Abstract
The neurite outgrowth of PC12 cells on collagen-coated glass plates under light emitting diode (LED) irradiation at several wavelengths (i.e., 455, 470, 525, 600, 630, 880 and 945 nm) was investigated. No neurite outgrowth was observed during cultivation under irradiation from the lamp of an inverted light microscope through filters (yielding mixed light at ca. 525 nm and more than 800 nm), whereas neurite outgrowth was observed during cultivation in the dark. When these cells were irradiated with monochromatic LED light, neurite outgrowth was slightly, but not completely, suppressed at 455, 525, 600, 630, 880 and 945 nm, as was observed in the case of mixed light. Long connected neuronal outgrowths (e.g., 3 mm length) were observed with LED light at 470 nm and 1.8 mW/cm2 intensity. No such outgrowths were observed at other LED light wavelengths (i.e., 455, 525, 600, 630, 880 and 945 nm). Irradiation at 470 nm may have caused specific responses to transductional signals in these cells that led to the connection of neuronal outgrowths between cells. Not only suppressed neurite outgrowth but also long connected neurite outgrowths were observed when PC12 cells were cultured under several different wavelengths of light.
Keywords: Cell, Nerve, Neurite outgrowth, Visible light, LED
Introduction
The use of visible light for therapy has been investigated for many years. Visible light has been used not only as a potential activator of photoactive therapeutic agents (Samia et al. 2003; Bakalova et al. 2004) but also as a therapeutic agent itself (Hogset et al. 2002; Prasmickaite et al. 2004; Chen et al. 2005), although it remains unclear how visible light interacts with tissue and cells. Several researchers have found that violet-blue light (380–500 nm) disrupts DNA integrity and cellular processes such as mitochondrial function and mitosis, which are probably mediated by light-induced reactive oxygen species (ROS) (Aggarwal et al. 1978; Gorgidze et al. 1998; Pflaum et al. 1998; Lewis et al. 2005).
The effect of light on tissues has been mainly studied from the standpoint of biological safety with respect to the skin or retina, where ROS induced by violet-blue or UV light are suspected as mediators of skin aging and age-related macular degeneration, respectively (O’Donovan et al. 2005; Sakurai et al. 2005). Furthermore, the impact of light on retinal pigment epithelial cells was studied with reference to how light influenced transcriptional regulation of apoptosis by activation of the redox-sensitive transcription factor NFκB (Legrand-Poels et al. 1998; Krishnamoorthy et al. 1999). Chen et al. (2005) have reported that phototherapy of jaundiced neonates using blue light affected the expression of the circadian genes Bmal1 and Cry1 in peripheral blood mononuclear cells. Their results suggested that phototherapy should be timed according to circadian rhythms when treating such infants (Chen et al. 2005).
These studies of both adverse and therapeutic effects of light have been mainly limited to skin, retinal, or oral cells, except for a few isolated reports (Edwards and Cline 1999; Ehrlicher et al. 2002; Khadra et al. 2005). Ehrlicher et al. (2002) have shown that the direction taken by the leading edge of a nerve cell could be guided by placing a laser spot in front of a specific area of the nerve’s leading edge. The laser spot guided the growth of nerve’s leading edge into the beam focus. This observation was explained by the fact that the laser beam biased actin polymerization, driving lamellipodia extension.
In our previous study, we found that neurite outgrowth of nerve-like PC12 cells in polystyrene tissue culture flasks and collagen-coated glass plates was suppressed by visible light irradiation from the lamp of an inverted light microscope (Higuchi et al. 2003, 2005). In those studies, PC12 cells were irradiated with light of wavelength ∼525 nm and greater than 800 nm through three filters (frost filter, daylight balancing filter and green interference filter) continuously or intermittently. We proposed the use of patterned light irradiation for the preparation of regulated, rewritable neuronal networks analogous to “CD−RW” or “CD + RW” devices. Previous methods such as lithography (Miyamoto et al. 1993; McFarland et al. 2000); laser ablation (Hammarback et al. 1985; Corey et al. 1991); elastomer stamping (Singhvi et al. 1994); and microcontact printing (Kam et al. 2001) yielded networks which were analogous to “CD−R” devices only.
Only suppression of neurite outgrowth was reported in our previous study (Higuchi et al. 2003, 2005). Here, we report the effect of different wavelengths (i.e., 455, 470, 525, 600, 630, 880 and 945 nm) of light emiting diode (LED) light on neurite outgrowth of PC12 cells. Not only light-regulated suppression but also extension of neurite outgrowth was investigated in this study.
Materials and methods
Preparation of collagen-coated glass plates
About 0.5 mg/mL of collagen solution (cellgen IPC-15, type I from calf skin, Koken, Tokyo) was cast on glass plates placed in a 24-well tissue culture flask and incubated for 24 h at 37 °C to prepare collagen-coated glass plates. After aspirating the collagen solution, the collagen-coated glass plates were washed three times with phosphate buffer saline (pH 7.4) (Higuchi et al. 2002).
Cell line and cultures
PC12 cells (Riken Cell Bank, Ibaraki, Japan) were maintained at 37 °C in RPMI1640 medium (JRH Bioscience, Lenexa, KS) supplemented with 5% heat-inactivated horse serum (JRH Biosciences, Lenexa, KS, USA), 10% fetal calf serum (JRH Biosciences, Lenexa, KS, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Oberdoerster and Rabin 1999; Hara et al. 2000; Higuchi et al. 2003, 2005).
Neurite regulation by visible light
PC12 cells (2 × 104 cells/cm2) were plated on the collagen-coated glass plates inserted into a 24-well polystyrene tissue culture flask under 100 U/mL penicillin, 100 μg/mL streptomycin, and 1% horse serum at 37 °C. After 2 days, the medium was changed to a medium composed of 100 ng/mL NGF, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.1% horse serum (Oberdoerster and Rabin 1999; Higuchi et al. 2003, 2005). PC12 cells on the collagen-coated glass plates in 24-well polystyrene tissue culture flasks were exposed to visible light using LED array (wavelength = 455 nm [BLAC-W9-CLP, Ikegami Tsusho Co., Ltd., Tokyo], 470 nm [BLAC-B9-CLP, Ikegami Tsusho Co., Ltd., Tokyo], 525 nm [BLAC-G9-CLP, Ikegami Tsusho Co., Ltd., Tokyo], 600 nm [BLAC-Y9-CLP, Ikegami Tsusho Co., Ltd., Tokyo], 630 nm [BLAC-R9-CLP, Ikegami Tsusho Co., Ltd., Tokyo], 880 nm [L2791-02, Hamamatsu Photonics K.K., Shizuoka], 945 nm [L2388-01, Hamamatsu Photonics K.K., Shizuoka]) equipped with a CO2 chamber to maintain a 5% CO2 atmosphere at 37 °C (IX-IBM2 and CO2 inject control timer, Olympus, Tokyo). Light intensity from LED light was controlled by using two types of voltage regulators (V-130-5, Yamabishi Co., Tokyo and 22-012, Sekisui Kinzoku Co., Ltd., Tokyo). PC12 cells on the collagen-coated glass plates in 24-well polystyrene tissue culture flasks were also exposed to the visible light using an inverted light microscope photo-lamp (IX70, Olympus, Tokyo) equipped with a CO2 chamber to maintain a 5% CO2 atmosphere at 37 °C. Light around 525 nm and more than 800 nm was attenuated by passing it through three filters attached to the microscope (frost filter, daylight balancing filter and green interference filter) in this case (Higuchi et al. 2005). Light intensity from LED light and microscope photo-lamp on the 24-well polystyrene culture flask was measured using an optical power meter (Q82017A, Advantest Co., Tokyo).
A neurite was identified when its length was more than 25 μm and had a clearly defined growth cone. A total of 200 cells per well was counted, and the percentage of these cells having neurites was calculated.
Temperature and pH measurement under light irradiation
The temperature and pH of the medium in a 24-well polystyrene culture flask in a CO2 chamber held in a constant 5% CO2 atmosphere at 37 °C was measured using a digital thermometer and a pH meter under light irradiation and in the absence of light irradiation from several LEDs to note if any rise in temperature or pH occurred due to 24 h of light irradiation.
Results and discussion
Dependence of neurite outgrowth on wavelength of light irradiation
PC12 cells, which display neuronal characteristics, differentiate their phenotype from a proliferating cell to a neurite-bearing neuron upon treatment with NGF in the dark (Damon et al. 1990; Oberdoerster and Rabin 1999). Neurite outgrowth of PC12 cells on collagen-coated glass plates under light irradiation of different wavelengths at constant light intensity (1.0 mW/cm2) using LEDs was investigated.
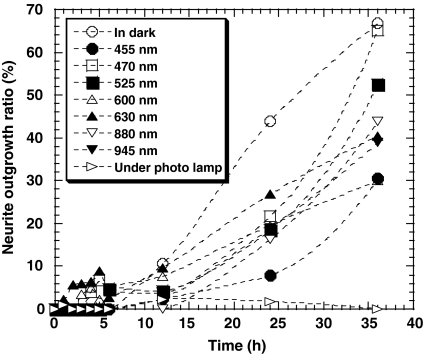
Figure 1 shows the time dependence of neurite outgrowth of PC12 cells under light irradiation of different wavelengths at a constant light intensity of 1.0 mW/cm2. The light source was an LED or the lamp of an inverted light microscope, filtered through three filters (frost filter, daylight balance filter, and green interference filter). No neurite outgrowth was observed when cells were cultivated under irradiation from the microscope lamp, as was observed in our previous studies (Higuchi et al. 2003, 2005), whereas such outgrowth was observed during cultivation in the dark. Filtered light from the microscope lamp of wavelength around 525 nm and more than 800 nm was thought to be responsible for the suppression of neurite outgrowth (Higuchi et al. 2005). Therefore, neurite outgrowth was investigated under light irradiation at different wavelengths using LEDs. When cells were irradiated under monochromatic LED light, neurite outgrowth was slightly suppressed at 455, 525, 600, 630, 880 and 945 nm, but such irradiation failed to suppress outgrowth completely, as was the case under mixed light irradiation from the microscope lamp at the intensity of light >0.25 mW/cm2, because the neurite outgrowth ratio of PC 12 cells under mixed light irradiation from 0.25 to 2 mW/cm2 was found to be less than 4% in this study. Furthermore, the neurite outgrowth ratio under irradiation at 470 nm was found to be the same as in the dark after 36 h of incubation with 100 ng/mL of NGF.
Fig. 1.
Time-course of PC12 cells with neurites after addition of 100 ng/mL NGF, cultured on collagen-coated glass plates under light irradiation of different wavelength at a constant light intensity (1.0 mW/cm2) using LEDs [wavelength = 455 nm (●), 470 nm (□), 525 nm (■), 600 nm (Δ), 630 nm (▲), 880 nm (∇), 945 nm (▼)], or under light irradiation using an inverted light microscope photolamp through three filters (frost filter, daylight balance filter, and green interference filter) (▹) at a constant light intensity (1.0 mW/cm2), or in dark place (◯)
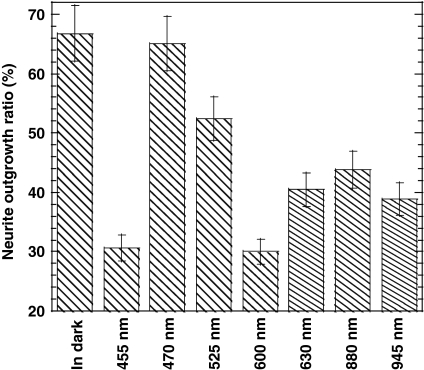
Figure 2 shows the effect of the wavelength of light on the neurite outgrowth ratio of PC12 cells 36 h after incubation with 100 ng/mL of NGF under light irradiation from LEDs. Irradiation at 600 and 455 nm effectively suppressed neurite outgrowth, compared to 470, 525, 630, 880 and 945 nm. The 455-nm light may have been too similar to UV light, therefore damaging cells, but it was unclear why 600-nm light suppressed neurite outgrowth. The neurite outgrowth ratio at 470 nm was found to be higher than that at other LED wavelengths in this study, and was the same as that measured in the dark.
Fig. 2.
Effect of wavelength on neurite outgrowth ratio of PC12 cells cultured on collagen-coated glass plates at 36 h after the addition of 100 ng/mL of NGF under LED irradiation at a constant light intensity (1.0 mW/cm2)
Effect of temperature and pH on suppression of neurite outgrowth
No increase in temperature and pH was observed in the culture medium under continuous light irradiation from LED light or microscope photo-lamp at an intensity of 1.0 mW/cm2. Therefore, suppression of neurite outgrowth under light irradiation from microscope photo-lamp and LED light is not due to the increase of the temperature nor pH in the culture medium. Thus, it is thought to be due to the effect of visible light having specific wavelength on PC12 cells; more specifically, the signal transduction of light on PC12 cells responding upon the light of specific wavelength.
Effect of light intensity on nerve growth ratio
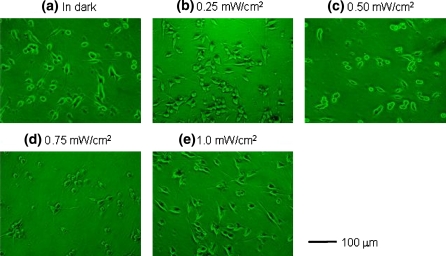
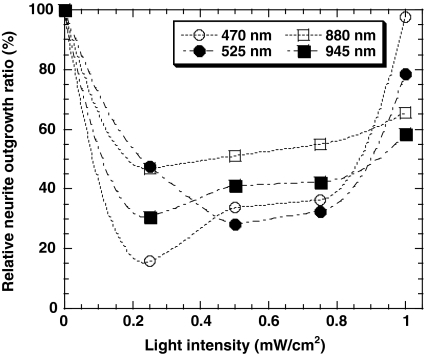
The effect of light intensity on the nerve growth ratio was also investigated. Figure 3 shows microphotographs of PC12 cells under LED irradiation at 525 nm, with a light intensity of 0, 0.25, 0.50, 0.75 and 1.0 mW/cm2. Neurite outgrowth was visibly suppressed at 0.50 and 0.75 mW/cm2. Figure 4 shows the effect of light intensity on the nerve growth ratio of cells in culture conditions identical to the above, under LED irradiation at 470, 525, 880 and 945 nm. The nerve growth ratio at 0.25, 0.50, 0.75 mW/cm2 was found to be lower than that in the dark, but neurite outgrowth was not suppressed completely as with light from the microscope lamp. Monochromatic light from LEDs failed to suppress neurite outgrowth completely at any intensity of light, as occurred with mixed light from the microscope lamp. However, light irradiation at lower power such as 0.2 mW/cm2 at each wavelength from 470 to 945 nm showed effective suppression of neurite outgrowth compared to that at higher power as found in Fig. 4. Therefore, the reason for the complete suppression of neurite outgrowth of PC12 cells under the irradiation of mixed light can be considered as each of light originated from different wavelength having low power in the mixed light suppress the neurite outgrowth independently. As the result, we might find the neurite outgrowth was suppressed completely under the mixed light irradiation from synergistic effects of each light at different wavelength in this study.
Fig. 3.
Microphotographs of PC12 cells, which are cultured on collagen-coated glass plates at 36 h after addition of 100 ng/mL NGF under 525 nm of light irradiation from LED
Fig. 4.
Effect of light intensity on the nerve growth ratio of PC12 cells cultured on collagen-coated glass plates at 36 h after the addition of 100 ng/mL NGF under irradiation by LED light at different wavelengths [wavelength = 470 nm (O), 525 nm (●), 880 nm (□), 945 nm (■)]
The neurite outgrowth ratio under light irradiation at 1 mW/cm2 was found to be higher than that at 0.25, 0.50, and 0.75 mW/cm2 when 470, 525, 880 or 945 nm LED light was used. The neurite outgrowth ratio under several wavelengths at 1.8 and 2.0 mW/cm2 was also investigated. The neurite outgrowth ratio under several wavelengths at 2.0 mW/cm2 showed less than 8%, while that at 1.8 mW/cm2 is approximately the same to that at 1.0 mW/cm2 within the experimental error. This indicates that the light irradiation at 2 mW/cm2 is too strong and generates the damage to PC12 cells. It was found that PC12 cells showed a specific response of neurite outgrowth ratio not only to the wavelength but also to the intensity of light.
Effect of blue light on neuronal outgrowth
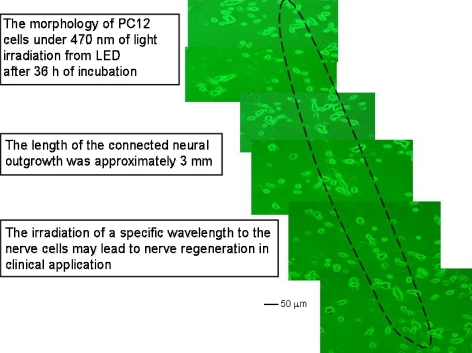
Light emitting diode (LED) irradiation at 470 nm produced unusual responses in PC12 cells at high light intensity. Figure 5 shows microphotographs of such cells, cultured as described above, and subjected to 1.8 mW/cm2 LED irradiation at this wavelength. Long (e.g., 3 mm length) connected neuronal outgrowths were observed under these connections, not observed at any other wavelength. The 470-nm light may have caused specific responses to transductional signals in PC12 cells that led to the connection of neuronal outgrowths between cells. Blue light (such as at 470 nm) is reported to induce many physiological responses in living cells (Christie et al. 1998; Mas et al. 2000; El-Assal et al. 2001; Kagawa et al. 2001; Harada et al. 2003; Lewis et al. 2005). It is known that it disrupts DNA integrity and cellular processes such as mitochondrial function and mitosis, and that light-induced ROS mediate these effects (Aggarwal et al. 1978; Gorgidze et al. 1998; Lewis et al. 2005). It is not clear at present why the exposure of PC12 cells to blue light (470 nm) induced the long neuronal outgrowth depicted in Fig. 5. Blue light is, however, responsible for the transductional signaling needed for the formation of elongated neuronal connections between PC12 cells. The generation of such outgrowths observed in this study suggests that blue light can be used as a therapeutic agent for the regeneration of nerve tissue.
Fig. 5.
Micrographs of PC12 cells cultured on collagen-coated glass plates at 36 h after the addition of 100 ng/mL NGF under 470 nm LED light irradiation at 1.8 mW/cm2
Conclusion
When PC12 cells were irradiated with monochromatic LED light, neurite outgrowth was slightly suppressed under 455, 525, 600, 630, 880 and 945 nm of light irradiation, but the light irradiation fails to suppress the neurite outgrowth of PC12 cells completely as the mixed light from the inverted light microscope photolamp does. Long connected neuronal outgrowth of PC12 cells (e.g., 3 mm length was observed in this study) was observed under 470 nm of light irradiation from LED at 1.8 mW/cm2. No long connected neuronal outgrowth was observed under light irradiation of another wavelength (i.e., 455, 525, 600, 630, 880 and 945 nm of light irradiation) from LEDs except 470 nm of light irradiation. Light irradiation of 470 nm may cause specific response to transductional signals in PC12 cells that lead to the connection of neuronal outgrowth between cells. Not only the suppressed neurite outgrowth but also long connected neurite outgrowth was observed when PC12 cells was cultured under several wavelength of light irradiation. Although it is not clear at this moment why the exposure of blue light (470 nm) to PC12 cells induces the long connection of neuronal outgrowth as found in Fig. 5, blue light is responsible for the transductional signaling for the long connection of neuronal outgrowth between PC12 cells. The generation of long connected neuronal outgrowth observed in PC12 cells in this study proposes that blue light can be used as a therapeutic agent for the regeneration of nerve in tissue.
Acknowledgments
This research was partially supported from a Grants-in-Aid for Scientific Research (No. 14655136 and 17560691) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. This work was also supported by “High-Tech Research Center” Project from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Asahi Glass Foundation, Iketani Science Technology Foundation, Salt Science Foundation, and National Science Council of Taiwan under the Grant No. NSC95-2221-E-008-086 and NSC96-2218-E-008-007 were also acknowledged.
Abbreviations
- LED
Light emitting diode
- ROS
Reactive oxygen species
- NGF
Nerve growth factor
References
- Aggarwal BB, Quintanilha AT, Cammack R, Packer L (1978) Damage to mitochondrial electron transport and energy coupling by visible light. Biochim Biophys Acta 502:367–382 [DOI] [PubMed]
- Bakalova R, Ohba H, Zhelev Z, Ishikawa M, Baba Y (2004) Quantum dots as photosensitizers? Nature Biotechnol 22:1360–1361 [DOI] [PubMed]
- Chen A, Du L, Xu Y, Chen L, Wu Y (2005) The effect of blue light exposure on the expression of circadian genes: bmal1 and cryptochrome 1 in peripheral blood mononuclear cells of jaundiced neonates. Pediatr Res 58:1180–1184 [DOI] [PubMed]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282:1698–1701 [DOI] [PubMed]
- Corey JM, Wheeler BC, Brewer GJ (1991) Compliance of hippocampal neurons to patterned substrate networks. J Neurosci Res 30:300–307 [DOI] [PubMed]
- Damon DH, D’Amore PA, Wagner JA (1990) Nerve growth factor and fibroblast growth factor regulate neurite outgrowth and gene expression in PC12 cells via both protein kinase C- and cAMP-independent mechanisms. J Cell Biol 110:1333–1339 [DOI] [PMC free article] [PubMed]
- Edwards JA, Cline HT (1999) Light-induced calcium influx into retinal axons is regulated by presynaptic nicotinic acetylcholine receptor activity in vivo. J Neurophysiol 81:895–907 [DOI] [PubMed]
- Ehrlicher A, Betz T, Stuhrmann B, Koch D, Milner V, Raizen MG, Kas J (2002) Guiding neuronal growth with light. Proc Natl Acad Sci USA 99:16024–16028 [DOI] [PMC free article] [PubMed]
- El-Assal SE, Alonso-Blanco C, Peeters AJ, Raz V, Koornneef M (2001) A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Nat Genet 29:435–440 [DOI] [PubMed]
- Gorgidze LA, Oshemkova SA, Vorobjev IA (1998) Blue light inhibits mitosis in tissue culture cells. Biosci Rep 18:215–224 [DOI] [PubMed]
- Hammarback JA, Palm SL, Furcht LT, Letourneau PC (1985) Guidance of neurite outgrowth by pathways of substratum-adsorbed laminin. J Neurosci Res 13:213–220 [DOI] [PubMed]
- Hara M, Asakura T, Cho CS, Akaike T, Higuchi A (2000) Morphologies of PC12 Cells cultured on some polymeric films—relationship between cell growth and surface physical properties. Nippon Kagaku Kaishi 4:257–266 [DOI]
- Harada A, Sakai T, Okada K (2003) Phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc Natl Acad Sci USA 100:8583–8588 [DOI] [PMC free article] [PubMed]
- Higuchi A, Takanashi Y, Ohno T, Asakura T, Cho CS, Akaike T, Hara M (2002) Production of interferon-β by fibroblast cells on the membranes prepared by extracellular matrix proteins. Cytotechnology 39:131–137 [DOI] [PMC free article] [PubMed]
- Higuchi A, Kitamura H, Shishimine K, Konishi S, Yoon BO, Hara M (2003) Visible light is able to guide neuronal networks in a micro-patterned manner. J Biomat Sci Polym Edn 14:1377–1388 [DOI] [PubMed]
- Higuchi A, Watanabe T, Matsubara Y, Matsuoka Y, Hayashi S (2005) Regulation of neurite outgrowth by intermittent irradiation of visible light. J Phys Chem B 109:11033–11036 [DOI] [PubMed]
- Hogset A, Ovstebo EB, Prasmickaite L, Berg K, Fodstad O, Maelandsmo GM (2002) Light-induced adenovirus gene transfer, an efficient and specific gene delivery technology for cancer gene therapy. Cancer Gene Ther 9:365–371 [DOI] [PubMed]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291:2138–2141 [DOI] [PubMed]
- Kam L, Shain W, Turner JN, Bizios R (2001) Axonal outgrowth of hippocampal neurons on micro-scale networks of polylysine-conjugated laminin. Biomaterials 22:1049–1054 [DOI] [PubMed]
- Khadra M, Lyngstadaas SP, Haanaes HR, Mustafa K (2005) Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials 26:3503–3509 [DOI] [PubMed]
- Krishnamoorthy RR, Crawford MJ, Chaturvedi MM, Jain SK, Aggarwal BB, Al-Ubaidi MR, Agarwal NJ (1999) Photo-oxidative stress down-modulates the activity of nuclear factor-kappaB via involvement of caspase-1, leading to apoptosis of photoreceptor cells. J Biol Chem 274:3734–3743 [DOI] [PubMed]
- Legrand-Poels S, Schoonbroodt S, Matroule JY, Piette J (1998) Nf-kappa B: an important transcription factor in photobiology. J Photochem Photobiol B 45:1–8 [DOI] [PubMed]
- Lewis JB, Wataha JC, Messer RLW, Caughman GB, Yamamoto T, Hsu SD (2005) Blue light differentially alters cellular redox properties. J Biomed Mater Res Part B Appl Biomater 72B:223–229 [DOI] [PubMed]
- Mas P, Devlin PF, Panda S, Kay SA (2000) Functional interaction of phytochrome B and cryptochrome 2. Nature 408:207–211 [DOI] [PubMed]
- McFarland CD, Thomas CH, DeFilippis C, Steele JG, Healy KE (2000) Protein adsorption and cell attachment to patterned surfaces. J Biomed Mater Res 49:200–210 [DOI] [PubMed]
- Miyamoto S, Ohashi A, Kimura J, Tobe S, Akaike T (1993) A novel approach for toxicity sensing using hepatocytes on a collagen-patterned plate. Sens Actuators B 13:196–199 [DOI]
- Oberdoerster J, Rabin RA (1999) NGF-differentiated and undifferentiated PC12 cells vary in induction of apoptosis by ethanol. Life Sci 64:267–272 [DOI] [PubMed]
- O’Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P (2005) Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science 309:1871–1874 [DOI] [PMC free article] [PubMed]
- Pflaum M, Kielbassa C, Garmyn M, Epe B (1998) Oxidative DNA damage induced by visible light in mammalian cells: extent, inhibition by antioxidants and genotoxic effects. Mutat Res 408:137–146 [DOI] [PubMed]
- Prasmickaite L, Hogset A, Olsen VM, Kaalhus O, Mikalsen SO, Berg K (2004) Photochemically enhanced gene transfection increases the cytotoxicity of the herpes simplex virus thymidine kinase gene combined with ganciclovir. Cancer Gene Ther 11:514–523 [DOI] [PubMed]
- Sakurai H, Yasui H, Yamada Y, Nishimura H, Shigemoto M (2005) Detection of reactive oxygen species in the skin of live mice and rats exposed to UVA light: a research review on chemiluminescence and trials for UVA protection. Photochem Photobiol Sci 4:715–720 [DOI] [PubMed]
- Samia AC, Chen X, Burda C (2003) Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc 125:15736–15737 [DOI] [PubMed]
- Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE (1994) Engineering cell shape and function. Science 264:696–698 [DOI] [PubMed]