Abstract
Background
This study tested the ability of a characterized extract of Polygonum cuspidatum (PCE) to inhibit mouse ear inflammation in response to topical application of 12-O-tetradecanoylphorbol-13-acetate (TPA).
Methods
A 50% (wt:vol) ethanolic solution of commercial 200:1 PCE was applied to both ears of female Swiss mice (n = 8) at 0.075, 0.15, 0.3, 1.25 and 2.5 mg/ear 30 min after TPA administration (2 μg/ear). For comparison, 3 other groups were treated with TPA and either 1) the vehicle (50% ethanol) alone, 2) indomethacin (0.5 mg/ear), or 3) trans-resveratrol (0.62 mg/ear). Ear thickness was measured before TPA and at 4 and 24 h post-TPA administration to assess ear edema. Ear punch biopsies were collected at 24 h and weighed as a second index of edema. Myeloperoxidase activity was measured in each ear punch biopsy to assess neutrophil infiltration.
Results
PCE treatment at all doses significantly reduced ear edema compared to the TPA control. The PCE response was dose-dependent and 2.5 mg PCE significantly inhibited all markers of inflammation to a greater extent than indomethacin (0.5 mg). MPO activity was inhibited at PCE doses ≥ 1.25 mg/ear. Trans-resveratrol inhibited inflammation at comparable doses.
Conclusion
PCE inhibits development of edema and neutrophil infiltration in the TPA-treated mouse ear model of topical inflammation.
Background
Polygonum cuspidatum Sieb et Zucc., commonly called Japanese knotweed or Mexican bamboo, is a member of the Polygonaceae family that is widely distributed in Asia and North America. Interest in Polygonum cuspidatum (PC) has increased owing to the high concentration of resveratrol and its glycosides in the root [1,2]. In traditional Chinese medicine, PC is called Hu Zhang and is used as an analgesic, antipyretic, diuretic, and an expectorant. Traditional uses include treatments for arthralgia, chronic bronchitis, jaundice, amenorrhea, and high blood pressure [3]. Several studies have evaluated the antioxidant capacity of Polygonum cuspidatum extract (PCE) [4,5], and anti-inflammatory activities such as inhibition of NF-kB have been reported [6-8]. At present, studies of PCE effects on classic symptoms of inflammation such as edema and neutrophil infiltration are lacking.
PCE is used as an ingredient in many nutraceutical product formulations because of its high concentration of trans-resveratrol, a polyphenolic trans-stilbene (3, 4'-5-trihydroxystilbene). Resveratrol and related phytochemicals produce antioxidant, cardioprotective, immunomodulatory, chemopreventive, anti-bacterial, anti-fungal, and anti-viral effects [9-14]. The concentration of resveratrol in sources such as grapes and red wine varies depending on environmental conditions [15]. Therefore, PCE is being used commercially as an additive to standardize resveratrol concentration in extracts of grape pomace (skins and seeds) that have low or variable natural concentrations [16,17]. Also, additive and synergistic effects have been noted for combinations of resveratrol and flavonoids such as quercetin and ellagic acid [18].
PCE has not been tested in the tetradecanoylphorbol acetate (TPA)-treated mouse ear model of inflammation. This model evaluates whether pharmaceutical agents or natural products may block the inflammatory response to topical TPA [19-21]. Because PCE is being used as an ingredient in cosmeceutical products that are applied to the skin and in nutraceutical products that are ingested, it is worthwhile to test PCE activity in this model. The skin and gastrointestinal mucosa are both subject to inflammation, but it is far easier to screen for anti-inflammatory effects on an accessible surface than on an internal epithelium. Therefore, the present study tested whether PCE has topical anti-inflammatory activities in the well-characterized TPA-induced mouse ear model of inflammation, edema, and PMN leukocyte infiltration [22,23]. Total phenolics and ferric reducing antioxidant power (FRAP values) were measured in the ethanolic PCE because both characteristics may reflect the degree of anti-inflammatory activity of the preparation. For example, Chung et. al. reported that edema formation in the TPA model may be regulated by H2O2 generation [24], as evidenced by anti-inflammatory activity of several antioxidant compounds [25,26].
Materials and methods
Materials
12-O-Tetradecanoylphorbol 13-acetate, hexadecyltrimethylammonium bromide, indomethacin (minimum 99% TLC), 3,3',5,5'-tetramethylbenzidine dihydrochloride, N, N-dimethylformamide, trans-3,4',5-trihydroxystilbene (trans-resveratrol), Folin-Ciocalteu reagent, gallic acid, and 10 mM 2,4,6-tripyridyl]-1,3,5-triazine (TPTZ) were all purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Polygonum cuspidatum 200:1 powdered extract was purchased from Supplemental Health Formulations (Mayer, AZ).
Preparation of ethanolic solution of Polygonum cuspidatum extract (PCE)
PCE used in this study was a 200-fold concentrate prepared from PC root grown in China. Chemical analysis from Supplemental Health Formulations reported that the trans-resveratrol complex was at least 500 mg/g and emodin content was < 20 mg/g. The 200:1 PC powder was dissolved in 50% ethanol (1 part PC to 9 parts ethanol) and stirred for 1 h at 23°C. The mixture was centrifuged (1500 rpm for 10 min, 4°C) and the supernatant was diluted for topical dose-response applications in this study. The majority of the powder was not soluble in 50% ethanol under these conditions.
Chromatographic analysis of the Polygonum cuspidatum ethanolic solution
The ethanolic extract was diluted 400-fold and subjected to HPLC analysis using an ESA (Chelmsford, MA) system consisting of a Model 582 Solvent Delivery Module, a Model 542 autosampler maintained at 6°C and a Model 5600A CoulArray detector at 250 mV. The column was an MCM C18 (4.6 × 150 mm, 5–120 A) from MC Medical, Japan. Mobile phase A was 75 mM citric acid, 25 mM ammonium acetate and 10% acetonitrile; Mobile phase B was similar to A but with 50% acetonitrile. The gradient was linear from 0–17 minutes from 10%A to 80%B. Flow rate was 1.0 ml/min and 20 μl of sample was injected. Resveratrol eluted between 16.2 and 16.8 minutes as judged by a standard obtained from Sigma-Aldrich (St. Louis, MO).
Measurement of total phenolic compounds
Total phenolic acid content of each extract was measured by the method of Slinkard and Singleton [27] with minor modifications. Triplicate samples of a 1:10 extract (wt/vol) (20 μL) were added to 1.58 mL of distilled water in 3 mL polystyrene cuvettes. 100 μL of Folin-Ciocalteu reagent was added and the sample was mixed well. Within 10 minutes, 300 μL of sodium carbonate solution (200 g Na2CO3 in 1 L distilled water) was added. Solutions were incubated for 2 h at room temperature. Absorbance was measured at 765 nm. Total phenolic acid concentration was calculated from a gallic acid standard curve (0–500 mg/L) and expressed as gallic acid equivalents per gram 200:1 PCE powder.
Measurement of FRAP values (Ferric Reducing Antioxidant Power)
The antioxidant activity of a 1:10 (wt/vol) extraction was determined in triplicate by the FRAP method [28]. 10 μL of the sample or standard, 30 μL of distilled water and 300 μL of FRAP reagent were mixed. FRAP reagent was made by mixing 25 mL acetate buffer (300 mM, pH 3.6), 2.5 mL of 10 mM TPTZ solution dissolved in 40 mM HCl, and 2.5 mL of 20 mM ferric chloride solution. The solutions were incubated at 37°C for six minutes then 340 μL of distilled water was added. The absorbance of the sample or standards was read immediately at 593 nm. FRAP value was calculated from a standard curve of ferrous sulfate (0–1 mmol/L) and the antioxidant power of the PCE was expressed as mmol ferrous sulfate equivalents/100 g dry weight of the 200:1 PCE powder.
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Georgia and conducted according to IACUC guidelines. The sample size of 8 animals for each test group was justified on the basis of a pilot experiment showing that the sample standard deviation (s) for measurements of ear edema was about 5% of the measured value and the average expected difference (d) between TPA treated ears and PCE-treated ears was about 0.2 mm. Assuming that α = 0.05 and 1 - β = 0.9, the formula used was n (sample size) = 1 + 21*(s/d)2 [29]. The formula gave 6.25, which was increased to 8 in case of unexpected experimental problems. Female Swiss Webster mice (Harlan Laboratories, Indianapolis, IN) weighing 22–25 g were housed in groups of 4 in large shoebox cages. All groups were fed a standard rodent diet (TestDiet® 570B, Purina Mills, St. Louis, MO) ad libitum with free access to water. Animals were in the fed condition throughout the experiment. Photoperiods equaled 12 h of light and 12 h of darkness daily, with the environmental temperature maintained at 21°C.
TPA-induced mouse ear edema
Edema was induced in both ears of each mouse by the topical application of 2 μg TPA dissolved in 20 μL of acetone to both the inner and outer ear surfaces. Thirty minutes after the application of TPA, the inner and outer surface of each ear was treated (10 μL to each side) with 50% ethanolic solutions of PCE in doses of 0.075, 0.15, 0.3, 1.25 and 2.5 mg PCE/ear (n = 8 at each dosage). Comparisons included equal volumes of 50% ethanol (vehicle control), indomethacin (0.5 mg/ear dissolved in 50% ethanol as an anti-inflammatory drug standard), or a 50% ethanol solution of trans-3, 5, 4'-trihydroxystilbene (resveratrol, 0.6 mg/ear). The thickness of each ear was measured using a micrometer (Mitutoyo Series IP65, Mitutoyo America, Aurora, IL) before and at 4 h and 24 h after TPA administration. The micrometer was applied near the top of the ear distal to the cartilaginous ridges. At 24 h each animal was sacrificed with CO2 inhalation by the IACUC approved protocol. Ear punch biopsies (6 mm diameter hole punch) were taken immediately, weighed, frozen and stored at -80°C. A single investigator performed all ear measurements and biopsies in order to standardize the procedure and reduce experimental error.
Myeloperoxidase assay
Tissue MPO (MPO, E.C. 1.11.1.7) activity was measured in biopsies taken from both ears 24 h after TPA administration using a method by Suzuki et. al. [30] and modified by De Young et. al. [31]. Each mouse ear biopsy was placed in 0.75 mL of 80 mM phosphate-buffered saline (PBS) pH 5.4 containing 0.5% hexadecyltrimethyl-ammonium bromide (HTAB). Each sample was homogenized for 45 s at 4°C with a small sample laboratory Tissue Tearor Homogenizer Model 985-370 (Biospec Products, Bartlesville, OK). The homogenate was transferred quantitatively to a microcentrifuge tube with an additional 0.75 mL HTAB in PBS. The 1.5 mL sample was centrifuged at 12,000 × g for 15 min, maintained at 4°C. Triplicate 30 μL samples of the resulting supernatant were added to 96-well microtiter plate wells. For the MPO assay, 200 μL of a mixture containing 100 μL of 80 mM PBS (pH 5.4), 85 μL of 0.22 M PBS (pH 5.4), and 15 μL of 0.017% hydrogen peroxide were added to each well. 20 μL of 18.4 mM tetramethylbenzidine HCl in 8% aqueous dimethylformamide was added to start the reaction. Microtiter plates were incubated at 37°C for 3 min, and then placed on ice. The reaction was stopped with the addition of 30 μL of 1.46 M sodium acetate, pH 3.0. MPO enzyme activity was assessed colorimetrically using a BioTek Microplate Reader (Winooski, VT) at an absorbance wavelength of 630 nm. MPO activity was expressed as optical density (OD)/biopsy.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). Statistical evaluations used t-tests and one-way analysis of variance (ANOVA) with post-hoc tests for significance of differences by the Student-Newman-Keuls Method. Statistical significance was considered at p < 0.05.
Results
Total phenolics and FRAP values in PCE
A 50% ethanolic extract (1:10 wt/vol) of the 200:1 PCE yielded 188 mg of total phenolics (gallic acid equivalents) per gram of PCE. Antioxidant power based on the FRAP assay was 85 mmol ferrous sulfate equivalents/100 g dry weight of PCE. Most of the solids in the commercial extract were not soluble in 50% ethanol. The dry weight of the ethanol-insoluble pellet remaining after centrifugation of the extract from 1.0 g of powder equaled 0.76 g, indicating that the majority was not soluble in 50% ethanol. Chromatography of the ethanol-soluble material as described in the Methods section showed only one major peak, which co-eluted with authentic trans-resveratrol (Figure 1).
Figure 1.
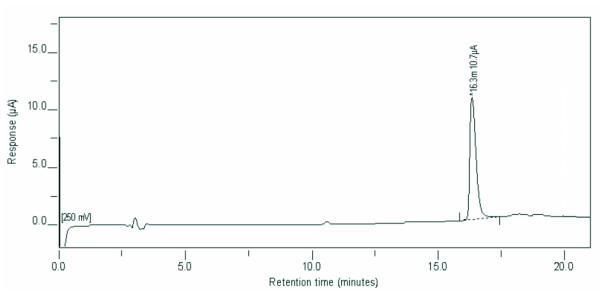
Chromatogram of ethanol-soluble PCE fraction. A single major peak was observed in the chromatogram of the 50% ethanol soluble fraction of PCE that eluted at 16.3 min with the same retention time as authentic trans-resveratrol (not shown).
Ear edema
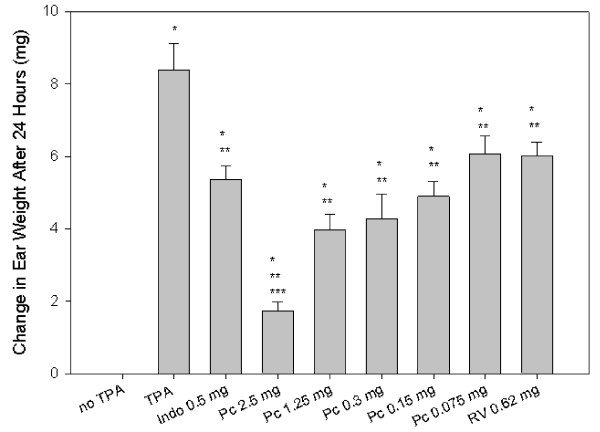
Ear edema was observed in all TPA-treated animals by 4 h and 24 h after treatment. In animals treated only with vehicle (50% ethanol), initial ear thickness equaled 0.27 ± 0.01 mm (mean ± SEM). Ear thickness increased to 0.42 ± 0.01 mm at 4 h and 0.46 ± 0.02 mm by 24 h after TPA treatment. PCE-treated experimental groups showed significantly reduced ear edema compared to TPA treatment alone. Dosages tested included 0.075, 0.15, 0.3, 1.25 and 2.5 mg PCE/ear (n = 8 at each dosage). PCE at 2.5, 1.25, and 0.3 mg per ear was as effective as indomethacin (0.5 mg/ear) in reducing edema (Figure 2). These treatments inhibited edema 61%, 55%, 52%, and 65% (Indo), respectively compared to TPA treated with vehicle controls. In comparison, 0.62 mg of commercially purified trans-resveratrol inhibited edema by only 35%. At 24 h, all experimental groups had significantly reduced ear edema compared to TPA alone except PCE at 0.075 mg per ear and the trans-resveratrol-treated groups. PCE at 1.25, 0.3, and 0.15 mg per ear inhibited edema as well as indomethacin (58%, 36%, 40%, respectively, vs 45% for indomethacin. PCE applied at 2.5 mg per ear was significantly more effective than indomethacin in reducing edema with a 73% reduction compared to the TPA treated vehicle control.
Figure 2.
Change in ear thickness 4 and 24 h after TPA application. Ear thickness was measured with a digital micrometer 4 and 24 h after application of 2 μg TPA. Abbreviations include Indo (indomethacin), PCE (Polygonum cuspidatum extract), and RV (resveratrol). Results represent means ± SEM. *p ≤ 0.05 compared to no TPA, **p ≤ 0.05 compared to TPA control, ***p ≤ 0.05 compared to indomethacin (Indo).
Edema was also indicated by changes in ear punch masses at 24 h, and the treatment effects were similar to the changes in ear thickness shown in Figure 2. Typical masses of ear punch biopsies at 24 h were 9.1 ± 0.3 mg in vehicle-treated controls compared to 17.5 ± 0.7 mg in TPA-treated animals. Ear punch biopsy weights were significantly lower in all PCE groups compared to the TPA-treated control group (data not shown). For example, 2.5 mg of PCE reduced the change in ear mass to 1.3 ± 0.25 mg (an 80% reduction), which was significantly greater than the reduction by 0.5 mg of indomethacin to 5.36 ± 0.39 mg (36% reduction). Resveratrol (0.62 mg) produced an effect similar to indomethacin, and reduced the change in ear thickness to 6.02 ± 0.38 mg.
Myeloperoxidase activity
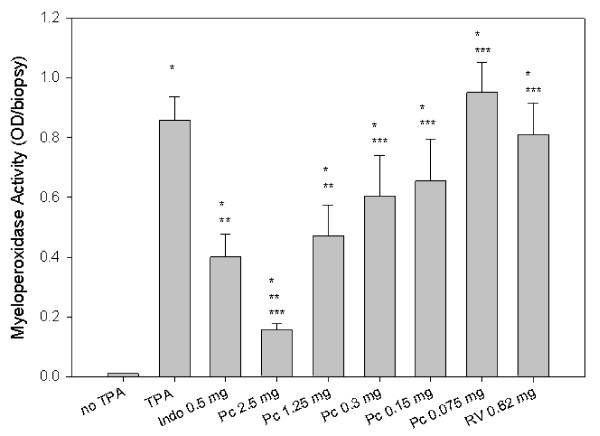
Myeloperoxidase activity was measured in the ear punch biopsies taken 24 h after TPA administration as an index of neutrophil infiltration (Figure 3). Biopsies from ears treated with indomethacin at 0.5 mg/ear and PCE at 1.25 and 2.5 mg/ear doses had significantly reduced MPO activity. The higher PCE dose (2.5 mg/ear) decreased MPO to 18% of the activity of the TPA-treated vehicle control group and was significantly more effective at decreasing MPO activity than indomethacin. Indomethacin (0.5 mg/ear) and PCE (1.25 mg/ear) inhibited MPO to the same extent at 53% and 45%, respectively.
Figure 3.
Myeloperoxidase activity. Myeloperoxidase activity (an index of neutrophil activation) was measured in ear punches 24 h after TPA administration. Abbreviations include Indo (indomethacin), PCE (Polygonum cuspidatum extract), and RV (resveratrol). Results represent means ± SEM. *p ≤ 0.05 compared to no TPA, **p ≤ 0.05 compared to TPA control, ***p ≤ 0.05 compared to indomethacin.
Discussion
An early hallmark of skin irritation and local inflammation in the TPA model is thickening within 1–4 h due to increased vascular permeability, edema and swelling within the dermis [32]. Topical application of PCE significantly inhibited ear edema at 4 h and 24 h after TPA treatment. Secondarily, PMN leukocytes migrate to the dermis within about 24 h and may be estimated by the MPO assay. Both of these inflammatory processes were blocked by topical application of PCE in a dose-dependent manner. PCE at a dose of 2.5 mg/ear reduced edema and inhibited leukocyte infiltration to a greater extent than indomethacin (0.5 mg/ear). Indomethacin is a potent non-steroidal, anti-inflammatory drug. It has an LD50 of 50 mg/kg in mice based on a 14 day mortality response [33]. This LD50translates to 1.25 mg indomethacin per 25 g mouse, just above the dose administered topically (1 mg/mouse). In contrast, no significant toxicity has been shown for PCE in this bioequivalence range. These data show that an ethanolic solution of PCE reduces inflammation to a similar extent as indomethacin or trans-resveratrol.
PCE is widely used in nutraceutical products because of consistently high concentration of resveratrol and its glucosides. Resveratrol derivatives in extracts of PC root include several glycosides [1,34,35]. In addition, PC contains emodin and a glycoside. However, Figure 1 shows that the ethanol-soluble fraction of the commercial concentrate used here was less complex than crude extracts of PC root [2,34,35]. The chromatogram agrees with the certificate of analysis of that PCE powder, 200:1, contains at least 50% trans-resveratrol and less than 2% emodin. In our tests, PCE was similar in activity to trans-resveratrol on a mass basis (Figures 2 and 3).
Our data are consistent with findings that trans-resveratrol and its derivatives have anti-inflammatory activity. For example, resveratrol and its glycosides inhibit human TNF-α and LPS-induced activation of NF-κB [36,37]. Resveratrol inhibits induced production of prostaglandin E2 release from human peripheral blood leukocytes [38]. In a model of early colonic inflammation in rats, resveratrol significantly decreases elevated plasma levels of prostaglandin D2 and the expression of COX-2 [39]. Resveratrol also inhibits the TPA-induced mouse dorsal skin inflammatory response by reducing NF-κB and activator protein-1 [40,41].
The TPA model of ear inflammation is useful for screening prospective topical anti-inflammatory compounds or botanical extracts that act at a variety of levels. In epidermal cell culture, TPA stimulates cell proliferation and increases the formation of leukotrienes and prostaglandins [42]. Phospholipase A2 inhibitors have proven effective against both leukocyte infiltration and edema in the TPA model of ear inflammation [43]. Products of arachidonic acid metabolism such as PGI2 and LTB4 increase vascular permeability leading to edema during the inflammatory response [23], and compounds inhibiting COX and LOX enzymes have been shown to inhibit TPA-induced inflammation [23]. TPA applied topically to mouse ears promotes mast cell infiltration with release of mediators that increase vascular permeability and promote neutrophil influx [22].
In addition to 50% resveratrol, PCE extract contains compounds such as quercetin and emodin that have anti-inflammatory activities. It is known that additive and syngergistic interactions of polyphenols occur in vitro [44,45]. For example, in human leukemia cells, ellagic acid and quercetin interact synergistically with resveratrol to induce apotosis and cell cycle arrest [18]. Emodin, an anthraquinone, is present in PC rhizomes at concentrations similar to resveratrol and piceid [2]. However, the emodin content in PCE is reduced during processing to achieve a final content of ≤ 20 mg/g. This is important because PCE is a constituent in products that are ingested, and it is desirable to reduce the risk of unpleasant gastrointestinal side effects in humans [46]. Emodin is a phytoestrogen with anti-viral and anti-inflammatory actions [47]. It inhibits NF-κB activation and IκB degradation, and decreases gene expression of cell surface adhesion proteins in vascular endothelial cells [6]. Emodin also effectively inhibits gene expression for TNF-α, iNOS, and IL-10 in RAW 264.7 macrophages by activating IκB [48]. Thus, even though emodin levels in PCE have been reduced from levels in crude extracts, it may contribute to the topical anti-inflammatory activity of PCE. The present work shows that PCE and trans-resveratrol are anti-inflammatory in the mouse ear model, and that PCE could provide anti-inflammatory properties to cosmeceutical and dermatological products.
Abbreviations used
TPA: 12-O-tetradecanoylphorbol-13-acetate, PMN: Polymorphonuclear, MPO: Myeloperoxidase, TNF-α: Tumor Necrosis Factor – alpha, IL-6, -1β, -8: Interleukin-6, -1β, -8, COX: Cyclooxygenase, LOX: Lipoxygenase, Indo: Indomethacin, PC: Polygonum cuspidatum, PCE: Polygonum cuspidatum extract in 50% ethanol
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
The study was conceived by DKH and LW. EB conducted the study as part of her doctoral research under the direction of PG, DKH and JLH. EB and JLH prepared the figures and manuscript, which was reviewed and approved by each of the coauthors.
Acknowledgments
Acknowledgements
The authors thank Ms. Linda Duncan for her technical assistance and help with animal care and Emily Kelso for obtaining the chromatogram. We thank Dr. Ron Pegg, Dept. of Food Science and Technology, University of Georgia, for analyzing the constituents of the extract used in this study.
Contributor Information
Eve E Bralley, Email: bralleye@rx.uga.edu.
Phillip Greenspan, Email: greenspn@rx.uga.edu.
James L Hargrove, Email: jhargrov@fcs.uga.edu.
Louise Wicker, Email: lwicker@uga.edu.
Diane K Hartle, Email: dhartle@rx.uga.edu.
References
- Vastano BC, Chen Y, Zhu N, Ho CT, Zhou Z, Rosen RT. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J Agric Food Chem. 2000;48:253–256. doi: 10.1021/jf9909196. [DOI] [PubMed] [Google Scholar]
- Chu X, Sun A, Liu R. Preparative isolation and purification of five compounds from the Chinese medicinal herb Polygonum cuspidatum Sieb. et Zucc by high-speed counter-current chromatography. Journal of chromatography. 2005;1097:33–39. doi: 10.1016/j.chroma.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Editor Committee of Jiangsu New Medical College . Encyclopedia of Traditional Chinese Medicine. Shanghai , Shanghai Science and Technology Press; 2001. p. 1329. [Google Scholar]
- Hsu CY, Chan YP, Chang J. Antioxidant activity of extract from Polygonum cuspidatum. Biological research. 2007;40:13–21. doi: 10.4067/s0716-97602007000100002. [DOI] [PubMed] [Google Scholar]
- Masaki H, Sakaki S, Atsumi T, Sakurai H. Active-oxygen scavenging activity of plant extracts. Biological & pharmaceutical bulletin. 1995;18:162–166. doi: 10.1248/bpb.18.162. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dhawan S, Aggarwal BB. Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene. 1998;17:913–918. doi: 10.1038/sj.onc.1201998. [DOI] [PubMed] [Google Scholar]
- Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- Zhao KS, Jin C, Huang X, Liu J, Yan WS, Huang Q, Kan W. The mechanism of Polydatin in shock treatment. Clinical hemorheology and microcirculation. 2003;29:211–217. [PubMed] [Google Scholar]
- Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, Russell RE, Barnes PJ. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–83. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- Pinto MC, Garcia-Barrado JA, Macias P. Resveratrol is a potent inhibitor of the dioxygenase activity of lipoxygenase. Journal of agricultural and food chemistry. 1999;47:4842–4846. doi: 10.1021/jf990448n. [DOI] [PubMed] [Google Scholar]
- Carluccio MA, Siculella L, Ancora MA, Massaro M, Scoditti E, Storelli C, Visioli F, Distante A, De Caterina R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler Thromb Vasc Biol. 2003;23:622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- Ferrero ME, Bertelli AA, Pellegatta F, Fulgenzi A, Corsi MM, Bertelli A. Phytoalexin resveratrol (3-4'-5-trihydroxystilbene) modulates granulocyte and monocyte endothelial adhesion. Transplant Proc. 1998;30:4191–4193. doi: 10.1016/S0041-1345(98)01388-8. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer. 2001;39:102–107. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- Roldan A, Palacios V, Caro I, Perez L. Resveratrol content of Palomino fino grapes: influence of vintage and fungal infection. J Agric Food Chem. 2003;51:1464–1468. doi: 10.1021/jf020774u. [DOI] [PubMed] [Google Scholar]
- Pastrana-Bonilla E, Akoh CC, Sellappan S, Krewer G. Phenolic content and antioxidant capacity of muscadine grapes. J Agric Food Chem. 2003;51:5497–5503. doi: 10.1021/jf030113c. [DOI] [PubMed] [Google Scholar]
- Yilmaz Y, Toledo RT. Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J Agric Food Chem. 2004;52:255–260. doi: 10.1021/jf030117h. [DOI] [PubMed] [Google Scholar]
- Mertens-Talcott SU, Percival SS. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Cancer Letters. 2005;218:141–151. doi: 10.1016/j.canlet.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Conney AH, Lysz T, Ferraro T, Abidi TF, Manchand PS, Laskin JD, Huang MT. Inhibitory effect of curcumin and some related dietary compounds on tumor promotion and arachidonic acid metabolism in mouse skin. Advances in enzyme regulation. 1991;31:385–396. doi: 10.1016/0065-2571(91)90025-H. [DOI] [PubMed] [Google Scholar]
- Gabor M. Models of acute inflammation in the ear. Methods in molecular biology (Clifton, NJ. 2003;225:129–137. doi: 10.1385/1-59259-374-7:129. [DOI] [PubMed] [Google Scholar]
- Griffiths RJ, Wood BE, Li S, Blackham A. Pharmacological modification of 12-0-tetradecanoylphorbol-13-acetate induced inflammation and epidermal cell proliferation in mouse skin. Agents Actions. 1988;25:344–351. doi: 10.1007/BF01965041. [DOI] [PubMed] [Google Scholar]
- Rao TS, Currie JL, Shaffer AF, Isakson PC. Comparative evaluation of arachidonic acid (AA)- and tetradecanoylphorbol acetate (TPA)-induced dermal inflammation. Inflammation. 1993;17:723–741. doi: 10.1007/BF00920477. [DOI] [PubMed] [Google Scholar]
- Carlson RP, O'Neill-Davis L, Chang J, Lewis AJ. Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions. 1985;17:197–204. doi: 10.1007/BF01966592. [DOI] [PubMed] [Google Scholar]
- Chung WY, Jung YJ, Surh YJ, Lee SS, Park KK. Antioxidative and antitumor promoting effects of [6]-paradol and its homologs. Mutat Res. 2001;496:199–206. doi: 10.1016/s1383-5718(01)00221-2. [DOI] [PubMed] [Google Scholar]
- Hara H, Sukamoto T, Ohtaka H, Abe K, Tatumi Y, Saito Y, Suzuki A, Tsukamoto G. Effects of baicalein and alpha-tocopherol on lipid peroxidation, free radical scavenging activity and 12-O-tetradecanoylphorbol acetate-induced ear edema. Eur J Pharmacol. 1992;221:193–198. doi: 10.1016/0014-2999(92)90700-E. [DOI] [PubMed] [Google Scholar]
- Cui XY, Kim JH, Zhao X, Chen BQ, Lee BC, Pyo HB, Yun YP, Zhang YH. Antioxidative and acute anti-inflammatory effects of Campsis grandiflora flower. J Ethnopharmacol. 2005. [DOI] [PubMed]
- Slinkard K, Singleton VL. Total phenolic analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- Benzie I, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. . Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Dell RB, Holleran S, Ramakrishnan R. Sample size determination. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2002;43:207–213. doi: 10.1093/ilar.43.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983;132:345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Young JM, Spires DA, Bedord CJ, Wagner B, Ballaron SJ, De Young LM. The mouse ear inflammatory response to topical arachidonic acid. J Invest Dermatol. 1984;82:367–371. doi: 10.1111/1523-1747.ep12260709. [DOI] [PubMed] [Google Scholar]
- De Vry CG, Valdez M, Lazarov M, Muhr E, Buelow R, Fong T, Iyer S. Topical application of a novel immunomodulatory peptide, RDP58, reduces skin inflammation in the phorbol ester-induced dermatitis model. J Invest Dermatol. 2005;125:473–481. doi: 10.1111/j.0022-202X.2005.23831.x. [DOI] [PubMed] [Google Scholar]
- Barnhart ER. Monography. Indomethacin. 43rd Ed. Physician's Desk Reference . In: Barnhart ER, editor. 43rd Ed Physician's Desk Reference. New Jersey , Medical Economics Co.; 1989. pp. 1345–1350. [Google Scholar]
- Matsuda H, Shimoda H, Morikawa T, Yoshikawa M. Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): structure-requirement of hydroxyanthraquinones for estrogenic activity. Bioorg Med Chem Lett. 2001;11:1839–1842. doi: 10.1016/S0960-894X(01)00318-3. [DOI] [PubMed] [Google Scholar]
- Chu Q, Peng Y, Ye J. Determination of Active Ingredients of Polygonum cuspidatum Sied. et Zucc. by Capillary Electrophoresis with Electrochemical Detection. Electroanalysis. 2004;16:1434–1438. doi: 10.1002/elan.200302968. [DOI] [Google Scholar]
- Heynekamp JJ, Weber WM, Hunsaker LA, Gonzales AM, Orlando RA, Deck LM, Jagt DL. Substituted trans-stilbenes, including analogues of the natural product resveratrol, inhibit the human tumor necrosis factor alpha-induced activation of transcription factor nuclear factor KappaB. J Med Chem. 2006;49:7182–7189. doi: 10.1021/jm060630x. [DOI] [PubMed] [Google Scholar]
- Ashikawa K, Majumdar S, Banerjee S, Bharti AC, Shishodia S, Aggarwal BB. Piceatannol inhibits TNF-induced NF-kappaB activation and NF-kappaB-mediated gene expression through suppression of IkappaBalpha kinase and p65 phosphorylation. J Immunol. 2002;169:6490–6497. doi: 10.4049/jimmunol.169.11.6490. [DOI] [PubMed] [Google Scholar]
- Richard N, Porath D, Radspieler A, Schwager J. Effects of resveratrol, piceatannol, tri-acetoxystilbene, and genistein on the inflammatory response of human peripheral blood leukocytes. Mol Nutr Food Res. 2005;49:431–442. doi: 10.1002/mnfr.200400099. [DOI] [PubMed] [Google Scholar]
- Martin AR, Villegas I, La Casa C, de la Lastra CA. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol. 2004;67:1399–1410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27:1465–1474. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- Kundu JK, Shin YK, Surh YJ. Resveratrol modulates phorbol ester-induced pro-inflammatory signal transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as prime targets. Biochem Pharmacol. 2006;72:1506–1515. doi: 10.1016/j.bcp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Fischer SM, Baldwin JK, Jasheway DW, Patrick KE, Cameron GS. Phorbol ester induction of 8-lipoxygenase in inbred SENCAR (SSIN) but not C57BL/6J mice correlated with hyperplasia, edema, and oxidant generation but not ornithine decarboxylase induction. Cancer Res. 1988;48:658–664. [PubMed] [Google Scholar]
- Tramposch KM, Steiner SA, Stanley PL, Nettleton DO, Franson RC, Lewin AH, Carroll FI. Novel inhibitor of phospholipase A2 with topical anti-inflammatory activity. Biochem Biophys Res Commun. 1992;189:272–279. doi: 10.1016/0006-291X(92)91554-4. [DOI] [PubMed] [Google Scholar]
- Pignatelli P, Di Santo S, Buchetti B, Sanguigni V, Brunelli A, Violi F. Polyphenols enhance platelet nitric oxide by inhibiting protein kinase C-dependent NADPH oxidase activation. Effect on platelet recruitment. Atherosclerosis Supplements. 2006;7:439–439. doi: 10.1016/S1567-5688(06)81773-3. [DOI] [PubMed] [Google Scholar]
- Mertens-Talcott SU, Talcott ST, Percival SS. Low concentrations of quercetin and ellagic acid synergistically influence proliferation, cytotoxicity and apoptosis in MOLT-4 human leukemia cells. Journal of Nutrition. 2003;133:2669–2674. doi: 10.1093/jn/133.8.2669. [DOI] [PubMed] [Google Scholar]
- Srinivas G, Babykutty S, Sathiadevan PP, Srinivas P. Molecular mechanism of emodin action: Transition from laxative ingredient to an antitumor agent. Med Res Rev. 2006. [DOI] [PubMed]
- Zhang C, Zhang X, Zhang Y, Xu Q, Xiao H, Liang X. Analysis of estrogenic compounds in Polygonum cuspidatum by bioassay and high performance liquid chromatography. J Ethnopharmacol. 2006;105:223–228. doi: 10.1016/j.jep.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Li HL, Chen HL, Li H, Zhang KL, Chen XY, Wang XW, Kong QY, Liu J. Regulatory effects of emodin on NF-kappaB activation and inflammatory cytokine expression in RAW 264.7 macrophages. Int J Mol Med. 2005;16:41–47. doi: 10.1007/s00894-004-0218-5. [DOI] [PubMed] [Google Scholar]