Abstract
Indoleamine 2,3-dioxygenase (IDO; EC 1.13.11.42) is a rate-limiting enzyme involved in the catabolism of tryptophan, which is an essential amino acid. It is induced under pathological conditions, such as the presence of viral infections or tumour cells. This enzyme is induced by IFN-γ in the mouse rectal carcinoma cell line CMT-93. It is known that both 1-methyl-l-tryptophan (1-MT) and methylthiohydantoin-dl-tryptophan (MTH-trp) are tryptophan analogues, and are authentic inhibitors of the enzymatic activity of IDO. In this study, we examined the effects of both 1-MT and MTH-trp on the IFN-γ inducible IDO expression of CMT-93. As a result, the IFN-γ inducible IDO mRNA and the protein levels in CMT-93 were suppressed by 1-MT and MTH-trp, independently. Moreover, tryptophan (Trp), as a substrate of IDO, also suppressed IDO induction by IFN-γ at the transcriptional level. These results suggest that 1-MT and MTH-trp are as inhibitors of IDO enzymatic activity, and Trp suppresses IDO induction by IFN-γ at the transcriptional level.
Keywords: CMT-93, IFN-γ, 1-Methyl-l-tryptophan, Methylthiohydantoin-dl-tryptophan
Introduction
Indoleamine 2,3-dioxygenase (IDO; EC 1.13.11.42) is a monomeric haemoprotein with a molecular mass of about 45 kDa. It is the rate-limiting, first enzyme in the kynurenine pathway and catabolizes l-tryptophan (l-Trp) to N-formylkynurenine (Shimizu et al. 1978; Takikawa et al. 1986; Werner et al. 1987; Taylor and Feng 1991). IDO induction can deplete l-Trp in the target cells, which is partially responsible for the anti-microbial, anti-vial and anti-proliferative activities of interferon (IFN)-γ (Burke et al. 1995; Friberg et al. 2002). L-Trp depletion is also involved in the inhibition of T-cell proliferation by IFN-γ-treated human monocytes derived from macrophage and dendritic cells (Munn et al. 1999). Several of the metabolites involved in the l-tryptophan-kynurenine pathway act to induce excitotoxic, neuronal death and may cause apoptosis (Morita et al. 2001). It is widely accepted that interferons, particularly IFN-γ, are essential factors in the IDO induction process, primarily because two interferon-stimulated response elements (ISREs) and IFN-γ-activated site (GAS) element sequences have been found in the 5′-flanking region of the IDO gene, and IFN-γ actually induces IDO in many cells (Kadoya et al. 1992; Hassanain et al. 1993; Chon et al. 1995; Konan and Taylor 1996). This enzyme is specifically induced by IFN-γ in the mouse rectal carcinoma line CMT-93. It is known that both 1-methyl-l-tryptophan (1-MT) and methylthiohydantoin-dl-tryptophan (MTH-trp) are tryptophan analogues, and are authentic inhibitors of the enzymatic activity of IDO (Muller et al. 2005). The inhibitor 1-MT has initially been used to block the immune privilege of the placenta (Munn et al. 1998). The treatment of pregnant mice with 1-MT induced the rejection of the allogeneic foetus by breaking the tolerance of the maternal T lymphocytes for the foetus. The maternal T-cell tolerance appeared to rely on the IDO-expressing cells in the maternal–foetal interface, which deprive the local microenvironment of Trp and inhibit T-cell proliferation. It is believed that 1-MT restores the local concentration of Trp in the placenta, allowing for T-cell activation. The tolerance towards tumour cells can also be broken by 1-MT (Friberg et al. 2002).
In this study, we examined the effects of 1-MT, MTH-trp and Trp on the IFN-γ inducible IDO expression in CMT-93.
Materials and methods
Mouse recombinant IFN-γ (specific activity = 1 × 107 U/mL) was purchased from Serotec Ltd. (Oxford, UK). L-Kynurenine (L-KYN) and p-dimethylaminobenzaldehyde were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The 1-methyl-l-tryptophan (1-MT) was obtained from Aldrich Chemical Co. (Milwaukee, WI, USA). To prepare the 1-MT, a stock solution (20 mM) was dissolved in 0.1 N NaOH, then adjusted to pH 7.4. The methylthiohydantoin-dl-tryptophan (MTH-trp) was obtained from Research Organics (Cleveland, Ohio, USA). To prepare the MTH-trp, a stock solution (100 mM) was dissolved in DMSO. The l-tryptophan (Trp) was obtained from Wako Pure Chemical Industries (Osaka, Japan). To prepare the Trp, a stock solution (20 mM) was dissolved in water. The L-Trp-free RPMI1640 medium was obtained from KOUJIN BIO Industries (Saitama, Japan).
Cells and growth conditions
The CMT-93 cells, which were obtained from a mouse rectal carcinoma, were a gift from Dr. Takikawa, of the National Institute for Longevity Science National Center for Geriatric and Gerontology. The CMT-93 cells were cultured in Dulbecco’s modified Eagle’s MEM medium supplemented with 10% heat-inactivated foetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/ml) at 37 °C in humidified air at 5% CO2.
Measurement of kynurenine level
The kynurenine level in the culture medium was measured with p-dimethylaminobenzaldehyde in acetic acid (Ehrlich reagent) at λmax 480 nm (Takikawa et al. 1988).
Reverse transcriptase PCR (RT-PCR) analysis of IDO mRNA
The total cellular RNA was isolated using the TRIZOL reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Following isolation, moloney murine leukemia virus (M-MLV)-reverse transcriptase (Promega, Madison, WI) was used in cDNA synthesis with the total RNA templates. The resultant cDNA samples were subjected to 30 cycles of PCR amplification in the presence of specific sense and antisense primers. The mouse IDO was amplified using PCR. The GAPDH was amplified as an internal control. The temperatures used were 94 °C for denaturation, 60 °C for annealing, and 72 °C for polymerization. The sequences for the PCR primers and the expected amplification products sizes are as follows for the GAPDH: sense 5′-GCCAAGGTCATCCATGACAACTTTGG-3′, and antisense 5′-GCCTGCTTCACCACCTTCTTGATGTC-3′ (348 bp); for IDO: sense 5′-AGGATCCTTGAAGACCACCA-3′, and antisense 5′-CCAATAGAGAGACGAGGAAG-3′ (398 bp). The amplified PCR products were subjected to electrophoresis in a 1.5% agarose gel and visualized by staining with ethidium bromide (Wako Pure Chemical Industries, Osaka, Japan) and digital images were taken with a Kodak digital science device (Eastman Kodak Company, Rochester, NY).
Northern-blot analysis of IDO
The total RNA (10 μg per lane) was electrophoresed in a formaldehyde gel (1.2% agarose), blotted on to nylon membranes (Hybond N, Amersham) and then probed with radiolabelled IDO cDNA fragments as described previously (Suzuki et al. 2001). In the case of the IDO Northern-blot analysis, the probe was made by RT-PCR (5′ primer, 5′-AGGATCCTTGAAGACCACCA-3′; 3′ primer, 5′-CCAATAGAGAGACGAGGAAG-3′, obtained from GenBank, accession no. NM_008324). The signal of IDO was quantified using an image analyzer (BAS 2000, Fuji Film, Tokyo, Japan).
Polyclonal antibody
The serum containing the anti-mouse IDO polyclonal antibody was obtained from rabbits that were immunized with a mixture of three peptides corresponding to amino acids 1–18, 156–175 and 359–377 of mouse IDO protein (Suzuki et al. 2001).
Western-blot analysis
The sample (20 μg of protein/lane) was subjected to electrophoresis in 12% (w/v) polyacrylamide gel in the presence of 0.1% SDS, then transferred onto a hydrophobic polyvinylidene difluoride membrane according to the method of Towbin et al. (1979). After blocking the nitrocellulose membrane with 100% Block ace for one night, the anti-mouse IDO polyclonal antibody was diluted to 500-fold with PBS plus 0.05% (w/v) Tween 20 (PBST). Next, the membrane was incubated with its anti-mouse IDO polyclonal antibody for 2 h at room temperature. The membrane was then washed extensively with PBST and incubated with horseradish peroxidase conjugated anti-rabbit IgG for 1 h at room temperature. Finally, the membrane was washed extensively with PBST and developed with ECL plus Western blotting detection reagent (Amersham, Little Chalfont, Bucks., UK).
Real time RT-PCR analysis of IDO mRNA
The total RNA was isolated from CMT-93 with Trizol Reagent (Invitrogen) following the manufacturer’s instructions. Next, the first-strand complementary DNA (cDNA) was synthesized using reverse transcription (RT) primed with ExScript RT reagent Kit (Cat. #RR035A; Takara, Japan) following the manufacturer’s instructions. The real-time polymerase chain reaction (real-time PCR) was prepared using SYBR Premix Ex Taq (Cat. #RR041A; Takara, Japan). The PCRs were performed on Mx3000P (Stratagene). The sequences of PCR primers (listed as forward primers (5′–3′) and reverse primers (5′–3′) are; IDO; ACTGTGTCCTGGCAAACTGGAAG and AAGCTGCGATTTCCACCAATAGAG and GAPDH; AAATGGTGAAGGTCGGTGTG and TGAAGGGGTCGTTGATGG. GAPDH gene served as an internal control. The PCRs were performed by repeating the following amplification cycle 40 times, then denaturing at 95 °C for 10 s, followed by annealing at 60 °C for 20 s. The results from the real-time PCR were presented as threshold cycles normalized to those of the GAPDH gene, according to the method of Overbergh et al. (1999). This result reversibly represented the concentration (number of copies) of the cDNA template of the individual gene.
Statistical analysis
The data are expressed as the means ± standard deviation. Differences between the groups were analyzed using the Student’s t-test. The data containing asterisk marks (*, **, ***) are significantly different from the values in the control group, with values of p < 0.05, p < 0.01, and p < 0.001, respectively.
Results and discussion
Effect of 1-MT on kynurenine induction in culture
Figure 1 shows the structure of tryptophan and its analogues. First, to test the action of 1-MT and IFN-γ on the kynurenine induction process in a culture medium, the CMT93 cells were treated with 1-MT and/or IFN-γ for 48 h (Fig. 2). IFN-γ was used as an inducer for IDO expression.
Fig. 1.
Tryptophan and its analogue. MW, molecular weight
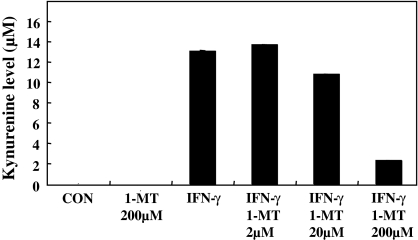
Fig. 2.
The CMT-93 cells were cultured for 48 h in a DMEM medium supplemented with IFN-γ 1,000 units/mL) and various concentrations of 1-MT
In this study, the kynurenine produced from tryptophan by IDO was converted into a yellow Schiff base using an Ehlrich reagent (p-dimethyl aminobenzaldehyde in acetic acid). The kynurenine level in the culture medium was increased by the addition of IFN-γ. But the kynurenine level in the culture medium was decreased by the addition of 1-MT at 200 μM. This result indicated that the IDO expression was induced by IFN-γ and that the IDO activity was suppressed by 1-MT, with an 85% inhibition at 200 μM 1-MT.
Cell growth and viability of CMT93 were not affected by the addition of 1-MT at 200 μM and IFN-γ at 1,000 units/mL (data not shown).
IFN-γ inducible IDO mRNA expression is inhibited by 1-MT
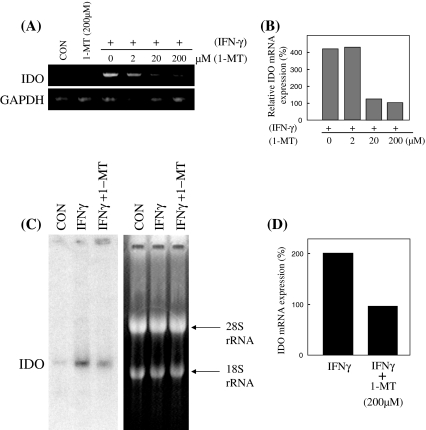
The total RNA of the CMT-93 cells was examined by RT-PCR and Northern-blot analysis in order to reveal the expression pattern of the IDO gene (Fig. 3A–D). Figure 3A, B shows that a strong IDO expression could be detected with IFN-γ stimulation and that the IDO mRNA expression was suppressed by the administration of 1-MT at 20 μM and 200 μM, as revealed by RT-PCR. Figure 3C, D shows the quantification of the Northern-blot analysis of IDO. These figures were obtained by normalizing the IDO signals with the amount of 18S rRNA. As a result, the IFN-γ inducible IDO mRNA expression was suppressed by 1-MT, with a half-maximal inhibition at 200 μM 1-MT.
Fig. 3.
(A) The semi-quantitation of IDO and GAPDH mRNA was performed by reverse transcription polymerase chain reaction. (B) The data were normalized to the mRNA expression of GAPDH. (C) The expression pattern of IDO at 28 S and 18 S rRNA. The IDO mRNA level was estimated by a Northern-blot analysis. (D) The data were normalized to the 18 S rRNA
IFN-γ inducible IDO protein expression is inhibited by 1-MT
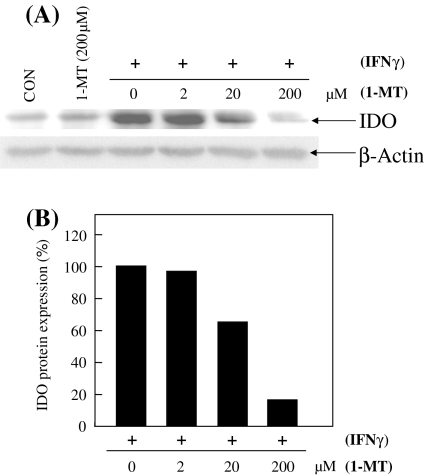
In order to examine the IDO protein levels, a Western-blot analysis was performed using an anti-IDO antibody (Fig. 4A, B). The IFN-γ inducible IDO protein level was suppressed by the addition of 1-MT at 20 μM and 200 μM in CMT-93. These facts suggest that 1-MT inhibits both the transcription and translation steps in the IDO process.
Fig. 4.
(A) In order to determine the IDO and β-Actin protein level, 20 μg of total protein from CMT-93 was subjected to a 12% polyacrylamide gel electrophoresis. The immunoblotting analysis was performed with an anti-IDO antibody and anti-β-Actin antibody, successively. (B) The data were normalized to the β-Actin
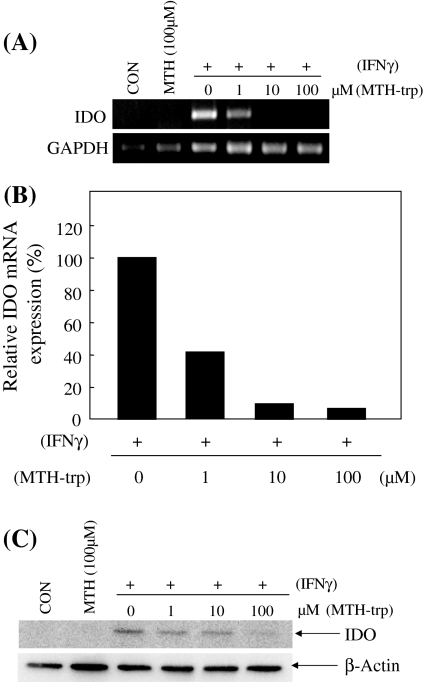
IFN-γ inducible IDO mRNA and protein expression is inhibited by MTH-trp
Next, we examined the effect of MTH-trp, as another analogue of tryptophan, and an IDO enzymatic inhibitor on the IFN-γ inducible IDO mRNA and protein expression (Fig. 5A–C). Similarly, the IFN-γ inducible IDO expression mRNA levels were suppressed by the addition of MTH-trp at 10 μM and 100 μM. These results suggest that both 1-MT and MTH-trp, which are inhibitors of IDO enzymatic activity, suppress IDO induction by IFN-γ at the transcriptional level. Moreover, these results imply that an indole-ring, which is a common structure of 1-MT and MTH-trp, may regulate the transcription of the IDO gene. Therefore we examined the effect of tryptophan (Trp) which also has an indole-ring structure.
Fig. 5.
(A) The semi-quantitation of IDO and GAPDH mRNA was performed by reverse transcription polymerase chain reaction. (B) The data were normalized to the mRNA expression of GAPDH. (C) An immunoblotting analysis was carried out with an anti-IDO antibody and anti-β-Actin antibody, successively
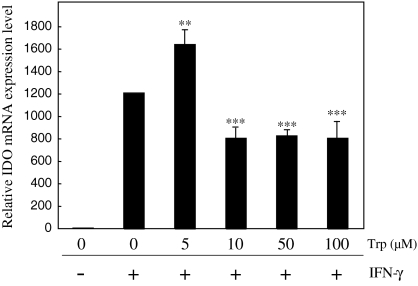
IFN-γ inducible IDO mRNA and protein expression is inhibited by Trp
Finally, we examined the effect of tryptophan (Trp) on the IFN-γ inducible IDO mRNA using real-time RT-PCR (Fig. 6). As a result, the IFN-γ inducible IDO expression mRNA level was suppressed by the addition of Trp at 10, 50, and 100 μM.
Fig. 6.
The CMT-93 cells were precultured for 48 h in RPMI1640 medium supplemented with 10% FBS. Following incubation for 24 h, cells were cultivated with tryptophan-free RPMI1640 medium alone or Trp in the presence or absence of IFN-γ for 24 h. The gene transcription level of IDO was determined by real-time PCR. The bar graph plotted from three independent experiments is presented; **p < 0.01, ***p < 0.001
These results demonstrate that Trp also suppresses IDO induction by IFN-γ at the transcriptional level and that Trp itself would be an effective transcriptional modulator of IDO. Moreover, several lines of publications support the notion that janus kinase-signal transducer and activator (JAK/STAT) signaling pathway mediated the induction of IDO expression by IFN-γ (Mellor and Munn 2004). Therefore, Trp may inhibit IDO expression at the transcriptional level by down-regulation of STAT1 activation in IFN-γ-stimulated mouse rectal carcinoma cell line.
Although the physiological significance of the transcriptional modulation of the IDO gene by Trp and its analogue is unknown at present, these findings may provide new insight regarding the novel role of this unique essential amino acid in gene control.
Acknowledgements
This study was supported in part by Research Project Grants (Nos. 17-509 and 18-502) from Kawasaki Medical School.
Abbreviations
- IDO
Indoleamine 2,3-dioxygenase
- IFN
Interferon
- 1-MT
1-Methyl-l-tryptophan
- MTH-trp
Methylthiohydantoin-dl-tryptophan
References
- Burke F, Knowles RG, East N, Balkwill FR (1995) The role of indoleamine 2,3-dioxygenase in the anti-tumor activity of human interferon-gamma in vivo. Int J Cancer 60:115–122 [DOI] [PubMed]
- Chon SY, Hassanain HH, Pine R, Gupta SL (1995) Involvement of two regulatory elements in interferon-gamma-regulated expression of human indoleamine 2,3-dioxygenase gene. J Interferon Cytokine Res 15:517–526 [DOI] [PubMed]
- Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, Mellow AL, Munn DH, Antonia SJ (2002) Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer 101:151–155 [DOI] [PubMed]
- Hassanain HH, Chon SY, Gupta SL (1993) Differential regulation of human indolamine 2,3-dioxygenase gene expression by interferons-gamma and -alpha Analysis of the regulatory region of the gene and identification of an interferon-gamma-inducible DNA-binding factor. J Biol Chem 268:5077–5084 [PubMed]
- Kadoya A, Tone S, Maeda H, Minatogawa Y, Kido R (1992) Gene structure of human indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun 189:530–536 [DOI] [PubMed]
- Konan KV, Taylor MW (1996) Importance of the two interferon-stimulated response element (ISRE) sequences in the regulation of the human indoleamine 2,3-dioxygenase gene. J Biol Chem 271:19140–19145 [DOI] [PubMed]
- Mellor AL, Munn DH (2004) IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4:762–774 [DOI] [PubMed]
- Morita T, Saito K, Takemura M, Maekawa N, Fujigaki S, Fujii H, Wada H, Takeuchi S, Noma A, Seishima M (2001) 3-Hydroxyanthranilic acid, an l-tryptophan metabolite, induces apoptosis in monocyte-derived cells stimulated by interferon-gamma. Ann Clin Biochem 38:242–251 [DOI] [PubMed]
- Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E (2005) Prendergast GC inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med 11:312–319 [DOI] [PubMed]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL (1998) Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281:1191–1193 [DOI] [PubMed]
- Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL (1999) Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 189:1363–1372 [DOI] [PMC free article] [PubMed]
- Overbergh L, Valckx D, Waer M, Mathieu C (1999) Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11:305–312 [DOI] [PubMed]
- Shimizu T, Nomiyama S, Hirata F, Hayaishi O (1978) Indoleamine 2,3-dioxygenase. J Biol Chem 253:4700–4706 [PubMed]
- Suzuki S, Tone S, Takikawa O, Kubo T, Kohno I, Minatogawa Y (2001) Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem J 355:425–429 [DOI] [PMC free article] [PubMed]
- Takikawa O, Kuroiwa T, Yamazaki F, Kido R (1988) Mechanism of interference-γ action. J Biol Chem 263:2041–2048 [PubMed]
- Takikawa O, Yoshida R, Kido R, Hayaishi O (1986) Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem 261:3648–3653 [PubMed]
- Taylor MW, Feng G (1991) Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 5:2516–2522 [PubMed]
- Towbin H, Staehlin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354 [DOI] [PMC free article] [PubMed]
- Werner ER, Bitterlich B, Fuchs D, Hausen A, Reibnegger G, Szabo G, Dierich MP, Wachter H (1987) Human macrophages degrade tryptophan upon induction by interferon-gamma. Life Sci 41:273–280 [DOI] [PubMed]