Abstract
Tryptophan alleles in COL9A2 (Trp2) and COL9A3 (Trp3) have been linked to lumbar disc diseases in the Finnish population. Although such diseases consist of various pathogenetically different conditions, detailed analysis of each has not been well documented. The aim of this study was to clarify whether the collagen IX tryptophan alleles influence the symptomatic degeneration of the lumbar disc in Japanese patients with herniated nucleus pulposus. We performed a prospective study of 84 patients who underwent lumbar discectomy. The degree of disc degeneration was evaluated by magnetic resonance images in relation to the collagen IX genotype. Twenty patients (21.4%) had the Trp2 allele and no patients had the Trp3 allele. Patients under 40 years with the Trp2 allele showed more severe disc degeneration at the surgical level than did those without the Trp2 allele (odds ratio 6.00, P=0.043). In contrast, patients aged 40 years or over did not show significant association between disc degeneration and collagen IX genotype. Our results suggest that the Trp2 allele is an age-dependent risk factor for the severity of disc degeneration in younger patients with symptomatic herniated nucleus pulposus of the lumbar spine.
Résumé
Les allèles tryptophane COL9A2 (Trp2) et COL9A3 (Trp3) ont été chainés à la pathologie discale dans la population finlandaise. Bien que cette pathologie reconnaisse des pathogénies bien différentes, l’analyse détaillée de chacune n’a pas été faite. Le but de cette étude était de clarifier si l’allèle tryptophane du collagène IX influençait la dégénérescence du disque lombaire chez les patients japonais porteurs de hernie discale. Le degré de dégénérescence discale était apprécié par IRM en relation avec leur génotype du collagène IX. Vingt patients (21,4%) avaient l’allèle Trp2 et aucun n’avait le Trp3. Les patients de moins de quarante ans avec le Trp2 avaient une dégénérescence discale plus marquée au niveau opéré que ceux sans l’allèle (odds ratio 6.00, p=0,043). En revanche les patients de plus de quarante ans ne montraient pas d’association entre la dégénérescence et le génotype du collagène IX. Nos résultats suggèrent que l’allèle Trp2 est un facteur de risque âge-dépendant pour l’importance de la dégénérescence discale chez les jeunes patients avec un hernie discale lombaire symptomatique.
Introduction
The aetiology of lumbar disc diseases is complicated, with various environmental risk factors such as age, gender, occupation (lifting heavy loads), cigarette smoking, height, weight, and exposure to vehicular vibration [3, 21]. Previous studies have also suggested a significant contribution of genetic factors, such as collagen IX, aggrecan and MMP3 [1].
Collagen IX is a relatively minor component of the nucleus pulposus, which is integral to the proper formation of the collagen types II/IX/XI heteropolymer [6]. Transgenic mice expressing mutant alpha 1(IX) collagen were reported to have accelerated disc degeneration [10]. Ala-Kokko and others reported the association between allelic variants in the collagen IX genes, COL9A2 and COL9A3, and degenerative lumbar disc diseases [2, 9, 11, 13, 14, 17]. Both of the variants predict introduction of a bulky tryptophan residue into the triple-helical region of COL9A2 (Trp2) and COL9A3 (Trp3). It is well known that genetic variations are influenced by the population examined. For example, the frequency of the Trp alleles in patients with intervertebral disc diseases was different among the Finnish, the Greeks and the Chinese [7, 8]. Although degenerative disc diseases (DDDs) consist of aetiologically and pathologically different conditions, detailed analysis of each has not been established. Therefore, we aimed to clarify whether collagen IX tryptophan alleles influence the symptomatic degeneration of the lumbar discs and studied Japanese patients with herniated nucleus pulposus who underwent discectomy.
Materials and methods
Patients
Between June 2004 and October 2005, 84 consecutive patients who underwent lumbar disc nucleotomy for lumbar nucleus pulposus at Takamatsu Red Cross Hospital were included in this study. Seventy-nine patients underwent micro-endoscopic discectomy according to the procedure described by Perez-Cruet et al. [15], while five patients underwent conventional discectomy. All patients were Japanese and had low back pain and/or sciatica after a series of conservative treatments. The study protocol was approved by the Ethics Committees of Takamatsu Red Cross Hospital and The University of Tokushima Graduate School. Written informed consent was obtained from all patients before participation in the study.
Genetic analysis
Blood samples were collected from the patients for analysis of COL9A2 and COL9A3. Genomic DNA was extracted from 2 ml of peripheral blood using gene trapping by liquid extraction (GenTLE; TaKaRa, Shiga, Japan). Primers for DNA amplification and sequencing were designed manually, according to the genomic sequence flanking exon 19 of the COL9A2 or exon 3 of the COL9A3[12]. The 20 μl of reaction mixture contained genomic DNA (10 ng), standard polymerase chain reaction (PCR) buffer, dNTPs (2 nM each), Ampli Taq Gold (0.1 μl), and the primer pair (10 μM each). PCRs were performed in a GeneAmp PCR system 9700 (Applied Biosystems, Foster City, Calif., USA) with an initial denaturation at 95°C for 10 min, followed by 35 cycles at 95°C for 30 s, 56°C for 10 s and 72°C for 30 s, and a final step at 72°C for 5 min. Five microliters of PCR products were treated with two units of shrimp alkaline phosphatase and 10 units of exonuclease I (Amersham, Buckinghamshire, UK) at 37°C for 15 min, followed by incubation at 80°C for 15 min for enzyme inactivation. Sequencing reactions were performed using ABI Prism BigDye Terminator kit (Applied Biosystems) in a GeneAmp PCR system 9700 with an initial denaturation at 96°C for 5 min, followed by 25 cycles at 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min. Products were purified using Sephadex G-50 Fine (Amersham) and MultiScreen-PCR filter plate (Millipore, Bedford, Mass., USA). Purified products were analysed on an ABI Prism 3100 multicapillary sequencer (Applied Biosystems).
Clinical assessment
The severity of the clinical symptoms was calculated using a scoring system for lumbar disease, which contains three categories: (1) subjective symptoms (low back pain, leg pain and gait); (2) clinical signs (straight-leg-raising test, sensory disturbance and motor disturbance); (3) urinary bladder function. The intensity of low back pain before surgery was assessed by 100-mm visual analog scale (VAS). We also evaluated environmental risk factors of disc degeneration, including occupation (lifting heavy loads), obesity [body mass index (BMI) over 25] and smoking.
Magnetic resonance imaging equipment and protocol
Magnetic resonance (MR) images of the lumbar spine were obtained with a 1.5-T instrument (Gyroscan ACS-NT, Philips, Holland) with a surface coil. T2-weighted sagittal images were obtained for evaluation, with repetition/echo times of 2,000/110 ms, an acquisition matrix of 256×256 pixels and a slice thickness/inter-slice gap of 5/0.5 mm.
Assessment of the magnetic resonance images and plain radiographs
The degree of the intervertebral disc degeneration was evaluated by MR images in all patients according to the classification described by Schneiderman et al. [16]. In this, signal intensity of the discs on the midline sagittal section was scored as follows: grade 1 (normal), normal height and signal intensity; grade 2 (intermediate), speckled pattern or heterogeneous, decreased, signal intensity; grade 3 (marked), diffuse loss of signal; grade 4 (absent), signal void (Fig. 1). All the MR images were analysed by experienced orthopaedic physicians with no information about the genetic analysis.
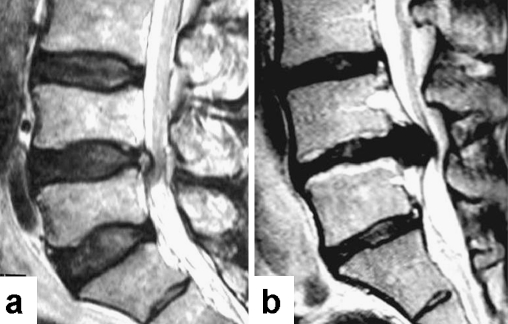
Fig. 1.
T2-weighted MR images of the lumbar spine. a A 54-year-old Trp2(−) patient showing grade 2 disc degeneration at the level of L4/5. b A 29-year-old Trp2(+) patient showing grade 4 disc degeneration at the level of L4/L5
Statistical analysis
The subjects were divided into two groups: Trp2(+) and Trp2(−). The statistical difference between two genotypes was analysed using the Mann–Whitney U test. The statistical differences of other assessments were analysed using the χ2 test. Fisher’s exact test was also used when any expected cell count was less than 5. A P value of less than 0.05 was considered significant.
Results
Study population and comparison of patients with and without the Trp2 allele
Eighteen of the 84 patients (21.4%) had the Trp2 allele, and no patient had the Trp3 allele (Table 1). Clinical characteristics were analysed in relation to the type IX collagen tryptophan allele. No significant differences were detectable between the presence of the Trp2 allele and age, gender, scoring system for lumbar disease, VAS, obesity (BMI over 25), occupation (lifting heavy loads), or smoking.
Table 1.
Distribution of the Trp2 allele according to selected characteristics of the study subjects
| Characteristics | Total | Trp2(+) | Trp2(−) | P |
|---|---|---|---|---|
| Patients (%) | 84 | 18 (21.4%) | 66 (78.6%) | |
| Age (years) | ||||
| Mean | 43.4 | 40.6 | 44.2 | 0.426 |
| Gender | ||||
| Female (%) | 19 (22.6%) | 5 (27.8%) | 14 (21.2%) | 0.540 |
| Male (%) | 65 (77.4%) | 13 (72.2%) | 52 (78.8%) | |
| BMI | ||||
| Mean | 23.8 | 22.6 | 24.2 | |
| <25 (%) | 26 (31.0%) | 4 (22.2%) | 22 (33.3%) | 0. 566 |
| VAS (1∼100 mm) | ||||
| Mean | 68.8 | 85.3 | 64.4 | 0.059 |
| Occupation | ||||
| Lifting heavy loads (%) | 30 (35.7%) | 4 (22.2%) | 26 (39.4%) | 0.268 |
| Smoking (%) | 44 (52.4%) | 9 (50.0%) | 35 (53.0%) | >0.999 |
Comparison of patients based on the disc degeneration at the surgical level
Environmental risk factors including obesity (BMI over 25), occupation (lifting heavy loads), smoking, and a genetic risk factor, the tryptophan allele, were analysed in relation to the grading of degenerative disc findings on MR images at the surgical level. Specifically, patients with grade 2 or 3 degeneration were compared with patients with grade 4 degeneration. As a total, no significant differences were observed (data not shown). However, patients aged under 40 years with the Trp2 allele had more severe disc degeneration at the surgical level (odds ratio 6.00, P=0.043) (Table 2). In contrast, patients aged 40 years or over showed no significant differences (Table 3).
Table 2.
Grading of degenerative disc findings on MR images at the surgical level in patients aged under 40 years
| Characteristics | Total | Grade 2 or 3 | Grade 4 | Odds ratioa | P |
|---|---|---|---|---|---|
| BMI | |||||
| 25<(%) | 13 (35.1%) | 9 (60.2%) | 4 (30.8%) | 0.74 | 0.734 |
| ≥25 (%) | 24 (64.9%) | 15 (62.5%) | 9 (37.5%) | ||
| Occupation | |||||
| Lifting heavy loads (%) | 13 (35.1%) | 7 (53.8%) | 6 (46.2%) | 2.08 | 0.302 |
| Desk workers (%) | 24 (64.9%) | 17 (70.8%) | 7 (29.2%) | ||
| Smoking | |||||
| Smokers (%) | 21 (56.8%) | 13 (61.9%) | 8 (38.1%) | 1.35 | 0.739 |
| Non-smokers (%) | 16 (43.2%) | 11 (68.7%) | 5 (31.3%) | ||
| Trp2 | |||||
| Trp2(+), (%) | 9 (24.3%) | 3 (33.3%) | 6 (66.7%) | 6.00 | 0.043b |
| Trp2(−), (%) | 28 (75.7%) | 21 (75.0%) | 7 (25.0%) |
aCalculated for 2×2 table for each risk factor (exposure) vs grading of degenerative disc findings, alpha=0.05
bFisher’s exact probability test, alpha=0.05
Table 3.
Grading of degenerative disc findings on MR images at the surgical level in patients aged 40 years and over
| Characteristics | Total | Grade 2 or 3 | Grade 4 | Odds ratioa | P |
|---|---|---|---|---|---|
| BMI | |||||
| <25 (%) | 13 (27.7%) | 8 (61.5%) | 5 (38.5%) | 0.89 | >0.999 |
| ≥25 (%) | 34 (72.3%) | 20 (58.8%) | 14 (41.2%) | ||
| Occupation | |||||
| Lifting heavy loads (%) | 17 (36.2%) | 9 (53.9%) | 8 (47.1%) | 1.54 | 0.486 |
| Desk workers (%) | 30 (63.8%) | 19 (63.3%) | 11 (36.7%) | ||
| Smoking | |||||
| Smokers (%) | 23 (48.9%) | 15 (65.2%) | 8 (34.8%) | 0.63 | 0.440 |
| Non-smokers (%) | 24 (51.1%) | 13 (54.2%) | 11 (45.8%) | ||
| Trp2 | |||||
| Trp2(+), (%) | 9 (19.1%) | 5 (55.6%) | 4 (44.4%) | 1.23 | >0.999 |
| Trp2(−), (%) | 38 (80.9%) | 23 (60.5%) | 15 (39.5%) |
aCalculated for 2×2 table for each risk factor (exposure) vs grading of degenerative disc findings, alpha=0.05
Discussion
In this study significant difference of the severity of disc degeneration at the surgical level was observed in the younger patients (aged under 40 years) when analysed for the Trp2 allele. Having the Trp2 allele resulted in a six-fold increase in the risk of severe disc degeneration in the Japanese population. In contrast, no patients had the Trp3 allele in our study, suggesting an ethnic difference of the polymorphism.
There are numerous qualitative methods of evaluating disc degeneration from MR images, such as disc space narrowing and reduction of signal intensity. MR findings of degenerative discs are clearly associated with age [4, 20]. Single-leveled disc space narrowing is more likely to reflect a traumatic or biomechanical origin than a systemic origin. Disc signal intensity seems to be one of the representative degenerative findings, on which Schneiderman’s classification stands, with a reduced signal intensity most highly associated with age. In this context our results, showing significant association between the Trp2 allele and reduction in signal intensity of the disc at the surgical level in younger patients, implying that Trp2 is an age-dependent risk factor for the severity of disc degeneration.
Our results, showing that the Trp2 allele predisposes carriers to develop severe disc degeneration under 40 years of age, contradict the Chinese study, which suggests that the predisposition relates to those over 40 years of age [7]. This difference might reflect the different subjects analysed in each study, i.e., Japanese patients with herniated discs who underwent discectomy in our study and Southern China volunteers (the general Chinese population of Hong Kong) in their study.
Despite many reports on an association between MRI-based disc degeneration and environmental risk factors, our study did not detect any significance for obesity (BMI over 25), occupation (lifting heavy loads) or smoking. These results may reflect the multifactorial nature of DDDs, in which various environmental factors and many genes are expected to act together to determine the degenerative phenotype. In some populations, the contribution of each factor for DDDs may not be recognised.
Since we exclusively analysed patients who underwent lumbar discectomy for herniated nucleus pulposus, we cannot tell whether the Trp2 allele is associated with an increased risk of the herniated nucleus pulposus in general. It is possible that the risk of herniated nucleus pulposus is much higher in Trp2(+) individuals than in Trp2(−) individuals, or that Trp2(+) patients are more likely to develop symptoms that require surgical treatment. The latter possibility is less likely, because the frequency of Trp2(+) patients in the study (21.4%) was similar to the population frequency in the Chinese (19.9%) [7]. Nevertheless, our findings that patients aged under 40 years with the Trp2 allele had more severe disc degeneration suggest that having Trp2 allele may accelerate the onset of the disc degeneration. The premature disc degeneration may lead to spondylolisthesis, which is linked to the tryptophan polymorphisms [11].
A number of limitations in the current study need to be considered, such as the small series of patients and the marginal significance in statistics. It is important to see whether our results are reproducible in a large series or in a different population.
The nucleus pulposus has a collagen network similar to that of hyaline cartilage. The collagen network is a cross-linked co-polymer of collagens type II, IX and XI [22]. Type IX collagen, a heterotrimer of genetically distinct α1(IX), α2(IX) and α3(IX) chains, consists of three triple-helical domains, COL1, COL2 and COL3, and four non-helical domains, NC1–NC4. Human mutations of collagen IX genes, which predict in-frame deletion of a part of the COL3 domain, produce a chondrodysplasia phenotype, multiple epiphyseal dysplasias, featuring early onset osteoarthritis [18]. In contrast, type IX collagen tryptophan polymorphisms are not linked to the degenerative joint disorders such as osteoarthritis and rheumatoid arthritis [19]. Although the reason for this gap is currently unknown, we have recently shown that Trp2-containing collagen IX is present in the cartilage matrix [12]. The Trp2 locates in the middle of α2(IX) COL2, within a few residues of the site of cross-linking between the α3(IX) chain and the type II collagen [5, 23], possibly interfering with this molecular interaction. Therefore, individuals with Trp2 allele may have a less cross-linked collagen network and may allow more enzymatic cleavage of the disc matrix.
In conclusion, our study demonstrates a novel association between the type IX collagen Trp2 allele and severe disc degeneration in younger patients with symptomatic herniated nucleus pulposus of the lumbar spine. Biochemical analysis of collagen IX containing disc matrix in relation to Trp2 allele may clarify the pathological bases of this genetic risk factor.
Acknowledgments
This work was supported, in part, by grants from the Japan Orthopedics and Traumatology Foundation (grant no. 0142), the Japan Society for Promotion of Science (grant no. 16591496), the Uehara Memorial Foundation, and the Nakatomi Foundation.
References
- 1.Ala-Kokko L (2002) Genetic risk factors for lumbar disc disease. Ann Med 34:42–47 [DOI] [PubMed]
- 2.Annunen S, Paassilta P, Lohiniva J, Perala M, Pihlajamaa T, Karppinen J, Tervonen O, Kroger H, Lahde S, Vanharanta H, Ryhanen L, Goring HH, Ott J, Prockop DJ, Ala-Kokko L (1999) An allele of COL9A2 associated with intervertebral disc disease. Science 285:409–412 [DOI] [PubMed]
- 3.Battie MC, Videman T, Gibbons LE, Manninen H, Gill K, Pope M, Kaprio J (2002) Occupational driving and lumbar disc degeneration: a case–control study. Lancet 360:1369–1374 [DOI] [PubMed]
- 4.Battie MC, Videman T, Parent E (2004) Lumbar disc degeneration: epidemiology and genetic influences. Spine 29:2679–2690 [DOI] [PubMed]
- 5.Diab M, Wu JJ, Eyre DR (1996) Collagen type IX from human cartilage: a structural profile of intermolecular cross-linking sites. Biochem J 314:327–332 [DOI] [PMC free article] [PubMed]
- 6.Eyre D (2002) Collagen of articular cartilage. Arthritis Res 4:30–35 [DOI] [PMC free article] [PubMed]
- 7.Jim JJ, Noponen-Hietala N, Cheung KM, Ott J, Karppinen J, Sahraravand A, Luk KD, Yip SP, Sham PC, Song YQ, Leong JC, Cheah KS, Ala-Kokko L, Chan D (2005) The TRP2 allele of COL9A2 is an age-dependent risk factor for the development and severity of intervertebral disc degeneration. Spine 30:2735–2742 [DOI] [PubMed]
- 8.Kales SN, Linos A, Chatzis C, Sai Y, Halla M, Nasioulas G, Christiani DC (2004) The role of collagen IX tryptophan polymorphisms in symptomatic intervertebral disc disease in Southern European patients. Spine 29:1266–1270 [DOI] [PubMed]
- 9.Karppinen J, Paakko E, Paassilta P, Lohiniva J, Kurunlahti M, Tervonen O, Nieminen P, Goring HH, Malmivaara A, Vanharanta H, Ala-Kokko L (2003) Radiologic phenotypes in lumbar MR imaging for a gene defect in the COL9A3 gene of type IX collagen. Radiology 227:143–148 [DOI] [PubMed]
- 10.Kimura T, Nakata K, Tsumaki N, Miyamoto S, Matsui Y, Ebara S, Ochi T (1996) Progressive degeneration of articular cartilage and intervertebral discs. An experimental study in transgenic mice bearing a type IX collagen mutation. Int Orthop 20:177–181 [DOI] [PubMed]
- 11.Matsui Y, Mirza SK, Wu JJ, Carter B, Bellabarba C, Shaffrey CI, Chapman JR, Eyre DR (2004) The association of lumbar spondylolisthesis with collagen IX tryptophan alleles. J Bone Joint Surg Br 86:1021–1026 [DOI] [PubMed]
- 12.Matsui Y, Wu JJ, Ann Weis M, Pietka T, Eyre DR (2003) Matrix deposition of tryptophan-containing allelic variants of type IX collagen in developing human cartilage. Matrix Biol 22:123–129 [DOI] [PubMed]
- 13.Noponen-Hietala N, Kyllonen E, Mannikko M, Ilkko E, Karppinen J, Ott J, Ala-Kokko L (2003) Sequence variations in the collagen IX and XI genes are associated with degenerative lumbar spinal stenosis. Ann Rheum Dis 62:1208–1214 [DOI] [PMC free article] [PubMed]
- 14.Paassilta P, Lohiniva J, Goring HH, Perala M, Raina SS, Karppinen J, Hakala M, Palm T, Kroger H, Kaitila I, Vanharanta H, Ott J, Ala-Kokko L (2001) Identification of a novel common genetic risk factor for lumbar disk disease. JAMA 285:1843–1849 [DOI] [PubMed]
- 15.Perez-Cruet MJ, Foley KT, Isaacs RE, Rice-Wyllie L, Wellington R, Smith MM, Fessler RG (2002) Microendoscopic lumbar discectomy: technical note. Neurosurgery 51:S129–S136 [PubMed]
- 16.Schneiderman G, Flannigan B, Kingston S, Thomas J, Dillin WH, Watkins RG (1987) Magnetic resonance imaging in the diagnosis of disc degeneration: correlation with discography. Spine 12:276–281 [DOI] [PubMed]
- 17.Solovieva S, Lohiniva J, Leino-Arjas P, Raininko R, Luoma K, Ala-Kokko L, Riihimaki H (2002) COL9A3 gene polymorphism and obesity in intervertebral disc degeneration of the lumbar spine: evidence of gene–environment interaction. Spine 27:2691–2696 [DOI] [PubMed]
- 18.Takahashi M, Matsui Y, Goto T, Nishimura G, Ikegawa S, Ohashi H, Yasui N (2006) Intrafamilial phenotypic diversity in multiple epiphyseal dysplasia associated with a COL9A2 mutation (EDM2). Clin Rheumatol Jan 27;:1–5 [Epub ahead of print] [DOI] [PubMed]
- 19.Takata Y, Matsui Y, Hamada D, Goto T, T. K, Egawa H, Nakano S, Shinomiya F, Inoue H, Itakura M, Yasui N (2005) The alpha 2 type IX collagen gene tryptophan polymorphism is not associated with rheumatoid arthritis in the Japanese population. Clin Rheumatol Oct 25;:1–4 [Epub ahead of print] [DOI] [PubMed]
- 20.Videman T, Battie MC, Gill K, Manninen H, Gibbons LE, Fisher LD (1995) Magnetic resonance imaging findings and their relationships in the thoracic and lumbar spine. Insights into the etiopathogenesis of spinal degeneration. Spine 20:928–935 [DOI] [PubMed]
- 21.Videman T, Sarna S, Battie MC, Koskinen S, Gill K, Paananen H, Gibbons L (1995) The long-term effects of physical loading and exercise lifestyles on back-related symptoms, disability, and spinal pathology among men. Spine 20:699–709 [DOI] [PubMed]
- 22.Wu JJ, Eyre DR (2003) Intervertebral disc collagen. Usage of the short form of the alpha1(IX) chain in bovine nucleus pulposus. J Biol Chem 278:24521–24525 [DOI] [PubMed]
- 23.Wu JJ, Woods PE, Eyre DR (1992) Identification of cross-linking sites in bovine cartilage type IX collagen reveals an antiparallel type II-type IX molecular relationship and type IX to type IX bonding. J Biol Chem 267:23007–23014 [PubMed]