Abstract
The water extracts of propolis (WEP) could inhibit growth of different cell lines namely McCoy, HeLa, SP2/0, HEp-2, and BHK21 and stimulate growth of normal cell named human lymphocyte, rat kidney, rat liver, and rat spleen. In these experiments 1 and 2 mg of WEP were added to 1 ml RPMI media with 5% FCS. Cell counts and cell viability of propolis-treated and propolis-free cells were assessed by Trypan blue dye exclusion test and MTT assay. The results showed that in case of McCoy, HeLa, SP20, HEp-2, and BHK21 cell lines, the water extracts of propolis could inhibit cell growth as well as reduction on size of the cells. In contrast the same amount of WEP could stimulate growth of normal cells up to 60% with the same concentration used for cell lines. Thus our study indicates that although WEP consists only of the soluble part of propolis, it enables to inhibit different cell lines and increase growth of normal cells. This indicates also that WEP contains the specific compounds with bioactivity against cell lines. Although propolis contain different number of compounds it is clear that WEP has enough biological compounds useful for the treatment of some diseases, medical and related applications.
Keywords: Cell line, Cell stimulation, MTT tests, Normal cells, Propolis, Water extracts of propolis (WEP)
Introduction
Propolis is the resinous substance collected by bees from the leaf buds and bark of trees, especially poplar and conifer trees. Bees use the propolis along with bees wax to construct their hives. It originates as a gum secretion gathered by bees from a variety of plants, and can vary in color depending on the plant species of origin. There are a number of reviews on propolis (Khalil 2006; Ribeiro et al. 2006) and its compounds have been reported by different people. More than 250 individual compounds have been established as the constituents of propolis. Propolis contains mainly resins, balsams and phenolic aldehydes (polyphenols), waxes and fatty acids, essential oils, pollen, other organics and minerals. Phenolic acids, esters, and flavonoids have been shown to account for most important of propolis composition. Bees modify propolis by glucodiases, enzymes from hypopharyngeal glands, during collection and processing. Results of this enzymatic modification are hydrolyzation of phenolic compounds like flavonoid heterosides to free flavonoid aglycones and sugars and enhancement of the pharmacological action of the resulting products. Chemically, flavonoid aglycones from propolis are flavones, flavonols, flavanones, dihydroflavonols and chalcones. Other phenolic compounds are phenolic aldehydes and polyphenolic derivates of cinnamic and benzoic acid, including caffeic acid esters, terpenes, steroids, sesquiterpenes, naphthalene and stilbene derivatives (Marcucci et al. 2001). Propolis has remarkable therapeutic qualities, and is much sought after in some countries for the treatment of a range of human ailments, and for cosmetic purposes. General medicinal uses of propolis include treatment of the cardiovascular and blood systems disorder (anemia), respiratory apparatus (for various infections), dental care (Ikeno et al. 1991), dermatology (tissue regeneration, ulcers, excema, wound healing––particularly burn wounds, mycosis, mucous membrane infections and lesions), cancer treatment (Grunberger et al. 1988; Scheller et al. 1989b; Hausen et al. 1992), immune system support and improvement (Scheller et al. 1989a), digestive tract disorders (ulcers and infections), liver protection and support and many others. Propolis compounds including flavonoids, phenolic acids and its esters have anti-inflammatory, antibacterial, antiviral, immunomodulatory, antioxidant and antiproliferative effects (Hu et al. 2005; Noelker et al. 2005; Orsi et al. 2005; Kim et al. 2006). Propolis is shown to inhibit cell division of unmoral cell and protein synthesis. However the exact mechanism underlying antitumor effect is not clearly described. The aim of this study was to show effect of the water extracts of propolis on different cell lines and normal cells.
Materials and methods
Extraction of propolis
Ethanol extraction
Propolis was collected from beehives located in Mashhad area in Iran. In the first step propolis was extracted by ethanol, propolis (90 g) was added into 400 ml of 96% ethanol and mixed for them for 18 h at 15 °C. The mixture was centrifuged at 7000 rpm for 15 min at 20 °C. The supernatant was collected and the pellet was re-extracted with 100 ml ethanol. After pooling the supernatants of both steps, they were used for the experiments.
Water extracts of propolis
About 200 ml of ethanol extracts of propolis from pervious step was poured into 500-ml flask and kept on a magnetic stirrer. 200 ml of 20 mM phosphate buffer was added to the ethanol extract of propolis and was mixed for 20 min at 20 °C. The mixture was centrifuged at 7000 rpm for 15 min and the supernatant was collected. Water-soluble compounds remained in the aqueous phase which was a solution with a light yellow color. The lower part was dark brown and very sticky. The water extracts of propolis were concentrated by freeze-drying method and used for the next step. Since there is a different number of compounds in the extract of propolis, a quick analysis of the amount of amino acids, flavonoids and sugar in each extraction step was performed by photometric methods and paper chromatography.
Protein and carbohydrate estimation
Protein concentration was measured with Lowry’s method (Lowry et al. 1951) using bovine serum albumin as standard. The total neutral sugar was determined by the phenol/sulphuric acid method (Dubois et al. 1956) using glucose as standard. The reducing sugar present in ethanol and water extracts of propolis was measured by using the DNSA method at 540 nm (Miller 1959) using glucose as standard.
Antibacteral activity
Antibacterial activity of the water extracs of propolis from the used propolis and Chinese’s propolis (Zhejiang Jiangshan Hengliang bee products Co., Ltd., China) was tested using two Gram positive bacteria (Brumfitt et al. 1990). The bacteria (Bacillus subtils and Staphylococcus aureus) were grown on agar medium, then the same amount of the water extracts of propolises was added to each well, buffer and ethanol were used as controls. The plates were kept at 37 °C for 20 h and then the zone of growth inhibition was monitored.
Total flavonoid assay
Total flavonoid content was measured by using the aluminum chloride photometry assay (Marinova et al. 2005). An aliquot (0.5 ml) of extracts was added to a tube containing 2 ml of deionized water and then 0.15 ml 5% NaNO2 was added to the tube. After 5 min, 0.15 ml 10% AlCl3 was added. After 6 min incubation and mixing, 1 ml 1 M NaOH was added and the total volume was adjusted up to 5 ml with deionized water. The solution was mixed well and the absorbance was measured against the control at 510 nm. Samples were analyzed in duplicates.
Paper chromatography
Carbohydrate
Equal volume of each sample (ethanol and water extracts of propolis) was subjected to paper chromatography on Whatmans paper No.1 using solvent system, containing n-Butanol, Pyridine and water (6:3:4). The spots were visualized by staining with silver nitrate (Robyt and French 1963).
Protein and amino acid
The protein and amino acids present in ethanol and water extracts of propolis were analyzed and compared by paper chromatography, solvent system was n-Butanol, acidic acid and water (4:1:5). The chromatogram was stained with 0.5% ninhydrine in acetone.
Flavonoids
Samples were spotted on Whatmans paper No. 3 and subjected to a solvent system containing n-Butanol, acetic acid and water (4:1:5). The chromatogram was viewed under ultraviolet light 260–350 nm for spot detection (Markham 1982).
Medium preparation
The water extracts of propolis (WEP) was sterilized by 0.22 μm Millipore syringe filter and then diluted to 1 and 2 mg/ml of final concentration in the culture medium. The medium used in this experiment was RPMI 1640 (Sigma) with 5% FCS (RPpro medium). The control was used without WEP as compliment.
Cell culture
Different cell types (McCoy, HeLa, SP2/0, HEp-2, BHK21) human lymphocyte, rat kidney, rat liver, and rat spleen were cultivated in RPMI 1640 medium with 10% FCS for pre-inoculation. After 72 h around 5,000–7,000 cells from different cells were obtained per well of 24-well plates. Different concentrations of WEP were added to each well and incubated at 37 °C in humidified 5% CO2 atmosphere for 48 h. Some wells were kept untreated as controls for comparison purposes. After different time intervals each well was visualized and monitored under an inverted microscope. Cell growth and viability was evaluated by MTT test. Photographs were taken from each cell at different time intervals.
MTT assay
Cell viability was evaluated by the MTT colorimetric technique (Mosmann 1983). Briefly, 100 μl of an MTT (Sigma) solution (5 mg/ml in PBS) was added to each well. The plates were incubated for 3 h at 37 °C, and then the supernatants were removed. For solubilization of the MTT crystals, 1 ml of DMSO plus 125 μl of 100 mM glycine (pH 10.5) were added to the wells. The plates were placed on a shaker for 15 min for complete solubilization of crystals and then the optical density of each well was determined at 492 nm.
Results
Extraction of propolis
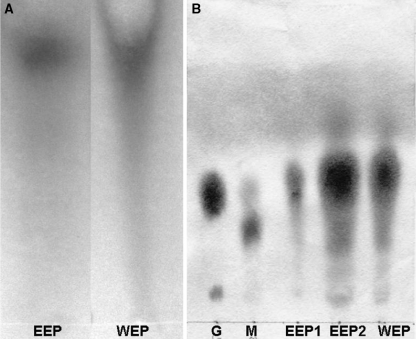
The ethanol extract of propolis was collected and consisted of near 35% propolis. The extract had a clear reddish color. Additions of buffer to the ethanol extract of propolis caused sedimentation of the low water-soluble materials, which are very sticky. After centrifugation of the mixture, the liquid phase was separated and consisted of near 7% of dry propolis and the sediment was weighted to be 28–30%. The paper chromatogram of the samples from different steps of extraction showed that 90% of carbohydrates in the ethanol extract of propolis were transferred into the water extract of propolis. Beside the colorimetric assay, the paper chromatogram for flavonoids confirmed in this experiment that near 80% of total flavonoids appeared in WEP (Fig. 1).
Fig. 1.
Paper chromatogram of flavonoids and sugars present in EEP and WEP samples. (A) Paper chromatogram of flavonoids, EEP = undiluted ethanol extract of propolis, WEP = undiluted water extract of propolis. Both samples were loaded in the same concentration according to the initial concentration of propolis. (B) paper chromatogram of sugars, G = glucose, M = maltose, EEP1 = EEP diluted 1/10, EEP2 = undiluted EEP, WEP = undiluted water extract
Antibacteral activity
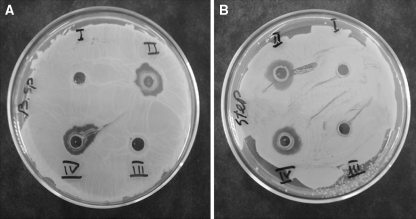
As shown in Fig. 2, both the water extracts of Chinese and Iranian propolis were able to inhibit growth of both bacteria (Bacillus subtilis and Staphylococcus aureus). The same results were observed for ethanol extracts of propolis (EEP) (data not shown). The aim of this experiment was to show that the water extracts of propolis in this method did not affect the biological activity of antibacterial compounds present in propolis.
Fig. 2.
Comparison of antibacterial activity of the water extracts of Chinese’s propolis with used propolis in this study. (A) Bacillus subtils, (B) Staphylococcus aureus were grown on agar medium and effect of both propolises on inhibition of the bacterium were studied. I = 10 μl 50 mM phosphate buffer, II = 10 μl of 10% the water extracts of Chinese’s propolis, III = 10 μl 96% ethanol, IV = 10 μl of 10% the water extracts of Iranian’s propolis. Incubation was performed at 37 °C for 20 h
Cell culture
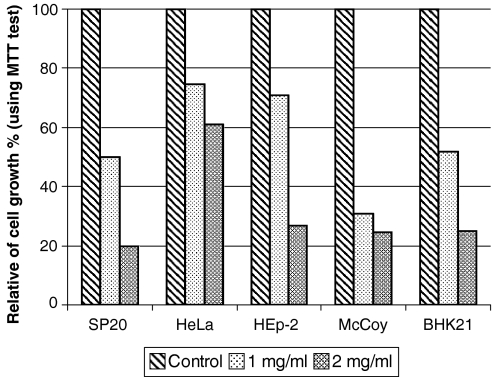
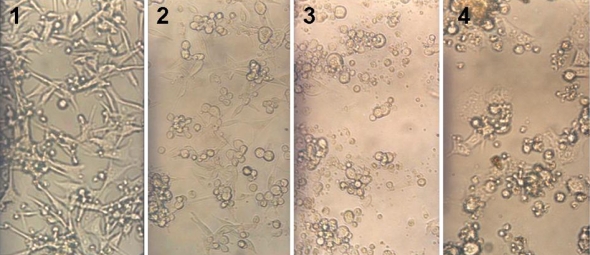
Cell culture results showed that there were differences between growths of different cells in WEP compare with the controls. In this experiment number of living cells was examined for McCoy, HeLa, SP2/0, HEp-2, and BHK21 cell growth (by MTT test) in RPpro medium showed a decrease in 1 and 2 mg/ml of WEP respectively, suggesting inhibitory effect of WEP on cell lines. As shown in Fig. 3 in 1 mg/ml of WEP maximum inhibition was seen for McCoy cells with near 70% and a minimum inhibition was observed for HeLa cells with near 30%. With increasing WEP concentration (2 mg/ml) maximum inhibition was seen for SP2/0 cells with 80% and minimum inhibition was seen for HeLa cells with near 40%. Direct observation of cells under inverted microscope showed the same results as the MTT test. For example in case of BHK21 as shown in Fig. 4, in 1 and 2 mg/ml of WEP reduction of cell number and change in cell morphology were observed clearly. Similar results were achieved for other cell lines (data not shown).
Fig. 3.
MTT test for different cell lines grown in RPMI 1640 medium and 5% FCS with incorporation of WEP. The cells were grown for 2 days at 37 °C
Fig. 4.
BHK21 cells were grown in RPMI 1640 medium and 5% FCS with incorporation of different concentrations of WEP. Cell cultures were performed for 2 days at 37 °C.1 = control, 2 = 0.2 mg/ml WEP, 3 = 1 mg/ml WEP, 4 = 2 mg/ml WEP
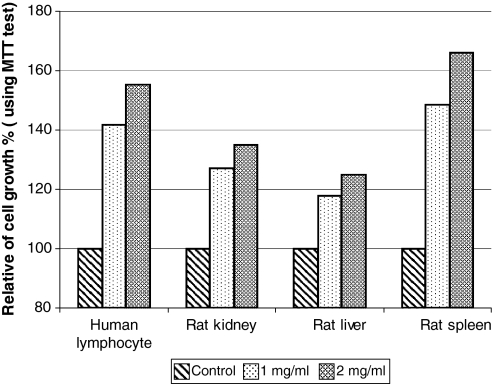
In case of normal cells used in this experiment (human lymphocyte, rat kidney, rat liver, and rat spleen) the results were different than with cell lines. As shown in MTT test results (Fig. 5) for normal cells, the same concentration of WEP used for treatment of cell lines could stimulate cell growth and final cell numbers. According to the MTT test in 1 mg/ml of WEP maximum increase was seen for rat spleen cell with 48% and minimum increase for cell growth was seen for rat liver cells with near 18%. When WEP concentration was increased up to 2 mg/ml in the medium, the normal cells showed faster rate of cell proliferation and increase in cell number. These results indicate that in 2 mg/ml of WEP the relative increase in cell number for rat spleen, human lymphocyte, rat kidney and rat liver cells were 65, 55, 35 and 25% respectively. Direct observations indicated that all normal cells grown in the presence of WEP had a longer life span compared to cell lines and the controls. These results showed that WEP enable to act as growth inhibitor for cell lines and growth stimulator for the normal cells.
Fig. 5.
MTT test for different normal cells grown in RPMI 1640 medium and 5% FCS with incorporation of two different concentrations of WEP for 2 days at 37 °C
Discussion
With respect to our best knowledge the water extraction method used in this work, is a new method for extraction of maximum water-soluble compounds in propolis. In this method most of flavonoids, vitamins, amino acids and other water-soluble compounds were released and remain free of wax and resin. Addition of buffer (under cold conditions) allows quick precipitation of wax and resin. This method is very simple when compared to other methods such as glycol extraction (GEP), aqueous (water) extraction (AEP), oil extraction (OEP), and water-soluble derivatives (WSD). Thus we believe that with this method not only most of the water-soluble parts of propolis are extracted but also that most of the resin and wax present in propolis are removed. The results can be used for various applications such as cell culture, injection, cosmetics etc. With respect to our results, since most of compounds in propolis are resin and wax, high concentrations of alcohol could be helpful for releasing soluble compounds from this sticky part. This experience was also reported previously (Sawaya et al. 2004). After complete solubilization of propolis, addition of water or buffer to decrease ethanol down to 50% helps to keep the soluble compounds in solution. In contrast almost all of the wax and resin present in propolis would precipitate in this concentration. There are advantages of this method in comparison to other methods, propolis solubilization is faster and more water-soluble compounds appear in the last step than for other methods. Although ethanol extracts of propolis have been found to be applicable for different purposes we believe that removing the wax, resin and alcohol would be very helpful for other applications such as cell culture and medical applications.
There are different reports, which indicate that the biological activity of propolis is due to the combination of different compounds present in propolis. Although each propolis has various biological activities, flavonoids are as key candidate compounds for evaluating the quality of propolis products. A convenient colorimetric method can be useful for the estimation of the real content of total flavonoids (Bankova et al. 2000; Chang et al. 2002; Salatino et al. 2005).
According to the results achieved in cell culture, it can be suggested that propolis with different types of compounds, specially phenolic acids and flavonoids, enable to control cell growth and distinguish the normal cell from the cancer cell. The effect of propolis on inhibition of different cell lines were reported for different cell lines such as K-562 (Aliyazicioglu et al. 2005) HL-60 cells (Akao et al. 2003; Mishima et al. 2005), MCF-7 human breast cancer cells (Luo et al. 2001) or some other leukemia cells (Hamblin 2006). There are number of reports indicating that some specific chemicals present in propolis such as caffeic acid phenethyl ester (CAPE) can inhibit growth of mutated cells without harming normal cells (Guarini et al. 1992; Rao et al. 1993). Propolis was also found to have a cytotoxic and cytostatic effect in vitro against hamster ovary cancer cells and sarcoma-type tumours in mice. The substance has also displayed cytotoxicity on cultures of human and animal tumour cells, including breast carcinoma, melanoma, colon, and renal carcinoma cell lines (Grunberger et al. 1988). The component producing these effects was identified as caffeic acid phenethy ester. A substance called Artepillin C has been isolated from propolis, and has been shown to have a cytotoxic effect on human gastric carcinoma cells, human lung cancer cells and mouse colon carcinoma cells in vitro (Kimoto et al. 2001). In addition, different researchers (Guarini et al. 1992; Rao et al. 1995) showed that propolis had strong cancer inhibitory effects against several cancers. Other reports related to these compounds indicated that inhibitory effects of propolis on cancer cells was due to an increase in apoptosis (Chiao et al. 1995; Su et al. 1995). The flavonoids present in propolis are powerful antioxidants, and have been shown to be capable of scavenging free radicals and thereby protecting lipids and other compounds such as Vitamin C from being oxidised or destroyed (Popeskovic et al. 1980). It is probable that active free radicals, together with other factors, are responsible for cellular ageing and degradation in such conditions as cardiovascular diseases, arthritis, cancer, diabetes, Parkinson’s disease and Alzheimer’s disease. Here we showed that if the extract of propolis would be used for some cancer treatment and can also act as cancer cell inhibitor, but it can also be useful as medium compliment for normal factors. Therefore if two groups of cells (cancer cells and normal cells) lay beside each other, treatment of the cells by WEP helps to kill the cancer cells and stimulate proliferation of normal cells during the treatment. By this method the dead cancer cells can be faster replaced with the normal cells than with other treatments.
Although there are number of reports for specific compounds in propolis responsible for the bioactivity of propolis against different diseases most scientists working with propolis believe that propolis contains a number of unidentified compounds which work together synergistically to create specificity for inhibition of cancer cells and proliferative support of normal cells. Although we don’t know what compounds are responsible for these phenomena and how these mechanisms work we can conclude that the extracts of propolis enable to act on each cell selectively and logically.
We also believe that each propolis has its own properties but all of them are useful for human life. If we look to history of propolis we can see that the Greek physician, Hippocrates (460–377 BC), found the healing properties of propolis and in different old medical books, propolis is known as natural medicine and was highly prized for its medicinal properties. However, it has only been in the last thirty years that scientists proved that propolis is an active and important medical substance. There are many open questions about propolis that need to be answered and we have to do more work to better define propolis and to find out what propolis is.
Acknowledgements
We thank Dr Kianizadeh and Dr Toroghi for their support and stimulating discussion. Also we are thankful from Kohe Dasht Company for preparation of natural and high quality of propolis.
References
- Akao Y, Maruyama H, Matsumoto K, Ohguchi K, Nishizawa K, Sakamoto T, Araki Y, Mishima S, Nozawa Y (2003) Cell growth inhibitory effect of cinnamic acid derivatives from propolis on human tumor cell lines. Biol Pharm Bull 26:1057–1059 [DOI] [PubMed]
- Aliyazicioglu Y, Deger O, Ovali E, Barlak Y, Hosver I, Tekelioglu Y, Karahan SC (2005) Effects of Turkish pollen and propolis extracts on respiratory burst for K-562 cell lines. Int Immunopharmacol 5:1652–1657 [DOI] [PubMed]
- Bankova VS, De Castro SL, Marcucci MC (2000) Propolis recent advances in chemistry and plant origin. Apidologie 31:3–15 [DOI]
- Brumfitt W, Hamilton-Miller JM, Franklin I (1990) Antibiotic activity of natural products: 1. Propolis. Microbios 62:19–22 [PubMed]
- Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Analysis 10:178–182
- Chiao C, Carothers AM, Grunberger D, Solomon G, Preston GA, Barrett JC (1995) Apoptosis and altered redox state induced by caffeic acid phenethyl ester (CAPE) in transformed rat fibroblast cells. Cancer Res 55:3576–3583 [PubMed]
- Dubois M, Gilles KA, Hamilton JK, Roberts DA, Smith F (1956) Colorimetric methods for determination of sugars and related substances. Anal Chem 28:350–356 [DOI]
- Grunberger D, Banerjee R, Eisinger K, Oltz EM, Efros L, Caldwell M, Estevez V, Nakanishi K (1988) Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia 44:230–232 [DOI] [PubMed]
- Guarini L, Su ZZ, Zucker S, Lin J, Grunberger D, Fisher PB (1992) Growth inhibition and modulation of antigenic phenotype in human melanoma and glioblastoma multiforme cells by caffeic acid phenethyl ester (CAPE). Cell Mol Biol 38:513–527 [PubMed]
- Hamblin T (2006) Natural products and the treatment of leukemia. Leuk Res 30:649–650 [DOI] [PubMed]
- Hausen BM, Evers P, Stuwe HT, Konig WA, Wollenweber E (1992) Propolis allergy (IV). Studies with further sensitizers from propolis and constituents common to propolis, poplar buds and balsam of Peru. Contact Derm 26:34–44 [DOI] [PubMed]
- Hu F, Hepburn HR, Li Y, Chen M, Radloff SE, Daya S (2005) Effects of ethanol and water extracts of propolis (bee glue) on acute inflammatory animal models. J Ethnopharmacol 100:276–283 [DOI] [PubMed]
- Ikeno K, Ikeno T, Miyazawa C (1991) Effects of propolis on dental caries in rats. Caries Res 25:347–351 [DOI] [PubMed]
- Khalil ML (2006) Biological activity of bee propolis in health and disease. Asian Pac J Cancer Prev 7:22–31 [PubMed]
- Kim JD, Liu L, Guo W, Meydani M (2006) Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. J Nutr Biochem 17:165–176 [DOI] [PubMed]
- Kimoto T, Koya-Miyata S, Hino K, Micallef MJ, Hanaya T, Arai S, Ikeda M, Kurimoto M (2001) Pulmonary carcinogenesis induced by ferric nitrilotriacetate in mice and protection from it by Brazilian propolis and artepillin C. Virchows Arch 438:259–270 [DOI] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed]
- Luo J, Soh JW, Xing WQ, Mao Y, Matsuno T, Weinstein IB (2001) PM-3, a benzo-gamma-pyran derivative isolated from propolis, inhibits growth of MCF-7 human breast cancer cells. Anticancer Res 21:1665–1671 [PubMed]
- Marcucci MC, Ferreres F, Garcia-Viguera C, Bankova VS, De Castro SL, Dantas AP, Valente PH, Paulino N (2001) Phenolic compounds from Brazilian propolis with pharmacological activities. J Ethnopharmacol 74:105–112 [DOI] [PubMed]
- Marinova D, Ribarova F, Atanassova M (2005) Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metall 40:255–260
- Markham KR (1982) Techniques of flavonid Identification. Academic press, London
- Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428 [DOI]
- Mishima S, Narita Y, Chikamatsu S, Inoh Y, Ohta S, Yoshida C, Araki Y, Akao Y, Suzuki KM, Nozawa Y (2005) Effects of propolis on cell growth and gene expression in HL-60 cells. J Ethnopharmacol 99:5–11 [DOI] [PubMed]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63 [DOI] [PubMed]
- Noelker C, Bacher M, Gocke P, Wei X, Klockgether T, Du Y, Dodel R (2005) The flavanoide caffeic acid phenethyl ester blocks 6-hydroxydopamine-induced neurotoxicity. Neurosci Lett 383:39–43 [DOI] [PubMed]
- Orsi RO, Sforcin JM, Funari SR, Bankova V (2005) Effects of Brazilian and Bulgarian propolis on bactericidal activity of macrophages against Salmonellatyphimurium. Int Immunopharmacol 5:359–368 [DOI] [PubMed]
- Popeskovic D, Kepcija D, Dimitijevic D, Stojanovic N (1980) The antioxidative properties of propolis and some of its components. Acta veterinaria (Beograd) 30:133–136
- Rao CV, Desai D, Rivenson A, Simi B, Amin S, Reddy BS (1995) Chemoprevention of colon carcinogenesis by phenylethyl-3-methylcaffeate. Cancer Res 55:2310–2315 [PubMed]
- Rao CV, Desai D, Simi B, Kulkarni N, Amin S, Reddy BS (1993) Inhibitory effect of caffeic acid esters on azoxymethane-induced biochemical changes and aberrant crypt foci formation in rat colon. Cancer Res 53:4182–4188 [PubMed]
- Ribeiro LR, Mantovani MS, Ribeiro DA, Salvadori DM (2006) Brazilian natural dietary components (annatto, propolis and mushrooms) protecting against mutation and cancer. Hum Exp Toxicol 25:267–272 [DOI] [PubMed]
- Robyt J, French D (1963) Action pattern and specificity of an amylase from Bacillus subtilis. Arch Biochem Biophys 100:451–467 [DOI] [PubMed]
- Salatino A, Teixeira EW, Negri G, Message D (2005) Origin and chemical variation of brazilian propolis. Evid Based Complement Alternat Med 2:33–38 [DOI] [PMC free article] [PubMed]
- Sawaya ACHF, Souza KS, Marcucci MC, Cunha IBS, Shimizu MT (2004) Analysis of the composition of Brazilian propolis extracts by chromatography and evaluation of their in vitro activity against Gram-positive bacteria. Braz J Microbiol 35:104–109 [DOI]
- Scheller S, Gazda G, Krol W, Czuba Z, Zajusz A, Gabrys J, Shani J (1989a) The ability of ethanolic extract of propolis (EEP) to protect mice against gamma irradiation. Z Naturforsch [C] 44:1049–1052 [DOI] [PubMed]
- Scheller S, Krol W, Swiacik J, Owczarek S, Gabrys J, Shani J (1989b) Antitumoral property of ethanolic extract of propolis in mice-bearing Ehrlich carcinoma, as compared to bleomycin. Z Naturforsch [C] 44:1063–1065 [DOI] [PubMed]
- Su ZZ, Lin J, Prewett M, Goldstein NI, Fisher PB (1995) Apoptosis mediates the selective toxicity of caffeic acid phenethyl ester (CAPE) toward oncogene-transformed rat embryo fibroblast cells. Anticancer Res 15:1841–1848 [PubMed]