was averaged, and maps were obtained for the conjunction of PH-W and PW-W contrasts. The regions, listed in Table 4, include two of the most prominent areas associated with reading (OT and IFG). It should be noted that while STG is active above baseline in the rhyming task for the majority of the participants, it is not one of the regions in Table 4.
Table 3.
Properties of the 3 functionally defined ROIs
| Region | Mean Coordinates (SD) |
t | Mean # of Voxels | N | Size threshold (voxels) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Occipito-temporal region | -44 (5) | -53 (5) | -13 (4) | 12.82 | 1918(1060) | 26 | < 2800 |
| Superior temporal gyrus | -52 (3) | -43 (6) | 3 (4) | 14.36 | 1702(691) | 24 | < 2200 |
| Inferior frontal gyrus | -49 (4) | 6 (5) | 18 (7) | 12.35 | 1751(656) | 25 | < 2200 |
Table 4.
Activation clusters showing differences (p<.0001, cluster size > 30 voxels) between pseudowords and words and between pseudohomophones and words (conjunction analysis) during “Does it sound like a word?”
| Region | Mean Coordinates (SD) |
t | Voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R frontal lobe | 42 | 1 | 30 | 6.43 | 342 |
| 35 | 18 | 7 | 6.87 | 1769 | |
| L frontal lobe | 0 | 11 | 47 | 7.55 | 6052 |
| -36 | 19 | 6 | 7.08 | 3822 | |
| -46 | 6 | 28 | 7.72 | 6507 | |
| -45 | 34 | 14 | 6.32 | 210 | |
| L thalamus | -8 | -14 | 10 | 6.22 | 132 |
| L precuneus | -23 | -61 | 41 | 6.19 | 60 |
| L fusiform gyrus | -46 | -56 | -11 | 6.32 | 180 |
fMRI - Effects of orthographic familiarity
Repeated measures ANOVA revealed a significant main effect of letter string type in each of the three functionally localized ROIs: (OT: Wilks’ Lambda F(2,24) = 16.76, p < 0.001; STG: Wilks’ Lambda F(2,22) = 14.72, p < 0.001; IFG: Huynh-Feldt F(1.64,39.35) = 74.50, p < 0.001). In OT, pairwise comparisons revealed significant mean differences between the following pairs: activation for the PH condition was greater than activation for the word condition (mean difference = 0.22 units of percent BOLD signal change; PBonferroni < 0.001) and activation for the PW condition was greater than activation for the word condition (mean difference = 0.17 units; PBonferroni < 0.001). The mean difference between activation for the PH and PW conditions was not significant (PBonferroni > 0.10). In STG, activation for the PH condition was greater than activation for the word condition (mean difference = 0.15 units; PBonferroni < 0.001) and activation for the PW condition was higher than for the word condition (mean difference = 0.22 units; PBonferroni < 0.001). The mean difference in activation between the PH and PW conditions was not significant (PBonferroni > 0.10). Every possible pairwise comparison was significant in IFG; activation for the PH condition was greater than activation for the word condition (mean difference = 0.26 units; PBonferroni < 0.001) but less than the PW condition (mean difference = 0.10 units; PBonferroni < 0.001). (See Figure 3b.)
A significant interaction was found between word type and ROI (Wilks’ Lambda F(4,16) = 9.86; p < 0.001), indicating that the pattern of activation in response to the different word types was dependent on the ROI. Whereas the activation for PH was slightly greater than activation for PW in OT, the order was reversed in both STG and IFG.
When analyzing the word frequency levels (see Figure 4), increased activation for low-frequency compared to high-frequency words was revealed for each ROI [OT: Wilks’ Lambda F (1,25) = 26.69 p < 0.001; STG: Wilks’ Lambda F (1,23) = 8.53 p < 0.01; IFG: Wilks’ Lambda F (1,24) = 16.62 p < 0.001]. Because word frequency was manipulated across runs, we verified that overall activity level did not differ between the runs with low frequency words and the runs with high frequency words, indicating that collapsing the data across runs was appropriate.
Figure 4.
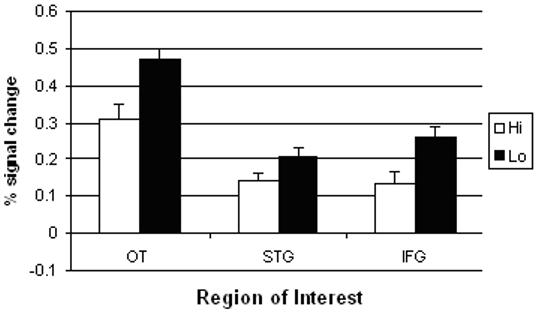
Peak BOLD response (average of time points 3 and 4 in the HRF curve) by levels of printed word frequency.
(Footer) OT, occipito-temporal region; STG, superior temporal gyrus; IFG, inferior frontal gyrus.
Activation related to behavioral measures
We used Pearson’s correlation to examine relationships between activation in each ROI, for each condition; performance during the activation task; and cognitive abilities measured outside the scanner. These values are reported in Table 5. Our measure of word reading efficiency (TOWRE-SWE) correlated positively with activation in the OT region for words. In contrast, TOWRE-SWE correlated negatively with activation in STG for the PW condition and IFG for the PH condition. Correlations with TOWRE-SWE showed the same negative trend for PH in STG and PW in IFG, but they were not significant. In contrast, TOWRE-SWE did not correlate with activation to words in either STG or IFG. TOWRE-SWE correlated with in-scanner reaction time for the phonological lexical decision task for each condition. Verbal comprehension scores (hereafter referred to as verbal IQ) correlated with activation in the OT region for PH (r = 0.44, p < 0.05, 2-tailed). In sum, the results indicate that faster readers tended to be faster in the magnet, but also that they tended to show higher activation in the OT region, and lower activation in the STG and IFG. Verbal IQ was associated with greater activation in the OT region, but only for PH.
Table 5.
Pearson correlations for BOLD signal, cognitive abilities, and task performance
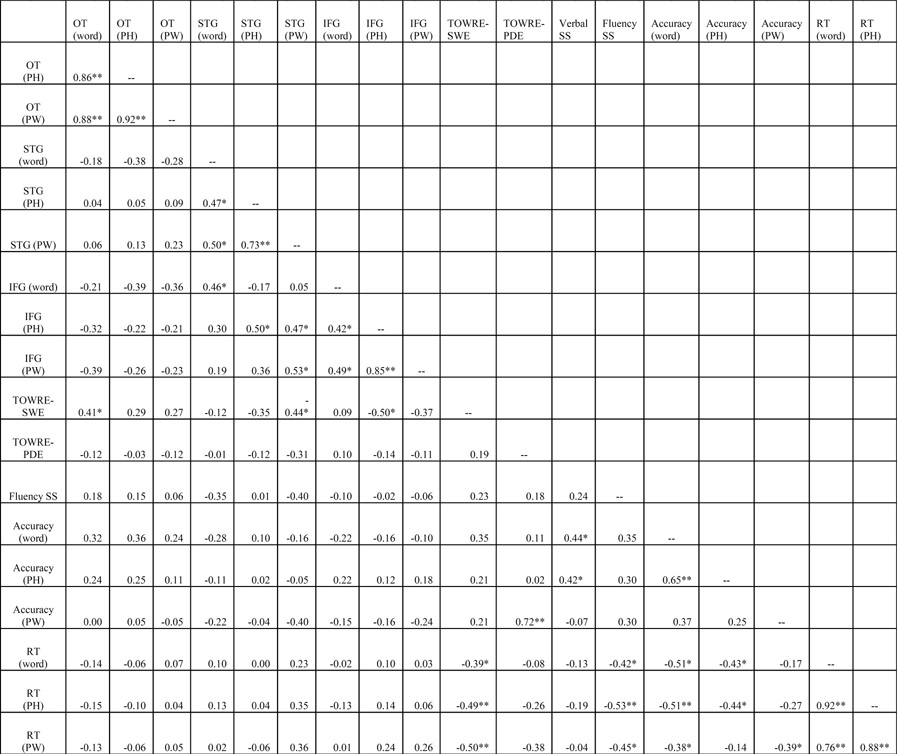 |
TOWRE-SWE, Test of Word Reading Efficiency—Sight Word Efficiency; TOWRE-PDE, Test of Word Reading Efficiency—Phonological Decoding Efficiency; Verbal SS and Fluency SS are Standardized scores from the Verbal and Fluency subtests of the Woodcock-Johnson III-Test of Cognitive Abilities; OT, occipito-temporal region; STG, superior temporal gyrus; IFG, inferior frontal gyrus; AG, angular gyrus; PH, pseudohomophones; PW, pseudowords.
Note. Total is the average of word, PH and PW conditions; accuracy, percent correct on phonological lexical decision task; reaction time, median reaction time on correct trials
p< .05, 2-tailed.
p<.01, 2-tailed.
Somewhat surprisingly, TOWRE-PDE did not correlate significantly with any activation measures. It correlated positively with accuracy on the phonological lexical decision task. We also noted that TOWRE-PDE did not correlate significantly with TOWRE-SWE (r=0.19). As expected, there was a high degree of intercorrelation between the TOWRE-SWE, verbal IQ and Reading Fluency.
The finding of a positive correlation between TOWRE word reading efficiency and OT activation to words, and trends in the same direction for OT activation to PH and PW, suggests that more skilled readers show larger responses to printed stimuli. This finding seems to contradict the finding of reduced activation to words relative to PH and PW found for the group as a whole. However, it is possible that more skilled readers show greater activation to printed stimuli overall, but also show greater differentiation between familiar and non-familiar printed letter strings. To find out, we divided the 28 subjects based on the median TOWRE-SWE score into low and high word reading efficiency groups (n = 14 per group). The effect of letter string type was significant in the higher TOWRE group (Wilks’ Lambda F[2,13] = 17.19, p < 0.01) but failed to reach conventional levels of significance in the lower TOWRE group (Wilks’ Lambda F[2,9] = 3.47, p > 0.05). Although the analyses were conducted on small samples, the results tentatively suggest that it was primarily the more skilled reader group that showed greater sensitivity to the orthographic familiarity in printed words. However, the comparison should be replicated in a larger sample varying more widely in reading skill.
When groups were median-split on estimated verbal IQ, the other variable showing a correlation with brain activity in OT, again it was only for the higher verbal IQ group that the letter string type effect was significant (lower: Wilks’ Lamda F(2,11) = 3.40; p > 0.05; higher: Wilks’ Lambda F(2,11) = 21.42; p < 0.01).
For STG and IFG, splitting the sample based on reading efficiency or on verbal IQ did not produce different results across the groups. In STG, the differences between words and PH (0.21; PBonferroni < 0.05) and between words and PW (0.32; PBonferroni < 0.01) were significant in the lower efficiency group, and those differences were also significant in the higher efficiency group (words and PH: 0.12, PBonferroni < 0.05; words and PW: 0.17, PBonferroni < 0.05). When the sample was split based on verbal IQ, the two patterns of activation were comparable across these groups, as well (lower: words and PH = 0.14, PBonferroni < 0.05; words and PW = 0.23, PBonferroni < 0.01; higher: words and PH = 0.16, PBonferroni < 0.01; words and PW = 0.22, PBonferroni < 0.05). The difference between PH and PW was not significant in any group (PBonferroni > 0.10). In IFG, the differences between words and PH (0.35; PBonferroni < 0.001), words and PW (0.45; PBonferroni < 0.001), and PH and PW (0.10; PBonferroni < 0.01) were significant in the lower efficiency group, and they were also significant in the higher efficiency group (words and PH = 0.20, PBonferroni < 0.001; words and PW = 0.30, PBonferroni < 0.001; PH and PW = 0.10, PBonferroni < 0.05). Groups based on verbal IQ were also similar (lower: words and PH = 0.25, PBonferroni < 0.01; words and PW = 0.34, PBonferroni < 0.001; PH and PW = 0.09, PBonferroni < 0.05; higher: words and PH = 0.28, PBonferroni < 0.001; words and PW = 0.39, PBonferroni < 0.001; PH and PW = 0.11, PBonferroni < 0.05). These findings suggest that faster readers, as well as those with higher verbal IQ, tend to be more sensitive to the task manipulations in OT, but not in the other ROIs, perhaps because higher achieving readers had more efficient processing of orthographic forms.
Discussion
Our primary findings replicate the effects reported by both Kronbichler et al. (2004) and Kronbichler et al. (2007) within regions that were functionally localized on an individual basis and in the context of English, which has an irregular orthography. We were able to demonstrate a clear effect of orthographic familiarity by including conditions which were identical in phonological frequency, as well as post lexical semantic and phonological processing. The response pattern within the OT region was consistent with sensitivity to orthographic familiarity, in that orthographically novel PH elicited greater activation than did familiar words. Activation patterns for PH and PW did not differ in the OT region, indicating that phonological familiarity did not have an impact on OT functioning. IFG, however, did show a pattern consistent with sensitivity to phonological familiarity, as indicated by the activation difference between PH and PW in this region. There was a positive correlation between word-reading efficiency outside the magnet and the BOLD activation in the OT region, in contrast to the negative correlations found between word reading efficiency and activation in IFG and STG. Together, these findings indicate a unique role for the OT region that is related to acquisition of skill in reading familiar words.
The Nature of Word Processing in OT
First, we consider the effect of orthographic familiarity within the OT region. Lexical access via the OT region appears to have been facilitated by previous and repeated exposure to the specific orthographic form of the printed words. Although a PH is familiar once decoded, its orthographic form has never been encountered and is treated as a novel string. The phonological form of each PH has been experienced (as many times as the phonology of its real-word counterpart, in fact). Therefore, we are able to claim that the PH and word conditions were identical in terms of phonological familiarity and that our activation difference is related to orthographic familiarity.
We intended to test the hypothesis that the OT region is responsive to orthographic familiarity at the whole word level. The findings of Binder et al. (2006) that the OT region responded to manipulations of orthographic familiarity at the sub-word level necessitated holding bigram frequency constant across conditions. Given that we controlled for bigram frequency, we are able to conclude that OT is sensitive to whole word orthographic familiarity. It is possible that OT has more than one function, and that it also is sensitive to subword variables, but this question was outside the scope of the current study.
Lending further support to the claim that the OT region is sensitive to orthographic familiarity at the whole word level is the pattern of results for printed word frequency, a property of the whole word. Due to constraints of English orthography, it was very difficult to vary print frequency while holding bigram frequency constant, especially while attempting to control for other orthographic statistics. Because low and high frequency words differed significantly in bigram frequency, it is with caution that we interpret the findings of printed word frequency.
In the case of printed word frequency, neither condition (low or high) is novel, but readers have had many more exposures to high-frequency words. A result of more experience is more efficient processing, which is the interpretation of the finding of reduced reaction time across numerous behavioral studies (e.g., Grainger, 1990; Connine, Mullennix, Shernoff & Yelen, 1990). Another correlate of efficient processing, reduced BOLD signal, is evidenced by studies involving priming (e.g., Fiebach, Gruber, and Supp, 2005) and training (e.g., Poldrack & Gabrieli, 2001). In the current study, this profile of efficiency was observed in two separate contrasts: words relative to PH, and high-frequency words relative to low-frequency words.
What is the cognitive basis for the difference observed in BOLD signal for PH and words? Kronbichler et al. (2007) interpreted higher activity for PH as the result of a longer search through the mental lexicon. This ‘search’ theory has been proposed in previous research to explain the differences in processing time on different string types for lexical and naming tasks (e.g., Forster & Chambers, 1973). For words, the search is relatively quick compared to less familiar stimulus types; search time also varies with printed word frequency (Kronbichler et al., 2004; 2007). The advantage for words seen in the present study and Kronbichler et al. (2007) is most likely a cumulative effect on the efficiency of orthographic processing. The more reading experience one has, the greater the expertise and therefore the greater the difference in activation between familiar and unfamiliar letter strings. Training studies could provide more direct evidence of the orthographic familiarity effect on a shorter time scale. Sandak, Mencl, Frost, Rueckl, et al. (2004) showed that activation in the OT region decreases following training on novel letter strings across experimental sessions, but it was unclear whether the advantage was due to semantic, phonological, and/or orthographic familiarity.
Our results are also consistent with priming studies. For example, Dehaene et al. (2004) compared the effect of two types of primes in French prime-target pairs involving the same word in different case (e.g., REFLET-reflet) and prime pairs involving a real-word anagram of the prime word (e.g., TREFLE-reflet). During a syllable-count judgment task, activation in OT related to the target word (reflet) was lower following same-word primes as opposed to anagram primes. The interpretation was that only the same-word primes were activating stored orthography. A similar result offered by Devlin et al. (2006) involved the comparison of word primes in a different case (e.g., CABIN-cabin) to pseudoword primes (SOLST-solst). Repeating the same word in different case resulted in a greater reduction of activation in the OT area than repeating the same pseudoword in different case. The authors claim that the word primes preactivate an orthographic word representation, whereas the pseudoword primes have nothing to preactivate. The pseudoword pairs did not produce a neural priming effect in the OT area, but the timecourse and limited exposure provided by this experimental design may not allow for the same learning/encoding seen in the training study by Sandak, Mencl, Frost, Rueckl, et al. (2004).
An important limitation of these priming studies is the inherent confound of orthographic with phonological and semantic effects. In the Dehaene et al. (2004) study, the anagram prime differs from the target word on all three properties. In the Devlin et al. (2006) study, in addition to familiar orthography, the word pairs also have associated phonological and semantic entries, while the pseudowords have none of these learned features. The use of pseudohomophones in the present study allowed for isolation of orthographic from phonological and semantic effects.
Patterns of Activation in Areas Other than OT
In contrast to the pattern of activity found in the OT region, the pattern found in IFG may reflect sensitivity to phonological familiarity. Of particular interest is the difference between activation during PH and PW conditions. We agree with Kronbichler et al. (2007) that the higher activation for PW than for both PH and words is due to PW requiring the “assembly of a new pronunciation, whereas for PH [and words] an existing phonological form can be accessed” (p. 17). Studies that have compared pseudowords to words in terms of activation in the IFG have offered similar interpretations regarding phonological processing demands (Fiez et al., 1999; Jobard et al., 2003; Mechelli et al., 2003). Previous results indicate that the portion of IFG selected in the current study is likely to be sensitive to phonological as opposed to semantic processing. According to Poldrak et al. (1999), the ventral part of the left IFG is involved in semantic processing while the more dorsal region (corresponding to the region localized in the present study) is involved in phonological processing.
The activation pattern exhibited by the STG was not identical to that of the IFG. Although PW elicited greater activation than did PH in STG, the difference was not significant. Therefore, although the ordering of conditions in terms of activation matched the ordering in the IFG, the pattern of significance is indistinguishable from that in the OT region (PH and PW are both greater than words). The role of STG in semantic processing, as indicated in McDermott et al. (2003), is not supported by the observed pattern, yet its participation in semantic processing cannot be entirely ruled out. The pattern in STG may reflect the amount of phonological decoding; PH and PW both require more decoding than words. It is also possible that the STG processes orthography in addition to phonology. The observed pattern in STG could be a result of multiple relays to and from the OT region and IFG, as well as other regions not considered in this paper. The functions of these areas may be best delineated by employing connectivity analyses (e.g., Pugh et al., 2000) and/or methods with higher temporal resolution.
Alternative Interpretations of the Data
A possible critique of our findings and subsequent interpretations is that the pattern of the BOLD responses may be confounded by reaction time, i.e., items that require more time to process evoke stronger activation. If this were the case, activation elicited by PW should be greater than activation elicited by PH, since the reaction time for the PW condition was significantly longer than that for the PH condition. In fact, the patterns of activation differences in the OT region and STG were clearly not the same as our pattern of reaction time differences, indicating that the activation differences are not solely the result of longer processing. Nevertheless, the possibility remains that the pattern in the IFG region (PW > PH > Words) is a result of increased processing time and/or cognitive effort. However, some evidence against this possibility is provided by a lack of significant correlations between participants’ median reaction times and activation scores within the IFG (r = 0.07, p > 0.10), as well as other regions (OT: r = -0.03, p > 0.10; STG: r = 0.18, p > 0.10). If activity in the OT region is related to greater cognitive effort, we would also expect activity in the reading and language areas as a whole to have an inverse relationship with cognitive skill (reading and/or IQ). In fact we found a positive and highly significant relationship between scores on behavioral measures and the BOLD signal in the OT region.
We note that the two unfamiliar conditions (PH and PW) are associated with greater signal change than words in all three of our ROIs. The results of the whole brain analysis (Table 5) indicate that this effect was seen in a limited number of regions, mostly within the language network, and it is not an effect of global brain functioning.
A potential critique of our functional tasks is their phonological nature, which may hinder our ability to compare these to other findings from studies using tasks that did not explicitly require phonological judgments (such as passive word reading or lexical decision). In the present study, the choice of a phonological judgment task was essential to the goal of distinguishing between familiar and unfamiliar orthography. This goal required that every property of the stimuli and the resulting response choice be held constant except orthographic familiarity. The phonological lexical decision task is an ideal design to test this question, because PH and words can be equated in every aspect except orthographic familiarity, and the response in each case is the same (“YES”). This task also provided us assurance that participants were actively reading, so that we could reliably assess variation in reading performance and relate it to the subsequent BOLD activation and cognitive abilities measured outside of the scanner (see also Kronbichler et al., 2007).
Similar points can be made about the choice of a localizer task. We chose a localizer task that involved phonological judgments to provide consistency across tasks and to activate a network of regions that reflected phonological and orthographic processing. Alternative tasks that were ostensibly purely orthographic in nature could have been used, but such tasks (e.g., case judgment or letter feature recognition) do not require explicit reading of the word and, therefore, would not reliably and strongly activate the reading network. While performing the rhyming task, the majority of participants showed a distinct ROI for the OT region (26 out of 28 participants), STG (24 participants), and IFG (25 participants), validating our use of the task.
An inherent limitation of this particular design is the inability to address questions regarding the specificity of the OT region. According to Devlin et al. (2006), p. 919, “as long as the stimulus affords higher order, non-visual properties that must be integrated with visual information,” this area could serve as an interface between levels of processing. This interpretation explains why the region is activated in response to pseudowords and pictures (Price & Devlin, 2003), which enlist both low- and high-level processing. We were not able to test the entire scope of the OT area’s selectivity within the current experiment, and this was not the aim of the study. Current and future directions in the ongoing investigation of this region might include connectivity studies that could model interactions between the OT region and other regions and address the role of the OT in a broader context.
Relationships between Brain Activation and Cognitive Abilities
As previously discussed, the correlational data were mixed. Positive correlations were found between word reading efficiency (TOWRE-SWE) and activity in OT for words (a trend for PH and PW conditions) and negative correlations were found between TOWRE-SWE and activity in STG and IFG for the PW and PH conditions respectively. An interesting exception to these trends was the absence of any correlation between TOWRE-SWE and activity for words in STG and IFG. Adding to the pattern was the failure to find any significant correlations of phonological decoding efficiency (TOWRE-PDE) with BOLD activation in any of the ROIs. This is surprising, given that the construct of phonological decoding efficiency would be expected, a priori, to relate to brain activation in reading tasks. We would also expect moderate to high correlations between PDE and SWE as indicated in the test manual, but our data produced a non-significant correlation (r = 0.19; p > 0.10). This result is puzzling, but it should be noted that the TOWRE is designed and normed on a broader range of ages than was represented in our sample. It seems within our sample of older readers these two tests function slightly differently. TOWRE-SWE may function primarily as a measure of word reading speed or efficiency, given that none of the word items would pose any difficulty for subjects of this age and reading skill. In contrast, TOWRE-PDE may function primarily as a measure of phonological decoding accuracy, rather than speed (hence the correlation with accuracy in the scanner). An explanation for the lack of correlation between TOWRE-PDE and BOLD, therefore, could be that activation levels reflect efficiency, rather than accuracy only.
Negative relationships between BOLD signal in IFG and reading skill (Hoeft et al., 2007; Shaywitz et al., 2007; Shaywitz et al., 2002) as well as age (Shaywitz et al., 2007) have been suggested to reflect a greater demand for phonological decoding and assembly for younger and less skilled readers. In addition, both adults and adolescents with reading disability have been found to show lower activation in OT than more skilled readers (Paulesu et al., 2001; Shaywitz et al., 2007; Shaywitz et al., 2002; Sandak, Mencl, Frost & Pugh, 2004). Our data add to this picture by revealing a positive correlation between TOWRE word reading efficiency and BOLD signal in OT in adult skilled readers, and negative correlations between TOWRE word reading efficiency and BOLD signal (for PH and PW, but not words) in IFG and STG. It should be noted that Turkeltaub, Gareau, Flowers, Zeffiro & Eden (2003) reported a positive correlation between reading skill and BOLD signal in IFG and no correlation between skill and BOLD signal in OT. These divergent results may be attributable to the implicit nature of their tasks and inclusion of only words in their stimulus set.
The lack of significant correlations might be due, in part, to the lack of variability in our participants’ reading and verbal abilities. Therefore, caution must be exercised when interpreting these findings. However, a tentative interpretation of the correlation pattern can be offered. TOWRE-SWE primarily assesses speed and accuracy in reading familiar words, as none of the words on the test would present particular challenges to skilled adult readers. The correlations support the skill zone interpretation of the OT region, in that higher skill in reading familiar printed words is associated with greater activation in OT. At first glance, this finding seems to contradict our finding that OT activation for words is lower than for PH and PW. If greater familiarity with words leads to lower activation in OT, why wouldn’t more skilled readers show less OT activation for words than less skilled readers?
A careful examination of the data indicated that more highly skilled readers within our sample showed a larger word familiarity effect than less skilled readers. A possible interpretation of the data is that OT is involved in processing orthographic information of all types, and it is more efficient at processing familiar letter strings, particularly for more skilled readers. This effect is analogous to the processing of faces in the right fusiform face area: familiar faces elicit less activation than do unfamiliar faces (Rossion, Schiltz, Crommelinck & 2003). We don’t have a specific interpretation of the results contrasting readers of lower and higher verbal ability, but note that these groups overlapped considerably with the groups sorted on the basis of word reading efficiency. The same individuals in our sample tended to be high in both word reading efficiency and knowledge of word meanings.
The results suggest that allocation of resources across the reading network depends on reading skill, even within a group of above average adult readers. Allocation differences have been suggested when comparing disabled readers to non-impaired readers or when considering reading development across childhood and adolescence (Paulesu et al., 2001; Shaywitz et al., 2007; Shaywitz et al., 2002; Sandak, Mencl, Frost & Pugh, 2004), but not yet within a group of skilled adult readers. We propose that more highly skilled readers rely more on OT during phonological lexical decision (hence the positive correlation between reading efficiency and OT activation), and that the tuning of OT to orthographic familiarity is related to reading ability - higher skill is associated with larger differences between responses to familiar and unfamiliar forms. More highly skilled readers and individuals with higher verbal IQ would have more experience with words so they would show a larger difference between words and PH. Less skilled but above average readers may rely more on IFG and STG to perform the phonological lexical decision task, as suggested by negative correlations between activity in these areas and reading ability and the fact that their OT regions appear to be less sensitive to orthographic familiarity. The negative correlations may also reflect greater processing effort or less neural efficiency. These conclusions must be regarded as tentative, as the boundary between significant and non-significant correlations is not an absolute one, and the pattern of correlations might differ with a wider range of reading ability and/or a larger sample.
In conclusion, this study extends the ground breaking findings of reduced activation to familiar words in OT by Kronbichler et al. (2004; 2007) to English, demonstrating that the finding generalizes across regular (German) and irregular (English) orthographies. Replication of the results in a wider range of orthographies would be valuable. In addition, our study offers two methodological improvements. First, the occipito-temporal region was functionally localized on an individual basis, and second, its function was investigated using two separate comparisons within the same ROI. We conclude that the occipito-temporal region is sensitive to orthographic information at the whole-word level.
Our study also extends previous findings of the relationship between OT activation and reading skill to an older, skilled population. We support the claim that the OT region is, indeed, a skill zone. The specific skill it seems to subtend is efficient processing of orthography. Also, correlations between activation and skill indicate that the more reading experience one has, the greater the expertise and therefore the greater the difference between familiar and unfamiliar letter strings. Future directions include replicating the activation-skill correlations on a sample with a broader range of cognitive and reading abilities. Individuals with reading disability may have lower BOLD activation in the OT region, which would continue the trend we have found among skilled readers. It is also possible that reading-disabled individuals will display qualitative differences in activation across the reading network (Shaywitz et al., 2007).
Acknowledgments
This work was supported by Grant # HD 29891 from the National Institute of Child Health and Human Development to F. Manis. We thank Jiancheng Zhuang and Xiangrui Li for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. Differential sensitivity to words and shapes in ventral occipito-temporal cortex. Cerebral Cortex. 2007;17:1604–1611. doi: 10.1093/cercor/bhl071. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. NeuroImage. 2006;33:739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Carroll JB, Davies P, Richman B. The American Heritage word frequency book. American Heritage; New York: 1971. [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. NeuroImage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Connine CM, Mullennix J, Shernoff E, Yelen J. Word familiarity and frequency in visual and auditory word recognition. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1990;16:1084–1096. doi: 10.1037//0278-7393.16.6.1084. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H. Cortical systems for the retrieval of concrete knowledge: the convergence zone framework. In: Koch C, editor. Large Scale Neuronal Theories of the Brain. MIT Press; Cambridge, MA: 1994. pp. 61–74. [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L. Letter binding and invariant recognition of masked words: behavioral and neuroimaging evidence. Psychological Science. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec’H G, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. NeuroReport. 2002;13:321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Riviere D. Cerebral mechanisms of word masking and unconscious repetition priming. Nature Neuroscience. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Contribution à l’étude anatomo-pathologique et clinique des différentes variétés de cécité verbale. Mém. Soc. Biol. 1892;4:61–90. [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. The role of the posterior fusiform gyrus in reading. Journal of Cognitive Neuroscience. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences USA. 1998;95:914–21. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Gruber T, Supp GG. Neuronal mechanisms of repetition priming in occipitotemporal cortex: Spatiotemporal evidence from functional magnetic resonance imaging and electroencephalography. Journal of Neuroscience. 2005;25:3414–3422. doi: 10.1523/JNEUROSCI.4107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster KI, Chambers SM. Lexical access and naming time. Journal of Verbal Learning and Verbal Behavior. 1973;12:627–635. [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clemenceau S, Volle E, Hasboun D, Dupont S, Baulac M, Dehaene S, Adam C, Cohen L. Direct intracranial, fMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50:191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Grainger J. Word frequency and neighborhood frequency effects in lexical decision and naming. Journal of Memory and Language. 1990;29:228–244. [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker P, Herskovits E, Degaonkar M. The roles of the “visual word form area” in reading. NeuroImage. 2005;24:548–559. doi: 10.1016/j.neuroimage.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, Siok WT, Reiss AL, Whitfield-Gabrieli S, Gabrieli JD. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences USA. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Taxi vs. Taksi: On orthographic word recognition in the left ventral occipitotemporal cortex. Journal of Cognitive Neuroscience. 2007;19:1–11. doi: 10.1162/jocn.2007.19.10.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. NeuroImage. 2004;21:946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41:293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. Journal of Cognitive Neuroscience. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Medler DA, Binder JR. MCWord: An On-Line Orthographic Database of the English Language. 2005 http://www.neuro.mcw.edu/mcword/
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Démonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa SF, Cossu G, Habib M, Frith CD, Frith U. Dyslexia: cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JD. Characterizing the neural mechanisms of skill learning and repetition priming: evidence from mirror reading. Brain. 2001;124:67–82. doi: 10.1093/brain/124.1.67. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. NeuroImage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, Skudlarski P, Marchione KE, Jenner AR, Fletcher JM, Liberman AM, Shankweiler DP, Katz L, Lacadie C, Gore JC. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychological Science. 2000;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Shankweiler DP, Katz L, Fletcher JM, Skudlarski P, Fulbright RK, Constable RT, Bronen RA, Lacadie C, Gore JC. Predicting reading performance from neuroimaging profiles: the cerebral basis of phonological effects in printed word identification. Journal of Experimental Psychology: Human Perception & Performance. 1997;23:299–318. doi: 10.1037//0096-1523.23.2.299. [DOI] [PubMed] [Google Scholar]
- Rossion B, Schiltz C, Crommelinck M. The functionally defined right occipital and fusiform “face areas” discriminate novel from visually familiar faces. Neuroimage. 2003;19:877–883. doi: 10.1016/s1053-8119(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Pugh KR. The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Scientific Studies of Reading. 2004;8:273–292. [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Rueckl JG, Katz L, Moore DL, Mason SA, Fulbright RK, Constable RT, Pugh KR. The neurobiology of adaptive learning in reading: A contrast of different training conditions. Cognitive Affective Behavioral Neuroscience. 2004;4:67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: a defense of functional localizers. NeuroImage. 2006;30:1088–1096. doi: 10.1016/j.neuroimage.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Holahan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biological Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Skudlarski P, Holahan JM, Marchione KE, Constable RT, Fulbright RK, Zelterman D, Lacadie C, Shaywitz SE. Age-related changes in reading systems of dyslexic children. Annals of Neurology. 2007;61:363–370. doi: 10.1002/ana.21093. [DOI] [PubMed] [Google Scholar]
- Starrfelt R, Gerlach C. The visual what for area: words and pictures in the left fusiform gyrus. NeuroImage. 2007;35:334–342. doi: 10.1016/j.neuroimage.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A co-planar stereotaxic atlas of a human brain. Thieme; New York: 1988. [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency Austin. Pro-Ed; TX: 1999. [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III. Riverside Publishing; Itasca, IL: 2001. [Google Scholar]


