Abstract
Treatment of non-unions and delayed unions often requires osteogenic material. Recently, a biomimetic bone matrix that simulates the cellular environment of hard tissue, identified as P-15, was introduced to the orthopaedic community. A total of 22 patients with mal-union or delayed union fractures was treated from June 2000 to October 2003 with P15- bone graft substitute (P15-BGS) in the site of fracture and mostly with internal fixation. Patients were examined by independent radiographic analysis. Assessment criteria included time elapsed until bone bridging and time to full consolidation. In addition, histological assessment of the callus was done at the time of recovery of metal implants in five patients. Full consolidation was achieved in 90% (20 out of 22) of the patients treated with P15-BGS. The average time for full consolidation was 4.2 months. Histological assessment of the fracture callus in five of the patients confirmed the positive clinical and radiographic results. P15-BGS appears to offer a safe, economical and clinically useful alternative to autograft in the repair of ununited fractures. These results compare favourably with those in the published literature as an alternative to autograft.
Résumé
le traitement des pseudarthroses ou des retards de consolidation nécessite le plus souvent l’utilisation de matériel ostéogénique. Récemment un nouveau composant stimulant l’environnement cellulaire osseux a été développé (P-15TM) et introduit en orthopédie. 22 patients présentant un retard de consolidation après fracture ont été traités entre juin 2000 et octobre 2003 avec ce substitut P15 (P15-BGS), implanté au niveau du site de la pseudarthrose et le plus souvent associé à une fixation interne. Les patients ont été évalués par une analyse radiographique indépendante. Les critères étudiés ont été le temps nécessaire à obtenir un pont d’ossification et le temps complet de la consolidation. De plus, une évaluation histologique du cal a été pratiquée chez 5 patients hospitalisés pour une ostéosynthèse secondaire. La consolidation complète a été obtenue dans 90% des cas (20 sur 22), patients traités avec P15-BGS. Le temps moyen de consolidation a été de 4,2 mois. Les examens histologiques chez ces 5 patients ont confirmé le bon résultat clinique et histologique. P15-BGS apparaît comme une possibilité relativement sûre, économique et utile cliniquement. Il s’agit d’une bonne alternative à l’autogreffe dans la réparation des pseudarthroses. Ces résultats comparés à la littérature confirment qu’il s’agit d’une alternative à l’autogreffe.
Introduction
Surgical treatment of non-unions and delayed unions often requires autogenous bone graft to re-establish skeletal integrity. A common problem is the limited quantity of material available. Even if the non-union is stabilised with a mechanical device, adequate osteogenic material is required to establish the hard tissue continuum [18].
Considerable research has gone into the development of bone graft substitute materials that can replace or augment autogenous bone grafts. These biocompatible materials fall into the broad categories of being either osteo-inductive or osteo-conductive. Some materials are able to support functional loads, while others, generally granular in nature, are intended for use in conjunction with mechanical support devices [4].
Recently, a biomimetic bone matrix that simulates the cellular environment of hard tissue, identified as P-15, was introduced to the orthopaedic community [12]. P-15 is a synthetic, 15-amino-acid residue, identical to the sequence (766)GTPGPQGIAGQRGVV(780) found in the a1(I) chain of type I collagen [1]. Bhatnagar et al. have demonstrated that P-15, containing the potent cell-binding domain of collagen, can be adsorbed onto a calcium phosphate substrate [3] and will enhance cell attachment and extracellular matrix and factor production, resulting in the formation of bone [2, 19].
P-15 bone graft substitute (P15-BGS) is a combination of mineral component of bone and a peptide representing the cell-binding domain of type I collagen. The inorganic bone mineral component provides the necessary calcium phosphate and the natural anatomical matrix needed for cellular invasion. The P-15 component modulates cell binding, migration, proliferation, and differentiation, as well as the synthesis and secretion of extracellular matrix elements and factors that facilitate the production of bone [9, 11, 15].
The aim of this pilot study was to demonstrate that a P-15 biomimetic of autogenous bone graft could be used safely in the surgical repair of ununited fractures.
Materials and methods
This paper includes two case series reports of patients with delayed union and non-union fractures. Patients were treated by two different surgeons in two hospitals from June 2000 to October 2003, with a total of 22 patients, using P15-BGS at the non-union site.
The study protocol was approved by the Spanish Notified Body (D.G.F.P.S.), local administration ethics committees and the ethics and research committees of both hospitals. All patients signed the “consent agreement”.
Criteria for patients to be included were: (1) men or non-fertile women over the age of 18 years; (2) failure of previous surgical treatment, with loss of internal fixation, or lack of consolidation after at least 6 months from initial treatment; (3) no pathological condition with the potential to modify bone consolidation; (4) no sign of local or general infection.
A commercially available bone substitute (OsteoGraf/N-700, Dentsply Friadent CeraMed, Lakewood, Colo., USA), an inorganic bone mineral (ABM), was irreversibly complexed with a synthetic 15-amino-acid peptide (P-15) and sterilised in 3 g vials as previously described [1]. The product, ABM/P-15 (P15-BGS), provides a biomimetic of autogenous bone for repair of osseous defects [2, 3]. In two patients in series I, particulate P15-BGS was suspended in an inert hydrogel (PepGen P-15 Flow, Dentsply Friadent CeraMed). The hydrogel formulation circumvented issues related to particulate migration and allowed ease of handling and delivery to the osseous defect.
Series I included nine patients, two with delayed unions with failure of the internal fixation and seven non-unions. There were seven men and two women, with average age of 50 years (range 18–72 years). Three non-unions occurred in the femoral diaphysis, two in the humeral diaphysis, one in the tibial diaphysis, two in the distal tibial metaphysis, one in the proximal tibial metaphysis and one in the radial diaphysis. Series II included 13 non-union patients, eight men and five women, with an average age of 52 years (range 26–74 years). Four non-unions occurred in the femoral diaphysis, four in the humeral diaphysis, three in the tibial diaphysis, one in the distal femoral metaphysis, one in the proximal tibial diaphysis, and one in the distal tibial metaphysis. All patients were treated with internal fixation and one vial (3 g) of P15-BGS. In five patients in series I, P15-BGS was placed only on one side of the non-union, either anterior or posterior, so that we could observe whether a different callus was formed with P15-BGS.
Fixation included screw-on plates, nail plates and plaster in one patient, who had presented with severe osteoporosis (Sudeck syndrome), making internal fixation impossible. The average time elapsed from initial fracture to treatment of the non-union with P15-BGS was 14 months (range 2–58 months). Patients’ demographics, pre-treatment(s), treatment and time to treatment are summarised in Table 1.
Table 1.
Summary of patients’ demographics, pre-treatment(s), treatment and time to treatment (M male, F female)
| Case no. | Age (years) | Gender | Site | Previous treatments for non-union | Treatment associated with P15 | Time to treatment from initial fracture (months) |
|---|---|---|---|---|---|---|
| Patient series I | ||||||
| I-1 | 58 | M | Femur | None | Plate | 2 |
| I-2 | 72 | M | Femur | None | 1° Nail-plate | 3 |
| Nail plate | 2° Nail plate | 15 | ||||
| I-3 | 44 | F | Femur | (5) 3 nails & 2 plates | 2 plates | 18 |
| I-4 | 57 | M | Humerus | (2) plate & explant | Plate | 24 |
| I-5 | 48 | F | Humerus | (3) nail | Nail plate | 58 |
| I-6 | 60 | M | Tibia | None | Plate | 14 |
| I-7 | 18 | M | Tibia | Plate recovered | Plate | 18 |
| I-8 | 40 | M | Radius | 1 plate | Plate | 16 |
| I-9 | 42 | M | Tibia | Plaster | Plaster | 7 |
| Patient series II | ||||||
| II-1 | 39 | M | Tibia | None | Plate | 15 |
| II-2 | 29 | M | Femur | None | Nail | 6 |
| II-3 | 69 | F | Humerus | None | Plate | 9 |
| II-4 | 67 | M | Tibia | None | Plate | 7 |
| II-5 | 26 | M | Tibia | None | Nail | 6 |
| II-6 | 55 | M | Humerus | None | Plate | 15 |
| II-7 | 47 | M | Tibia | None | Plate | 7 |
| II-8 | 59 | F | Femur | None | Nail | 7 |
| II-9 | 61 | F | Tibia | None | Plate | 15 |
| II-10 | 74 | F | Femur | None | Plate | 24 |
| II-11 | 47 | M | Femur | None | Plate | 12 |
| II-12 | 50 | F | Humerus | None | Hacketal nailing | 6 |
| II-13 | 56 | M | Humerus | None | Hacketal nailing | 6 |
Post-operative X-rays were used to assess fixation. This factor was considered important to help establish the effect of P-15 when adverse conditions of insufficient immobilisation were present.
All patients were examined clinically and radiographically every 2 weeks until the first callus appeared; beyond this point examination was performed monthly until full consolidation. Pre- and post-operative X-rays were evaluated by an independent surgeon with emphasis on: (1) fixation method used to treat the non-union; (2) time elapsed until the presence of bone bridges between main fragments; (3) time elapsed until full consolidation. Time elapsed until appearance of the initial bone bridge was assumed when some X-ray projections presented continuity between the main fragments. Full consolidation was assumed when X-rays showed callus across the full bone thickness.
We had the opportunity to histologically assess the efficacy of P15-BGS in patients I-1, I-2, I3, and II-2 and II-4. Samples of fracture callus were taken for histological examination at the time of removal of the metal implants. Samples were decalcified, stained with haematoxylin and eosin and examined by an independent histopathologist at the University of Barcelona. In patient I-1 biopsy was obtained at 4 months; in patient I-2, when the second plate was implanted at 12 months. In patients I-3, II-2 and II-4 the biopsy was obtained at 13 months.
Results
Series I
Full healing was achieved in eight of nine non-union patients. The exception was patient I-2, with a distal femoral fracture. At 52 weeks the plate failed, and a new plate, with additional P15-BGS material, was required; consolidation was achieved 3 months later. In the healed fractures, the average time elapsed until the presence of a first bone bridge was 2 months (range 1–3 months); in patient I-6 the time elapsed could not be determined because of a partial lack of follow-up. When patient I-2 was excluded, the average time for full consolidation was 5 months (range 2–9 months). (Table 2).
Table 2.
Fixation method used to treat the non-union, time elapsed until presence of bone bridges between main fragments and time elapsed till full consolidation
| Case no. | Age (years) | Gender | Site | Bone bridging (months) | Consolidation healing (months) |
|---|---|---|---|---|---|
| Patient series I | |||||
| I-1 | 58 | M | Femur | 2 | 3 |
| I-2 | 72 | M | Femur | None | None |
| 2.5 | 4 | ||||
| I-3 | 44 | F | Femur | 1 | 5 |
| I-4 | 57 | M | Humerus | 2.5 | 9 |
| I-5 | 48 | F | Humerus | 2 | 6 |
| I-6 | 60 | M | Tibia | Lost for 6 months | 6 |
| I-7 | 18 | M | Tibia | 3 | 4.5 |
| I-8 | 40 | M | Radius | 1 | 3 |
| I-9 | 42 | M | Tibia | 1.5 | 4.5 |
| Patient series II | |||||
| II-1 | 39 | M | Tibia | 1.5 | 3 |
| II-2 | 29 | M | Femur | 1 | 3.5 |
| II-3 | 69 | F | Humerus | None | None |
| II-4 | 67 | M | Tibia | 1.5 | 2 |
| II-5 | 26 | M | Tibia | 1 | 2 |
| II-6 | 55 | M | Humerus | 2 | 3 |
| II-7 | 47 | M | Tibia | 1 | 3 |
| II-8 | 59 | F | Femur | 1.5 | 2.5 |
| II-9 | 61 | F | Tibia | 1.5 | 2.75 |
| II-10 | 74 | F | Femur | 1.5 | 5 |
| II-11 | 47 | M | Femur | 2 | 3 |
| II-12 | 50 | F | Humerus | 2 | 3 |
| II-13 | 56 | M | Humerus | 2 | 3 |
When the non-union site was treated with internal fixation and P15-BGS, in six patients internal fixation was considered correct, and in two patients (I-4 and I-6), treated with plates and screws, the number of screws was considered insufficient in at least one of the fragments.
For the five patients where P15-BGS was placed only on one side of the non-union site, in order to observe differences in callus formation between the P15-BGS zone and the rest, no conclusions could be drawn because the X-ray density image of ABM (carrier for P-15) did not allow detailed visualisation of newly formed bone. At the time of consolidation no significant differences in the volume of callus could be observed between the P15-BGS side and the rest of the callus.
In the bone biopsy of patient I-1, 4 months after operation, many newly formed trabeculae were observed, with membrane ossification among the P15-BGS particles, sometimes surrounding them completely. A predominantly osteogenic tissue response existed, with few osteoclasts. Representative radiographic and histological assessments of the P15-BGS are illustrated in Figs. 1, 2, 3 and 4, demonstrating non-union stabilisation, follow-up, and histological sections from patient I-1. The biopsy in patient I-2, at 12 months of follow up, showed mature and dense bone, almost completely filling the space between the P15-BGS particles. The number of P15-BGS particles per field was smaller than in the previous case, with much smaller particles and numerous osteoclasts around the remaining P15-BGS. Biopsy of patient I-3 at 13 months demonstrated particles of P15-BGS surrounded by dense mature bone, although the newly formed bone was less dense than in patient I-2. Osteoclastic activity was less evident with less resorption of the P15-BGS particles.
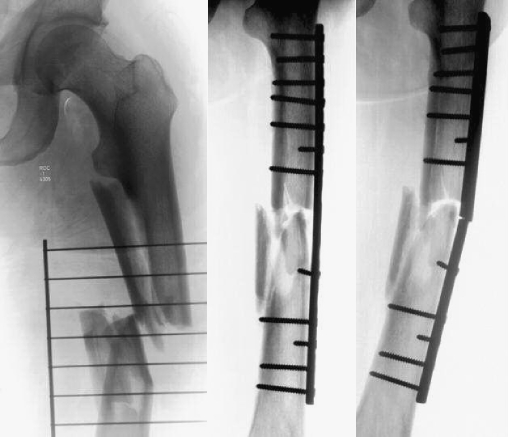
Fig. 1.
Radiographs of a 58-year-old male patient with initial treatment and subsequent plate failure at 2 months
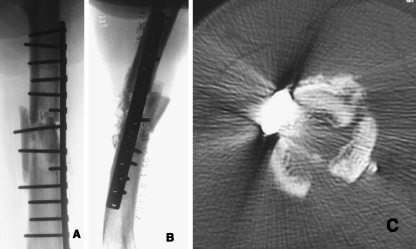
Fig. 2.
a,b Post-operative X-rays; c 2-week CT showing P15-BGS in the anterior cortical area
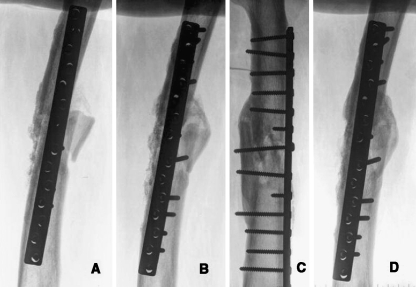
Fig. 3.
a One month after operation; b 3 months after operation; c,d 15 months after operation following treatment with P15-BGS
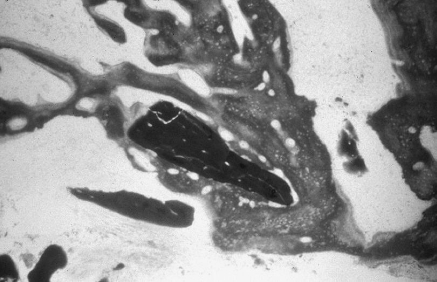
Fig. 4.
Histological section at 4 months showing a resorbing particle of P15-BGS surrounded by newly formed bone
In the two patients (I-7, I-8) treated with the particulate P15-BGS suspended in an inert hydrogel formulation, there appears to be no difference in the time to consolidation when compared with the other patients in this series.
Series II
Full consolidation was attained in 12 of the 13 non-unions treated with internal fixation and P15-BGS; one patient (II-3) had not achieved consolidation at 1 year and was lost to follow-up. The initial bone bridge was observed at an average time of 1.5 months (range 1–3.5 months). Consolidation time was 3.3 months (range 2–5 months) for the patients that achieved full fracture healing (Table 2). The bone callus observed was larger than in series I. The post-operative X-ray evaluation of internal fixation for treatment of the non-union indicated that, in the patients with intramedullary nailings (II-2, II-5 and II-9), fixation was correct; in all others, including those with plates or Hacketal nailings, internal fixation was insufficient, while, in one patient, no consolidation was achieved. Eleven of the patients with fracture healing achieved a very good functional result, with only one patient’s healing classified as poor.
Histological analysis of patients II-2 and II-4 revealed a mature callus undergoing active bone remodelling, with only a few small P15-BGS particles remaining.
Tibial non-unions (series I and II)
Although this study included a variety of non-union fractures, it is interesting to examine the reports in the literature specifically on tibial non-union treatment with autograft and recombinant bone morphogenetic proteins (rhBMPs). The average time to bone bridging was 1.5 months (range 1–3 months), and average time to consolidation was 3 months (range 2–4.5 months) for seven of the eight tibial non-unions (Table 3). Patient I-6 was not included in this analysis, owing to lack of follow-up.
Table 3.
Fixation method used to treat the non-union, time elapsed till presence of bone bridges between main fragments and time elapsed till full consolidation, for tibial non-union or delayed union
| Case no. | Age (years) | Gender | Site | Bone bridging (months) | Consolidation healing (months) |
|---|---|---|---|---|---|
| Patient series I | |||||
| I-6 | 60 | M | Tibia | Lost for 6 months | 6 |
| I-7 | 18 | M | Tibia | 3 | 4.5 |
| I-10 | 42 | M | Tibia | 1.5 | 4.5 |
| Patient series II | |||||
| II-1 | 39 | M | Tibia | 1.5 | 3 |
| II-4 | 67 | M | Tibia | 1.5 | 2 |
| II-5 | 26 | M | Tibia | 1 | 2 |
| II-7 | 47 | M | Tibia | 1 | 3 |
| II-9 | 61 | F | Tibia | 1.5 | 2.75 |
Discussion
Treatment of non-unions and delayed unions requires appropriate internal fixation and, in most cases, the addition of bone grafting materials that will enhance new bone formation. Historically, autogenous bone graft from the iliac crest has been selected because it provides an osteo-conductive structure, osteogenic cells (osteoblasts) and osteo-inductive proteins to enhance mitosis, as well as differentiation of non-differentiated cells into osteogenic type cells [17]. However, quantities of autogenous bone are limited, and, further, harvesting of autogenous bone is not without post-operative complications [20].
Numerous materials with osteo-conductive or osteo-inductive capacity have been used to improve new bone formation. These materials are available in unlimited quantities in the hope of eliminating or limiting the need for harvesting iliac crest bone grafts. In the past 10 years, osteo-inductive materials, such as bone morphogenetic proteins, have received much attention, mostly as a result of their capacity to improve proliferation of osteo-transforming cells derived from undifferentiated cells [7, 13, 14]. The rhBMPs have demonstrated healing rates in tibial non-unions similar to those in autogenous bone grafts from the iliac crest [8]. However, the results have not been as promising in other types of non-unions [16]. P15-BGS is a new approach to bone grafting, providing the specific sequence of type I collagen that is responsible for cell attachment, in particular fibroblasts and osteoblasts. The interaction between cells and the extracellular matrix plays a key role in the growth and differentiation of cells [10]. Basically, the P-15 on the ABM allows increased cellular viability in the inorganic bone matrix. The principal advantage of P15-BGS is that it includes only the specific cell-binding sequence of collagen, eliminating the immunologically reactive portion of the collagen structure [6]. P15-BGS was well tolerated in this pilot study, with no signs of inflammation or foreign body reactions observed in the histological studies. Whenever abundant osteoclasts were histologically observed, they were related to the mechanism of active resorption of the P15-BGS particles.
Histologically, the fracture callus observed in series II had a larger volume than that seen in series I. In addition, the appearance of initial bone bridging was earlier (1.5 months vs 2 months in series I), while full consolidation was also seen earlier (4 months vs 5 months in series I). These results are surprising, and the difference in expected outcome involves a number of factors: (1) non-unions in series II were older and had had multiple previous operations with very little success; (2) insufficient osteosynthesis in series II patients produced micromovements in the non-union site that stimulated external callus; (3) P15-BGS was uniformly distributed around the site, whereas, in series I, P15-BGS was placed only on one side in half of the patients.
In series I, where P15-BGS was placed on only one side of the non-union site, increased callus on the P15-BGS area was not observed, probably because more soft tissue had been released to ensure contact of the P15-BGS with the bone. Further experimental studies would be needed to confirm this hypothesis. The radiographic evaluation by the independent surgeon did not differ substantially from that of the operating surgeons, despite the added difficulty of evaluating initial bone bridging with X-ray image interference of radiopaque inorganic bone matrix. The graft interference was particularly difficult in series I, where the P15-BGS was concentrated on one side of the non-union site. In these patients, the first indication of union was the bone bridge that appeared on the side with no graft. When total consolidation time was determined, density and volume of bone callus were evaluated, together with the clinical situation of the patient. As a general rule, full healing was determined when dense fracture callus exhibited the same diameter as the neighbouring bone and the patient could perform full pain-free weight bearing on the lower extremity or exert a strong pain-free pulling force with the upper extremity.
The inorganic bone matrix (hydroxylapatite) to which the P-15 is bound creates the ideal conditions of biocompatibility, biodegradability and osteo-conduction. The use of an osteo-conductive bone substitute, however, may raise questions as to whether the result obtained could be due to the osteo-conductive effect of the matrix itself. The osteo-conductive capacity of ABM could, itself, induce callus formation, but it has been shown that its efficacy in non-unions is limited and the probabilities bringing about union are very slight [5]. The presence of P15-BGS particles in the histological samples from patients in this study with over 1 year of follow-up, indicates that the ABM maintains its scaffold role over long time periods.
In this series we used P15-BGS as a stand-alone material in order to evaluate its bone-forming capabilities. In one case, not included in this series, a female patient with a non-union of the femur, 6 months from fracture, P15-BGS was used with a bone autograft, and consolidation healing was achieved in 3 months, with no differences from similar patients II9, II10, II11. A prospective study will be needed to evaluate the possible synergistic capacity of P15-BGS with other substances such as autograft or BMP. It is worthy of note that P15-BGS was used in all patients, regardless of the fractured bone, age of the non-union, or delayed union, and no distinction has been made between atrophic and hypertrophic forms in failures of consolidation. The use of P15-BGS suspended in an inert hydrogel improves the handling characteristics of the graft, with no apparent differences in healing from that of the P15-BGS in particulate form. In contrast to growth factors, P15-BGS seems to be dose independent, but this needs to be confirmed.
P15-BGS was used in a case of periprosthetic osteolysis around the femoral stem, and bone density similar to that of the neighbouring bone was obtained in 4.5 months.
Although P15-BGS has been found to be statistically and clinically superior to both demineralised bone matrix (DBM) and synthetic calcium phosphate bone graft substitutes in the oral cavity, its use in clinical orthopaedics has not been explored [21, 22]. This pilot study is the first published report of the use of P15-BGS in human orthopaedic indications. It is worthy of note that the results of the subset of tibial non-union patients in this pilot study are comparable, if not superior, to the results reported by Friedlaender et al. [8] A randomised clinical trial will be necessary to confirm the increased efficacy of P15-BGS. In summary, in this pilot clinical study, P15-BGS appears to offer a safe, economical, and clinically useful alternative to autograft in the repair of ununited fractures.
References
- 1.Bhatnagar RS, Qian JJ, Gough CA (1997) The role in cell binding of a beta-bend within the triple helical region in collagen alpha 1 (I) chain: structural and biological evidence for conformational tautomerism on fiber surface. J Biomol Struct Dyn 14:547–560 [DOI] [PubMed]
- 2.Bhatnagar RS, Qian JJ, Wedrychowska A, Smith N (1998) In: Thomas, Mooney, Healy, Lkada, Mikos (eds) Construction of biomimetic environments with a synthetic analogue of collagen. Proceedings of the Materials Research Society, vol 530
- 3.Bhatnagar RS, Qian JJ, Wedrychowska A, Sadeghi M, Wu YM, Smith N (1999) Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng 5:53–65 [DOI] [PubMed]
- 4.Boden SD, Stevenson S (1999) Bone grafting and bone grafting substitutes. Orthop Clin N Amer 30:xi [DOI]
- 5.Cornell CN (1999) Osteoconductive materials and their role as substitutes for autogenous bone grafts. Orthop Clin North Am 30:591–598 [DOI] [PubMed]
- 6.DeLustro F, Dasch J, Keefe J, Ellingsworth L (1990) Immune responses to allogeneic and xenogeneic implants of collagen and collagen derivatives. Clin Orthop Relat Res 260:263–279 [PubMed]
- 7.Djapic T, Kusec V, Jelic M, Vukicevic S, Pecina M (2003) Compressed homologous cancellous bone and bone morphogenetic protein (BMP)-7 or bone marrow accelerate healing of long-bone critical defects. Int Orthop 27:326–330 [DOI] [PMC free article] [PubMed]
- 8.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S (2001) Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Jt Surg Am 83 [Suppl]:151–158 [PMC free article] [PubMed]
- 9.Hanks T, Atkinson BL (2004) Comparison of cell viability on anorganic bone matrix with or without P-15 cell binding peptide. Biomaterials 25:4831–4836 [DOI] [PubMed]
- 10.Jones PL, Schmidhauser C, Bissel MJ (1993) Regulation of gene expression and cell function by extracellular matrix. Crit Rev Eukaryot Gene Expr 2:137–154 [PubMed]
- 11.Kubler A, Neugebauer J, Oh JH, Scheer M, Zoller JE (2004) Growth and proliferation of human osteoblasts on different bone graft substitutes: an in-vitro study. Implant Dent 13:171–179 [DOI] [PubMed]
- 12.Orozco R (2004) A report on 10 difficult patients treated with P15. 41 Congresso Nacional SECOT, Madrid, Spain, 6 October 2004
- 13.Pecina M, Giltaij LR, Vukicevic S (2001) Orthopaedic applications of osteogenic protein-1 (BMP-7) (review). Int Orthop 25:203–208. No abstract available. PMID: 11561491 [PubMed - indexed for MEDLINE] [DOI] [PMC free article] [PubMed]
- 14.Pecina M, Haspl M, Jelic M, Vukicevic S (2003) Repair of a resistant tibial non-union with a recombinant bone morphogenetic protein-7 (rh-BMP-7). Int Orthop 27:320–321 [DOI] [PMC free article] [PubMed]
- 15.Qian JJ, Bhatnagar RS (1996) Enhanced cell attachment to anorganic bone mineral in the presence of a synthetic peptide related to collagen. J Biomed Mater Res 31:545–554 [DOI] [PubMed]
- 16.Suratwala S, Sinicropi S, Lee HJ et al (2005) Recombinant bone morphogenetic proteins are not sufficient to induce healing in difficult human non-unions. Poster no. 0785. Annual Meeting of the Orthopaedic Research Society, Washington, DC
- 17.Termaat MF, Den Boer FC, Bakker FC, Patka P, Haarman HJ (2005) Bone morphogenetic proteins. Development and clinical efficacy in the treatment of fractures and bone defects. J Bone Joint Surg Am 87:1367–1378 [DOI] [PubMed]
- 18.Wiss DA, Stetson WB (1996) Tibial nonunion: treatment alternatives. J Am Acad Orthop Surg 4:249–257 [DOI] [PubMed]
- 19.Yang XB, Bhatnagar RS, Li S, Oreffo RO (2004) Biomimetic collagen scaffolds for human bone cell growth and differentiation. Tissue Eng 10:1148–1159 [DOI] [PubMed]
- 20.Younger EM, Chapman MW (1989) Morbidity at bone graft donor sites. J Orthop Trauma 3:192–195 [DOI] [PubMed]
- 21.Yukna RA, Callan DP, Krauser JT, Evans GH, Aichelmann-Reidy ME, Moore K, Cruz R, Scott JB (1998) Multi-center clinical evaluation of combination anorganic bovine derived hydroxyapatite matrix (ABM)/cell-binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. J Periodontol 69:655–663 [DOI] [PubMed]
- 22.Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M (2000) Multi-center clinical comparison of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell-binding peptide (P-15) and ABM in human periodontal osseous defects: 6-month results. J Periodontol 71:1671–1679 [DOI] [PubMed]